Facile Synthesis and Characterization of Novel CoFe2O4@MgO@(Mg0.23Co0.77)(Mg0.35Co1.65)O4@C and CoFe2O4@MgO@C Nanocomposites for Efficient Removal of Zn(II) Ions from Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
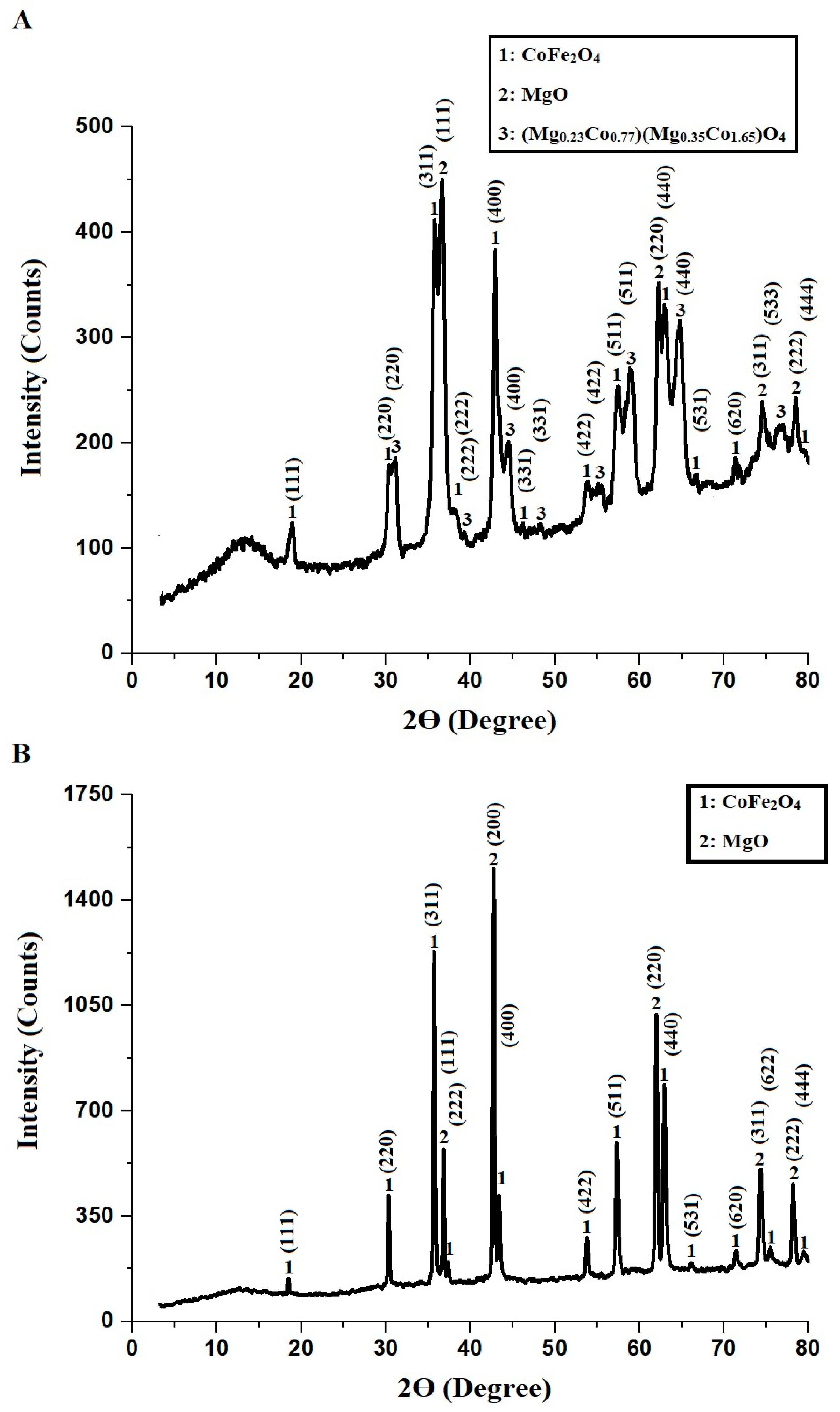
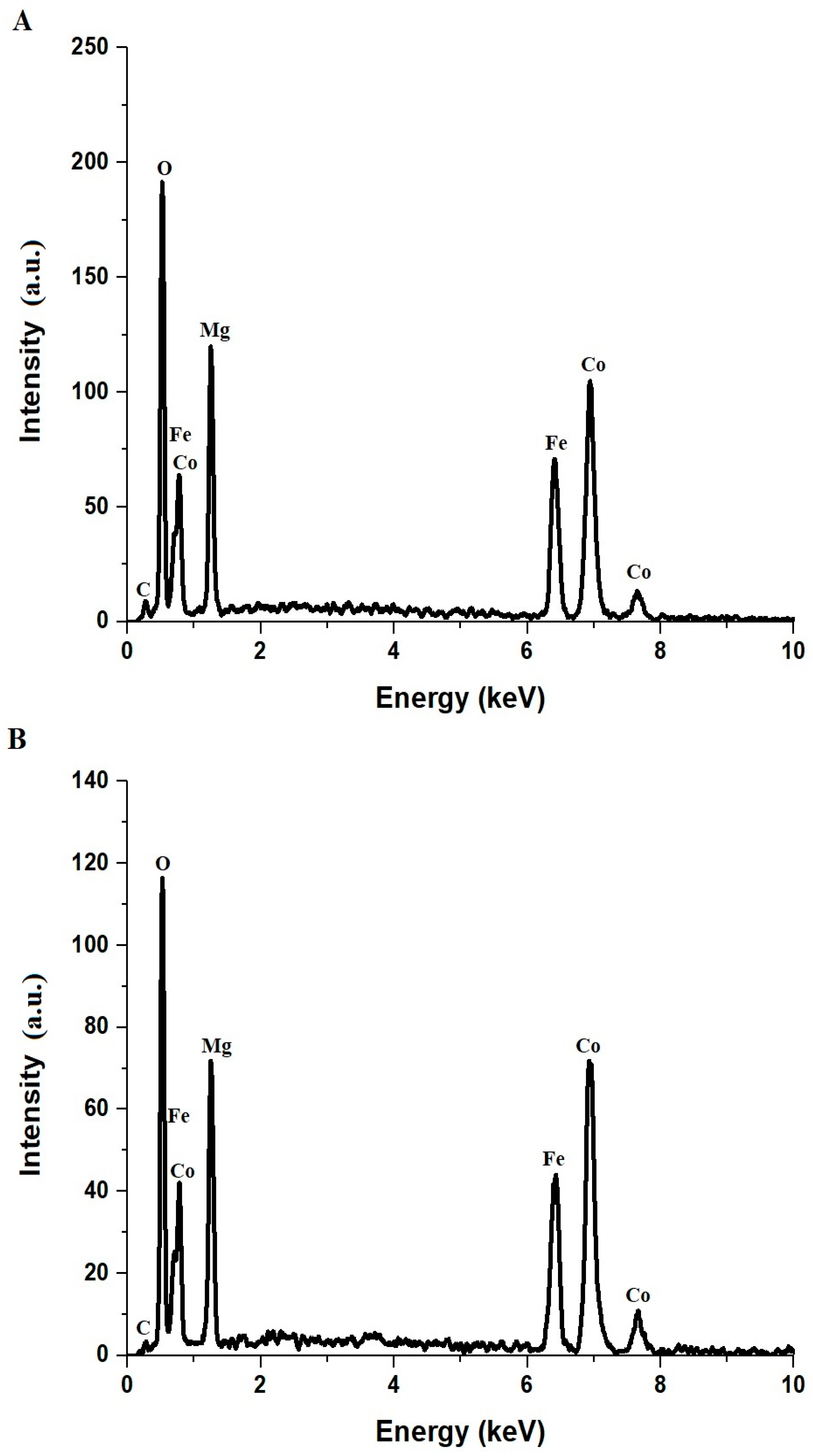
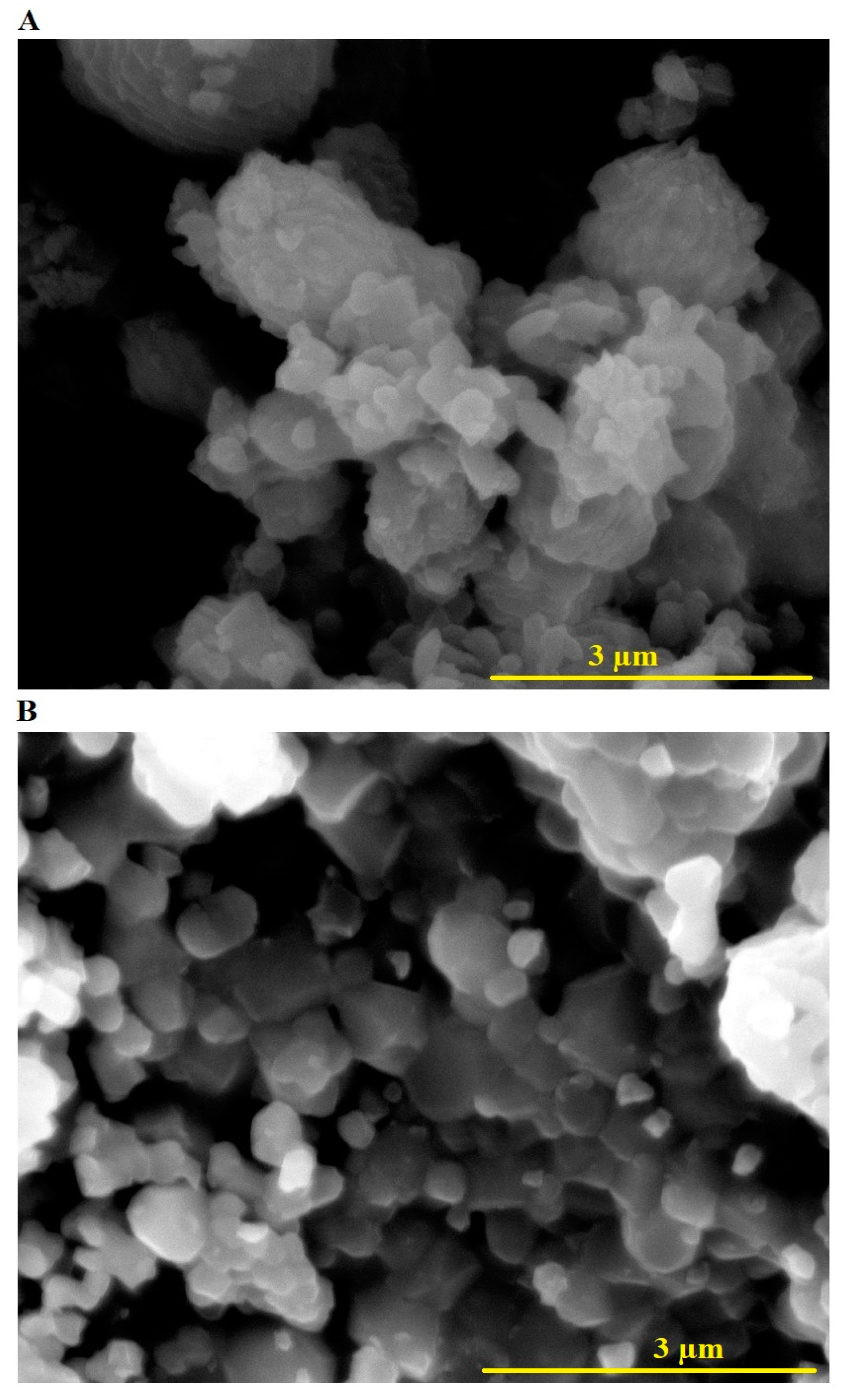
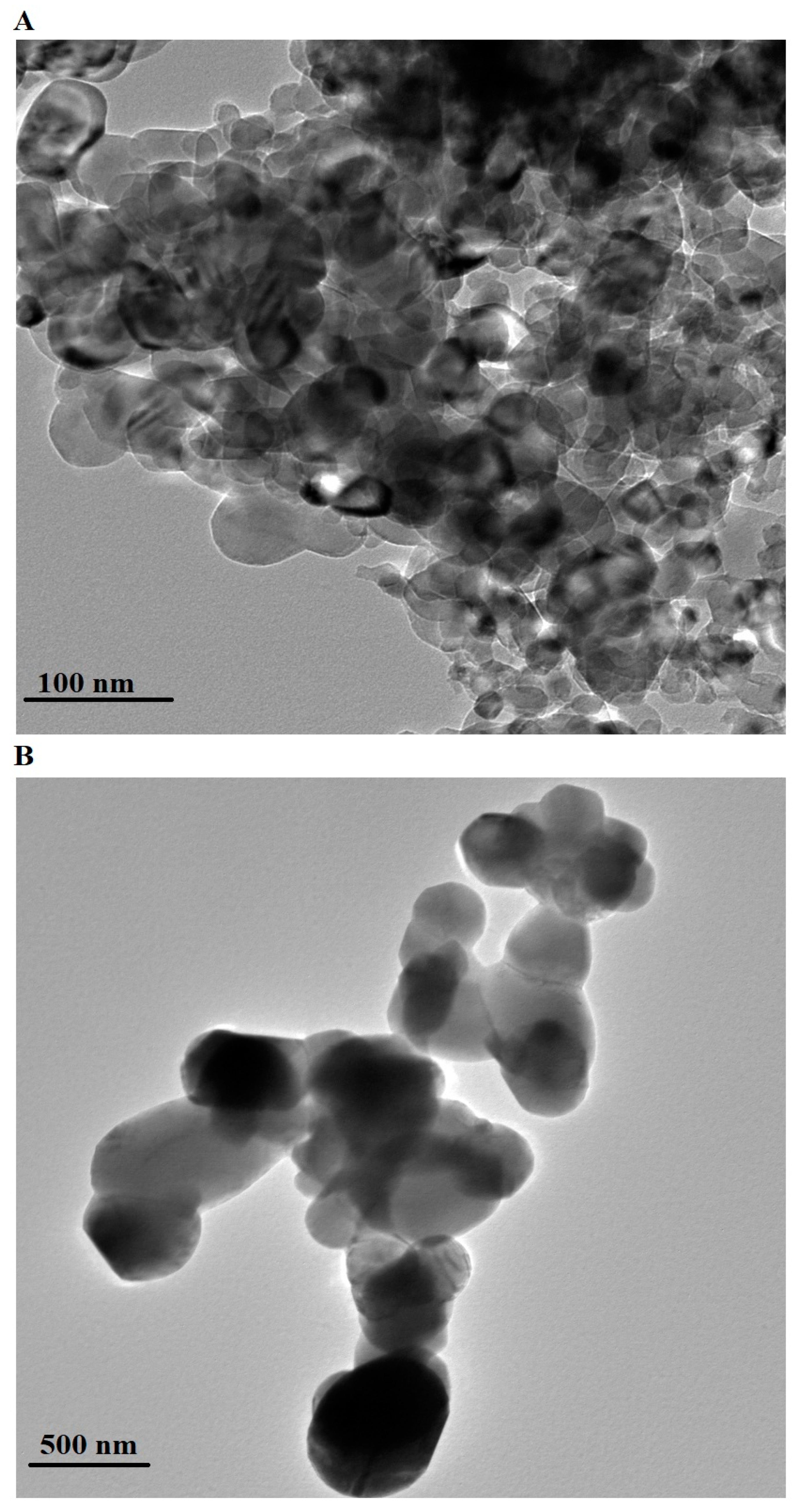
2.1. Characterization
2.2. Removal of Zn(II) Ions from Aqueous Media
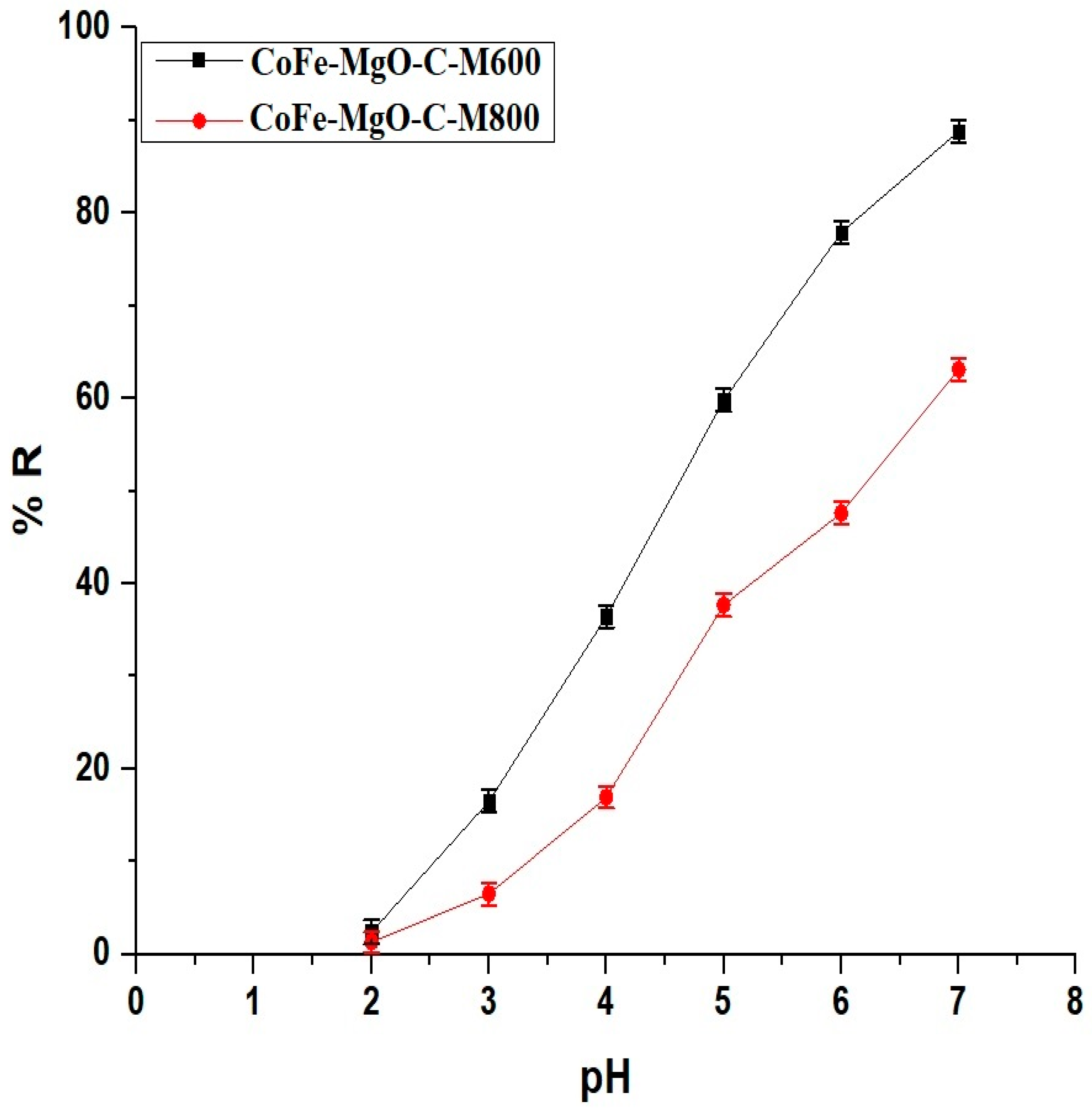
2.2.1. Effect of pH
Removal Mechanism of Zn(II) Ions Using CoFe-MgO-C-M600 and CoFe-MgO-C-M800 Nanocomposites
2.2.2. Effect of Contact Time
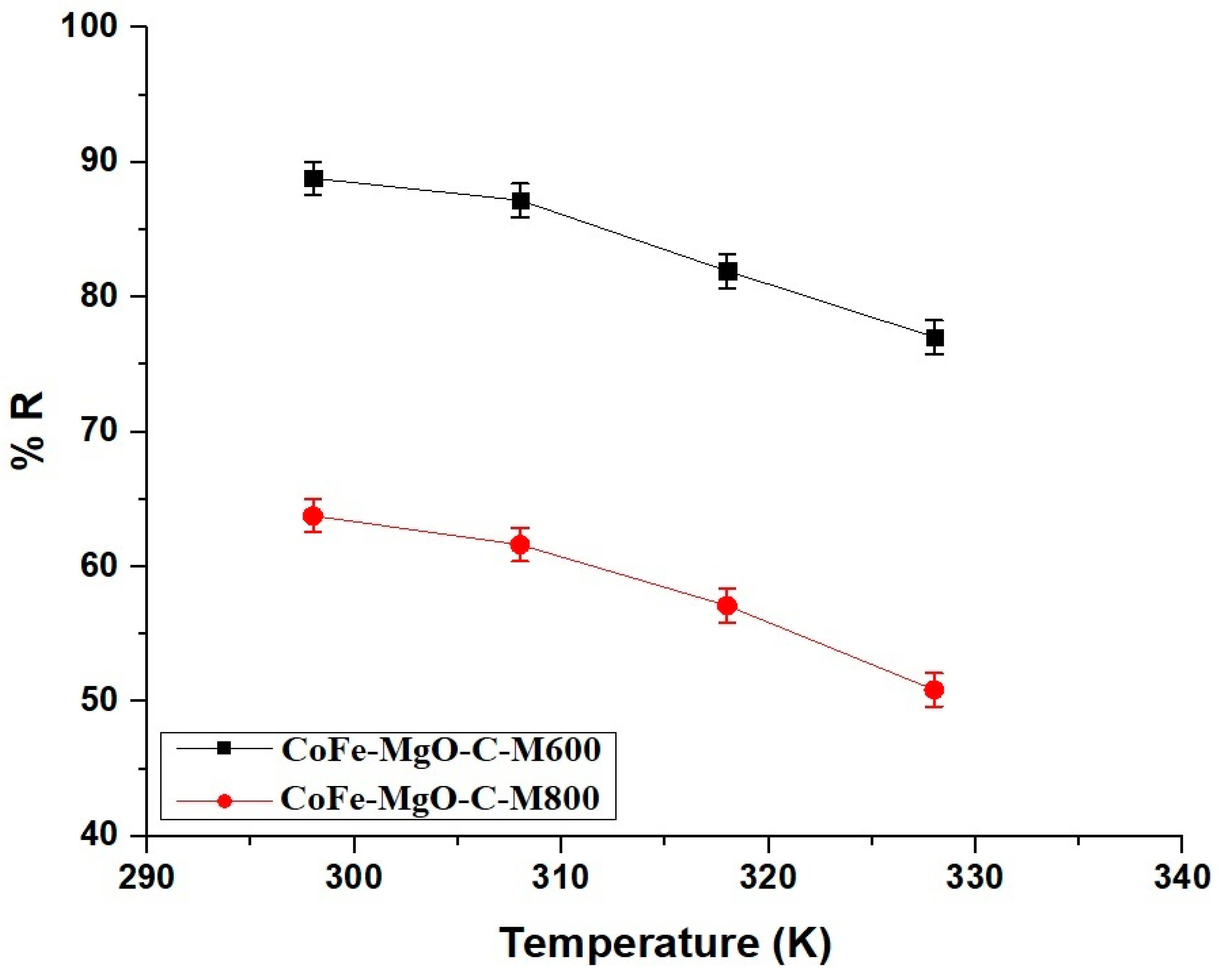
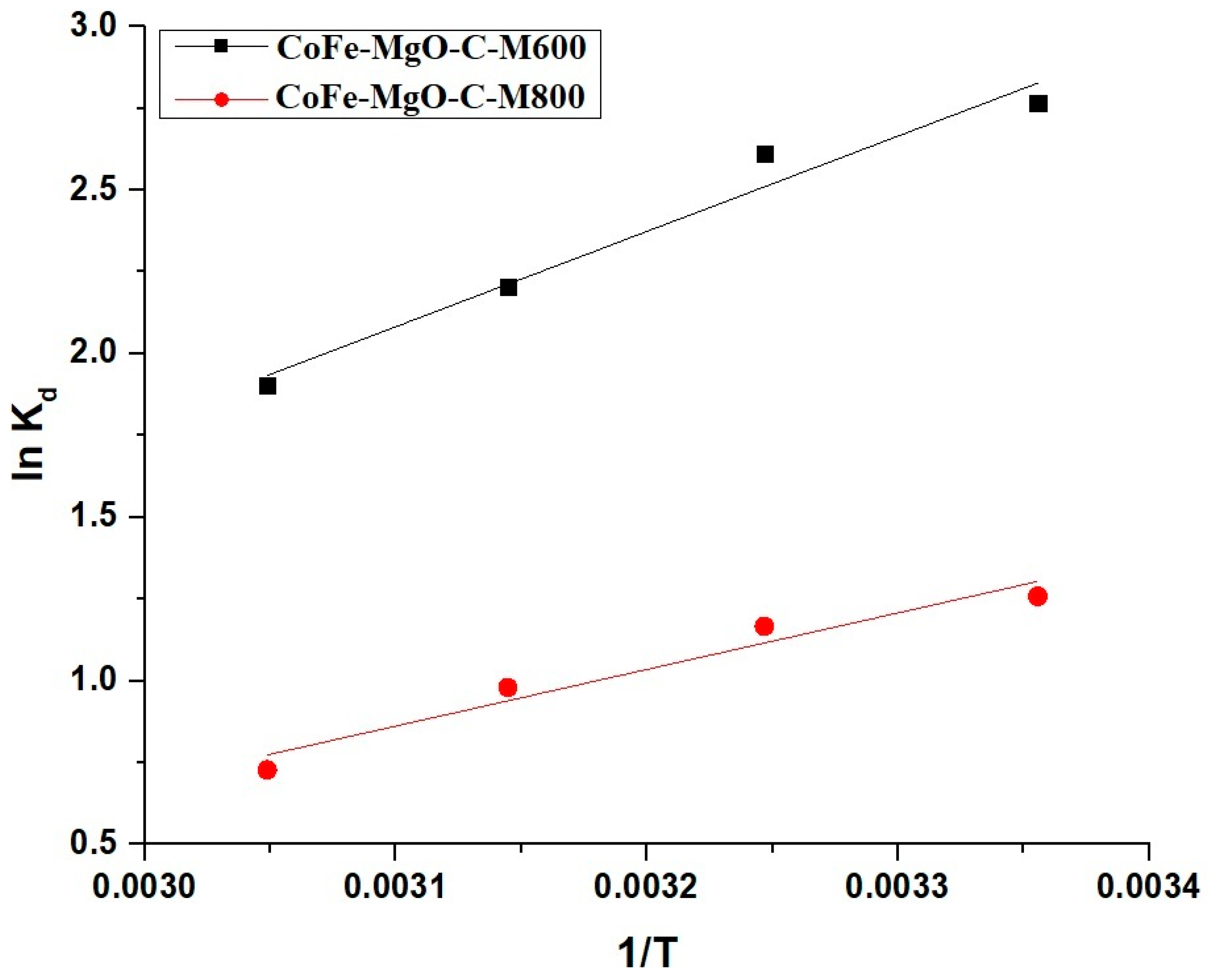
2.2.3. Effect of Temperature
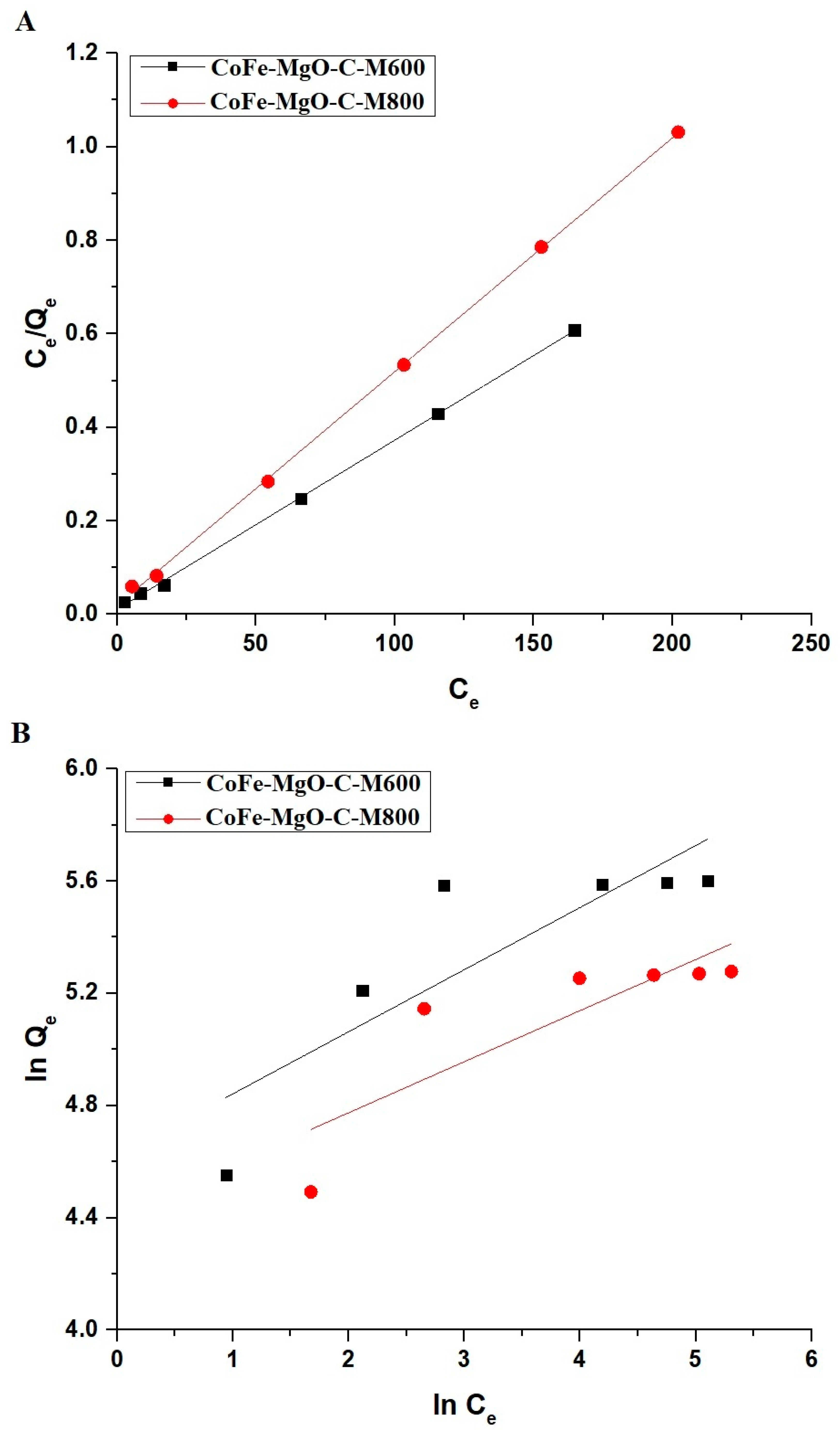
2.2.4. Effect of Concentration
3. Experimental
3.1. Materials
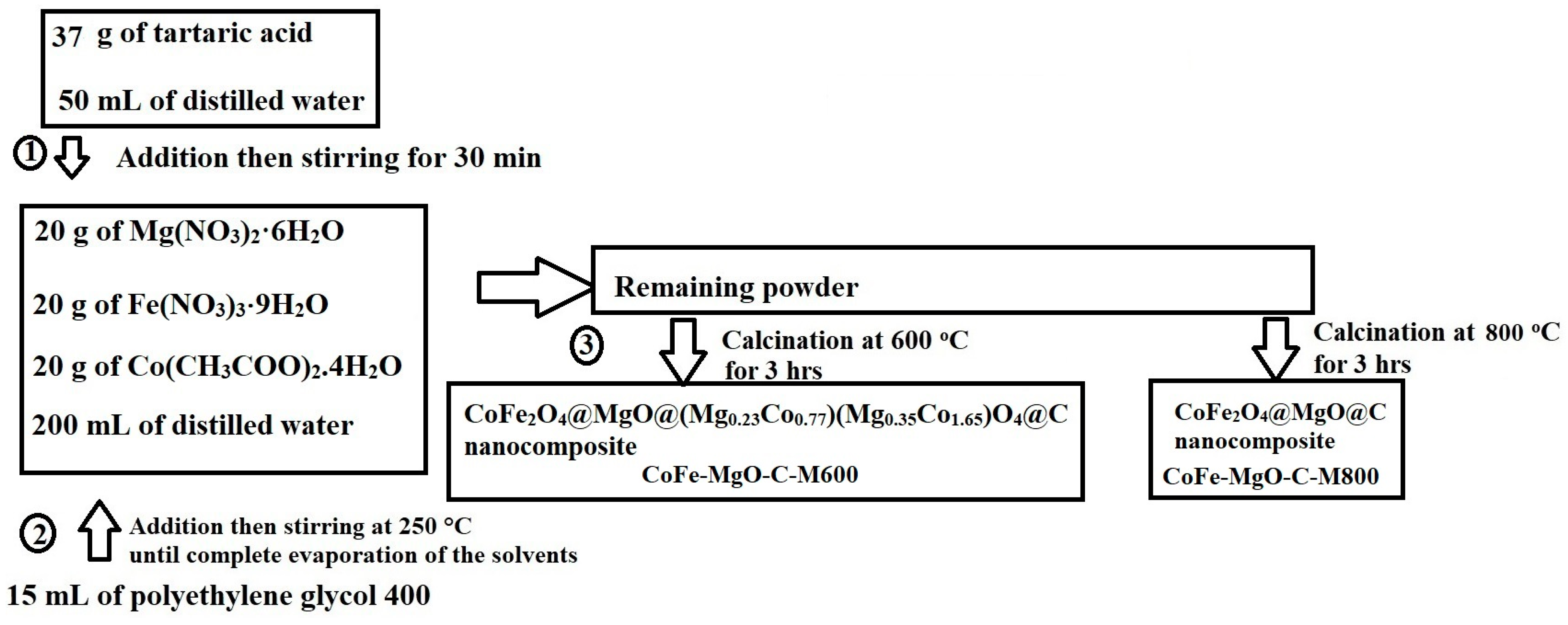
3.2. Synthesis of Nanocomposites
3.3. Instrumentation
3.4. Removal Procedure of Zn(II) Ions from Aqueous Media
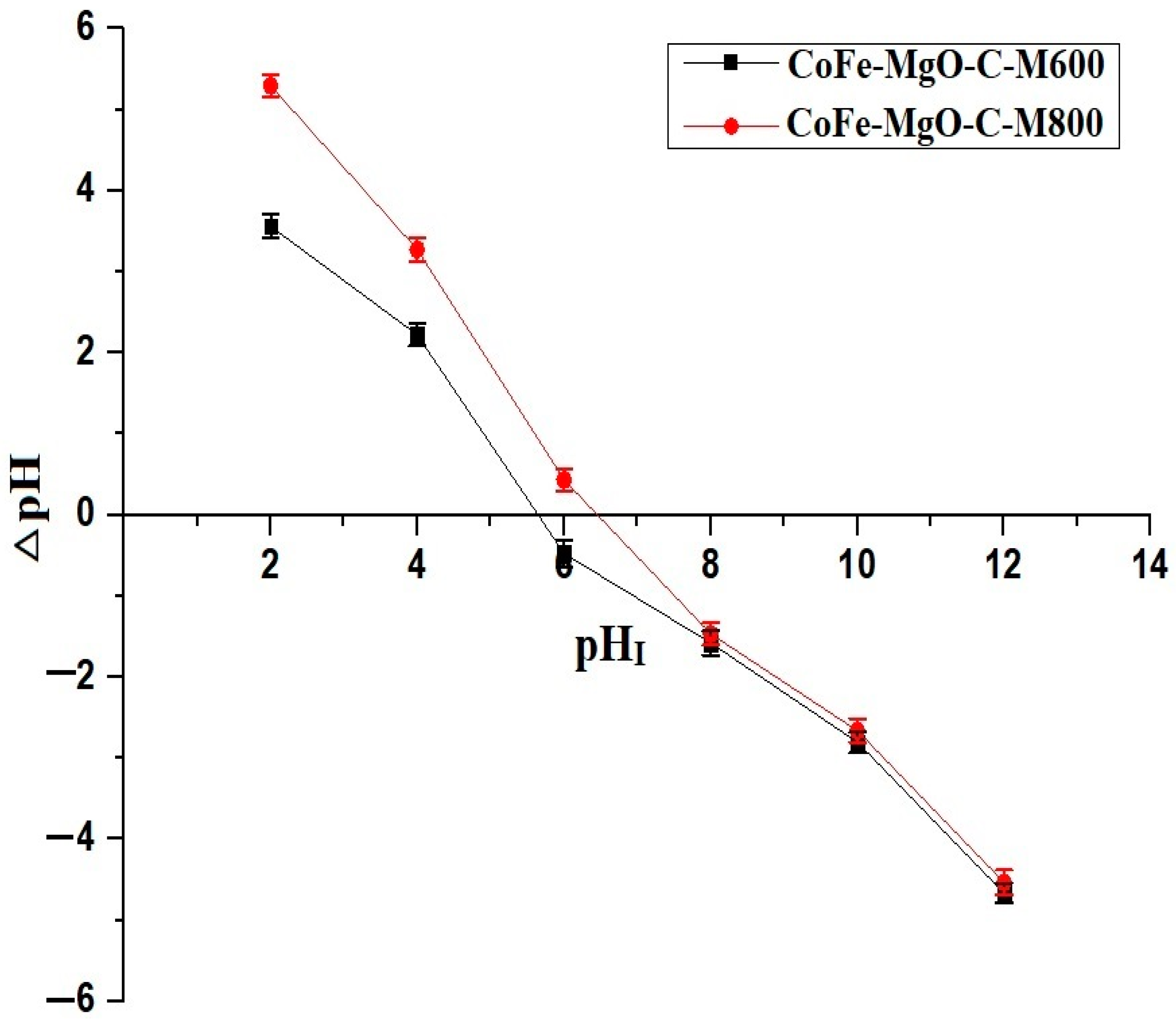
3.5. Point of Zero Charge (pHPZC) of Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, J.; Yu, Y.; Huang, S.; Zhu, N.; Mojiri, A.; Ge, D. Tannic Acid as a Green Chemical for the Removal of Various Heavy Metals: A Critical Review of Recent Developments. J. Environ. Manag. 2025, 375, 124390. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Mustafa, F.S.; Hamarawf, R.F.; Omer, K.M. Adsorptive Removal of Toxic Heavy Metals from Aquatic Environment by Metal Organic Framework (MOF): A Review. J. Water Process Eng. 2025, 70, 106867. [Google Scholar] [CrossRef]
