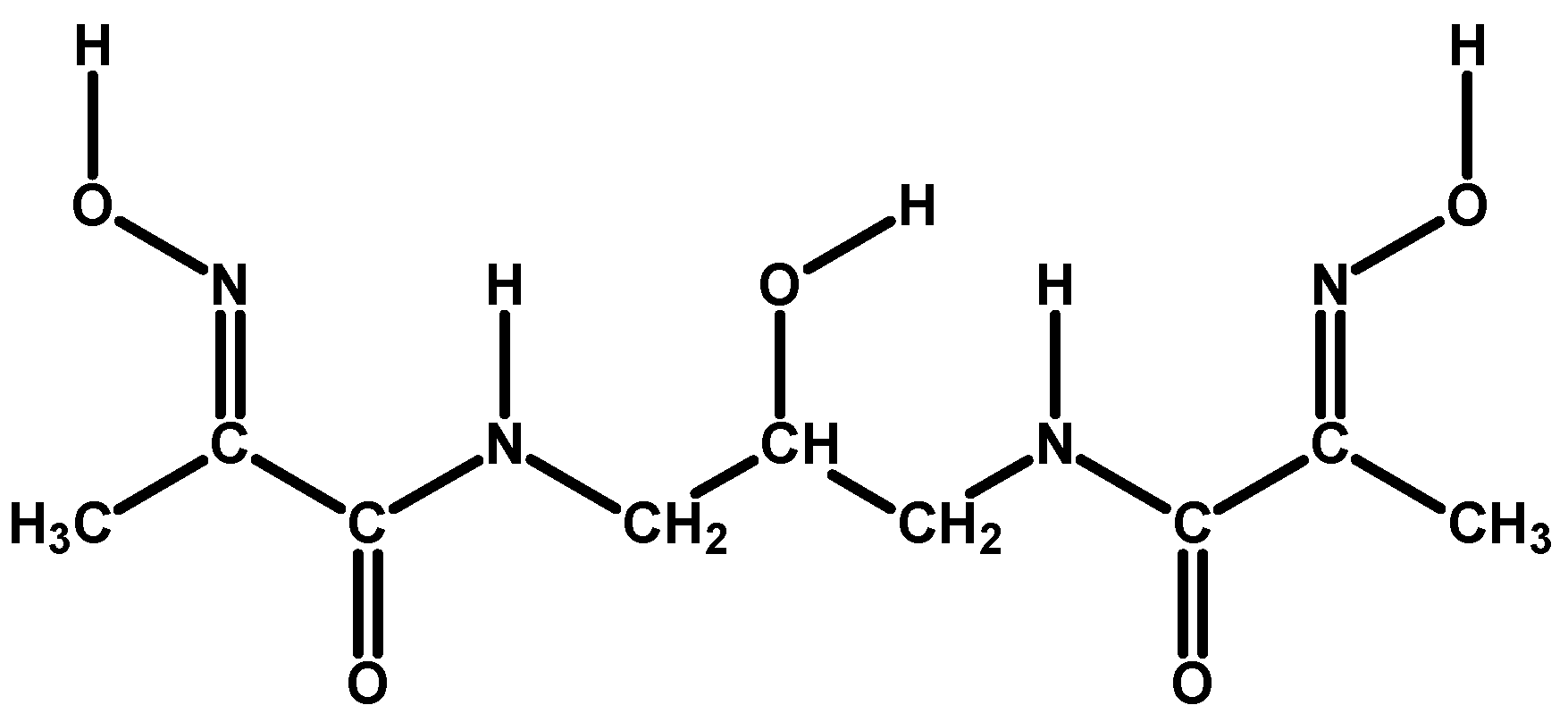
Ni(II) and Cu(II) Ion Coordination by the Novel (2E,2′E)-N,N′-(2-Hydroxypropane-1,3-diyl)bis[(2-hydroxyimino)propanamide] Ligand in the Solid State and in Aqueous Medium
Abstract
1. Introduction
2. Results and Discussion
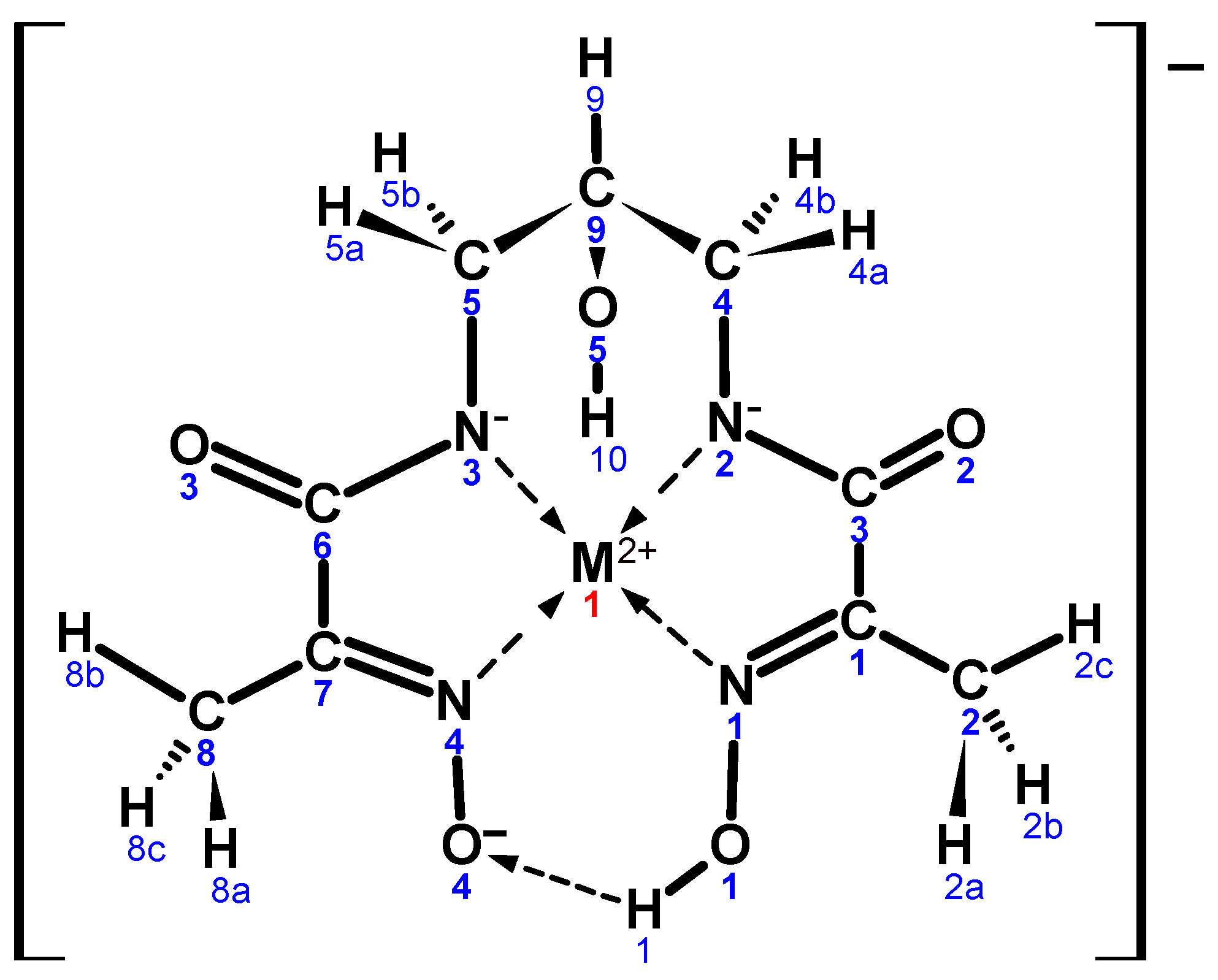
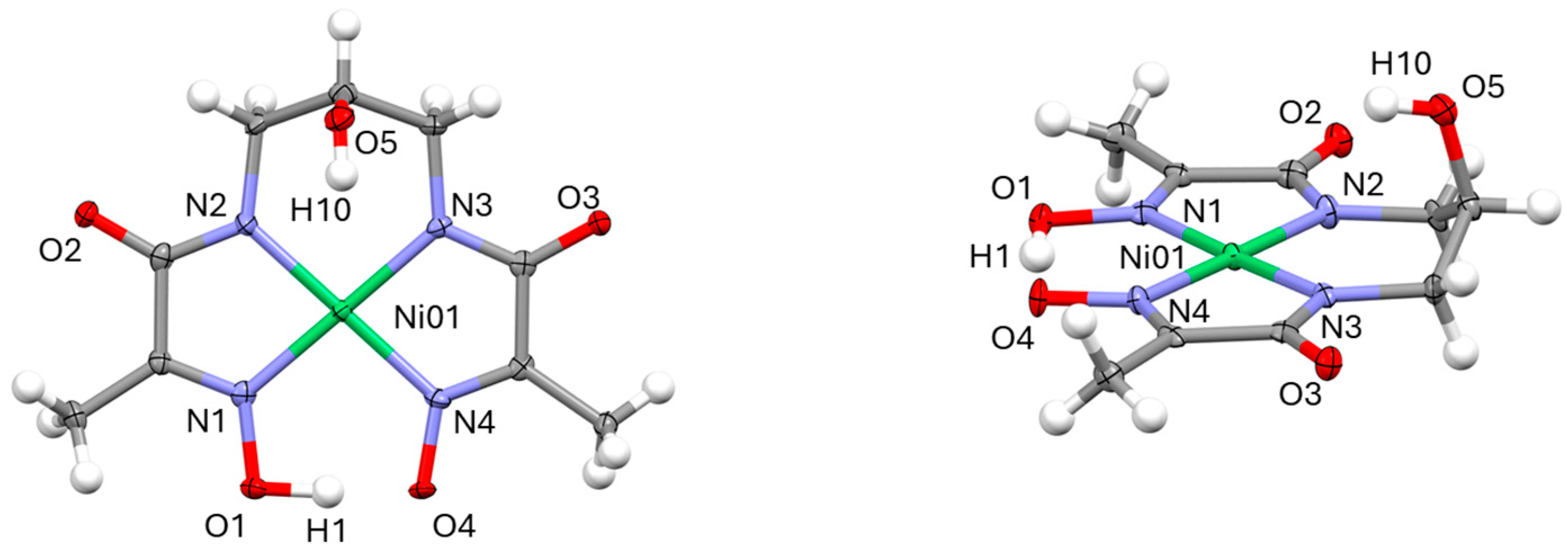
2.1. X-Ray Diffraction
2.2. Potentiometric Titrations and Solution Equilibria
2.3. ESR Spectra of Selected Cu(II)-Mhiea2poh Solutions
2.4. The Ligand 1H NMR Spectrum
3. Materials and Methods
3.1. Materials
3.2. Instrumental
3.3. Synthesis and Characterisation
3.4. Solution Preparation
3.5. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univer. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lesnikov, V.K.; Golovanov, I.S.; Nelyubina, Y.V.; Aksenova, S.A.; Sukhorukov, A.Y. Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis. Nat. Commun. 2023, 14, 7673. [Google Scholar] [CrossRef] [PubMed]
- Mezgebe, K.; Mulugeta, E. Synthesis and pharmacological activities of Schiff bases with some transition metal complexes: A review. Med. Chem. Res. 2024, 33, 439–463. [Google Scholar] [CrossRef]
- Nikolayenko, I.V.; Barry, J.R.; Manival, A.; Theron, T.-J.; Grimmer, C. Peculiar sequence of the thermodynamic protonation parameters for bis-chelate ligands with methylhydroxyiminoethanamide moieties. Curr. Inorg. Chem. 2015, 5, 83–97. [Google Scholar] [CrossRef][Green Version]
- Gutsche, C.D.; Mei, G.C. Polyfunctional catalysis of acetyl phosphate decomposition. J. Am. Chem. Soc. 1978, 100, 1857–1865. [Google Scholar] [CrossRef]
