Abstract
Gas-phase studies of fullerenes and metallofullerenes, though less well explored compared to condensed-phase research in recent years, offer critical insights into the mechanisms governing their formation and behavior. In this study, we re-examined the coalescence reactions of fullerenes using a high-resolution Fourier transform ion cyclotron resonance (FT ICR) mass spectrometer, especially the effect of electric fields in the source region on the formation of large-sized fullerenes. By varying the voltages on the metal plate where the C60 was deposited, we achieved enhanced control over the coalescence process, revealing distinct distributions of fullerene products that differ from those reported in earlier studies. What is the most attractive is that a negative voltage applied on the metal plate is actually more conducive to the production of large-sized fullerene cations. Notably, we identified previously unobserved species, including doubly charged fullerene cations (e.g., C1602+) and metallofullerene ions (e.g., Y1–2C94–124+), providing new evidence for the complexity of gas-phase fullerene chemistry. These findings underscore the importance of source region electric fields in shaping coalescence outcomes and highlight the potential of gas-phase approaches for synthesizing novel metallofullerenes.
1. Introduction
The coalescence reactions of fullerenes, first investigated by Yeretzian et al., represent an interesting phenomenon in the field of carbon cluster chemistry. Under laser ablation or laser desorption/ionization (LDI) conditions, C60 and C70 molecules can undergo coalescence to form larger fullerenes, such as C118, C178, and beyond [1,2]. This process was attributed to high-energy collisions and subsequent fusion of fullerene cages in the gas phase, facilitated by the high-temperature environment of the laser plasma. Their work not only provided the experimental evidence of fullerene coalescence but also highlighted the critical role of carrier gases, such as helium, in enhancing the reaction efficiency by cooling the excited clusters and promoting collisions.
Subsequent research further elucidated the mechanisms and conditions governing fullerene coalescence, but there are also inconsistencies in these results [3,4,5,6,7,8]. For instance, Liu et al. explored the coalescence of fullerene anions, as well as that of cations [3]. Although their experimental distributions of the large-size fullerene cations consist with those of Yeretzian et al. quite well, those of anions are very different. In Yeretzian’s results, no coalesced anions were observed in the negative ion mode, while in Liu’s results, the distribution of coalesced anions is reported to be very close to that of cations, suggesting a universal mechanism independent of charge state [1,3]. The later experiments by Zeegers et al. also failed to observe coalesced anions under various experimental conditions [6].
