Abstract
In order to design antimicrobial species, a series of methacrylate (Macr) complexes, [Cu(HBzIm)2(Macr)2] (1), [Cu2(HBzIm)2(Macr)4] (2), [Cu(2-MeBzIm)2(Macr)2] (3), [Cu2(2-MeBzIm)2(Macr)4] (4), and [Cu(5,6-Me2BzIm)2(Macr)2] (5) (HBzIm = benzimidazole, 2-MeBzIm = 2-methylbenzimidazole, and 5,6-Me2BzIm = 5,6-dimethylbenzimidazole) were synthesized and characterized by several spectral techniques, as well as by single crystal X-ray diffraction. The mononuclear species exhibit a distorted octahedral stereochemistry, while the binuclear types, with a paddle-wheel structure, adopt a square pyramidal surrounding. The methacrylate acts either as a chelate or a bridge, while all benzimidazole derivatives are coordinated as unidentate. The supramolecular networks are developed by both intermolecular π–π stacking interactions and hydrogen bonds. The antimicrobial assays provided both complexes the ability to inhibit planktonic strain proliferation, as well as to adhere on inert substratum. All complexes exhibit a moderate antimicrobial activity, both in regards to standard and clinical isolate strains, the most active being compound 5 against Candida albicans, with a minimum inhibitory concentration (MIC) of 0.156 mg/mL. It is worth mentioning that complex 1 inhibited the microbial adhesion of the clinical Escherichia coli strain and complex 2 constrained that of the clinical C. albicans strain.
1. Introduction
Benzimidazole is an organic compound formed by the fusion of a benzene ring with a five-membered imidazole unit. Due to its structure, it has become an interesting ligand for coordination chemistry, being incorporated into complexes, where it adopts a unidentate coordination mode through one nitrogen atom [1,2,3] or acts as a bridge between metallic ions through both nitrogen atoms of the imidazole ring [4].
Benzimidazole and its derivatives are subjects of research in the pharmaceutical industry, supported by their ability to mimic purines and induce biological responses [5]. Due to their antiviral, antimicrobial, antidiabetic, antifungal, and antitumor activities, they serve as building blocks for many clinical drugs [5,6,7,8]. Therefore, over the next decade, many new benzimidazole-based pharmaceutical products are expected to become available [9].
Among the reported plethora of benzimidazole or benzimidazole derivative complexes with biological applications [10], Cu(II) complexes containing such ligands stand out. For example, the complexes [Cu(HSal)2(HBzIm)2] (H2Sal = salicylic acid; HBzIm = benzimidazole) and [Cu(CH3COO)2(5,6-Me2BzIm)2] (5,6-Me2BzIm = 5,6-dimethylbenzimidazole) exhibit excellent superoxide dismutase (SOD) mimetic activity [11]. Considering that Cu(II) is an endogenous metal involved in many physiological processes, copper-based complexes are considered better alternatives for cancer treatment compared to non-essential metal-based drugs [12].
For instance, three Cu(II) complexes with benzimidazole-derived scaffolds, obtained from the condensation reaction between 2-aminobenzimidazole and o-vanillin with 1,10-phenanthroline/2,2′-bipyridyl, were tested for in vitro cytotoxicity against MCF-7 human breast cancer cell lines, proving to be promising candidates as antitumor drugs [13]. Furthermore, a novel Cu(II) complex, formulated as [Cu(BzImCF3)2(bipy)(ClO4)] (BzImCF3 = 1-(4-trifluoromethyl)benzyl-1H-benzimidazole), exhibited antiproliferative and apoptotic effects on prostate cancer (DU145) and mesothelioma cells (SPC212) [14]. Additionally, a series of Cu(II) complexes with 2-(2-pyridyl)benzimidazole and its derivatives of the type [Cu(Hpbz)Cl2] (Hpbz = 2-(2-pyridyl)benzimidazole) showed antitumor activity against adenocarcinoma alveolar basal epithelial cells (A549) [12].
Besides their biological activities, Cu(II) complexes with benzimidazole and different carboxylates often combine the coordination versatility of both ligands, yielding a wide variety of coordination modes and geometries, leading to interesting structural features. For example, in the polymeric complex [Cu(HBzIm)2(adp)(H2O)]∞ (H2adp = adipic acid), Cu(II) ions are seven-coordinated, adopting an elongated tetragonal pyramidal geometry with a double-capped base. In addition, a three-dimensional hydrogen bond system is formed through O···O and O···N interactions [15].
Other similar Cu(II) complexes with benzimidazole and dicarboxylates, such as [Cu(HBzIm)2(adc)(H2O)]∞ and [Cu(HBzIm)2(fum)(H2O)]∞ (H2adc = acetylene dicarboxylic acid; H2fum = fumaric acid), exhibit similar structures, where dicarboxylates act as bridges between metallic centers, while benzimidazole units act as monodentate ligands. An interesting aspect is the Cu···Cu separation of less than 10 Å, providing an opportunity to analyze the interactions between Cu(II) centers [16]. Furthermore, in the case of the polymeric Cu(II) complex [Cu(HBzIm)2(Hsal)2]n, π–π stacking interactions between benzimidazole rings of neighboring polymeric chains, which are connected via hydrogen bonds between benzimidazole and carboxylate groups, were observed [17].
Considering the importance of Cu(II) ions in biological systems and their versatility in forming different coordination geometries, as well as our ongoing interest in synthesizing new complexes using unsaturated carboxylates and azole-type ligands [18,19,20,21], we hereby report the synthesis and structural characterization of new methacrylate and benzimidazole derivative complexes: [Cu(HBzIm)2(Macr)2] (1), [Cu2(HBzIm)2(Macr)4] (2), [Cu(2-MeBzIm)2(Macr)2] (3), [Cu2(2-MeBzIm)2(Macr)4] (4), and [Cu(5,6-Me2BzIm)2(Macr)2] (5) (2-MeBzIm = 2-methylbenzimidazole, HMacr = methacrylic acid). Furthermore, this research aims to develop new species that incorporate both methacrylate and benzimidazole derivatives in order to modulate biological activity.
2. Results and Discussions
This paper presents the results obtained in the synthesis, physico-chemical, and biological characterization of five Cu(II) complexes with benzimidazole, 2-methylbenzimidazole, 5,6-dimethylbenzimidazole, and methacrylate anion. Although the structures of the two benzimidazole-containing compounds have been previously published [22], they are included in this article for the following reasons: (i) the mononuclear compound (1) has been reformulated as octahedral (unlike the previous paper, where it was considered square-planar); (ii) the formation of the dinuclear compound (2) demonstrates the tendency to obtain multiple species from the same reaction system, and (iii) new findings, specifically biological activity, are presented for both compounds.
2.1. Description of the X-Ray Crystal Structures of the Complexes
All five new compounds are mixed ligands complexes containing Cu(II), benzimidazole derivatives, and methacrylate anions. Crystallographic data for all five compounds are provided in Table S1, while the bond lengths and angles are displayed in Supplementary Tables S2–S11. There are some relationships and analogies of the crystal structures concerning non-covalent interactions and Cu(II) stereochemistry. Still, there is also some heterogeneity in the packing of the compounds due to the different benzimidazole derivatives. The main difference between the compounds comes from the axial positions, so the complexes can be grouped into two types of families.
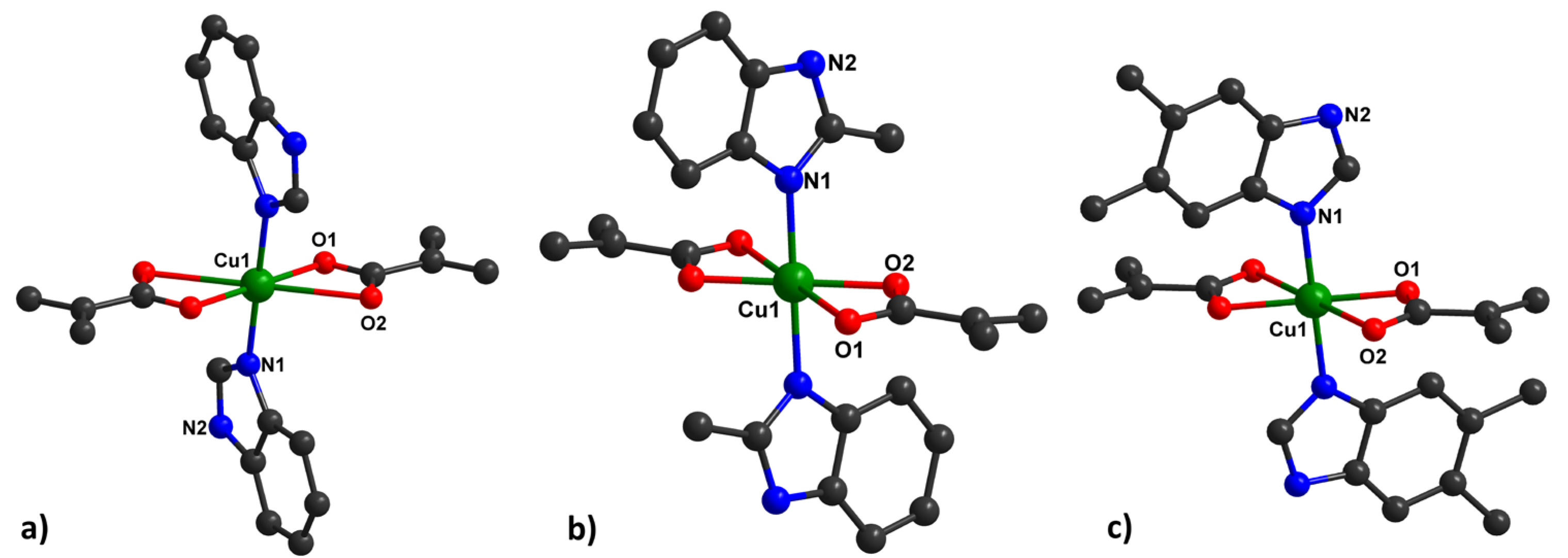
Type 1 of the complexes presents a slightly distorted octahedral geometry around the metal ion, containing benzimidazole derivatives in the axial positions, with a general formula of [Cu(HBzIm)2(Macr)2] (1) (Figure 1a), [Cu(2-MeBzIm)2(Macr)2] (3) (Figure 1b), and [Cu(5,6-Me2BzIm)2(Macr)2] (5) (Figure 1c).
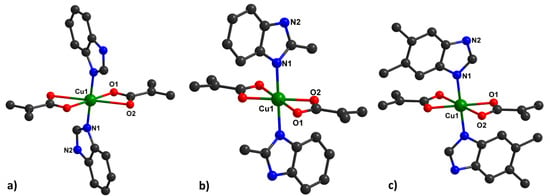
Figure 1.
Mononuclear units in 1, (a), 3, (b), and 5, (c), together with the numbering scheme.