- Lau, H.-P.; Gutsche, C.D. Mixed chelate and comicellar catalysis of acetyl phosphate “olysis” reactions. J. Am. Chem. Soc. 1985, 107, 7964–7967. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Kozlowski, H.; Sadler, P.J.; Yefetova, O.P.; Swatek-Kozlowska, J.; Kalibabchuk, V.A.; Glowiak, T. Template synthesis of square-planar nickel(II) and copper(III) complexes based on hydrazide ligands. Dalton Trans. 1998, 19, 3269–3274. [Google Scholar] [CrossRef]
- Hanss, J.; Beckmann, A.; Krueger, H.-J. Stabilization of copper(III) ions with deprotonated hydroxyiminoamide ligands. Syntheses, structures, and electronic properties of copper(II) and copper(III) complexes. Eur. J. Inorg. Chem. 1999, 1, 163–172. [Google Scholar] [CrossRef]
- Kanderal, O.M.; Kozlowski, H.; Dobosz, A.; Swiatek-Kozlowska, J.; Meyer, F.; Fritsky, I.O. Effect of metal ionic radius and chelate ring alternation motif on stabilization of trivalent nickel and copper in binuclear complexes with double cis-oximato bridges. Dalton Trans. 2005, 1428–1437. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Kozlowski, H.; Kanderal, O.M.; Haukka, M.; Swiatek-Kozlowska, J.; Gumienna-Kontecka, E.; Meyer, F. Efficient stabilization of copper(III) in tetraaza pseudo-macrocyclic oxime-and-hydrazide ligands with adjustable cavity size. Chem. Commun. 2006, 39, 4125–4127. [Google Scholar] [CrossRef]
- Cervera, B.; Ruiz, R.; Lloret, F.; Julve, M.; Cano, J.; Faus, J.; Bois, C.; Mrozinski, J. Tuning the nature of the exchange interaction in out-of-plane oximato-bridged dinuclear copper(II) complexes. J. Chem. Soc. Dalton Trans. 1997, 395–401. [Google Scholar] [CrossRef]