- Baldev; Kumar, G.; Sharma, V.; Nemiwal, M. Biomass-Derived Zirconium Composite: An Adsorbent for Preferential Removal of Heavy Metals and Contaminants in Wastewater. J. Water Process Eng. 2025, 69, 106778. [Google Scholar] [CrossRef]
- Yu, K.; Yang, L.; Zhang, S.; Zhang, N. Nanocellulose-Based Aerogels for the Adsorption and Removal of Heavy-Metal Ions from Wastewater: A Review. Mater. Today Commun. 2025, 43, 111744. [Google Scholar] [CrossRef]
- Ávila, F.G.; Cabrera-Sumba, J.; Valdez-Pilataxi, S.; Villalta-Chungata, J.; Valdiviezo-Gonzales, L.; Alegria-Arnedo, C. Removal of Heavy Metals in Industrial Wastewater Using Adsorption Technology: Efficiency and Influencing Factors. Clean. Eng. Technol. 2025, 24, 100879. [Google Scholar] [CrossRef]
- Rana, P.; Kaur, B.; Poonia, K.; Soni, V.; Singh, P.; Thakur, S.; Huang, C.W.; Nguyen, V.H.; Raizada, P. Recent Advancements in Polythiophene-Based Adsorbents for Heavy Metal Ion Removal: Modification, Kinetics and Mechanistic Insights. Inorg. Chem. Commun. 2025, 172, 113657. [Google Scholar] [CrossRef]
- Yang, T.; Gao, H.; Chen, H.; Xiao, X.; Zhao, C.; Gong, H.; Li, X.; Liu, L.; Liu, Y. Insights and Perspectives of Chitosan-Based Hydrogels for the Removal of Heavy Metals and Dyes from Wastewater. Int. J. Biol. Macromol. 2025, 292, 139280. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Faruque, M.O.; Uddin, S.; Hossain, M.M.; Hossain, S.M.Z.; Shafiquzzaman, M.; Razzak, S.A. A Comprehensive Review on Microalgae-Driven Heavy Metals Removal from Industrial Wastewater Using Living and Nonliving Microalgae. J. Hazard. Mater. Adv. 2024, 16, 100492. [Google Scholar] [CrossRef]