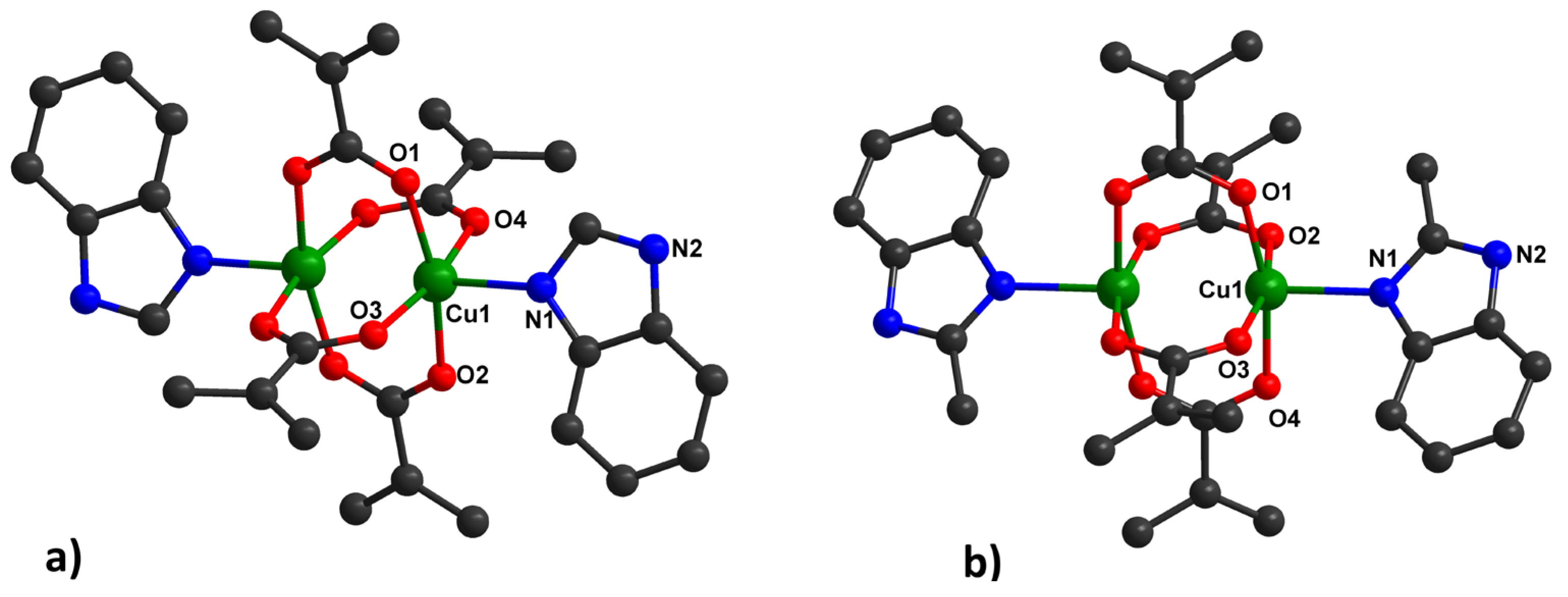
Type 2, [Cu2(HBzIm)2(Macr)4] (2) (Figure 2a) and [Cu2(2-MeBzIm)2(Macr)4] (4) (Figure 2b), appear as green crystals and adopt a binuclear paddle-wheel structure. The Cu(II) ion is pentacoordinated, exhibiting a square pyramidal stereochemistry (with a continuous shape measure (CShM) value of 0.321 for 2 and 0.441 for 4), arising from four bridging carboxylate groups and a nitrogen atom from the benzimidazole molecule in the apical position.
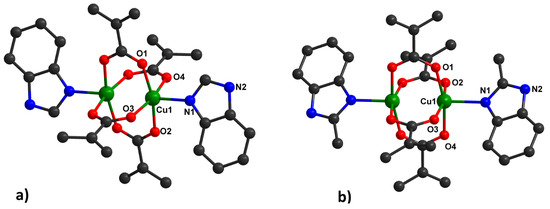
Figure 2.
Binuclear units in 2 (a) and 4 (b), together with the numbering scheme.
The Cu∙∙∙O distances in the equatorial positions are similar in all investigated compounds, but there are some differences in Cu∙∙∙O distances for type I of the complex species (Figure 1, Tables S2, S6, and S10). The Cu∙∙∙O distances vary between 1.9702(14)Å and 2.640(3)Å for methacrylate anions.
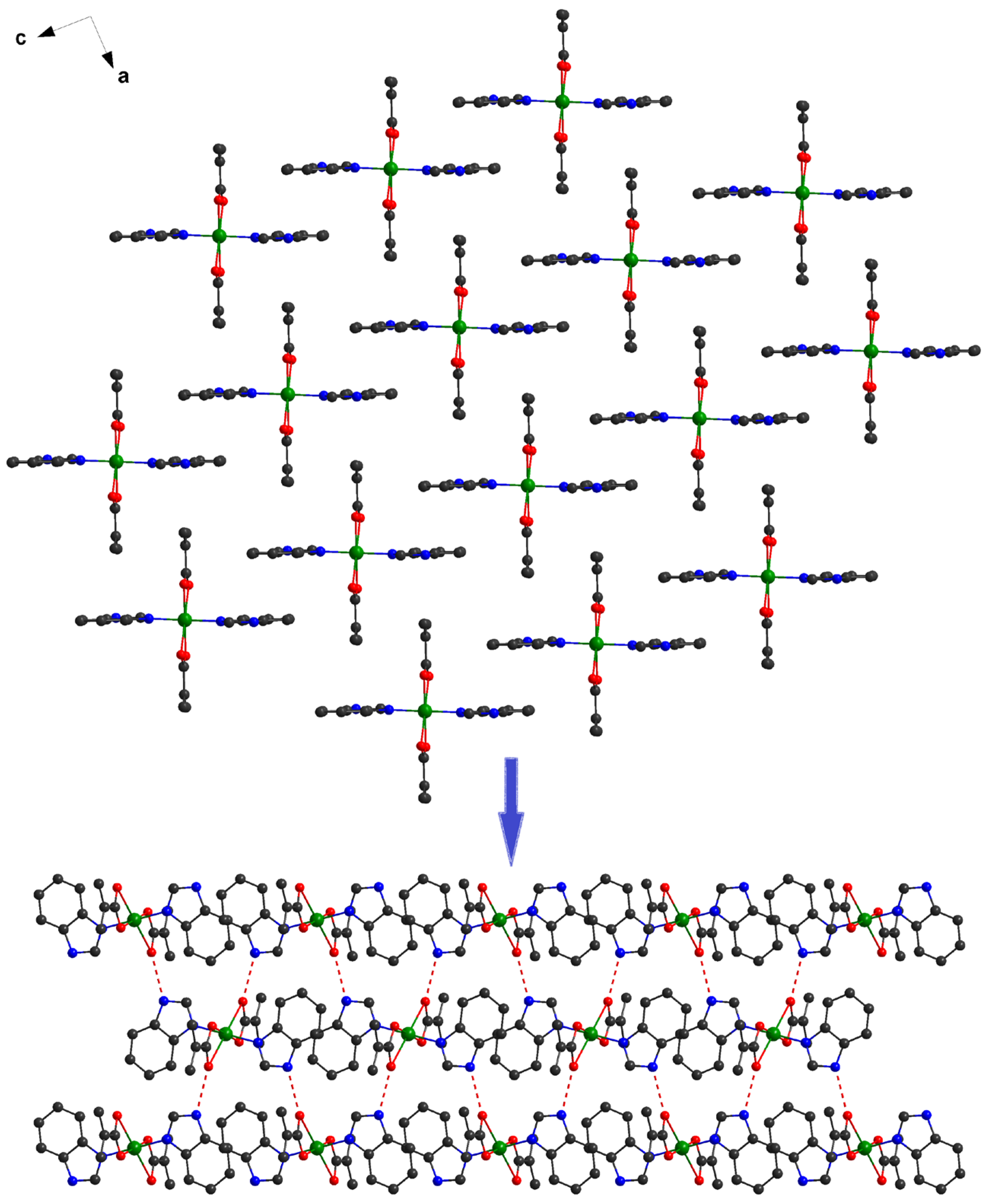
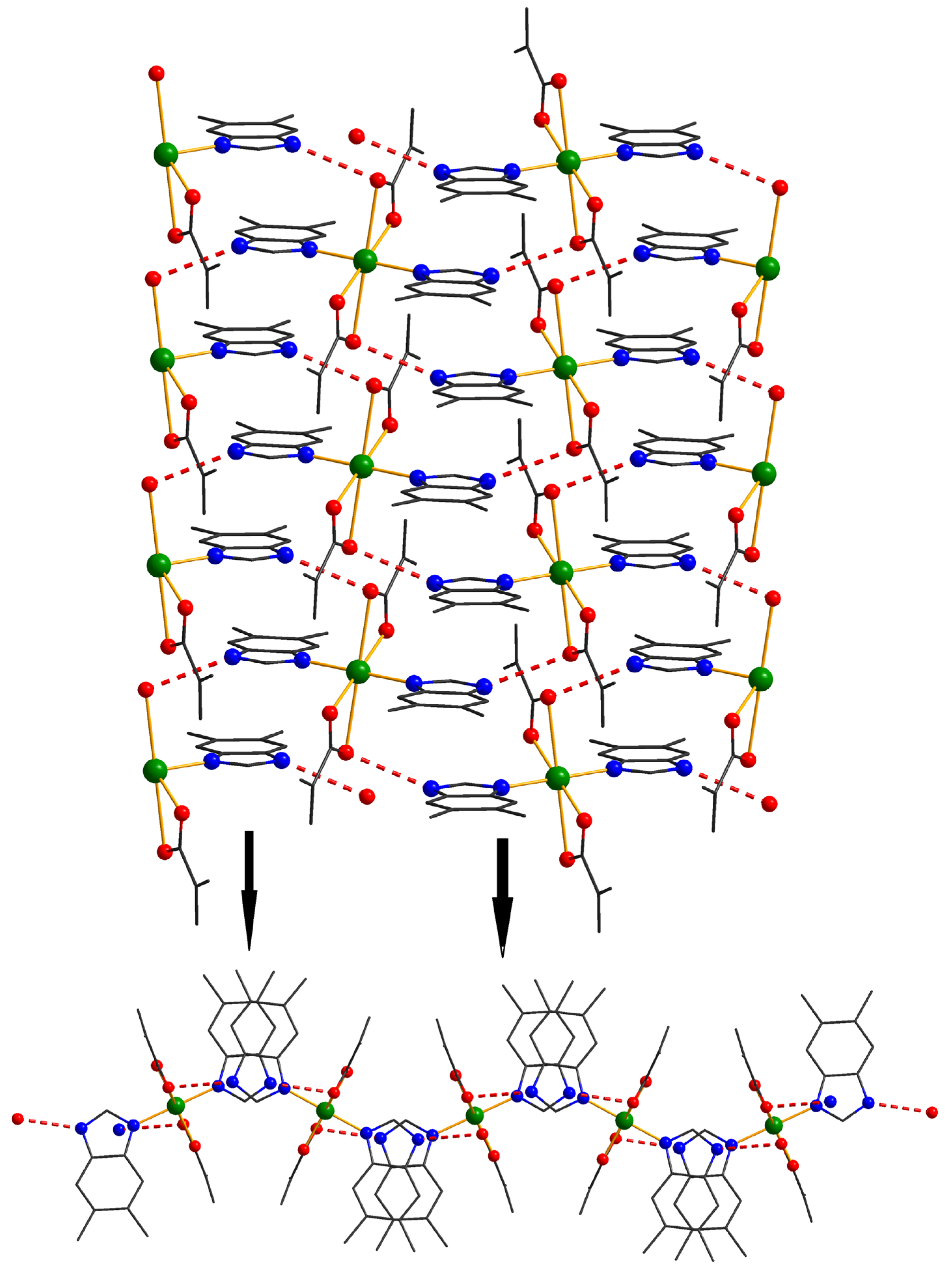
The investigation of the packing diagram for compound 1 reveals the formation of a supramolecular 2D network, although intermolecular π–π interactions (distances varying between 3.86 and 3.95 Å) were established between benzimidazole molecules. The additional 2D structure is expanded to the third direction by hydrogen bonds, which implies the presence of N-H groups from the benzimidazole residues and oxygens atoms from carboxylate anions (Figure 3).
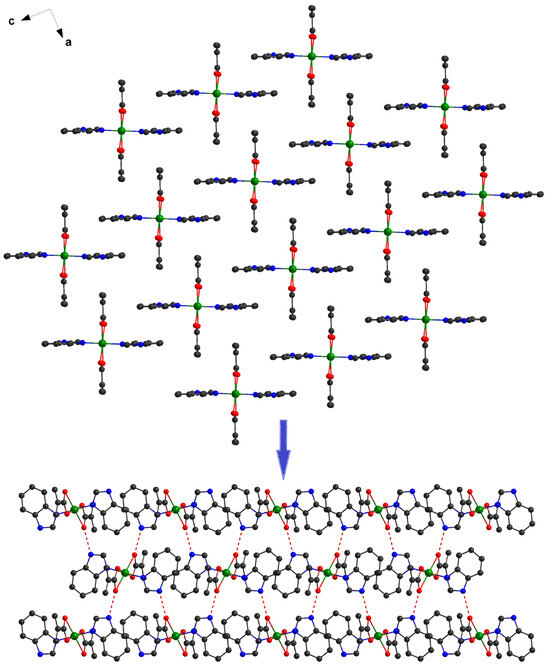
Figure 3.
Formation of the 3D supramolecular network in 1.