- Colacio, E.; Dominguez-Vera, J.M.; Escuer, A.; Kivekas, R.; Klinga, M.; Moreno, J.-M.; Romerosa, A. Bis(didentate) open-chain and tetradentate pseudo-macrocyclic bridging co-ordination modes of N,N′-bis(1,3-dimethyl-5-nitroso-2,4-dioxopyrimidin-6-yl)butane-1,4-diamine. Dalton Trans. 1997, 1685–1689. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Bridging ability of a novel polydentate ligand (H2L) comprising an oxime function. Structures of a mononuclear precursor [NiL] and a dinuclear CuII2 complex. Magnetic properties of mononuclear (NiII and CuII), dinuclear (CuII2, NiII2, NiIICuII and CuIICrIII) and trinuclear (CuII3, CuIIMnIICuII and CuIIZnIICuII) complexes. Dalton Trans. 1998, 1307–1314. [Google Scholar] [CrossRef]
- Chaudhuri, P. Homo- and hetero-polymetallic exchange coupled metal-oximates. Coord. Chem. Rev. 2003, 243, 143–190. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Turner, D.R.; Moubaraki, B.; Murray, K.S.; Deacon, G.B.; Batten, S.R. Nucleophilic addition of water and alcohols to dicyanonitrosomethanide: Ligands with diverse bonding modes in magnetically coupled d-block complexes. Eur. J. Inorg. Chem. 2010, 2010, 59–73. [Google Scholar] [CrossRef]
- Razali, M.R.; Urbatsch, A.; Langley, S.K.; MacLellan, J.G.; Deacon, G.B.; Moubaraki, B.; Murray, K.S.; Batten, S.R. Linear trinuclear copper(II) complexes derived from the nucleophilic addition products of dicyanonitrosomethanide [C(CN)2(NO)]−: Syntheses, structures, and magnetic properties. Aust. J. Chem. 2012, 65, 918–925. [Google Scholar] [CrossRef]
- Giri, S.; Maity, D.; Godsell, J.F.; Roy, S.; Drew, M.G.; Ghosh, A.; Mukhopadhyay, G.; Saha, S.K. A new tetranuclear copper(II) complex with oximate bridges: Structure, magnetic properties and DFT study. Inorg. Chim. Acta 2011, 377, 99–104. [Google Scholar] [CrossRef]