Moreover, theoretical studies have complemented experimental findings by providing insights into the energetics and dynamics of fullerene fusion [9,10,11,12,13,14,15]. Typically, these calculations and simulations have revealed that coalescence involves the breaking of carbon–carbon bonds in the fullerene cages, followed by the rearrangement of carbon atoms to form larger, stable structures. Based on all this progress, new requirements have been put forward to experimental scientists: how can we better explain the observed experimental results (including those inconsistencies) based on existing results, and conduct better experimental designs for the precise preparation of large-sized fullerenes or new endohedral fullerenes? In fact, previous results have shown that both experimental conditions and setups have significant impacts on the observed ion distribution. For example, studies have shown that carrier gases like helium or nitrogen not only enhance collision rates but also act as cooling agents, stabilizing the nascent coalesced clusters and preventing their fragmentation [1,2]. However, experiments conducted in high vacuums, such as those by Liu and others, could still yield strong coalescence signals, even without the cooling and collision-promoting effects due to the carrier gas [3].
Despite the above-related studies on the topic, the research on the effects of the source region electrical field on the formation of the coalesced clusters still needs further study. Thus, we believe that it is meaningful to use our laboratory’s methods to reinvest this process, especially focusing on the effect of the electrical field in the source region [16,17,18,19,20]. With the help of the high resolution of the applied Fourier transform ion cyclotron resonance (FT ICR) mass spectrometer, the species generated under different electrical fields can be clearly identified. Since the voltage applied on the sample plate can be adjusted readily, providing new controllable experimental parameters in such processes. Our findings reported herein further underscore the importance of the electrical field in shaping coalescence outcomes and highlight the potential of gas-phase approaches for synthesizing metallofullerenes.
2. Experimental Results
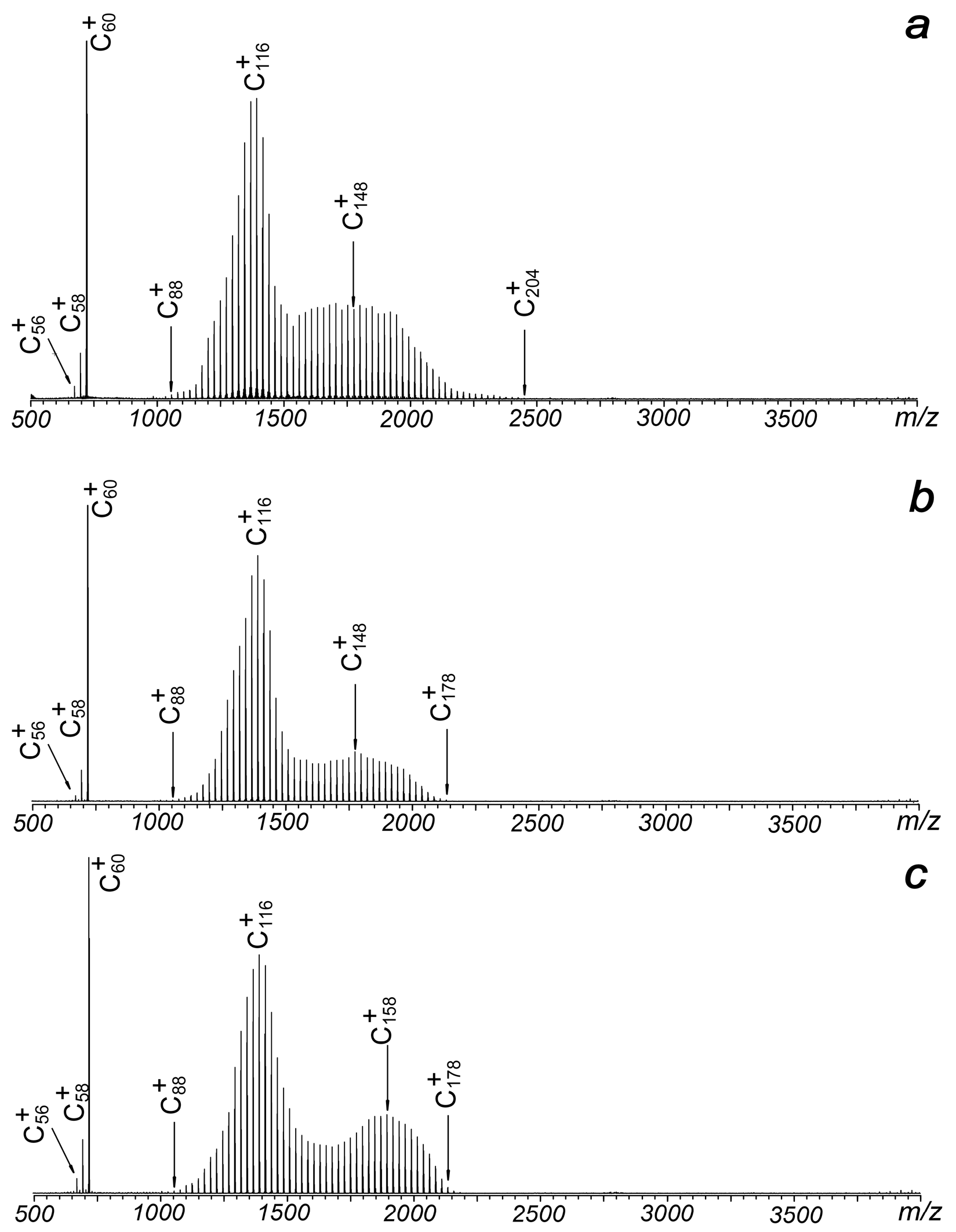
Figure 1 illustrates the typical laser ablation mass spectra of C60 in the positive ion mode under different voltages applied to the plate. It is observed that the plate voltage has minimal impact on the distribution of observed ions as it varies from 0 to 95 volts. In all cases, the signals of C60 cations are consistently accompanied by a strong oscillation centered at C116 and another oscillation near C148 or C158. When a positive voltage is applied to the metal plate (Figure 2b,c), the largest observed cluster ions are C178+ or C180+, which are smaller than the C60 trimer. When the positive voltage is removed, the size of the observed clusters increases to C204+, although the two observed oscillations remain largely unchanged.
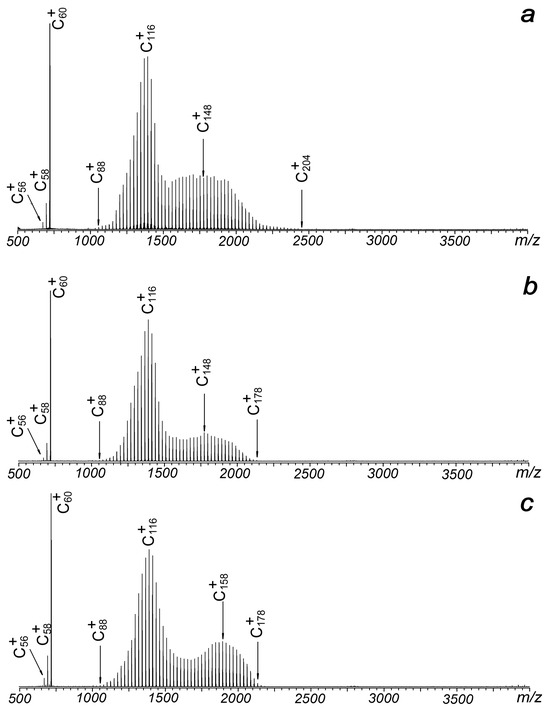
Figure 1.
Laser ablation mass spectra of C60 under different plate voltages: (a) 0 V, (b) 50 V and (c) 95 V. The mass spectra are collected under the positive ion mode.