- Jaffar, F.H.; Othman, M.H.D.; Ismail, N.J.; Puteh, M.H.; Kurniawan, T.A.; Abu Bakar, S.; Abdullah, H. Hydroxyapatite-Based Materials for Adsorption, and Adsorptive Membrane Process for Heavy Metal Removal from Wastewater: Recent Progress, Bottleneck and Opportunities. J. Taiwan Inst. Chem. Eng. 2024, 164, 105668. [Google Scholar] [CrossRef]
- Miyah, Y.; El Messaoudi, N.; Benjelloun, M.; Georgin, J.; Franco, D.S.P.; El-habacha, M.; Ali, O.A.; Acikbas, Y. A Comprehensive Review of β-Cyclodextrin Polymer Nanocomposites Exploration for Heavy Metal Removal from Wastewater. Carbohydr. Polym. 2025, 350, 122981. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Alam, O.; Zhou, Y.; Du, D.; Li, G.; Zhu, W. Heavy Metals Detection and Removal from Contaminated Water: A Critical Review of Adsorption Methods. J. Environ. Chem. Eng. 2024, 12, 114366. [Google Scholar] [CrossRef]
- Alnasra, O.A.; Khalili, F.I.; Alhnafat, F.A. Enhanced Removal of Pb(II), Zn(II) and Cd(II) Ions by Insolubilized Humic Acid: Characterization and Sorption Behaviors. Desalin. Water Treat. 2024, 320, 100604. [Google Scholar] [CrossRef]
- Nizam, T.; Krishnan, K.A.; Joseph, A.; Krishnan, R.R. Isotherm, Kinetic and Thermodynamic Modelling of Liquid Phase Adsorption of the Heavy Metal Ions Zn(II), Pb(II) and Cr(VI) onto MgFe2O4 Nanoparticles. Groundw. Sustain. Dev. 2024, 25, 101120. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Liu, S.; Zhang, J.; Yang, X. Molecular Simulation of Reverse Osmosis for Heavy Metal Ions Using Functionalized Nanoporous Graphenes. Comput. Mater. Sci. 2017, 139, 65–74. [Google Scholar] [CrossRef]
- Hou, R.; Bao, S.; Dai, J.; Yan, W.; Yao, Y.; Zhou, Y.; Gao, C. Development of Novel Nanofiltration Membrane via Cross-Linking a Natural Polymer and Its Application in Heavy Metals Removal from Groundwater. J. Memb. Sci. 2025, 719, 123768. [Google Scholar] [CrossRef]