- Pagonda, H.; Yogesh, P.P.; Katreddi, H.R.; Munirathinam, N. Novel tetranuclear distorted open-cubane copper complex contain-ing oximate bridges: Synthesis, crystal structure, DNA binding and cleavage activity. Inorg. Chim. Acta 2012, 392, 478–482. [Google Scholar] [CrossRef]
- Dass, L.K.; Biswas, A.; Kinyon, J.S.; Dalal, N.S.; Zhou, H.; Ghosh, A. Di-, tri-, and tetranuclear nickel(II) complexes with oximato bridges: Magnetism and catecholase-like activity of two tetranuclear complexes possessing rhombic topology. Inorg. Chem. 2013, 52, 11744–11757. [Google Scholar] [CrossRef]
- Afati, T.; Zaleski, C.M.; Dendrinou-Samara, C.; Mezei, G.; Kampf, J.W.; Pecoraro, V.; Kessissoglou, D.P. Di-2-pyridyl ketone oxime in copper chemistry: Di-, tri-, penta- and hexanuclear complexes. Dalton Trans. 2007, 2658–2668. [Google Scholar] [CrossRef]
- Alcazar, L.; Cordero, B.; Esteban, J.; Tangoulis, V.; Font-Bardia, M.; Calvet, T.; Escuer, A. Manganese clusters derived from 2-pyridylcyanoxime: New topologies and a large spin ground state in pyridyloximate chemistry. Dalton Trans. 2013, 12334–12345. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Grimmer, C.; Nikolayenko, I.V. Old acquaintances and novel complex structures for the Ni(II) and Cu(II) complexes of bis-chelate oxime–amide ligands. Molecules 2024, 29, 522. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 637, 3192–3210. [Google Scholar] [CrossRef]
- Banks, C.V.; Barnum, D.W. Intermolecular Metal-Metal Bonds and Absorption Spectra of Some Nickel(II) and Palladium(II) Complexes of vic-Dioximes1. J. Am. Chem. Soc. 1958, 80, 4767–4772. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Available online: http://www.hyperquad.co.uk/ (accessed on 1 November 2024).