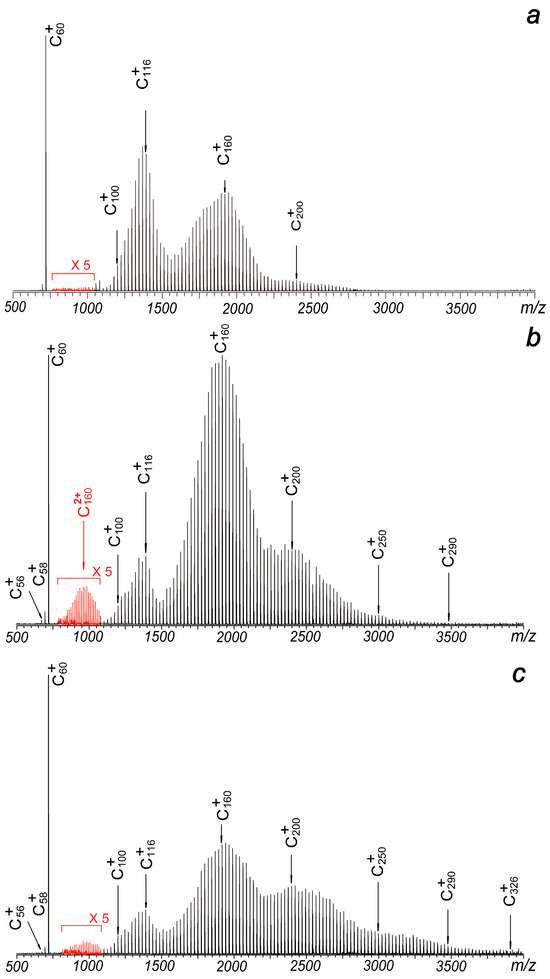
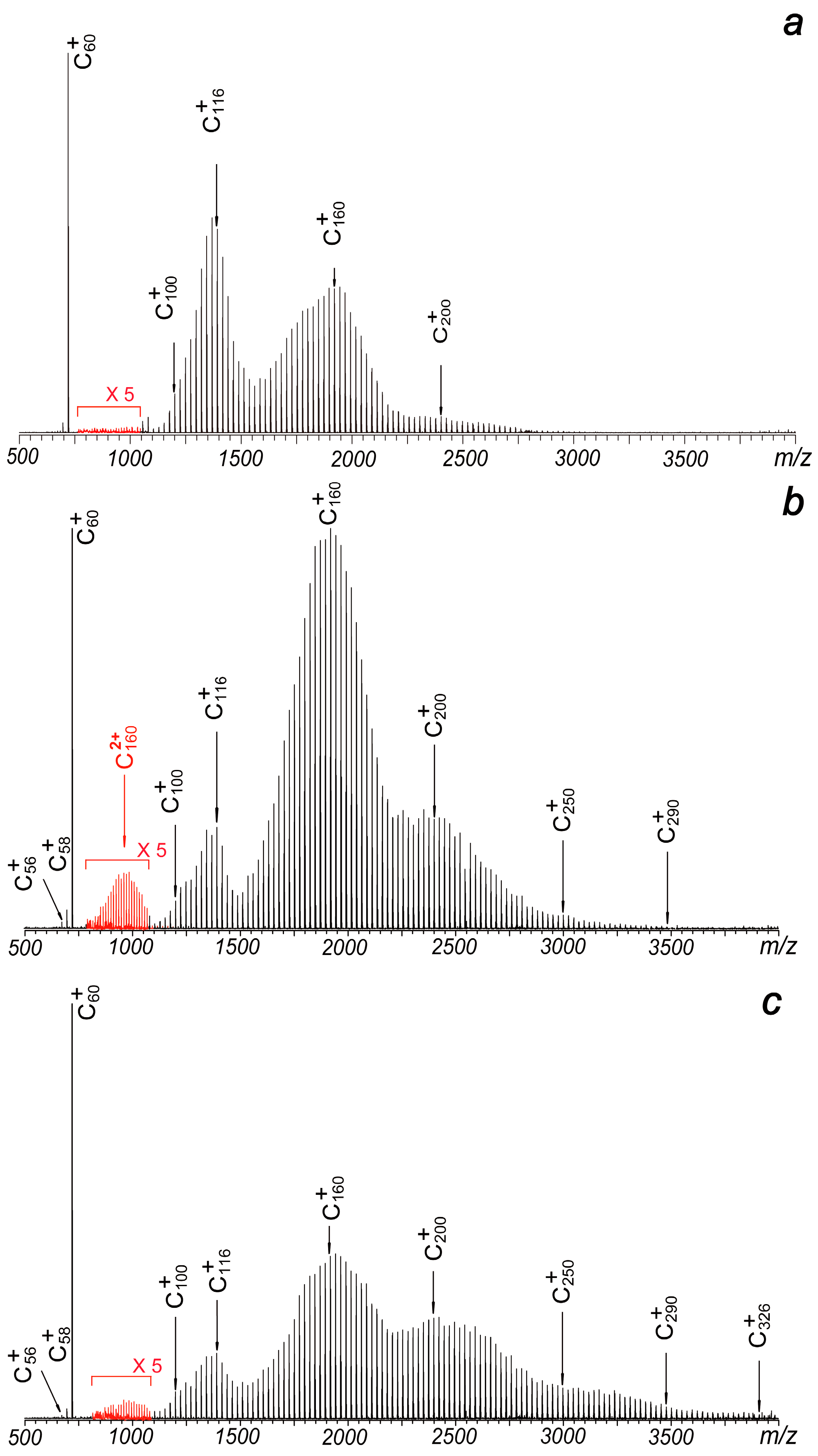
Figure 2.
Laser ablation mass spectra of C60 under different plate voltages: (a) −15 V, (b) −50 V and (c) −95 V. The mass spectra are collected under the positive ion mode. Doubly charged ions can be observed in some of them (the red peaks of which intensities have been amplified to 5 times their original values).
Compared with previously reported results, both similarities and differences are observed [1,2,3,4,5,6,7,8]. First, there is no evidence of oscillations centered near the tetramer or pentamer of C60. Even for the second distribution, which is centered at C148+ or C158+, it is distinct from the previously reported centers at C178+ or C168+, which were clearly generated from the coalescence of three C60 units [1,2,3]. However, the strongest oscillation reported here, centered at C116+, is very close to the previously reported centers at C118+ [1,2,3]. This suggests that it originates from the coalescence of two C60 units, followed by the emission of small even-numbered fragments. The probabilities of fragment capture are lower than those of fragment loss, as indicated by the asymmetrical distribution in the region spanning from C100+ to C140+. Second, fragment ions of C58+ and C56+ were clearly observed in all experiments, consistent with previous results [1,2,3]. Coalescence reactions between these fragments and C60 can also contribute to the formation of ions such as C118+ or C116+, although their contributions are still challenging to distinguish. The results also provide insight into the temperature of the laser-induced plasma, as the energy required for the fragmentation of C60 is approximately 11 eV [1]. Previous molecular dynamics (MD) simulations by Wang et al. about C118+ indicated that C58+ has a much higher reactivity than C60+ [11]. The species of C58+ and C56+, although weak, are very important in the formation of large-sized fullerene ions for low-energy collisions.