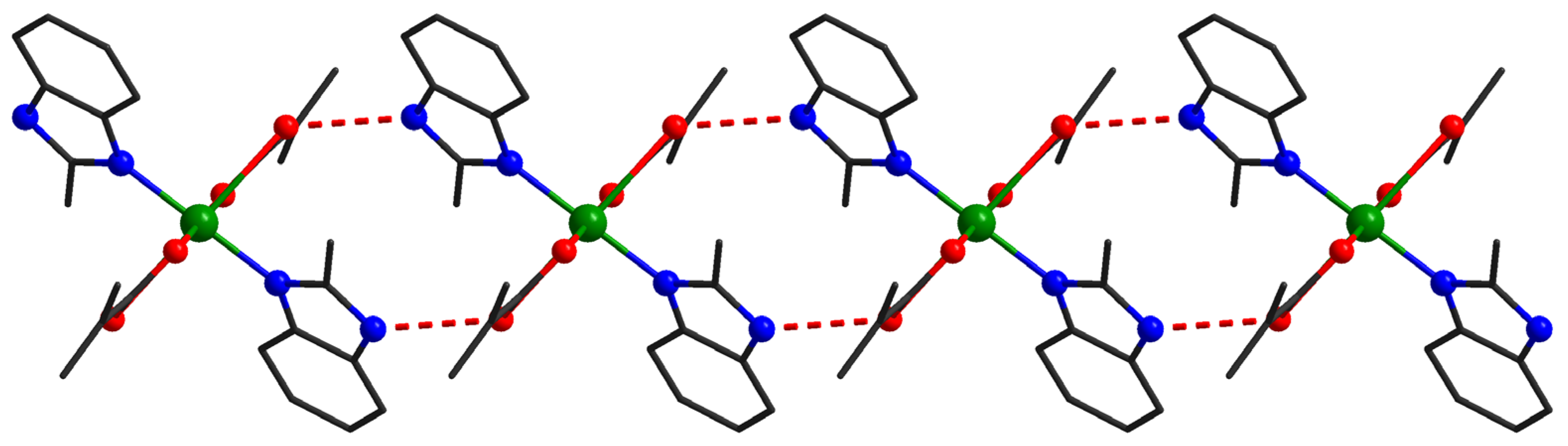
At the supramolecular level in the case of compound 3, the mononuclear units interact through hydrogen bonds established between N-H groups from 2-methylbenzimidazole and oxygen atoms from methacrylate ligands (O1–H1 = 1.963Å O1–N2 = 2.761Å, N2-H2-O1 = 153.65°). The resulting supramolecular chains run along the c crystallographic axis (Figure 4).
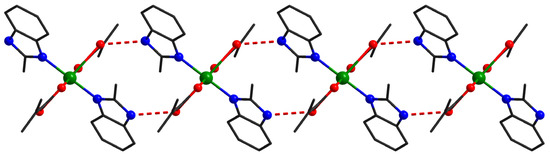
Figure 4.
Supramolecular chains in compound 3.
For compound 5 (Figure 5), the formation of bidimensional crystal structures was observed, driven by hydrogen bonding interactions between the N-H groups of the benzimidazole moieties and the oxygen atoms of the methacrylate ligands, as well as π–π interactions between the benzimidazole aromatic rings.
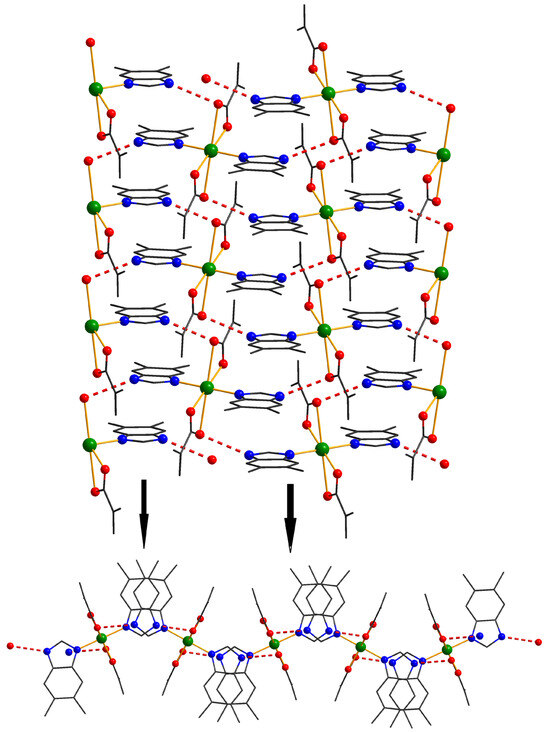
Figure 5.
View of a 1D motif in crystal 5 and perspective view of the layers resulting from connecting the chains via benzimidazol bridges.
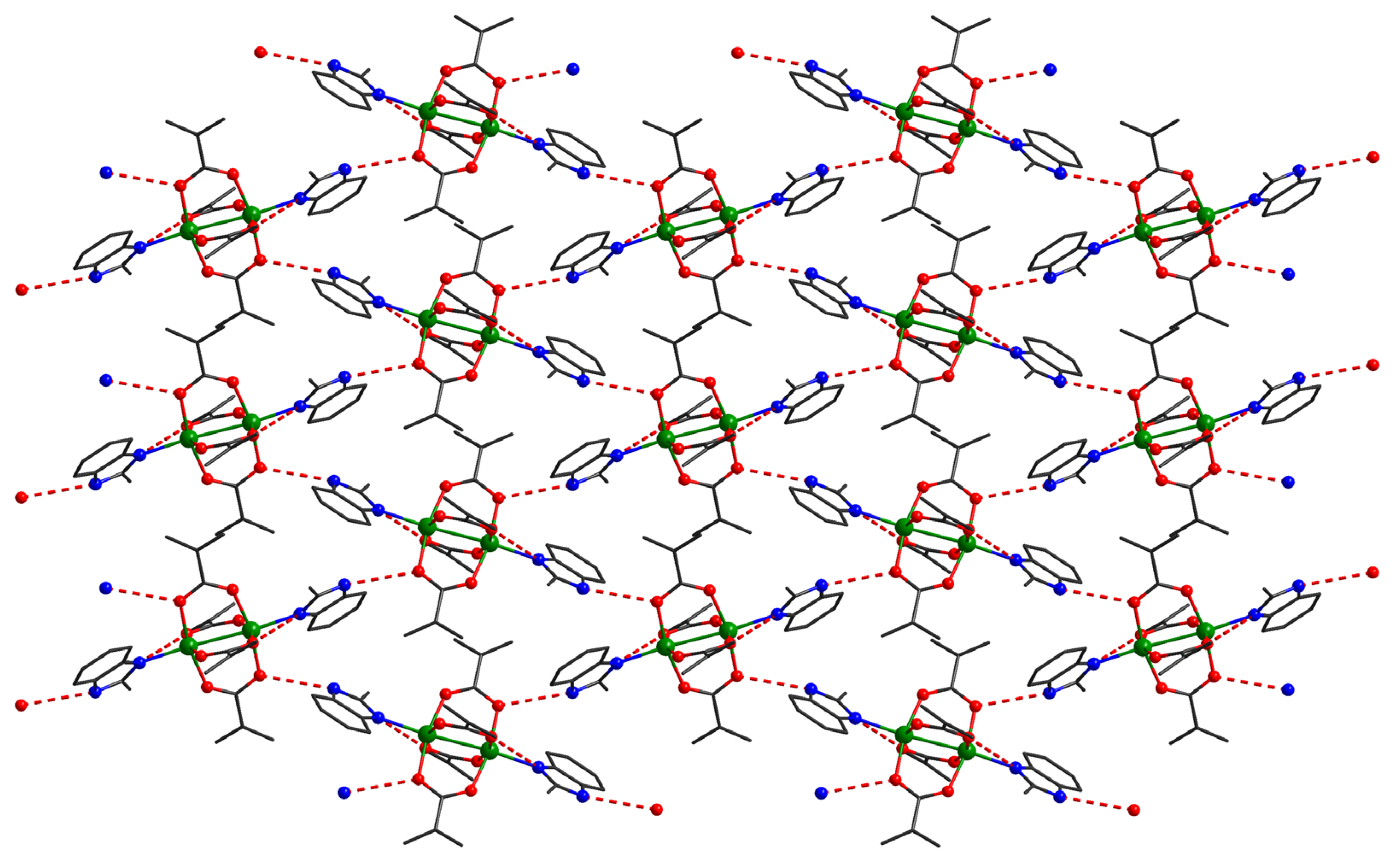
In the case of compound 4, the binuclear units act as four connected nodes in the supramolecular herringbone structure (Figure 6). These dimers interact through four hydrogen bonds interactions: two oxygen atoms from the carboxylate anion act as acceptors and two nitrogen atoms from benzimidazole derivatives act as donors for hydrogen bond interactions.
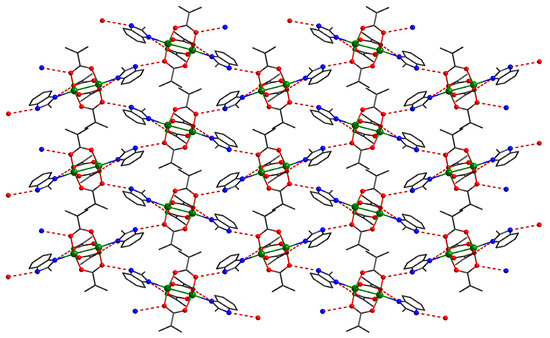
Figure 6.
Detail of a supramolecular layer in crystal 4, assembled from neutral units through hydrogen interactions.
2.2. Spectral Characterization
2.2.1. Fourier Transform Infrared Spectroscopy
The IR spectra of the complexes were compared with those of benzimidazole derivatives, sodium methacrylate, and Cu(II) methacrylate. The most important bands and their assignments are presented in Table 1. A common feature of all the complex spectra is the presence of characteristic bands of benzimidazole derivatives. Moreover, the characteristic stretching vibration band of the C=N bond is shifted to higher wavenumbers in the complex spectra compared to those of the free ligands, indicating their coordination through the imine nitrogen atom.

Table 1.
IR absorption bands (cm−1) for benzimidazole derivatives and complexes.
For the carboxylate anion, the coordination mode can be established, according to the literature [23,24], based on the parameter Δ = νas − νs of the free ion, which, in the case of sodium methacrylate, is 136 cm−1. If this value is lower in the complexes, the carboxylate anion is coordinated in a bidentate manner. This behavior was observed for the mononuclear compounds 1, 3, and 5 (Figure S1). However, the binuclear compounds 2 and 4 do not follow this rule, as the Δ parameter value is higher than that of the free ion. To confirm the paddle-wheel structure, with bridging methacrylate anions, the spectra of the complexes were compared with that of copper methacrylate, [Cu2(Macr)4(H2O)2] (CuMacr) (Figure S2). A good agreement was observed regarding the position and intensity of the characteristic stretching vibration bands of these anions. Moreover, a literature survey concerning paddle-wheel Cu(II) complexes [25,26,27] indicated similar values for the Δ parameter as those found in the case of compounds 2 and 4. As a result, this characteristic appears to be a common pattern for copper complexes with a paddle-wheel structure, regardless of the nature of the carboxylate anions.