- Duda, A.M.; Karaczyn, A.; Kozłowski, H.; Fritsky, I.O.; Głowiak, T.; Prisyazhnaya, E.V.; Sliva, T.Y.; Świątek-Kozłowska, J. Co-ordination of copper(II) and nickel(II) ions by a novel open chain oxime ligand. Dalton Trans. 1997, 3853–3859. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Kozłowski, H.; Prisyazhnaya, E.V.; Karaczyn, A.; Kalibabchuk, V.A.; Głowiak, T. A short intramolecular hydrogen bond is a key factor in the self-assembly of a dimeric complex with a 22-membered metallamacrocyclic cavity. J. Chem. Soc. Dalton Trans. 1998, 10, 1535–1536. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Kozłowski, H.; Prisyazhnaya, E.V.; Rzączyńska, Z.; Karaczyn, A.; Sliva, T.Y.; Głowiak, T. Co-ordination ability of novel tetradentate amide-and-oxime ligands: Differential binding to CuII and NiII. J. Chem. Soc. Dalton Trans. 1998, 21, 3629–3633. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Swiatek-Kozlowska, J.; Kapshuk, A.A.; Kozlowski, H.; Sliva, T.Y.; Gumienna-Kontecka, E.; Prisyazhnaya, E.V.; Iskenderov, T.S. Preparation and crystal structure of a mixed metal assembly [Ni(phen)3][Cu(H-1pap)]2(NO3) · 8 H2O featuring octahedral cationic and square-planar anionic modules. Z. Naturforsch. 2000, 55, 966–970. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Swiatek-Kozlowska, J.; Dobosz, A.; Sliva, T.Y.; Dudarenko, N.M. Hydrogen bonded supramolecular structures of cationic and anionic module assemblies containing square-planar oximate complex anions. Inorg. Chim. Acta 2004, 357, 3746–3752. [Google Scholar] [CrossRef]
- Nomkoko, E.T.; Jackson, G.E.; Nakani, B.S. In vitro and in vivo stability investigations of Cu(II), Zn(II), Ca(II) and Gd(III) complexes with N,N′-bis(2-hydroxyiminopropionyl) propane-1,3-diamine. Dalton Trans. 2004, 9, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Nomkoko, E.T.; Jackson, G.E.; Nakani, B.S.; Bourne, S.A. Computer simulation of nickel in blood-plasma following the in vitro investigations of complex formation chemistry with polyamine(amide) ligands. Dalton Trans. 2004, 12, 1789–1796. [Google Scholar] [CrossRef][Green Version]
- Kufelnicki, A.; Tomyn, S.V.; Nedelkov, R.V.; Haukka, M.; Jaciubek-Rosińska, J.; Pająk, M.; Jaszczak, J.; Świątek, M.; Fritsky, I.O. Synthesis of cobalt(III) complexes with novel open chain oxime ligands and metal–ligand coordination in aqueous solution. Inorg. Chim. Acta 2010, 363, 2996–3003. [Google Scholar] [CrossRef]
- Sawada, T.; Fukumaru, K.; Sakurai, H. Coordination-Dependent ESR Spectra of Copper(II) Complexes with a CuN4 Type Coordination Mode: Relationship between ESR Parameters and Stability Constants or Redox Potentials of the Complexes. Chem. Pharm. Bull. 1996, 44, 1009–1016. [Google Scholar] [CrossRef]
- El-Tabl, A.S.; Shakdofa, M.M.E.; El-Seidy, A.M.A. Synthesis, characterisation and ESR studies of new copper(II) complexes of vicinal oxime ligands. J. Korean Chem. Soc. 2011, 55, 603–611. [Google Scholar] [CrossRef]
- Marat, K. SpinWorks 4.2.8.0. University of Manitoba: Winnipeg, MB, Canada, 2017. [Google Scholar]
- Quirt, A.R.; Martin, J.S. NMR spectra of symmetric molecules. I. The spin Hamiltonian for twofold symmetry. J. Magn. Reson. 1971, 5, 318–327. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.34.44, Oxford Diffraction Ltd.: Abingdon, UK, 2010.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Lübben, J.; Wandtke, C.M.; Hübschle, C.B.; Ruf, M.; Sheldrick, G.M.; Dittrich, B. Aspherical scattering factors for SHELXL—Model, implementation and application. Acta Crystallogr. Sect. A Found. Adv. 2019, A75, 50–62. [Google Scholar] [CrossRef]
- Mercury 2024.2.0 (Build 415171). Copyright © CCDC, 2001–2023. Available online: https://www.ccdc.cam.ac.uk/solutions/software/mercury/ (accessed on 1 November 2024).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- enCIFer 2024.2.0 (Build 415171). Copyright © CCDC, 2001–2024. Available online: https://www.ccdc.cam.ac.uk/solutions/software/encifer/ (accessed on 1 November 2024).
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Available online: http://www.metrohm.com/com/Produkte2/Titration/PotentiometricTitration/ (accessed on 1 November 2024).
- Gran, G. Determination of the equivalence point in potentiometric titrations. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. Potentiometric titrations using Gran plots: A textbook omission. J. Chem. Educ. 1965, 42, 375–378. [Google Scholar] [CrossRef]
- Gans, P.; O’Sullivan, B. GLEE, a new computer program for glass electrode calibration. Talanta 2000, 51, 33–37. [Google Scholar] [CrossRef]
- Available online: http://www.metrohm.com/com/Produkte2/Titration/PotentiometricTitration/tiamo.html (accessed on 1 November 2024).