- Vairamuthu, M.; Nidheesh, P.V.; Anantha Singh, T.S. Treatment of Unregulated Open Dumping Site Soil by Combined Aloe Vera Gel Washing and Electrocoagulation for the Removal of Microplastics and Heavy Metals. J. Environ. Chem. Eng. 2025, 13, 115555. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseini, S.M.; Shabanian, M. Novel Electrodialysis Cation Exchange Membrane Prepared by 2-Acrylamido-2-Methylpropane Sulfonic Acid; Heavy Metal Ions Removal. J. Hazard. Mater. 2017, 337, 90–104. [Google Scholar] [CrossRef]
- Zhang, L.; Su, J.; Liu, S.; Huang, T.; Wang, Z.; Liu, Y.; Hou, C.; Wang, X. Calcium Self-Release Bioremediation System Combined with Microbially Induced Calcium Precipitation for the Removal of Ammonium Nitrogen, Phosphorus and Heavy Metals. J. Environ. Chem. Eng. 2024, 12, 114190. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, D.; Song, S.; Zhang, J.; Yang, Y.; Chen, Z.; Lu, J.; Wang, S.; Sun, Y. Removal of Heavy Metals from Water by Adsorption on Metal Organic Frameworks: Research Progress and Mechanistic Analysis in the Last Decade. Chem. Eng. J. 2025, 506, 160063. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic Reductive Preparation of Magnetic Inverse Spinel Iron Oxide Nanoparticles for the Adsorption Removal of Heavy Metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Precious, N.; Patience, T.; Kweinor, E.; Rathilal, S. Review on Advancing Heavy Metals Removal: The Use of Iron Oxide Nanoparticles and Microalgae-Based Adsorbents. Clean. Chem. Eng. 2025, 11, 100137. [Google Scholar] [CrossRef]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent Advances in Application of Iron-Manganese Oxide Nanomaterials for Removal of Heavy Metals in the Aquatic Environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef]