Typically, in standard matrix-assisted laser desorption ionization (MALDI) operations with this setup, the metal plate is added with positive voltages under the positive ion mode, since a negatively charged metal plate can greatly suppress the extraction efficiency of positive ions, reducing the collection efficiency of positive cations. However, in this experiment, when the metal plate is subjected to −15 V, which means the source region between the plate and the hexapole (with a bias voltage of −15 V) is free of electric field, strong signals of C60+, and coalesced clusters centered at C116+ and C200+ can be clearly identified. The result is shown in Figure 2a. The total intensities of all observed cations in Figure 2a are just slightly lower than those in Figure 1b. Even more, when the metal plate is subjected to more negative voltages while keeping other experimental conditions constant, strong signals of C60+ and widely distributed product ions extending up to C290+ or C326+ become distinctly observable. As illustrated in Figure 2b, a −50 V plate voltage leads to four distinct ion distributions centered at C116+, C160+, C200+ and C250+. These intensity patterns differ significantly from earlier reports [1,2,3,4,5]. As the plate voltage increases to −95 V, the distributions expand further (Figure 2c). The notable enhancement in both signal strength and distribution width observed in Figure 3 suggests that ions with higher translational energy persist longer during the detection process, allowing their survival and identification. Furthermore, the broader distributions and stronger signals in high m/z regions compared to positive electric field conditions (Figure 1) indicate a higher likelihood of coalescence reactions among fullerenes. This phenomenon can be attributed to the delayed extraction effect in the laser plasma.
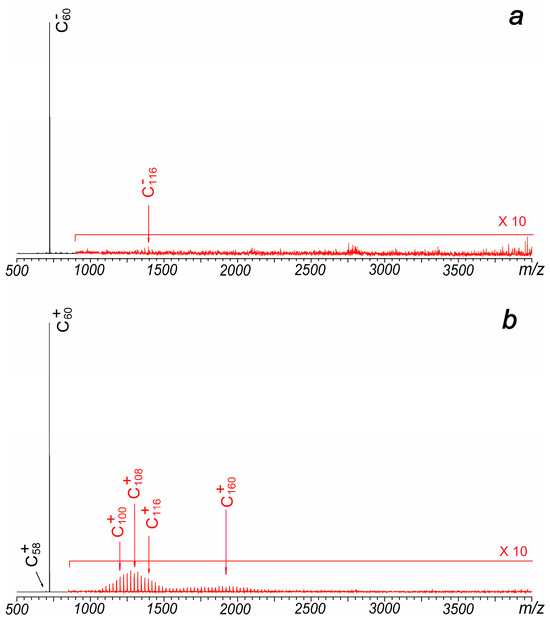
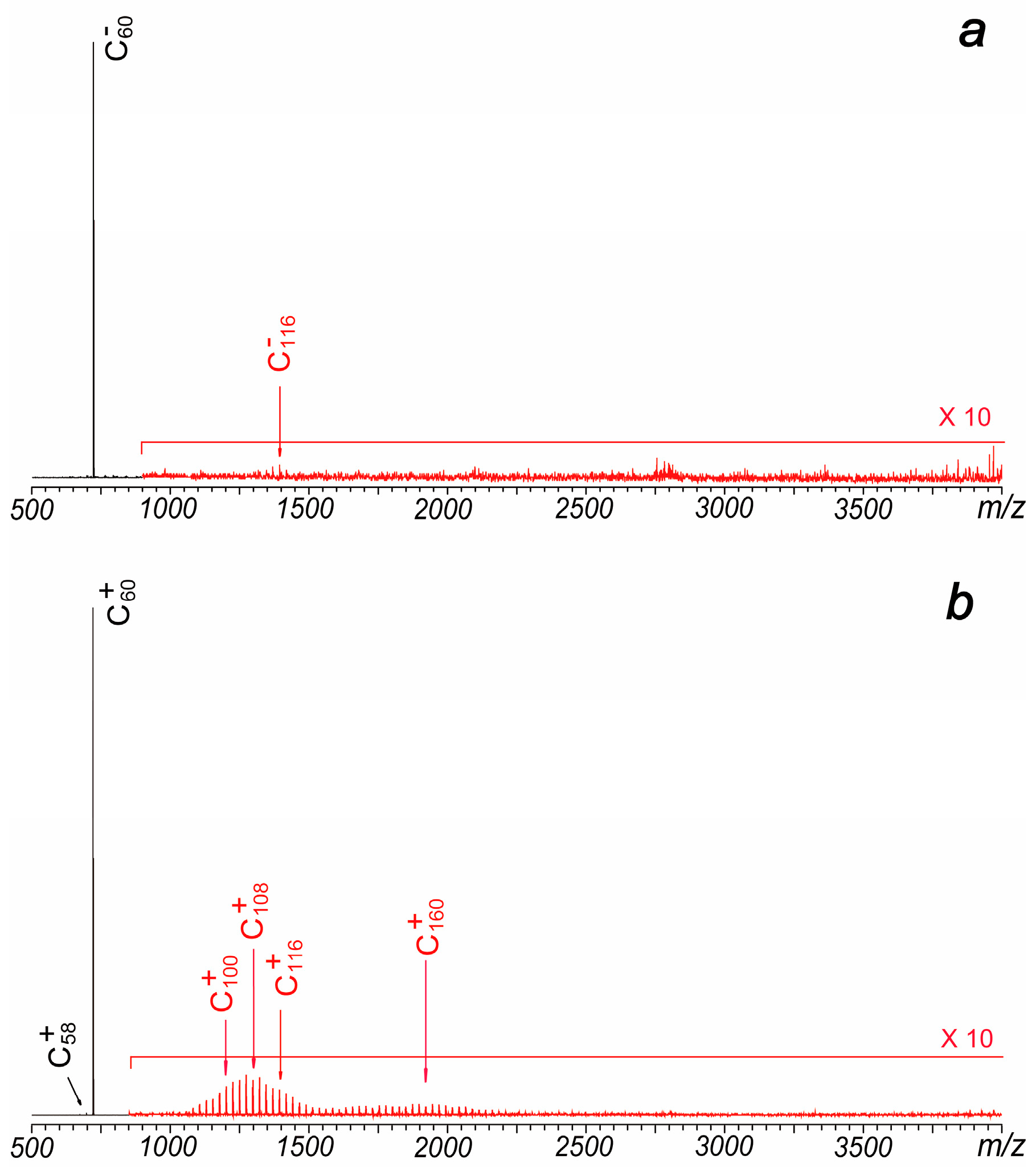
Figure 3.