- Lebruška, V.; Dobrovolná, T.; Gemperle, T.; Kubíček, V.; Susanne Kossatz, S.; Hermann, P. A UV-Vis method for investigation of gallium(iii) complexation kinetics with NOTA and TRAP chelators: Advantages, limitations and comparison with radiolabelling. Dalton Trans. 2024, 53, 17554–17564. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 46. NIST Critically Selected Stability Constants of Metal Complexes Database, Version 8.0 for Windows; Users’ Guide Data collected and selected by: R. M. Smith and A. E. Martell, Program developed by: R. J. Motekaitis, Texas A&M University, College Station, TX; U.S. Department of Commerce, Technology Administration, National Institute of Standards and Technology, Standard Reference Data Program: Gaithersburg, MD, USA, 2004.
- Nikolayenko, I.; Barry, J.; Chalmers, C. The nature and solution thermodynamics of Ni(II) and Cu(II) complexes with tetradentate oxime-and-amide ligands. In Proceedings of the 37th International Conference on Coordination Chemistry, Cape Town, South Africa, 13–18 August 2006; p. 245. [Google Scholar]
















| Complex | (1) | (2) |
|---|---|---|
| Empirical formula | C18H42N8O18Ni3 | C33H41N4O9PCu |
| M | 834.72 | 732.21 |
| T/K | 293 (2) | 293 (2) |
| Crystal system | monoclinic | triclinic |
| Space group | P 21/c | P |
| a/Å | 7.4351(2) | 11.3575(7) |
| b/Å | 12.8172(4) | 12.880(1) |
| c/Å | 16.0660(7) | 12.900(1) |
| α/° | 90 | 77.163(7) |
| β/° | 94.436(3) | 68.123(7) |
| γ/° | 90 | 82.197(6) |
| V/Å−3 | 1526.46(9) | 1704.5(2) |
| Z | 2 | 2 |
| dc/g cm−3 | 1.816 | 1.427 |
| μ/mm−1 | 2.985 | 1.851 |
| F(0 0 0) | 868 | 766 |
| Crystal size/mm | 0.25 × 0.2 × 0.2 | 0.15 × 0.15 × 0.1 |
| Colour | tea yellow | pale red |
| λ/Å | 1.54180 | 1.54180 |
| θrange/° | 4.418 to 72.461 | 4.538 to 71.309 |
| Index range | −9 ≤ h ≤ 7, | −11 ≤ h ≤ 13, |
| −15 ≤ k ≤ 15, | −14 ≤ k ≤ 15, | |
| −19 ≤ l ≤ 15 | −15 ≤ l ≤ 14 | |
| Reflections collected | 11,085 | 13,080 |
| Independent Reflections/Rint | 2956/0.0371 | 6339/0.0585 |
| Data/ | 2553 | 4769 |
| restraints/ | 1 | 2 |
| parameters | 254 | 463 |
| Goodness of fit on F2 | 1.161 | 1.063 |
| R indices | ||
| [I > 2σ(I)] | ||
| R1 | 0.0720 | 0.0603 |
| wR2 | 0.1914 | 0.1582 |
| R indices | ||
| (all data) | ||
| R1 | 0.0825 | 0.0849 |
| wR2 | 0.1962 | 0.1783 |
| Max/min electron density/e Å−3 | 1.2/−0.8 | 1.7/−0.9 |
| (1) | (2) | ||||||
|---|---|---|---|---|---|---|---|
| Ni01 N1 | 1.866(7) | N3 C5 | 1.466(9) | Cu01 N1 | 1.953(4) | N3 C5 | 1.463(7) |
| Ni01 N2 | 1.868(6) | C4 C9 | 1.520(10) | Cu01 N2 | 1.923(4) | C4 C9 | 1.537(5) |
| Ni01 N3 | 1.867(7) | C5 C9 | 1.520(10) | Cu01 N3 | 1.917(3) | C5 C9 | 1.527(7) |
| Ni01 N4 | 1.878(6) | C9 O5 | 1.414(9) | Cu01 N4 | 1.951(4) | C9 O5 | 1.383(5) |
| C3 O2 | 1.259(2) | O1 O4 | 2.436 | C3 O2 | 1.265(6) | O1 O4 | 2.472 |
| C6 O3 | 1.261(8) | BP 1–Ni01 | 0.023(1) | C6 O3 | 1.267(4) | BP 1–Cu01 | 0.0413(6) |