2.2.2. Electronic Spectroscopy
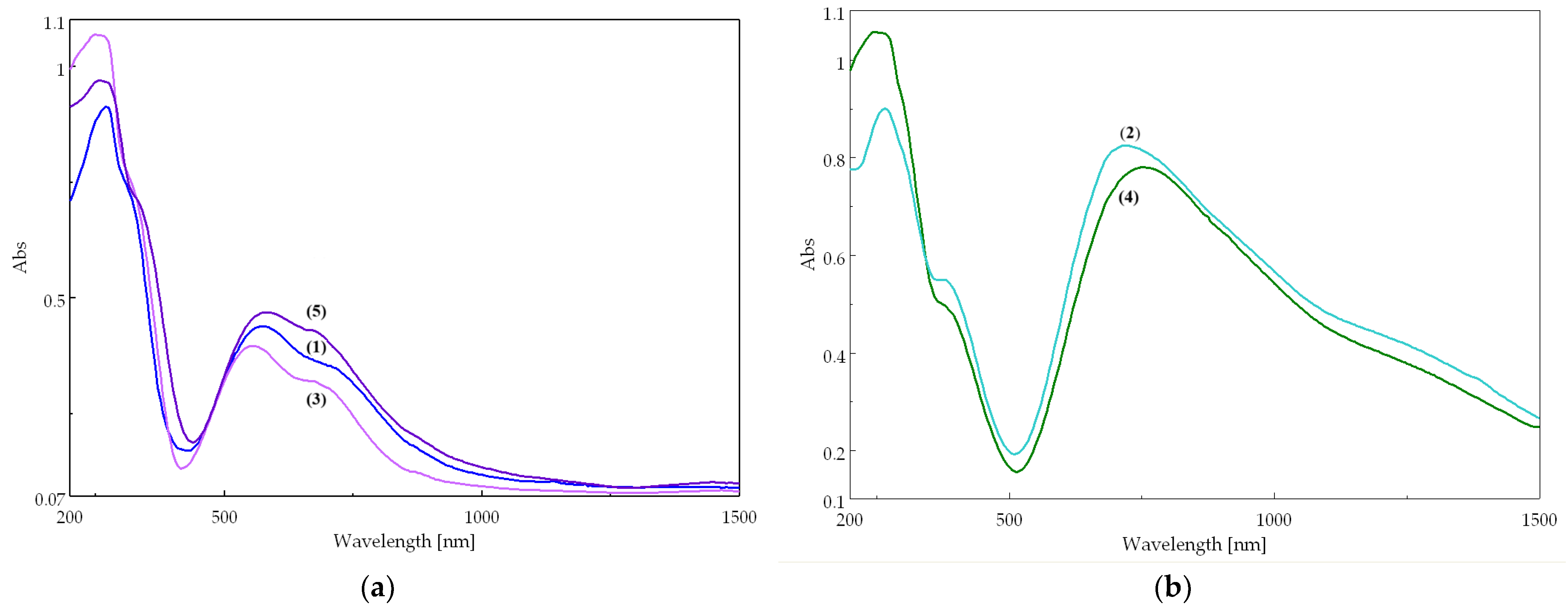
The two types of complexes display different electronic spectra (Figure 7). The mononuclear (purple) compounds 1, 3, and 5 (Figure 7a) exhibit two characteristic bands corresponding to the d–d transitions of the Cu(II) ion in an octahedral stereochemistry, tetragonal distorted (Table S12). The binuclear (green) compounds 2 and 4 display a broad and asymmetric band, shifted to higher wavenumbers, characteristic of a square pyramidal stereochemistry (Figure 7b) [28]. The different colors of the two types of compounds is due to their distinct stereochemistries, which determine different crystal field splitting parameters. Consequently, the bands in the electronic spectra appear at different wavelengths, leading to different colors.
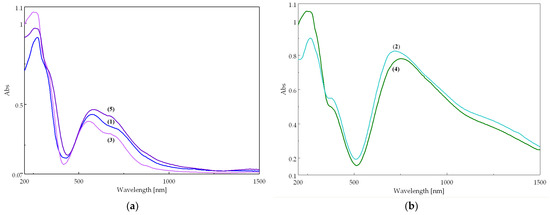
Figure 7.
Electronic spectra of complexes: (a) mononuclear compounds 1, 3, and 5; (b) binuclear compounds 2 and 4.
Since the antimicrobial assay was performed in DMSO solutions, the stability of the complexes in this solvent was evaluated using the same technique. Notably, no significant changes were observed, indicating that all compound solutions remain stable for at least 48 h (Supplementary Figure S3). The solution spectra are similar for all complexes, with the absorption maxima shifting to higher wavelengths in every case. This shift may result from DMSO coordination, accompanied by a change in the coordination mode of the methacrylate ion.
2.3. Evaluation of the Antimicrobial Activity Complexes
The discovery of effective remedies against resistant infections requires the investigation of novel complexes with antimicrobial properties. The chemical characteristics and structure of these compounds have a direct impact on their efficacy, and improving these factors can lead to the creation of novel antimicrobial treatments. Cu(II) species [29], methacrylate [30], and benzimidazole [31] exhibit great potential in medicine due to their biological activities as individual therapeutic agents.
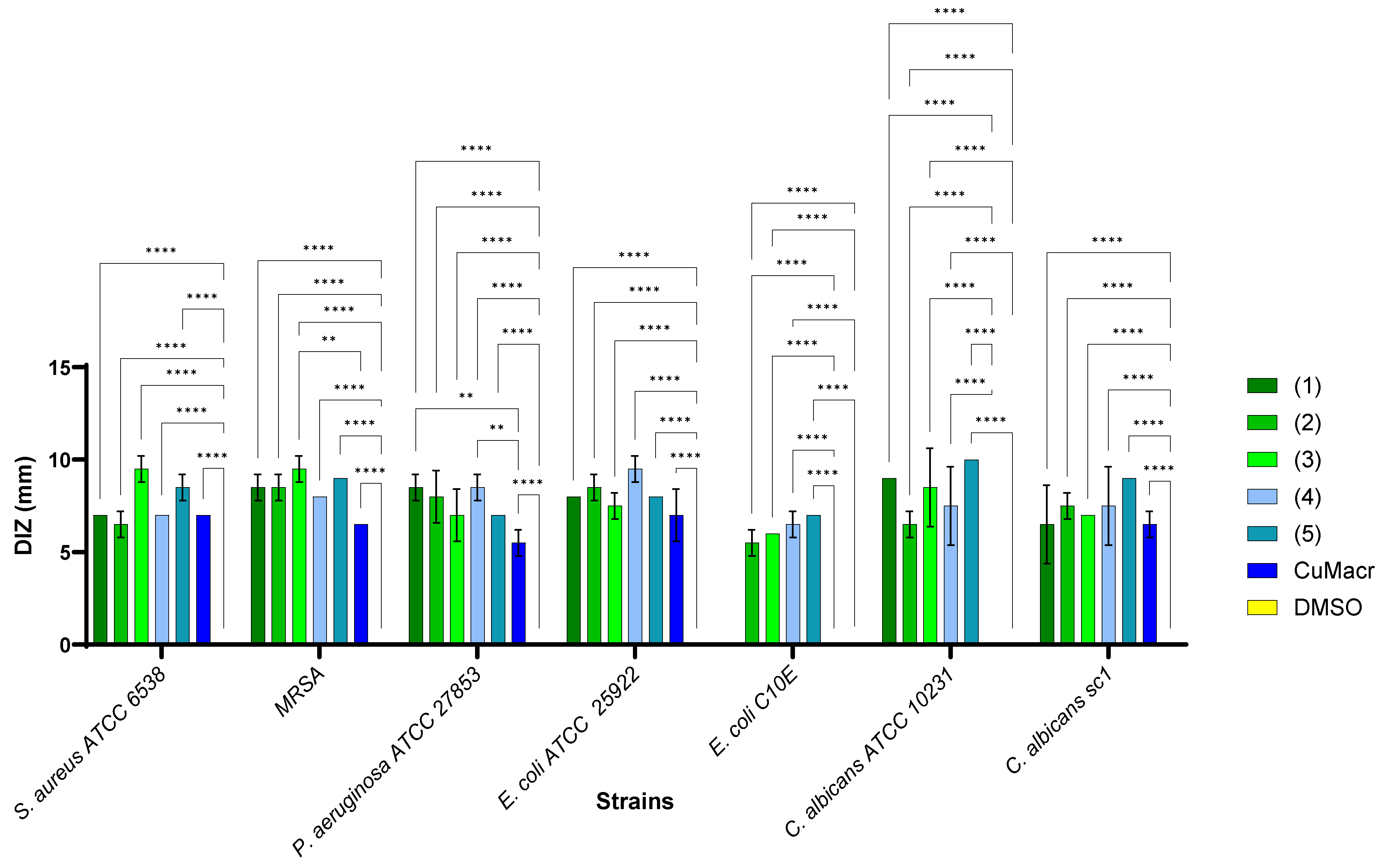
The studied complexes 1–5 exhibit a different spectrum of antimicrobial activity, depending on the chemical structure and the tested microbial strains (Figure 8). The solvent control (DMSO, yellow) did not show any antimicrobial activity for any strain, which implies that the qualitative antibacterial activity was due to the tested compounds and not the solvent. The raw material (CuMacr, blue) generated inhibition zones for most strains, but with smaller diameters compared to those of the synthesized compounds. The differences compared to the results for Cu(II) methacrylate proved to be significant, especially for compound 3 against MRSA (p < 0.01), compounds 1 and 4 against P. aeruginosa (p < 0.01), as well as for all compounds in the case of E. coli C10E and C. albicans ATCC 10231 (p < 0.0001).
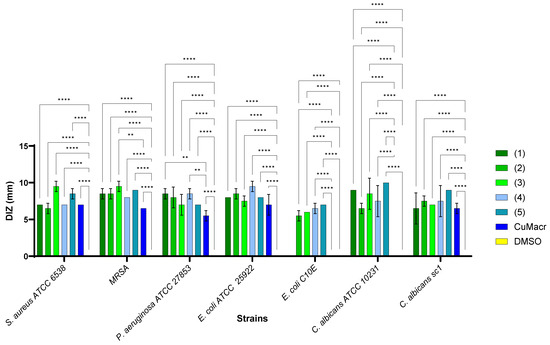
Figure 8.
Comparative qualitative evaluation (diameter of inhibition zone (DIZ)) of antimicrobial activity between complexes 1 ÷ 5, and Cu(II) methacrylate/solvent against microbial strains (** p < 0.01, **** p < 0.0001).
There are significant differences between the tested compounds, suggesting that some of them have a greater potential against certain microorganisms. Thus, compound 5, a mononuclear compound, demonstrated the best activity against C. albicans, while compound 3, also mononuclear, exhibited superior efficacy against Gram-positive bacteria. On the other hand, compound 4, a binuclear compound, proved to be more active against Gram-negative bacteria.
This difference in activity can be explained by the specific interactions of the compounds with microbial cellular structures. C. albicans displays a membrane rich in ergosterol, which allows [Cu(5,6-Me2BzIm)2(Macr)2] (5) to diffuse more efficiently, affect membrane permeability, and induce oxidative stress, respectively [32,33]. The [Cu(2-MeBzIm)2(Macr)2] (3) complex has proven to be more active against Gram-positive bacteria strains; the result correlates with the effect noted for 2-methyl-benzimidazole [34].
On the other hand, Gram-negative bacteria (E. coli, P. aeruginosa) exhibit an outer membrane composed of lipopolysaccharides (LPS), which gives them increased resistance to antimicrobial agents [35].
Thus, the mechanism of action of the compounds is closely related to their molecular structure and the physiological characteristics of each microbial strain, which explains the variations observed in regards to antimicrobial activity. Moreover, in the diffusion method, the physico-chemical characteristics of the compounds significantly influence diffusion in agar medium and implicitly, the evaluation of antimicrobial activity [36]. Thus, the quantitative antimicrobial activity was further evaluated.
The minimal inhibitory concentration (MIC) represents the lowest concentration of a compound that inhibits 90% of the visible growth of a microorganism. Lower MIC values indicate stronger antimicrobial activity. From Table 2, it can be observed that compound 5 is the most active, displaying a broad spectrum against S. aureus ATCC 25923 (0.313 mg/mL), MRSA (0.313 mg/mL), C. albicans ATCC 10231 (0.156 mg/mL), C. albicans sc1 (0.156 mg/mL), E. coli ATCC 25922 (0.625 mg/mL), and E. coli C10E (0.625 mg/mL).

Table 2.
The quantitative evaluation of antimicrobial activity expressed by the minimum inhibitory concentration (MIC), the minimum microbicidal concentration (MMC), and the minimum concentration for inhibiting microbial adhesion (MBEC).
Compounds 1–4 exhibit similar activity; for Gram-positive bacteria, they were active at MIC values of 1.25 mg/mL, but they showed somewhat greater activity against P. aeruginosa, which was due to the solvent utilized. However, they were substantially more effective against C. albicans (MIC = 0.625–0.313 mg/mL). DMSO demonstrated lesser antibacterial activity than that of the studied complexes (MIC ≥ 1.25 mg/mL), suggesting that the observed effects came from the tested compounds, except for the Gram-negative bacterial strains. The raw material CuMacr exhibits weaker activity than that of the tested compounds in the case of 1 against S. aureus and C. albicans sc1, 2 against S. aureus and C. albicans, and 3 and 4 against only S. aureus strains. Compound 5 proved to be the most active against yeast (C. albicans), with an MIC value of 0.156 mg/mL.
The study of the minimum microbicidal concentration (MMC) is essential for assessing new anti-infective drugs and developing new disinfectants. This is the lowest concentration of an antimicrobial agent that will fully eradicate a microorganism. Complex 5 exhibited the best microbicidal impact on most bacteria at a dose of 0.625 mg/mL, with the exception of the P. aeruginosa strain. From Table 2, it can be observed that for complexes 1 and 2 against the S. aureus ATCC 25923, and 3 against the P. aeruginosa, the microbicidal effect was provided by the solvent. In the case of the E. coli, P. aeruginosa, and C. albicans, the effect of complexes 1 ÷ 4 was similar to CuMacr.