(a) Typical negative ion mode laser ablation mass spectrum of C60, which is obtained under a plate voltage of −25 V. (b) Typical positive ion mode laser ablation mass spectrum of C60 without the introduction of the trapping gas during the hexapole accumulation period.
Another interesting observation is the presence of weak signals corresponding to doubly charged ions centered at C1602+ (Figure 2b). Although these cations can be attributed to the coalescence reactions of singly charged fullerenes, they have not been reported in previous studies. This absence may be explained by the relatively low stability of doubly charged ions, which can readily dissociate into singly charged species under the high-temperature conditions of the laser plasma. However, the detection of such species under negative plate voltage conditions remains puzzling, as the electron density in the laser plasma is expected to be significantly higher than under positive plate voltage conditions.
Although previously reported cations generated in coalescence reactions by different groups are quite similar, their anionic mass spectrometry results exhibit significant differences. Yeretzian et al. failed to detect coalesced anions in their experiments [1,2], whereas Liu et al. observed distributions of coalesced cluster anions that closely resembled those of the cations [3]. Similarly, Zeegers et al. did not observe coalesced anions in their LDI experiments, even when using different substrate materials, including stainless steel [6]. In this study, experiments were also conducted under negative ion mode. When a metal plate with a negative voltage was used, the signal of C60− was clearly identified. However, only very weak signals (less than 0.5%) of C116− and C114− were identified in the experiments (Figure 3a). Conversely, when the plate was positively charged, the signal of C60− decreased rapidly, and no coalesced anions were detected.
It is well known that the carrier gas of helium significantly affects the intensities of coalesced ions. The observation that coalescence products are strongly enhanced by the presence of a confining external gas not only suggests that collisions induced by the carrier gas promote coalescence reactions and cooling processes of the generated clusters but also indicates that the formation of large-size carbon clusters is a result of gas-phase coalescence reactions rather than aggregation of samples on the solid surface. In Liu’s experiments, the use of a carrier gas was not feasible because the experiments were conducted in a vacuum, and introducing a carrier gas would significantly increase the pressure, leading to failure in ion detection [3]. In our experiments, laser irradiation occurs under high vacuum conditions too. However, in a typical MALDI experiment, a pulsed valve connected to a small gas tank (containing 100 mTorr N2) is rapidly opened and closed after the laser pulse to slow down the velocity of generated ions and facilitate ion accumulation and transfer in the hexapole region. Compared to the results shown in Figure 2, the absence of the pulsed gas in our experiments significantly reduced the signals of coalesced ions (Figure 3b). This suggests that the pulsed gas serves a dual function: first, to enhance ion accumulation and transfer in the hexapole, and second, to influence the laser plasma and lower its temperature.
The coalescence reactions not only provide a unique perspective on the mechanisms of fullerene formation and growth, distinct from the widely proposed bottom-up or top-down pathways, but also offer a novel synthetic route to large-size fullerenes and encapsulation species. This naturally raises the question of whether such methods can be extended to the formation of metallofullerenes. However, to date, no metallofullerene ions generated through coalescence reactions have been reported, despite the observation of various endohedral metallofullerene (EMF) ions in the laser ablation mass spectra of La-loaded graphite or La+-loaded graphene using different ion sources [13,15,16,17,18,19,20].