| N1 C1 | 1.290(10) | N1 Ni01 N2 | 82.7(3) | N1 C1 | 1.287(6) | N1 Cu01 N2 | 82.7(2) |
| N4 C7 | 1.297(9) | N2 Ni01 N3 | 97.0(3) | N4 C7 | 1.278(5) | N2 Cu01 N3 | 98.8(2) |
| O1 N1 | 1.366(8) | N3 Ni01 N4 | 83.1(3) | O1 N1 | 1.363(5) | N3 Cu01 N4 | 83.2(2) |
| O4 N4 | 1.347(8) | N4 Ni01 N1 | 97.2(3) | O4 N4 | 1.354(5) | N4 Cu01 N1 | 95.2(2) |
| C3 N2 | 1.319(9) | N1 Ni01 N3 | 177.8(3) | C3 N2 | 1.304(5) | N1 Cu01 N3 | 176.2(2) |
| C6 N3 | 1.326(9) | N2 Ni01 N4 | 179.4(3) | C6 N3 | 1.319(6) | N2 Cu01 N4 | 177.4(2) |
| N2 C4 | 1.458(9) | C9 O5 H10 | 112(5) | N2 C4 | 1.464(6) | C9 O5 H10 | 109.5(4) |
| q | r | log10βqr (σ) | log10Kqr (σ) |
|---|---|---|---|
| 1 | 1 | 11.303 (10) | 11.303 (10) |
| 1 | 2 | 21.75 (2) | 10.44 (3) |
| 1 | 3 | 31.49 (2) | 9.74 (4) |
| 1 | 4 | 40.70 (3) | 9.48 (5) |
| p | q | r | log10βpqr (σ) |
|---|---|---|---|
| 1 | 1 | 0 | 13.8 (6) |
| 1 | 1 | −1 1 | 2.59 (1) |
| 1 | 1 | −2 | −8.5 (6) |
| 2 | 2 | 3 | 61.3 (8) |
| 2 | 2 | 2 | 54 (2) |
| 2 | 2 | 1 | 41.3 (5) |
| 2 | 1 | −1 | 10.4 (9) |
| 2 | 1 | −2 | −0.28 (15) |
| 4 | 2 | 3 | 66.55 (8) |
| 4 | 2 | 2 | 60.4 (9) |
| 4 | 2 | 1 | 53.86 (5) |
| 4 | 2 | 0 | 45.72 (8) |
| p | q | r | log10βqr (σ) |
|---|---|---|---|
| 1 | 1 | 1 | 28.85 (10) |
| 1 | 1 | −2 | 1.2 (7) |
| 2 | 2 | 6 | 81.89 (9) |
| 2 | 2 | 1 | 53.5 (4) |
| 2 | 1 | −2 | 16.14 (13) |
| 2 | 1 | −4 | 0.5 (6) |
| 4 | 2 | 2 | 72.1 (3) |
| 4 | 2 | 0 | 62.5 (3) |
| Reference | [29] | [34] | [35] | [36] | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | p q r | log10β (σ) | Medium | log10β (σ) | Medium | log10β (σ) | Medium | log10β (σ) | Medium |
| H+ | 0 1 1 0 1 2 | 10.383 (9) 19.933 (7) | 0.1 M KNO3 | 10.408 (1) 20.072 (1) | 0.15 M KCl | 10.54 (1) 20.31 (1) | 0.1 M KNO3 | ||
| Ni2+ | 1 1 1 1 1 −1 1 1 −2 | 1.14 (9) −7.05 (11) | 0.1 M KNO3 | 15.460 (35) 2.842 (10) −8.368 (35) | 0.15 M KCl | ||||
| Cu2+ | 1 1 1 1 1 0 1 1 −1 1 1 −2 | 19.61 (4) 9.95 (4) 2.08 (8) | 0.1 M KNO3 | 14.076 (30) 9.903 (30) 1.625 (53) | 0.15 M KCl | ||||
| This Work | Literature | |||
|---|---|---|---|---|
| Reaction | log10K | Reaction | log10K′ | Reference |
| Ni + LH4 + 4OH ⇌ NiL + 4H2O | 28.3 (9) | Ni + L′H2 + 4OH ⇌ NiL′H−2 + 4H2O | 28.2 (2) | [29] |
| Ni + L′H2 + 4OH ⇌ NiL′H−2 + 4H2O | 26.80 (9) | [35] | ||
| Cu + LH4 + 3OH ⇌ CuLH + 3H2O | 29.3 (4) | Cu + L′H2 + 3OH ⇌ CuL′H−1 + 3H2O | 31.39 (8) | [29] |
| Cu + L′H2 + 3OH ⇌ CuL′H−1 + 3H2O | 31.26 (7) | [34] | ||
| Target Complex Stoichiometry, pqr a | 224 | 223 | 222 | 111 |
|---|---|---|---|---|
| [L]0/mL | 0.998 | 0.998 | 0.998 | 0.998 |
| H2O/mL | 3.942 | 3.892 | 3.842 | 3.842 |
| [HCl]0/μL | 25.2 | 25.2 | 25.2 | 25.2 |
| [NiCl2]0/μL | 34.8 | 34.8 | 34.8 | 34.8 |
| [KOH]0/μL | 117 | 153 | 185 | 500 |
| [TPPB]0/μL | 0 | 50.1 | 100.1 | 100.1 |
| Target pH | 6.21 | 6.96 | 10.00 | 11.46 |
| Observed solution colour | yellow | yellow | yellow | yellow |
| Observed solids | clustered honey-yellow prisms | bright-yellow needles and honey-yellow prisms | tea-yellow slabs and prisms | tea-yellow prisms and branched colourless crystals |