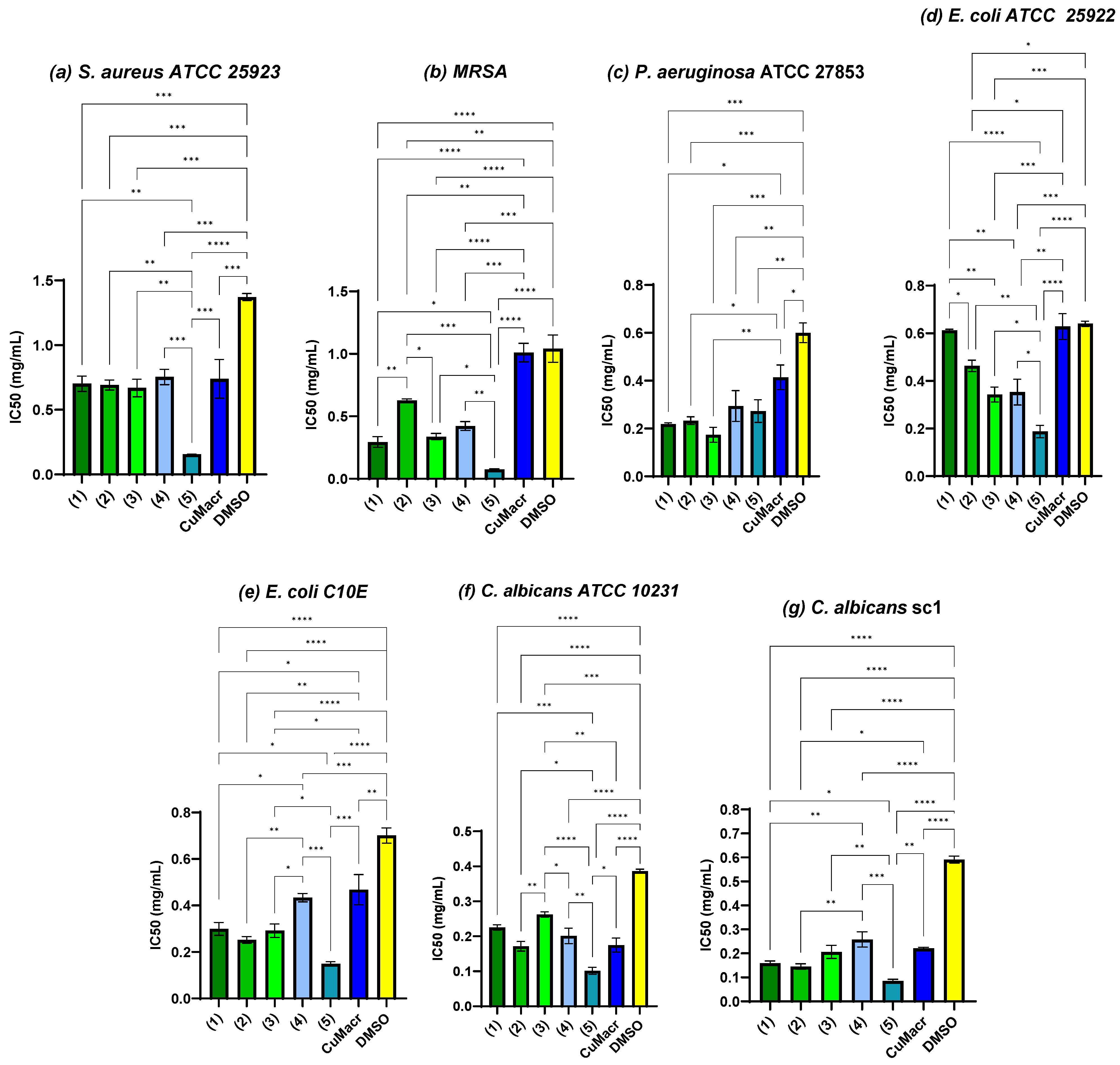
To evaluate the improvement in antimicrobial activity, statistical analysis was performed concerning the raw material (CuMacr) and the solvent used, and the half maximal inhibitory concentration (IC50) was determined for each variant. Figure 9a shows that compounds 1 ÷ 4 exhibited IC50 values against S. aureus, similar to those of the CuMacr (p > 0.05), and were more active than was DMSO. The antimicrobial activity trend for MRSA was 5 > 1 > 3 > 4 > 2. All complexes showed considerably decreased IC50 values relative to those of CuMacr and DMSO (p < 0.01, Figure 9b), even though the determination of the MIC values did not show a change compared to the solvent control in the case of the P. aeruginosa strain. From Figure 9c, it can be observed that all the tested compounds significantly reduced microbial viability; thus, the IC50 value was significantly lower compared to that of DMSO (p < 0.0001 for compounds 1, 2, and 3, and p < 0.01 for compounds 4 and 5). The lowest IC50 value against P. aeruginosa was for complex 3 (p < 0.001 compared to the solvent); however, the differences were not significant compared to the results for complexes 1 and 2 (p > 0.05). From Figure 9d, it can be observed that complex 5 shows the lowest IC50 value against E. coli, followed by complexes 3 and 4, with moderate activity, while complexes 1 and 2 exhibit the weakest activity, probably due to the HBzIm ligand, which is more polar and reduces diffusion through the cell membranes [37,38].
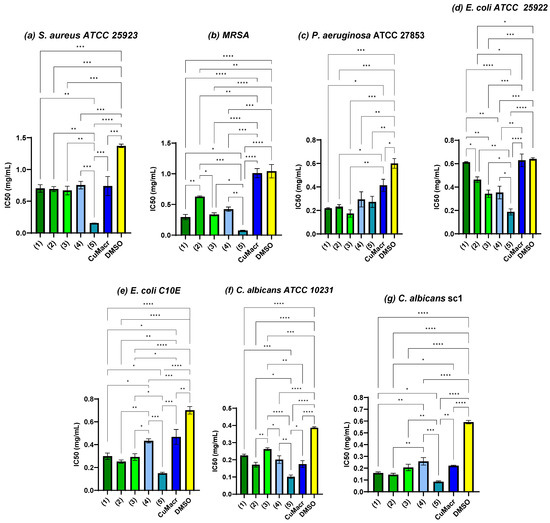
Figure 9.
Antimicrobial activity expressed as IC50 and the correlation between samples and methacrylate/solvent against S. aureus ATCC 25923 (a), MRSA (b), P. aeruginosa ATCC 27853 (c), E. coli ATCC 25922 (d), E. coli C10E (e), C. albicans ATCC 10231 (f), and C. albicans sc1 (g) strains (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
The E. coli C10E strain proved to be more sensitive when compared to the reference type (Figure 9e). Complex 4 was found to exhibit reduced activity compared to the other complexes, with an insignificant value compared to that of CuMacr (p > 0.05). This significantly different effect compared to that of complex 5 may be due to the fact that the lipophilicity was lower (with only one methyl group) and the complex is binuclear, which makes diffusion through the membrane more difficult [39]. In the case of yeast strains (Figure 9f,g), it is observed that the clinical strain was more sensitive than the reference strain to the tested complexes, except for compound 4, for which the IC50 value was 0.20 ± 0.02 mg/mL for the reference strain and 0.25 ± 0.03 mg/mL for the clinical type.
Compound 5 displays the lowest IC50 value, being the most active against S. aureus (Figure 9a,b), E. coli (Figure 9d,e), and C. albicans (Figure 9f,g), probably due to the methyl substituents in positions 5 and 6, which leads to increased lipophilicity and stability, favoring interaction with the cell membranes, increasing the complex’s ability to cross the bacterial cell wall or fungal membrane, but it may also influence the bioavailability and the ability to release the Cu(II) ion [40,41]. For a detailed statistical analysis of the IC50 values for each microbial strain, Tukey’s multiple comparison test was performed, and the results are presented in Table S13 in the Supplementary Material. Mononuclear complexes, characterized by distorted octahedral geometry and Cu(II) coordination by benzimidazole derivatives and methacrylate anions, exhibit moderate IC50 values, with complex 5 being the most active. The higher antimicrobial activity of complex 5 for all tested microorganisms may be attributed to the chelation theory and the effect of hypertonic conditions on cellular permeability [42]. Chelation reduces the polarity of the copper ion and increases the delocalization of the π electrons, allowing the complexes to penetrate the lipid layer of the cell membrane [41,43,44]. Moreover, the presence of methyl groups at positions 5 and 6 increases the lipophilicity of the compound. The difference in the activity of various copper complexes against different microorganisms depends on cell permeability or variations in the ribosomes of microbial cells [45]. Factors such as the chelation effect of ligands, donor atoms, the nature of the metal ion, and the stereochemistry of the complex increase the hydrophobic character and liposolubility of the molecule, favoring its permeability through the microbial cell membrane [46,47]. Binuclear complexes with square pyramidal coordination enhance redox activity, reducing reactive oxygen species and thereby generating lower activity against microbial cells [48,49]. Bimetallic complexes with methacrylate bridges can facilitate electron transfer between the two metal centers, increasing antioxidant efficiency [50].
The impact of the newly synthesized compounds on microbial adhesion, the first stage of biofilm growth [51], was also evaluated. The minimum concentration values for inhibiting microbial adhesion (MBEC) are presented in Table 2. Complexes with MBEC values lower than their MIC values were considered effective in inhibiting microbial adhesion. However, a few variants meet this criterion: complex 1 inhibited the microbial adhesion of clinical E. coli strain (0.625 mg/mL) and complex 2 of clinical C. albicans strain (0.156 mg/mL), while the other complexes showed MBEC values similar to those of MIC. This finding implies that the compounds most likely impair microbial adherence by interfering with cellular survival rather than targeting a specific adhesion pathway. The type of Cu(II) coordination and the nature of the ligand can influence the interaction with the microbial cell surface. Thus, mononuclear complexes are more mobile and can penetrate Gram-negative bacteria more easily, affecting membrane structure and adhesion [52,53], while binuclear complexes are more stable, interacting with the fungal cell wall, blocking adhesion proteins, and limiting access to essential metals [54,55].
Dimethyl sulfoxide (DMSO) is a versatile solvent used in pharmaceutical industry for cryopreservation and drug delivery [56]. Despite being believed to be harmless, small doses can influence cellular macromolecules and gene expression. According to studies [57,58,59], DMSO affects bacterial cells by influencing DNA architecture, gene transcription, and altering membrane potential. This underscores the necessity of using it as a solvent control in all biological tests. While DMSO does not exert substantial antibacterial effects at doses commonly employed in laboratories, research has demonstrated that it can alter the efficiency of antimicrobial drugs [60]. For example, DMSO has been demonstrated to limit the efficiency of antibiotics, including ampicillin, kanamycin, and quinolones, by shielding bacteria against ROS-mediated programmed cell death [61].