- Xu, C.; Shi, S.; Wang, X.; Zhou, H.; Wang, L.; Zhu, L.; Zhang, G.; Xu, D. Electrospun SiO2-MgO Hybrid Fibers for Heavy Metal Removal: Characterization and Adsorption Study of Pb(II) and Cu(II). J. Hazard. Mater. 2020, 381, 120974. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Shah, R.K.; Abou-krisha, M.M.; Saad, F.A.; Munshi, A.M. Facile Synthesis and Characterization of Novel Fe0.65Mg0.35Cr2O4@C Nanocomposite for Efficient Removal of Cd(II) Ions from Aqueous Media. Inorganics 2025, 13, 82. [Google Scholar] [CrossRef]
- Afriyie, E.; Arthur, E.K.; Gikunoo, E.; Boateng, A. Bentonite-Assisted Carbonisation of Waste Polyethylene Terephthalate Bottles for the Removal of Heavy Metals from Polluted Water. Results Surf. Interfaces 2025, 18, 100406. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, Y.; Xue, L.; Lu, Y.; Hu, R.; Yu, J.; Jiang, X.; Sun, J. International Journal of Biological Macromolecules Phosphorylated Chitosan-Lignin Composites for Efficient Removal of Pb ( II ) and Cu ( II ) from Aqueous Environments and Sustainable Upcycling of Spent Adsorbents. Int. J. Biol. Macromol. 2025, 304, 140840. [Google Scholar] [CrossRef]
- Ehab, N.S.A.; Fowzia, A.A.; Fawaz, S.A.; Doaa, A.S. Facile Synthesis of MnCO3/ZrO2/MgCO3 Nanocomposite for High-Efficiency Malachite Green Dye Removal. J. Inorg. Organomet. Polym. Mater. 2025, in press. [Google Scholar] [CrossRef]