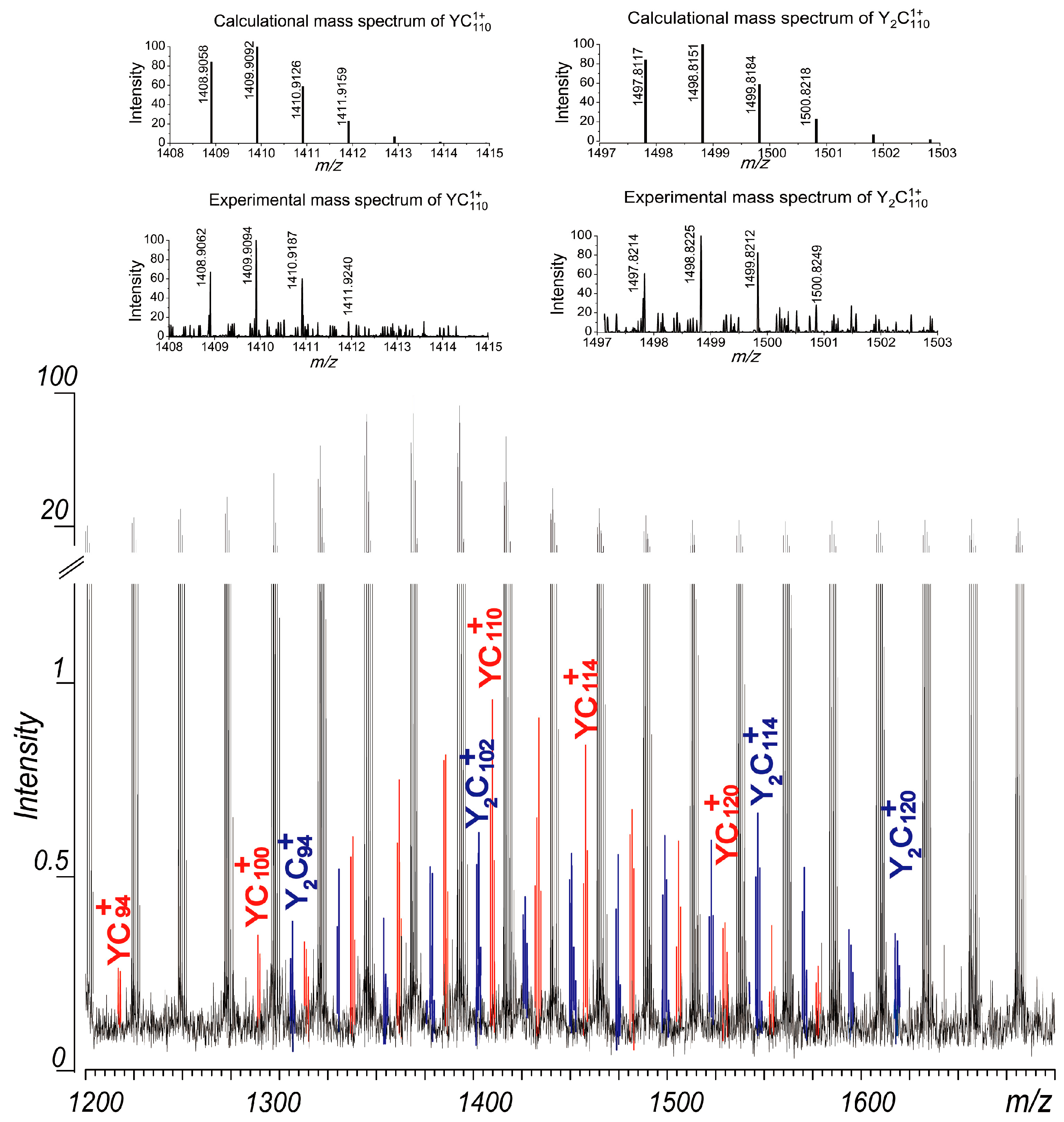
In this study, a C60 solution was mixed with a solution of Y(NO3)3 and dried in air. The laser ablation mass spectra of the resulting mixture revealed the presence of corresponding metallofullerene ions. As shown in Figure 4, metallofullerene ions of YC2n+ (2n = 94, 100–124) and Y2C2n+ (2n = 94–120) were observed in the mass spectrum obtained under suitable positive plate voltages. These peaks were weak, with intensities less than 1% of those of the coalesced carbon clusters. Due to their low intensities, the endohedral or extrohedral nature of these metallofullerenes was not studied. The distributions of these metallofullerenes are centered around C110, slightly smaller than those of the carbon clusters observed in Figure 2. Given that their distributions align with those of the coalesced carbon clusters, it is reasonable to deduce that the observed Y1–2C2n+ ions likely possess endohedral structures. Further experiments, including improvements in sample preparation methods and optimization of experimental conditions, are currently underway in our laboratory to enhance the yield of these metallofullerenes. However, while negative plate voltages facilitated the formation of high-mass coalescence product ions (Figure 2), no metallofullerene ions were detected under these conditions. This is attributed to the suppression of La+ cations in the plasma under negative plate voltages, where the coalescence reaction happens.
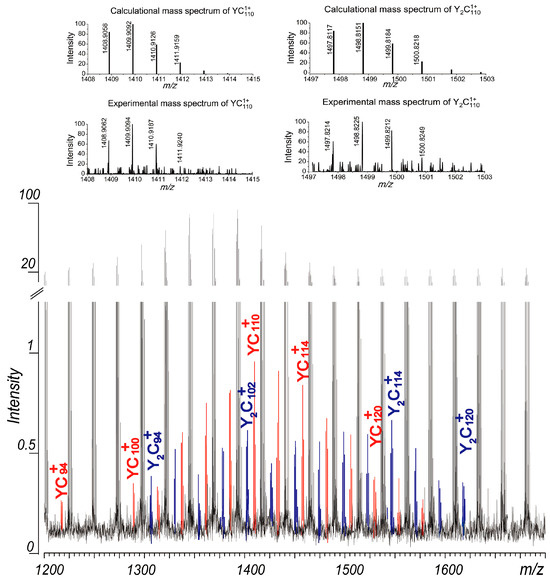
Figure 4.
Part of the laser ablation mass spectrum of C60/Y(NO3)3. Please notice a break in the y-axis is applied. And the comparison between the experimental mass spectra of YC110+ and Y2C110+ obtained with the absorption mode and their calculated mass spectra are displayed at the top of the figure.
3. Discussion
Since gas aggregation of fullerenes into larger clusters upon evaporation of fullerene powder into an inert gas has been successfully demonstrated by the group of T.P. Martin [21], we need to make sure that these cluster ions were not just the aggregation of individual fullerenes here. Tandem mass spectrometry experiments are also tried here based on the method of sustained off-resonance irradiation (SORI) collision-induced dissociation (CID) [22]. However, due to the wide distribution of the clusters, a clear selection of the mass-selected cluster cations is very difficult. For example, the isolation peak of C116+ is always accompanied by weak signals of C114+, C112+, and so on. Despite this, no large-sized fragment ions (such as C60+) were observed in such CID experiments conducted under these loosely isolated conditions. Further, the size distributions of ions observed in the experiments (Figure 2 and Figure 3) are very wide, and their centers are significantly offset by integer multiples of C60, which are quite different from the previously reported results formed through aggregations [23]. All these results support that these observed lager-sized clusters are coalesced ions, instead of the simple aggregations of fullerenes.
The results here show the effect of the source region electric field on the formation of the coalesced carbon clusters. To understand the unexpected finding that the larger cation cluster ions can be formed for negative voltages applied to the metal plate, some analysis and discussion are still needed.
First, the results here show that under vacuum conditions, some of the positive ions of C60 generated after laser ablation have high kinetic energy. Considering that other conditions were the same in all experiments, the result means that most of the generated C60+ cations are characterized by their high translational energies, which can counteract the negative electric field induced by the −95 V voltage applied on the metal plate.