2.4. Biocompatibility
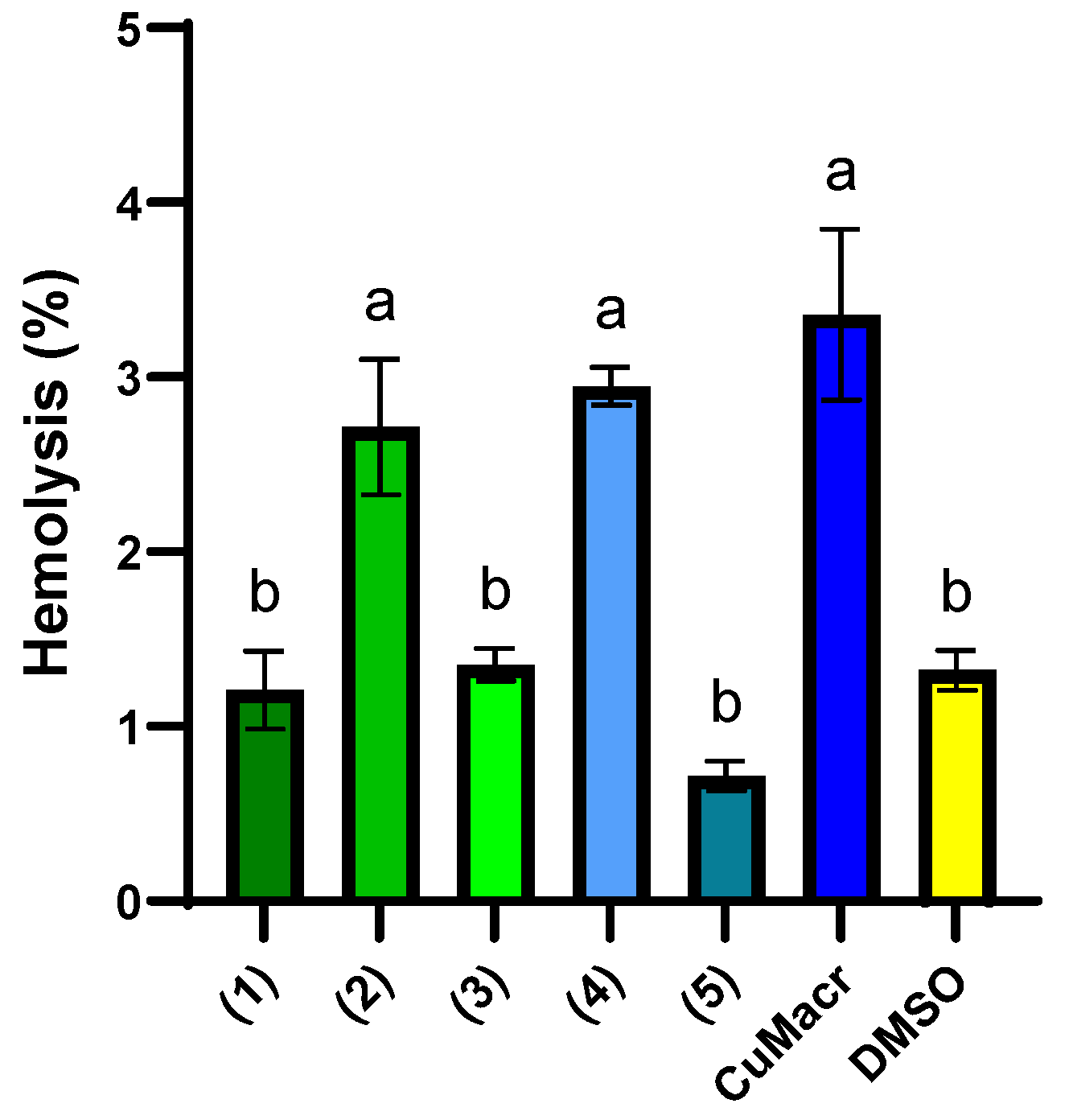
Biocompatibility testing is critical for evaluating materials and newly synthesized compounds for medical usage, guaranteeing their safety [62]. Hemocompatibility, or how chemicals interact with erythrocytes, is also important. Hemolysis is a hemocompatibility evaluation method that evaluates a substance’s potential to break down erythrocytes and release hemoglobin into the blood plasma. Hemolysis tests provide information about a compound’s safety and biocompatibility prior to pre-clinical usage by testing its ability to impair the integrity of blood cells. Compounds with a hemolytic index between 0–2% are considered non-hemolytic, while compounds with a hemolytic capacity between 2–10% are often considered mildly hemolytic [63].
In Figure 10, the percentages of hemolysis induced by the investigated Cu(II) complexes are presented, both in comparison to each other and to CuMacr and DMSO. The complexes 1, 3, and 5 display a low hemolysis percentage (~1%) that is similar to that of the solvent used (p > 0.05), indicating good biocompatibility. DMSO exhibits low hemolysis (1.32 ± 0.12%), which confirms that the solvent does not significantly contribute to the observed effects. Complexes 2 and 4 exhibit higher hemolysis (~3%), similar to that of Cu(Macr), which suggests a more aggressive interaction with erythrocyte membranes, but with values below 5%, in accordance with the threshold set for medical devices [64].
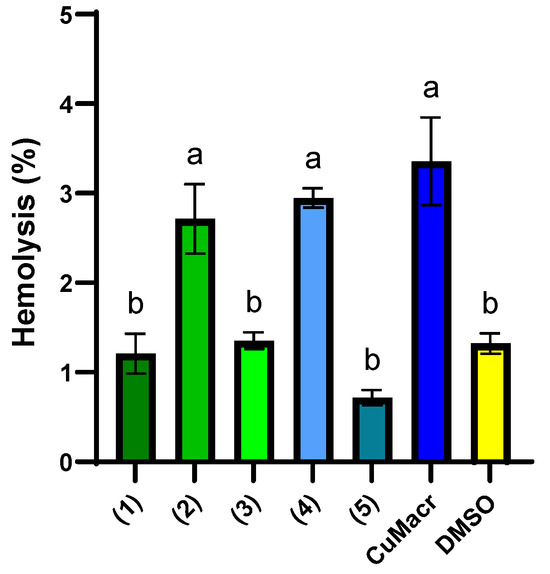
Figure 10.
Hemolysis (%) induced by 5 mg/mL stock solution of each compound in DMSO. Distinct letters (a, b) placed above the bars in the graph indicate significant differences between samples (p < 0.05). The presented data represent the average of three independent experiments (n = 3), and the error bars illustrate the standard deviation of the means.
The binuclear complexes 2 and 4 exhibit increased hemolysis, although the differences between the two compounds are not statistically significant (p > 0.05). Mononuclear complexes 1, 3, and 5 showed considerably lower hemolysis than did the binuclear complexes, which might be explained by their more compact structure and minimal contact with cells. Cu(Macr) exhibits the maximum hemolysis (3.36 ± 0.49%), indicating that complexation with benzimidazole ligands decreases toxicity.
3. Materials and Methods
3.1. General Information
High purity reagents were purchased and used without further purification from Merk Schuchardt OHG (Hohenbrunn, Germany, methacrylic acid), Fluka (Saint Louis, MO, USA, CuCO3·Cu(OH)2), and Sigma-Aldrich (Saint Louis, MO, USA, benzimidazole derivatives).
The content of carbon, nitrogen, and hydrogen was determined using a PE 2400 analyser (Perkin Elmer, Waltham, MA, USA). FTIR spectra were recorded in KBr pellets with a Tensor 37 spectrometer (Bruker, Billerica, MA, USA) in the range of 400–4000 cm−1. Electronic spectra were recorded on solid samples (diffuse reflectance technique) in the range of 200–1500 nm on a V670 spectrophotometer (Jasco, Easton, MD, USA), using Spectralon as a standard. The DMSO solution UV–VIS spectra were recorded on a Jasco V530 spectrophotometer (Jasco, Easton, MD, USA) in the range of 400–800 nm. The solution concentration for each complex was 10−3 M.
X-ray diffraction data for the crystals of compounds 1 and 3 were collected at 293 K on a STOE IPDS II diffractometer (STOE, Darmstadt, Germany) using a graphite-monochromator Mo Kα radiation source (λ = 0.71073 Å). For compounds 2, 4, and 5, data were collected at 293 K on a Rigaku XtaLAB Synergy S device (Rigaku, Wroclaw, Poland), with a single source at offset/far, and a HyPix diffractometer using a graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å). The structure was solved using direct methods and refined via full-matrix least squares techniques, based on F2. The non-H atoms were refined using anisotropic displacement parameters. Calculations were performed using the SHELX-2018 crystallographic software package. A summary of the crystallographic data and the structure refinement are presented in Supplementary Table S1. The crystallographic data (excluding structure factors) have been deposited with the Cambridge Crystallographic Data Center, with CCDC reference numbers 2422541–2422545. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk (accessed on 17 February 2025), or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: (+44)-1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. The XRPD patterns were recorded on a PROTO AXRD Benchtop Powder Diffractometer (CuKα radiation, Proto Manufacturing Ltd., Windsor, ON, Canada).
3.2. Synthesis of Complexes
First, copper methacrylate was synthesized from basic copper (II) carbonate and methacrylic acid in a methanolic solution, as described in reference [21].
All complexes have been synthesized by the interface slow diffusion method using three layers. The syntheses were performed in two systems, as follows:
- The first layer (from down to up) consists of a 10 mL methanol + ethanol solution (1:1 in volume) containing copper metacrylate (0.10 g), the second one of a 10 mL ethanol, and the last one of an 8 mL methanol solution of benzimidazole derivatives (0.15 g).
- The first layer (from down to up) consists of a 10 mL methanol solution containing copper metacrylate (0.10 g), the second one of a 10 mL methanol, and the last of an 8 mL methanol solution of benzimidazole derivatives (0.15 g).
After four weeks, crystals suitable for X-ray analysis were obtained as follows:
- From system I, a mixture of green and purple crystals were obtained in the case of benzimidazole and 2-methylbenzimidazole (with more green crystals obtained in the middle of the tube, while the purple crystals were concentrated in the lower part). These were separated mechanically. In case of 5,6-dimethylbenzimidazole, solely violet crystals were obtained.
- From system II, only purple crystals were obtained for all benzimidazole derivatives.
- [Cu(HBzIm)2(Macr)2] (1) (purple crystals). Anal. Calc. for Cu0.5C11H11N2O2: Cu, 13.52; C, 56.22; H, 4.72; N, 11.92; Found: Cu, 13.58; C, 56.19; H, 4.78; N, 12.02.
- [Cu2(HBzIm)2(Macr)4] (2) (green crystals); Anal. Calc. for CuC15H16N2O4: Cu, 18.06; C, 51.20; H, 4.58; N, 7.96; Found: Cu, 18.11; C, 51.16; H, 4.61; N, 8.04.
- [Cu(2-MeBzIm)2(Macr)2] (3) (purple crystals). Anal. Calc. for Cu0.5C12H13N2O2: Cu, 12.76; C, 57.88; H, 5.26; N, 11.25; Found: Cu, 12.81; C, 57.84; H, 5.31; N, 11.31.
- [Cu2(2-MeBzIm)2(Macr)4] (4) (green crystals). Anal. Calc. for CuC16H18N2O4: Cu, 17.37; C, 52.52; H, 4.96; N, 7.66; Found: Cu, 17.43; C, 52.58; H, 5.05; N, 7.73.
- [Cu(5,6-Me2BzIm)2(Macr)2] (5) (purple crystals). Anal. Calc. for Cu0.5C13H15N2O2: Cu, 12.08; C, 59.36; H, 5.75; N, 10.65; Found: Cu, 12.15; C, 59.44; H, 5.83; N, 10.73.
All compounds were also obtained as crystalline materials. The XRPD analysis of the polycrystalline samples revealed that compounds 1, 2, 3, 4, and 5 present the diffraction pattern in agreement with the one simulated using the single crystal data for 1, 2, 3, 4, and 5 (Supplementary Figure S4).
3.3. Antimicrobial Activity
3.3.1. Microbial Strains
Reference microbial strains (Staphylococcus aureus ATCC 25923, MRSA 43300, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Candida albicans ATCC 10231) and clinical isolates (E. coli C10E, C. albicans 1) were used to evaluate the antimicrobial activity and inhibition of microbial adhesion. The microbial strains used are part of the strain collection of the Faculty of Biology, University of Bucharest.
3.3.2. Qualitative Evaluation of Antimicrobial Activity
Microbial suspensions were prepared to a density of 1.5 × 108 CFU/mL, corresponding to the 0.5 McFarland standard. These suspensions were obtained from 18–24 h cultures grown on solid media (Mueller–Hinton for bacteria, Sabouraud for yeasts). The antimicrobial activity was evaluated using a diffusion method adapted for stock solutions of 10 mg/mL prepared in DMSO for each compound, according to the CLSI (Clinical and Laboratory Standards Institute). A solvent control was included for all variants. Each stock solution (10 μL) was dispensed in a spot on the medium inoculated with the strain of interest. The diameter of the inhibition zone (DIZ) around the spot was measured (mm), and the results were expressed as mean ± standard deviation for each substance, tested in duplicate.
3.3.3. Quantitative Evaluation of Antimicrobial Activity
The quantitative analysis was performed using the serial two-fold dilution method in liquid medium (Tryptone Soy Broth for bacteria and Sabouraud for yeasts) in a 96-well plate. Stock solutions of 10 mg/mL in DMSO were prepared, and the concentration range was 5000–0.16 µg/mL. Simultaneously, serial dilutions with DMSO were prepared under the same conditions to obtain the negative control. Each well was inoculated with 10 μL of a microbial suspension adjusted to 1.5 × 108 CFU/mL, prepared from 18–24 h cultures. The final volume in each well was 100 μL. The 96-well plates were incubated for 24 ± 2 h at 37 °C. The MIC values were determined both macroscopically, as the last concentration at which no microbial growth was observed, and spectrophotometrically. The absorbance was measured at 620 nm using the FlexStation 3 UV-Vis spectrophotometer (Molecular Devices, LLC, San Jose, CA, USA). The obtained data were analyzed using the inhibitor vs response—variable slope (four parameters) analysis function, with the help of Prism GraphPad 10.0 software, in order to calculate IC50 (the concentration of the sample that inhibits the growth of 50% of the untreated microbial inoculum).