- Saberi-Zare, M.; Bodaghifard, M.A. A Schiff Base-Functionalized Chitosan Magnetic Bio-Nanocomposite for Efficient Removal of Pb (II) and Cd (II) Ions from Aqueous Solutions. Int. J. Biol. Macromol. 2025, 296, 139794. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) Ions from Wastewater Using Polyethyleneimine (PEI) Cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Yusop, M.F.; Mohd Johan Jaya, E.; Ahmad, M.A. Single-Stage Microwave Assisted Coconut Shell Based Activated Carbon for Removal of Zn(II) Ions from Aqueous Solution—Optimization and Batch Studies. Arab. J. Chem. 2022, 15, 104011. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Naglah, A.M.; Saad, F.A.; Abdelrahman, E.A. Modification of Silica Nanoparticles with 1-Hydroxy-2-Acetonaphthone as a Novel Composite for the Efficient Removal of Ni(II), Cu(II), Zn(II), and Hg(II) Ions from Aqueous Media. Arab. J. Chem. 2022, 15, 104010. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous Removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) Ions from Aqueous Solutions via Adsorption on FAU-Type Zeolites Prepared from Coal Fly Ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Fadhel, S.R. Chitosan-NiFe2O4 Nanocomposite Synthesis for Effective Removal of Pb(II) and Zn(II) from Aqueous Solution. Results Eng. 2024, 24, 103293. [Google Scholar] [CrossRef]
- Panahandeh, A.; Parvareh, A.; Moraveji, M.K. Synthesis and Characterization of γ-MnO2/Chitosan/Fe3O4 Cross-Linked with EDTA and the Study of Its Efficiency for the Elimination of Zinc(II) and Lead(II) from Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 9235–9254. [Google Scholar] [CrossRef]
- Al-kadhi, N.S.; Abdelrahman, E.A.; Alamro, F.S.; Shah, R.K. Synthesis of Novel Magnesium Ferrite Schiff Base Chitosan Nanocomposite for Efficient Removal of Pb (II) Ions from Aqueous Media. Sci. Rep. 2025, 15, 4153. [Google Scholar] [CrossRef]