Second, a high negative voltage on the metal plate can decrease the velocity of C60 cations, resulting in longer residence time and increased collision frequency of these ions in the plasma region with high-density species, thereby promoting the occurrence of large-sized coalesced clusters. Similarly, the applied inert gas can also slow down the generated species with high velocity and increase their collision probability in high-density species areas (as shown in Figure 2b). After the coalesced reactions, the thermionic emission of hot neutral fullerene clusters can happen. Considering the existence of the C58+ in the plasma (Figure 1), the following reactions can occur:
C60+ + C60 → C120+ → C118+ + C2 → C116+ + 2C2 → ······
C58+ + C60 → C118+ → C116+ + C2 → C114+ + 2C2 → ······
And the formation of large-sized clusters after multiple collisions:
C116+ + C60 → C176+ → C174+ + C2 → C172+ + 2C2 → ······
C172+ + C60 → C232+ → C230+ + C2 → C228+ + 2C2 → ······
These reactions will result in the observation of large-sized carbon cluster ions in Figure 2 compared to Figure 1.
Third, for the small number of divalent ions observed under negative voltage, the possible sources of these ions include the reaction of two positive ions and their subsequent reactions:
C60+ + C58+ → C1182+ → C1162+ + C2 → ······
These divalent ions may react with the C60 anions (Figure 3) that are present at the negative voltage conditions to generate larger-sized monovalent positive ions:
C1602+ + C60− → C220+ → ······
Fourth, for experiments involving EMF formation, the negative voltage may not be conducive to the effective generation of metal ions. Therefore, finding suitable electric field conditions or using programmable pulsed electric fields is a more effective method to generate such species.
4. Experimental Method
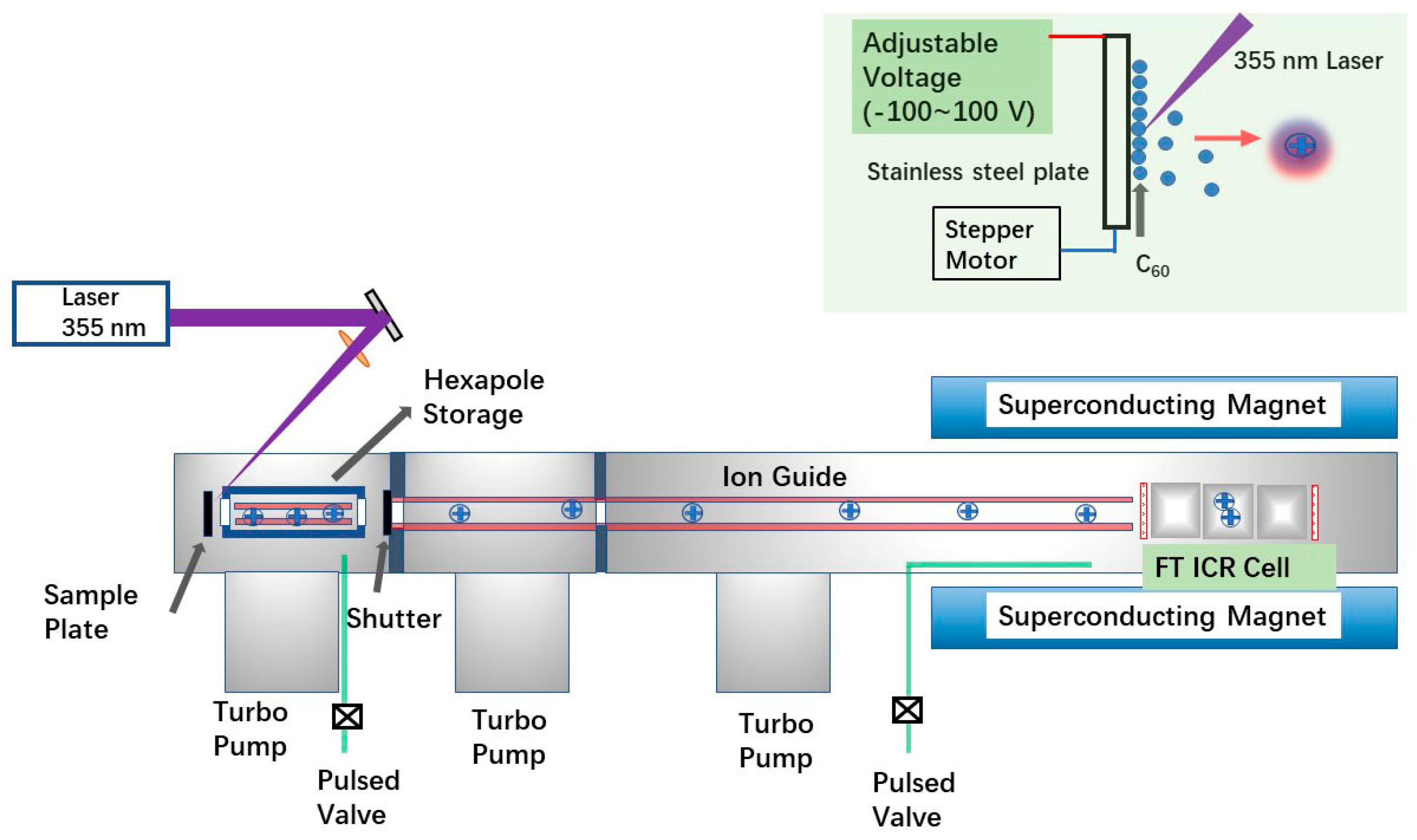
Instead of using the Smalley-type laser-vaporization supersonic expansion cluster beam source, a direct laser ablation in vacuum setup was employed here (Figure 1). The solid sample of C60 (from Sigma, St. Louis, MO, USA) was first dispersed in water by sonication for 5 min prior to use, achieving a concentration of 1 mg/mL. Then, 1 µL of the solution was dropped onto a stainless-steel plate (the commercial ProMALDI source target, from Varian IonSpec, Lake Forest, CA, USA) and dried at laboratory temperature before being placed into the source region of the mass spectrometer. For EMF experiments, a 0.5 μL aqueous solution of Y(NO3)3 at a concentration of 1 mM was added together with 0.5 μL of the C60 solution on the metal plate. All mass spectrometry (MS) experiments were performed using a 7.0 T FT ICR mass spectrometer equipped with the Varian IonSpec ProMALDI source (Varian IonSpec, Lake Forest, CA, USA). Mass spectra in the m/z range of 250–5000 were recorded in both positive and negative ion modes.