3.3.4. Evaluation of Microbicidal Activity
To determine the minimum microbicidal concentrations (MMC), 5 µL of culture were taken from each well and spread onto solid medium. The plates were incubated for 20–24 h at 37 °C. The last concentration at which no microbial colonies were observed was considered the MMC.
3.3.5. Microbial Adherence
Following the quantitative analysis of antimicrobial activity, microbial adherence was evaluated after fixation with 120 μL methanol and staining after drying with 120 μL crystal violet (0.1%) using the slime assay. The absorbance of the adhered biomass stained with crystal violet and resuspended in 33% acetic acid (120 μL) was measured at 490 nm.
3.4. Hemocompatibility
A hemolysis assay was performed using sheep red blood cells (RBCs), according to Geana et al. [65], with slight modifications. To prevent clotting, 9 mL of blood was combined with 1 mL of 10% citric acid dextrose. The mixture was centrifuged at 5000 rpm for 10 min at 4 °C, and the plasma was discarded. The RBC pellet was washed three times and resuspended in phosphate-buffered saline (PBS, 0.1 M, pH 7.4). For the assay, 0.1 mL of 5–0.625 mg/mL of each samples prepared in DMSO were combined with 0.4 mL of RBC suspension, incubated at 37 °C for 60 min, and centrifuged for 10 min at 5000 rpm. The supernatant was transferred to a 96-well plate, and absorbance at 540 nm was measured. The positive and negative controls used 1% Triton X-100 (Sigma-Aldrich, Munich, Germany) and PBS (Sigma-Aldrich, St Louis, MO, USA), respectively.
3.5. Statistical Analysis
The data were expressed as means ± standard deviation (SD) (determined by duplicate analysis). The statistical analysis was performed using GraphPad Prism 10.0. For comparing the IC50 values with the solvent used, a two-way ANOVA, followed by the Tukey multiple comparisons test with a single combined variance, was applied. The significance level was set at p < 0.05.
4. Conclusions
A series of copper (II) complexes with mixed ligands (benzimidazole derivatives and methacrylate ions) was synthesized and characterized. The structure of all complexes was determined by single-crystal X-ray diffraction. These adopt either mononuclear or binuclear units, which are arranged at the supramolecular level through noncovalent interactions (π–π stacking and hydrogen bonding). The electronic spectra pattern is consistent with their stereochemistry, while IR spectroscopy indicates the unidentate behavior of benzimidazole derivatives and the bidentate nature (chelate or bridging mode) of the methacrylate ion.
The results concerning antimicrobial activity highlight that the compound’s characteristics (e.g., nuclearity, lipophilicity, stereochemistry) have a significant impact on their antimicrobial activity, namely their ability to interact with microbial membranes. Compound 5 exhibited the highest potency against all studied strains, with MIC values up to 0.156 mg/mL for yeast strains. The IC50 results showed that all the investigated complexes display an enhanced antimicrobial activity compared to that of either solvent or copper (II) methacrylate species. Complexes 1 and 2 led to the inhibition of microbial adhesion and consequently, to the inhibition of microbial biofilm formation, which is a crucial factor in the persistence and resistance of infections. Mononuclear complexes display reduced hemolysis, indicating good compatibility with blood cells, while complexes 2 and 4 exhibit a higher hemolytic effect but with values lower than 5%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13040109/s1, Figure S1: IR spectra of complexes 1, 3, and 5; Figure S2: IR spectra of complexes 2, 4, and [Cu2(Macr)4(H2O)2]; Figure S3: Electronic spectra in 10 μM DMSO solution of complexes 1–5: (dark blue—0 min, green—1 h, red—6 h, yellow—12h, black—24 h, magenta—48 h); Figure S4: Comparison of the simulated and recorded PXRD patterns for compounds 1 ÷ 5; Table S1: Crystallographic data, details of data collection, and structure refinement parameters for compounds 1 ÷ 5; Table S2: Bond lengths for 1; Table S3: Bond angles for 1; Table S4: Bond lengths for 2; Table S5: Bond angles for 2; Table S6: Bond lengths for 3; Table S7: Bond angles for 3; Table S8: Bond lengths for 4; Table S9: Bond angles for 4; Table S10: Bond lengths for 5; Table S11: Bond angles for 5; Table S12: Absorption maxima in UV–Vis–NIR spectra of complexes 1–5; Table S13: Tukey’s multiple comparisons test for statistical analysis of IC50 values for each microbial strain.
Author Contributions
Conceptualization, M.B. and R.O.; formal analysis, A.-G.A., G.V.S., C.M., M.-D.G., I.-C.M., R.O. and M.B.; data curation, A.-G.A., M.-D.G., C.M., I.-C.M., R.O. and M.B.; writing—original draft preparation, M.B., R.O., G.V.S., C.M. and I.-C.M.; writing—review and editing, M.B., R.O., G.V.S., C.M. and I.-C.M.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olar, R.; Vlaicu, I.D.; Chifiriuc, M.C.; Bleotu, C.; Stănică, N.; Vasile Scăețeanu, G.; Silvestro, L.; Dulea, C.; Badea, M. Thermal behavior of new nickel (II) complexes with unsaturated carboxylates and heterocyclic N-donor ligands. J. Therm. Anal. Calor. 2017, 127, 731–741. [Google Scholar]
- Vlaicu, I.D.; Olar, R.; Maxim, C.; Chifiriuc, M.C.; Bleotu, C.; Stănică, N.; Vasile Scăețeanu, G.; Dulea, C.; Avram, S.; Badea, M. Evaluating the biological potential of some new cobalt (II) complexes with acrylate and benzimidazole derivatives. Appl. Org. Chem. 2019, 33, e4976. [Google Scholar] [CrossRef]
- Ajibola, A.A.; Perveen, F.; Jan, K.; Anibijuwon, I.I.; Shaibu, S.E.; Sieroń, L.; Maniukiewicz, W. A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity. Crystals 2020, 10, 991. [Google Scholar] [CrossRef]
- Pashchevskaya, N.V.; Nazarenko, M.A.; Bolotin, S.N.; Oflidi, A.I.; Panyushkin, V.T. Effect of the condition of synthesis on the composition and structure of copper (II) complexes with benzimidazole. Russ. J. Inorg. Chem. 2010, 55, 1425–1432. [Google Scholar]
- Mahurkar, N.; Gawhale, N.; Lokhande, M.; Uke, S.; Kodape, M. Benzimidazole: A versatile scaffold for drug discovery and beyond—A comprehensive review of synthetic approaches and recent advancements in medicinal chemistry. Results Chem. 2023, 6, 101139. [Google Scholar] [CrossRef]
- Satija, G.; Sharma, B.; Madan, A.; Iqubal, A.; Shaquiquzzaman, M.; Akhter, M.; Parvez, S.; Khan, M.A.; Alam, M.M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heterocycl. Chem. 2022, 59, 22–66. [Google Scholar]
- Wu, K.; Peng, X.; Chen, M.; Li, Y.; Tang, G.; Peng, J.; Peng, Y.; Cao, X. Recent progress of research on anti-tumor agents using benzimidazole as the structure unit. Chem. Biol. Drug Des. 2022, 99, 736–757. [Google Scholar] [PubMed]
- Al Awadh, A.A. Biomedical applications of selective metal complexes of indole, benzimidazole, benzothiazole and benzoxazole: A review (from 2015 to 2022). Saudi Pharm. J. 2023, 31, 101698. [Google Scholar] [CrossRef]
- Ebenezer, O.; Oyetunde-Joshua, F.; Omotoso, O.; Shapi, M. Benzimidazole and its derivatives: Recent advances (2020–2022). Results Chem. 2023, 5, 100925. [Google Scholar] [CrossRef]
- Hernandez-Romero, D.; Rosete-Luna, S.; Lopez-Monteon, A.; Chavez-Pina, A.; Perez-Hernandez, N.; Marroquin-Flores, J.; Cruz-Navarro, A.; Pesado-Gomez, G.; Morales-Morales, D.; Colorado-Peralta, R. First-row transition metal compounds containing benzimidazole ligands: An overview of their anticancer and antitumor activity. Coord. Chem. Rev. 2021, 439, 213930. [Google Scholar] [CrossRef]
- Devereux, M.; O’Shea, D.; O’Connor, M.; Grehan, H.; Connor, G.; McCann, M.; Rosair, G.; Lyng, F.; Kellet, A.; Walsh, M.; et al. Synthesis, catalase, superoxide dismutase and antitumour activities of copper (II) carboxylate complexes incorporating benzimidazole, 1,10-phenantroline, and bipyridine ligands: X-ray crystal structures of [Cu(BZA)2(bipy(H2O)], [Cu(SalH)2(BZDH)2] and [Cu(CH3COO)2(5,6-DMBZDH)2] (SalH2 = salicylic acid; BZAH = benzoic acid; BZDH = benzimidazole and 5,6-DMBZDH = 5,6-dimethylbenzimidazole). Polyhedron 2007, 26, 4073–4084. [Google Scholar]
- Prosser, K.; Chang, S.; Saraci, F.; Le, P.; Walsby, C. Anticancer copper pyridine benzimidazole complexes: ROS generation, biomolecule interactions, and cytotoxicity. J. Inorg. Biochem. 2017, 167, 89–99. [Google Scholar]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.; Khan, R.A. Copper(II) complexes as potential anticancer and nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237. [Google Scholar]
- Kacar, S.; Unver, H.; Sahinturk, V. A mononuclear copper (II) complex containing benzimidazole and pyridyl ligands: Synthesis, characterization, and antiproliferative activity against human cancer cells. Arab. J. Chem. 2020, 13, 4310–4323. [Google Scholar]
- Tosik, A.; Sieron, L.; Bukowska-Strzyzewska, M. Heptacoordinate CuII in catena-poly[aquabis(benzimidazole-N3)copper(II)-μ-adipato-O,O′:O″,O‴. Acta Crystallogr. 1995, C51, 1985–1987. [Google Scholar]
- Sieron, L.; Bukowska-Strzyzewska, M. Poly[bis(benzimidazole-N3)copper(II)-μ-acetylenedicarboxylato-O:O′-μ-aqua] and poly[bis(benzimidazole-N3)copper(II)-μ-aqua-μ-fumarato-O:O′. Acta Crystallogr. 1998, C54, 1431–1435. [Google Scholar]
- Li, H.; Yin, K.-L.; Xu, D.-J. Catena-poly[[bis(1H-benzimidazole-kN3)(salicylate-kO)copper(II)]-μ-salicylato-O,O′:O″]. Acta Crystallogr. 2005, C61, m19–m21. [Google Scholar]
- Vlaicu, I.D.; Constand, M.; Olar, R.; Marinescu, D.; Grecu, M.N.; Lazar, V.; Chifiriuc, M.C.; Badea, M. Thermal stability of new biologic active copper (II) complexes with 5,6-dimethylbenzimidazole. J. Therm. Anal. Calorim. 2013, 113, 1369–1377. [Google Scholar]
- Badea, M.; Vlaicu, I.D.; Olar, R.; Constand, M.; Bleotu, C.; Chifiriuc, M.C.; Marutescu, L.; Lazar, V.; Grecu, M.N.; Marinescu, D. Thermal behaviour and characterisation of new biologically active Cu(II) complexes with benzimidazole as main ligand. J. Therm. Anal. Calor. 2014, 118, 1119–1133. [Google Scholar]
- Vlaicu, I.D.; Borodi, G.; Vasile Scăețeanu, G.; Chifiriuc, M.C.; Maruţescu, L.; Popa, M.; Stefan, M.; Mercioniu, I.F.; Maurer, M.; Daniliuc, C.G.; et al. X-ray Crystal Structure, Geometric Isomerism, and Antimicrobial Activity of New Copper(II) Carboxylate Complexes with Imidazole Derivatives. Molecules 2018, 23, 3253. [Google Scholar] [CrossRef] [PubMed]