| Sample | Atomic Percentages | Weight Percentages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| %C | %O | %Mg | %Fe | %Co | %C | %O | %Mg | %Fe | %Co | |
| CoFe-MgO-C-M600 | 4.3 | 44.5 | 18.3 | 11.6 | 21.3 | 1.6 | 22.9 | 14.3 | 20.8 | 40.4 |
| CoFe-MgO-C-M800 | 1.6 | 42.6 | 19.9 | 13.9 | 22.0 | 0.6 | 21.0 | 14.9 | 23.8 | 39.7 |
| Sample | QExp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| K1 (1/min) | R2 | Qe (mg/g) | K2 (g/mg.min) | R2 | Qe (mg/g) | ||
| CoFe-MgO-C-M600 | 266.44 | 0.0444 | 0.9986 | 85.63 | 0.001232 | 0.9994 | 269.54 |
| CoFe-MgO-C-M800 | 191.32 | 0.02835 | 0.9720 | 106.53 | 0.000542 | 0.9994 | 196.46 |
| Sample | △S° (KJ/molK) | △H° (KJ/mol) | △G° (KJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| CoFe-MgO-C-M600 | 0.05804 | −24.34 | −41.63 | −42.22 | −42.79 | −43.38 |
| CoFe-MgO-C-M800 | 0.03758 | −14.45 | −25.65 | −26.02 | −26.40 | −26.78 |
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | R2 | K3 (L/mg) | K4 (mg/g)(L/mg)1/n | Qmax (mg/g) | 1/n | R2 | |
| CoFe-MgO-C-M600 | 276.24 | 0.9993 | 0.3181 | 101.59 | 328.02 | 0.2212 | 0.6674 |
| CoFe-MgO-C-M800 | 200.00 | 0.9995 | 0.2548 | 82.32 | 215.94 | 0.1820 | 0.6374 |
| Adsorbent | Qmax (mg/g) | Ref. |
|---|---|---|
| Polyethyleneimine | 24.39 | [31] |
| Activated carbon | 7.87 | [32] |
| Silica/1-hydroxy-2-acetonaphthone composite | 45.13 | [33] |
| FAU Zeolite | 36.77 | [34] |
| NiFe2O4/Chitosan composite | 90.70 | [35] |
| γ-MnO2/chitosan/Fe3O4/EDTA composite | 103.40 | [36] |
| CoFe-MgO-C-M600 | 276.24 | This study |
| CoFe-MgO-C-M800 | 200.00 | This study |
| Effect | V (L) | CI (mg/L) | M (mg) | T (K) | t (min) | pH |