| Target Complex Stoichiometry, pqr | 420, 224 | 111, 221 | 11-2 | 420 | 21-4 |
|---|---|---|---|---|---|
| [L]0/mL | 0.998 | 0.998 | 0.998 | 0.998 | 0.998 |
| H2O/mL | 3.951 | 3.851 | 3.751 | 3.725 | 3.925 |
| [HCl]0/μL | 25.2 | 25.2 | 25.2 | 25.2 | 25.2 |
| [CuCl2]0/μL | 25.7 | 25.7 | 25.7 | 25.7 | 25.7 |
| [KOH]0/μL | 135 | 180 | 300 | 178 | 226 |
| [TPPB]0/μL | 0 | 100.1 | 200.2 | 0 | 0 |
| [STPB]0/μL | 0 | 0 | 0 | 199.7 (a) | 0 |
| [PHFA]0/μL | 0 | 0 | 0 | 200.0 (b) | 0 |
| Target pH | 6.25 | 7.85 | 11.02 | 6.57 | 8.73 |
| Observed solution colour | pale green yellow | light terracotta | light terracotta | (a) sea green + white ppt (b) sea green | brown green |
| Observed solids | small fused pink, yellow, green, and colourless crystals | branched pink-red poorly shaped crystals | pale red prisms and colourless very long thin needles | (a) colourless prisms + pink and green powder (b) green rosettes + white fine powder | colourless prisms + brown ppt and green glass |
| Target Species | Desired Ratio | Concentrations of Stock Solutions | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.9823 mM | 0 | 0.1987 M | 0.1942 M | 0.1250 M | ||||
| p | q | r | [L]:[M] | L/μL | H2O/μL | H/μL | Cu/μL | NMe4OH/μL |
| 4 | 2 | 2 | 1:1 | 3054 | 1832 | 25.2 | 15.5 | 73 |
| 4 | 2 | 0 | 1:1 | 3054 | 1817 | 25.2 | 15.5 | 88 |
| 1 | 1 | 1 | 1:1 | 3054 | 1793 | 25.2 | 15.5 | 112 |
| 2 | 1 | −4 | 1:1 | 3054 | 1735 | 25.2 | 15.5 | 170 |
| 4 | 2 | 2 | 1:2 | 3054 | 1779 | 25.2 | 30.9 | 111 |
| 4 | 2 | 0 | 1:2 | 3054 | 1752 | 25.2 | 30.9 | 138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolayenko, I.V.; Bazzicalupi, C.; Theron, T.-J.; Grimmer, C. Ni(II) and Cu(II) Ion Coordination by the Novel (2E,2′E)-N,N′-(2-Hydroxypropane-1,3-diyl)bis[(2-hydroxyimino)propanamide] Ligand in the Solid State and in Aqueous Medium. Inorganics 2024, 12, 330. https://doi.org/10.3390/inorganics12120330
Nikolayenko IV, Bazzicalupi C, Theron T-J, Grimmer C. Ni(II) and Cu(II) Ion Coordination by the Novel (2E,2′E)-N,N′-(2-Hydroxypropane-1,3-diyl)bis[(2-hydroxyimino)propanamide] Ligand in the Solid State and in Aqueous Medium. Inorganics. 2024; 12(12):330. https://doi.org/10.3390/inorganics12120330
Chicago/Turabian StyleNikolayenko, Igor Vasyl, Carla Bazzicalupi, Thomas-John Theron, and Craig Grimmer. 2024. "Ni(II) and Cu(II) Ion Coordination by the Novel (2E,2′E)-N,N′-(2-Hydroxypropane-1,3-diyl)bis[(2-hydroxyimino)propanamide] Ligand in the Solid State and in Aqueous Medium" Inorganics 12, no. 12: 330. https://doi.org/10.3390/inorganics12120330
APA StyleNikolayenko, I. V., Bazzicalupi, C., Theron, T.-J., & Grimmer, C. (2024). Ni(II) and Cu(II) Ion Coordination by the Novel (2E,2′E)-N,N′-(2-Hydroxypropane-1,3-diyl)bis[(2-hydroxyimino)propanamide] Ligand in the Solid State and in Aqueous Medium. Inorganics, 12(12), 330. https://doi.org/10.3390/inorganics12120330