- Teodoru, D.V.; Olar, R.; Maxim, C.; Bacalum, M.; Răileanu, M.; Iorgulescu, E.-E.; Vasile Scăețeanu, G.; Badea, M. Copper(II) Methacrylate Complexes with Imidazole Derivatives—Structural, Spectral and Antitumor Features. Molecules 2024, 29, 4010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Q.; Yang, B.; Shi, Q.; Gao, Y.; Zhou, Z. Synthesis, crystal structure and forming mechanism of two novel copper (II) α-methacrylate complexes with benzimidazole. Sci. China Ser. B 1999, 42, 363–372. [Google Scholar] [CrossRef]
- Oldham, C. Carboxylates, squarates and related species. In Comprehensive Coordination Chemistry, 1st ed.; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 435–460. [Google Scholar]
- Deacon, G.B.; Philips, J.R. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Marques, L.; Marinho, M.V.; Correa, C.; Speziali, N.; Diniz, R.; Machado, F. One-dimensional copper (II) coordination polymers based on carboxylate anions and rigid pyridyl-donor ligands. Inorg. Chim. Acta 2011, 368, 242–246. [Google Scholar] [CrossRef]
- Edelsbacher, P.; Redhammer, G.; Monkowius, U. Copper (II) complexes bearing cyclobutanecarboxylate and pyridine ligands: A new series of dinuclear paddle-wheel complexes. Monatsh. Chemie 2020, 151, 543–547. [Google Scholar] [CrossRef]
- Bivián-Castro, E.Y.; Flores-Alamo, M.; Escudero, R.; Gómez-Vidal, V.; Segoviano-Garfias, J.J.N.; Castañeda-Contreras, J.; Saavedra-Arroyo, Q.E. Synthesis and Characterization of a New Cu(II) Paddle-Wheel-like Complex with 4-Vinylbenzoate as an Inorganic Node for Metal–Organic Framework Material Design. Materials 2023, 16, 4866. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1984; pp. 481–505. ISBN 0444416994. [Google Scholar]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Da Silva Barboza, A.; Fang, L.; Ribeiro, J.; Cuevas-Suarez, C.; Moraes, R.; Lund, R. Physicomechanical, optical, and antifungal properties of polymethyl methacrylate modified with metal methacrylate monomers. J. Prosthet. Dent. 2021, 125, 706.e1–706.e6. [Google Scholar] [CrossRef]
- Pathare, B.; Bansode, T. Review- biological active benzimidazole derivatives. Results Chem. 2021, 3, 100200. [Google Scholar] [CrossRef]
- Frota, H.F.; Barbosa, P.F.; Lorentino, C.M.A.; Affonso, L.R.F.; Ramos, L.S.; Oliveira, S.S.C.; Souza, L.O.P.; Abosede, O.O.; Ogunlaja, A.S.; Branquinha, M.H.; et al. Unveiling the antifungal mechanisms of CTP, a new copper(II)-theophylline/1,10-phenanthroline complex, on drug-resistant non-albicans Candida species. Biometals 2024, 37, 1237–1253. [Google Scholar] [CrossRef] [PubMed]
- Savić, N.D.; Vojnovic, S.; Glišić, B.Đ.; Crochet, A.; Pavic, A.; Janjić, G.V.; Pekmezović, M.; Opsenica, I.M.; Fromm, K.M.; Nikodinovic-Runic, J.; et al. Mononuclear silver(I) complexes with 1,7-phenanthroline as potent inhibitors of Candida growth. Eur. J. Med. Chem. 2018, 156, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Tiwari, M. Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole derivatives. Asian J. Chem. 2017, 29, 838–842. [Google Scholar] [CrossRef]
- Alexander, M.K.; Miu, A.; Oh, A.; Reichelt, M.; Ho, H.; Chalouni, C.; Labadie, S.; Wang, L.; Liang, J.; Nickerson, N.N.; et al. Disrupting Gram-Negative Bacterial Outer Membrane Biosynthesis through Inhibition of the Lipopolysaccharide Transporter MsbA. Antimicrob. Agents Chemother 2018, 62, e01142-18. [Google Scholar] [CrossRef]
- Al-Matarneh, C.M.; Nicolescu, A.; Marinas, I.C.; Chifiriuc, M.C.; Shova, S.; Silion, M.; Pinteala, M. Novel antimicrobial iodo-dihydro-pyrrole-2-one compounds. Future Med. Chem. 2023, 15, 1369–1391. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2023, 13, 478–497. [Google Scholar] [CrossRef]
- Smułek, W.; Kaczorek, E. Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review. Molecules 2022, 27, 6579. [Google Scholar] [CrossRef]
- Niu, M.; Li, Z.; Li, H.; Li, X.; Dou, J.; Wang, S. DNA/protein interaction, cytotoxic activity and magnetic properties of amino-alcohol Schiff base derived Cu(II)/Ni(II) metal complexes: Influence of the nuclearity and metal ions. RSC Adv. 2015, 5, 37085–37095. [Google Scholar] [CrossRef]
- Matsson, P.; Kihlberg, J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017, 60, 1662–1664. [Google Scholar] [CrossRef]
- Umba-Tsumbu, E.; Hammouda, A.N.; Jackson, G.E. Evaluation of Membrane Permeability of Copper-Based Drugs. Inorganics 2023, 11, 179. [Google Scholar] [CrossRef]
- Elhusseiny, A.F.; El-Dissouky, A.; Mautner, F.; Tawfik, E.M.; El-Sayed, D.S. An insight into non-covalent interactions in binary, ternary and quaternary copper (II) complexes: Synthesis, X-ray structure, DFT calculations, antimicrobial activity and molecular docking studies. Inorg. Chim. Acta 2022, 532, 120748. [Google Scholar] [CrossRef]
- Noreen, S.; Sumrra, S.H. Aminothiazole-Linked Metal Chelates: Synthesis, Density Functional Theory, and Antimicrobial Studies with Antioxidant Correlations. ACS Omega 2021, 6, 33085–33099. [Google Scholar]
- El-Sayed, D.S.; Tawfik, E.M.; Elhusseiny, A.F.; El-Dissouky, A. A perception into binary and ternary copper (II) complexes: Synthesis, characterization, DFT modeling, antimicrobial activity, protein binding screen, and amino acid interaction. BMC Chem. 2023, 17, 55. [Google Scholar]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Daben, M.; Libardo, J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121, 2648–2712. [Google Scholar]
- Syaima, H.; Prasetyo, W.E.; Rahardjo, S.B.; Suryanti, V. Semi-coordination Cu–O bond on a copper complex featuring O,O-donor ligand as potential antibacterial agent: Green synthesis, characterization, DFT, in-silico ADMET profiling and molecular docking studies. Struct. Chem. 2024, 35, 721–737. [Google Scholar]
- Zalevskaya, O.A.; Gur’eva, Y.A. Recent Studies on the Antimicrobial Activity of Copper Complexes. Russ. J. Coord. Chem. 2021, 47, 861–880. [Google Scholar]
- Bellia, F.; Lanza, V.; Naletova, I.; Tomasello, B.; Ciaffaglione, V.; Greco, V.; Sciuto, S.; Amico, P.; Inturri, R.; Vaccaro, S.; et al. Copper(II) Complexes with Carnosine Conjugates of Hyaluronic Acids at Different Dipeptide Loading Percentages Behave as Multiple SOD Mimics and Stimulate Nrf2 Translocation and Antioxidant Response in In Vitro Inflammatory Model. Antioxidants 2023, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Caro-Ramirez, J.Y.; Parente, J.E.; Gaddi, G.M.; Martini, N.; Franca, C.A.; Urquiza, N.M.; Lezama, L.; Piro, O.E.; Echeverría, G.A.; Williams, P.A.M.; et al. The biocatalytic activity of the “lantern-like” binuclear copper complex with trisulfide bridges mimicking SOD metallo-proteins. Polyhedron 2022, 221, 115879. [Google Scholar]
- Kupcewicz, B.; Sobiesiak, K.; Malinowska, K.; Koprowska, K.; Czyz, M.; Keppler, B.; Budzisz, E. Copper(II) complexes with derivatives of pyrazole as potential antioxidant enzyme mimics. Med. Chem. Res. 2013, 22, 2395–2402. [Google Scholar]
- Cegelski, L.; Smith, C.L.; Hulgren, S.J. Microbial adhesion. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Academic Press: New York, NY, USA, 2009; pp. 1–10. [Google Scholar]
- Beeton, M.L.; Aldrich-Wright, J.R.; Bolhuis, A. The antimicrobial and antibiofilm activities of copper(II) complexes. J. Inorg. Biochem. 2014, 140, 167–172. [Google Scholar]
- Nagarasu, P.; Gayathri, P.; Sri, S.N.; Saisubramanian, N.; Dhanaraj, P.; Moon, D.; Savarimuthu, P.A.; Madhu, V. Synthesis, antibiofilm activity and molecular docking study of new water-soluble copper(II)-pincer complexes. Inorg. Chem. Commun. 2022, 139, 109316. [Google Scholar]
- Gomes da Silva Dantas, F.; Araújo de Almeida-Apolonio, A.; Pires de Araújo, R.; Regiane Vizolli Favarin, L.; Fukuda de Castilho, P.; De Oliveira Galvão, F.; Inez Estivalet Svidzinski, T.; Antônio Casagrande, G.; Mari Pires de Oliveira, K. A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug Against Yeast Infection. Molecules 2018, 23, 1856. [Google Scholar] [CrossRef]
- Dimitrijević, J.D.; Solovjova, N.; Bukonjić, A.M.; Tomović, D.L.; Milinkovic, M.; Caković, A.; Bogojeski, J.; Ratković, Z.R.; Janjić, G.V.; Rakić, A.A.; et al. Docking Studies, Cytotoxicity Evaluation and Interactions of Binuclear Copper(II) Complexes with S-Isoalkyl Derivatives of Thiosalicylic Acid with Some Relevant Biomolecules. Int. J. Mol. Sci. 2023, 24, 12504. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.X.; Han, B.O.; Fang, W.H.; Tran, H.D.; Hoang, C.; Shaw, D.G.; Nguyen, T.Q. The Rationality of Implementation of Dimethyl Sulfoxide as Differentiation-inducing Agent in Cancer Therapy. Cancer Diagn. Progn. 2023, 3, 1–8. [Google Scholar] [CrossRef]
- Çağlayan, S.T.; Gurbanov, R. Modulation of bacterial membranes and cellular macromolecules by dimethyl sulfoxide: A dose-dependent study providing novel insights. Int. J. Biol. Macromol. 2024, 267, 131581. [Google Scholar]
- Fedorka-Cray, P.J.; Cray, W.C., Jr.; Anderson, G.A.; Nickerson, K.W. Bacterial tolerance of 100% dimethyl sulfoxide. Can. J. Microbiol. 1988, 34, 688–689. [Google Scholar] [CrossRef]
- Tunçer, S.; Gurbanov, R. Non-growth inhibitory doses of dimethyl sulfoxide alter gene expression and epigenetic pattern of bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 299–312. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of control: The need for standardised solvent approaches and data reporting in antibiofilm assays incorporating dimethyl-sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar]
- Mi, H.; Wang, D.; Xue, Y.; Zhang, Z.; Niu, J.; Hong, Y.; Drlica, K.; Zhao, X. Dimethyl Sulfoxide Protects Escherichia coli from Rapid Antimicrobial-Mediated Killing. Antimicrob. Agents Chemother. 2016, 60, 5054–5058. [Google Scholar] [CrossRef]
- Kanďárová, H.; Pôbiš, P. The “Big Three” in biocompatibility testing of medical devices: Implementation of alternatives to animal experimentation—Are we there yet? Front. Toxicol. 2024, 5, 1337468. [Google Scholar] [CrossRef]
- Saleem, K.; Wani, W.A.; Haque, A.; Lone, M.N.; Hsieh, M.F.; Jairajpuri, M.A.; Ali, I. Synthesis, DNA binding, hemolysis assays and anticancer studies of copper(II), nickel(II) and iron(III) complexes of a pyrazoline-based ligand. Future Med. Chem. 2013, 5, 135–146. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [PubMed]
- Geana, E.-I.; Ciucure, C.T.; Tamaian, R.; Marinas, I.C.; Gaboreanu, D.M.; Stan, M.; Chitescu, C.L. Antioxidant and Wound Healing Bioactive Potential of Extracts Obtained from Bark and Needles of Softwood Species. Antioxidants 2023, 12, 1383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).