|---|---|---|---|---|---|---|
| pH | 100 | 150 | 50 | 298 | 360 | 2–7 |
| Time | 100 | 150 | 50 | 298 | 10–100 | 7 |
| Temperature | 100 | 150 | 50 | 298–328 | 50 (CoFe-MgO-C-M600) 70 (CoFe-MgO-C-M800) | 7 |
| Concentration | 100 | 50–300 | 50 | 298 | 50 (CoFe-MgO-C-M600) 70 (CoFe-MgO-C-M800) | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, E.A.; Shah, R.K.; Abou-Krisha, M.M.; Saad, F.A.; Khedr, A.M. Facile Synthesis and Characterization of Novel CoFe2O4@MgO@(Mg0.23Co0.77)(Mg0.35Co1.65)O4@C and CoFe2O4@MgO@C Nanocomposites for Efficient Removal of Zn(II) Ions from Aqueous Media. Inorganics 2025, 13, 101. https://doi.org/10.3390/inorganics13040101
Abdelrahman EA, Shah RK, Abou-Krisha MM, Saad FA, Khedr AM. Facile Synthesis and Characterization of Novel CoFe2O4@MgO@(Mg0.23Co0.77)(Mg0.35Co1.65)O4@C and CoFe2O4@MgO@C Nanocomposites for Efficient Removal of Zn(II) Ions from Aqueous Media. Inorganics. 2025; 13(4):101. https://doi.org/10.3390/inorganics13040101
Chicago/Turabian StyleAbdelrahman, Ehab A., Reem K. Shah, Mortaga M. Abou-Krisha, Fawaz A. Saad, and Abdalla M. Khedr. 2025. "Facile Synthesis and Characterization of Novel CoFe2O4@MgO@(Mg0.23Co0.77)(Mg0.35Co1.65)O4@C and CoFe2O4@MgO@C Nanocomposites for Efficient Removal of Zn(II) Ions from Aqueous Media" Inorganics 13, no. 4: 101. https://doi.org/10.3390/inorganics13040101
APA StyleAbdelrahman, E. A., Shah, R. K., Abou-Krisha, M. M., Saad, F. A., & Khedr, A. M. (2025). Facile Synthesis and Characterization of Novel CoFe2O4@MgO@(Mg0.23Co0.77)(Mg0.35Co1.65)O4@C and CoFe2O4@MgO@C Nanocomposites for Efficient Removal of Zn(II) Ions from Aqueous Media. Inorganics, 13(4), 101. https://doi.org/10.3390/inorganics13040101