In the experiments, ions generated by laser pulses were injected into an open-ended cylindrical Penning trap via a quadrupole ion guide. Notably, the ion source used here is a commercial MALDI source, which differs from the typical commercial MALDI sources used in MALDI-time of flight (TOF) instruments. In this design, no high voltage (in typical MALDI TOF experiments, the value is 20 kV) is applied on the metal plate, and the voltage on the metal plate (as shown in Figure 1) is adjustable only within the range of −100 V to 100 V. In normal MALDI experiments performed with the instrument, the plate voltage is set to positive or negative depending on the mode of operation (positive or negative ion mode), respectively. To effectively extract ions, the bias voltage of the hexapole located in front of the metal plate (refer to Figure 5) was set to −15 V and 15 V for the positive-ion and negative-ion modes, respectively.
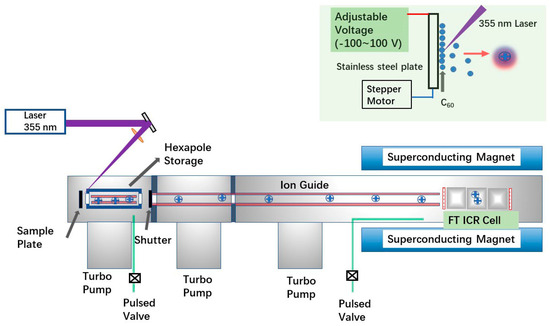
Figure 5.
The experimental setup applied here. A 7.0 T FT ICR mass spectrometer (Varian IonSpec ProMALDI) with the commercial ion source of ProMALDI was applied. The precursor of C60 is deposited on the metal plate and irradiated by the 355 nm laser. The metal plate is controlled by a stepper motor, and its voltage is adjustable from −100 V to 100 V.
For experiments reported here, ions produced by eight consecutive laser pulses were accumulated in the hexapole before being induced into the cell. The accumulation period of these ions in the cell was optimized to 2.5 ms for all experiments. During the accumulation process, N2 gas was pulsed into the hexapole region to assist in ion trapping. A 355 nm Nd:YAG laser (Orion, New Wave) was used for the laser ablation experiments. The laser operated at a frequency of 10 Hz, with an energy of 2 mJ/pulse and a pulse width of 5 ns. After collecting the image current signals, the time-domain data were converted to frequency domain data via fast Fourier transform (FFT). Generally, the magnitude mode was applied to the reported mass spectra. However, for the weak signals of metallofullerenes, the absorption-mode spectra were reported using the method of phase correction [24,25]. In tandem mass spectrometry experiments, the ions were selected in the cell of the FT ICR with the method of stored waveform inverse Fourier transform (SWIFT) [26]. Then, selected ions were fragmented by the methods of SORI-CID [22].
5. Conclusions
To further elucidate the coalescence reactions of fullerenes, we revisited the experiment using a high-resolution FT ICR mass spectrometer equipped with a commercial ProMALDI source. Significant progress has been made in understanding the influence of the electric field in the ion source region, enabling better control over the coalescence process. In contrast to previous studies, we observed distinct distributions of generated fullerenes, which were found to depend on the polarity of the applied plate voltages. Contrary to the general assumption, a negative voltage applied on the metal plate is actually more conducive to the production of large-sized fullerene cations. Additionally, new species, such as doubly charged fullerene cations (e.g., C1602+) and metallofullerene ions (e.g., Y1–2C94–124+), were detected for the first time in coalescence reaction experiments. Future research should focus on the design of programmable electric field and leverage the controlled coalescence process to explore its potential applications in the synthesis of novel metallofullerenes.
Author Contributions
Conceptualization, X.Z. and X.K.; Methodology, S.Y., X.Z. and X.K.; Software, J.Y. and X.K.; Validation, S.Y., J.Y., J.L., G.Y., X.Z. and X.K.; Investigation, S.Y., J.L. and X.Z.; Data curation, S.Y.; Writing—original draft, S.Y., J.Y., J.L. and G.Y.; Writing—review & editing, X.Z. and X.K.; Supervision, G.Y. and X.Z.; Funding acquisition, X.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (No. 2023YFF0713802).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeretzian, C.; Hansen, K.; Diederich, F.; Whetten, R.L. Coalescence reactions of fullerenes. Nature 1992, 359, 44–47. [Google Scholar] [CrossRef]
- Yeretzian, C.; Hansen, K.; Diederich, F.; Whetten, R.L. Coalescence reactions of fullerenes. Z. Phys. D 1993, 26, 300–304. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, C.R.; Huang, R.B.; Zheng, L.S. Mass-distribution of C-60 and C-70 coalescence products produced by direct laser vaporization. Int. J. Mass Spectrom. Ion Process. 1995, 145, 1–7. [Google Scholar] [CrossRef]
- Mitzner, R.; Winter, B.; Kusch, C.; Campbell, E.E.B.; Hertel, I.V. Coalescence reactions in laser-induced fullerene desorption: The role of fragments. Z. Phys. D 1996, 37, 89–95. [Google Scholar] [CrossRef]
- Rohmund, F.; Glotov, A.V.; Hansen, K.; Campbell, E.E. Experimental studies of fusion and fragmentation of fullerenes. J. Phys. B 1996, 29, 5143. [Google Scholar] [CrossRef][Green Version]
- Zeegers, G.P.; Günthardt, B.F.; Zenobi, R. Target plate material influence on fullerene-C60 laser desorption/ionization efficiency. J. Am. Soc. Mass Spectrom. 2016, 27, 699–708. [Google Scholar] [CrossRef]
- Barrow, M.P.; Drewello, T. Significant interferences in the post source decay spectra of ion-gated fullerene and coalesced carbon cluster ions. Int. J. Mass Spectrom. 2000, 203, 111–125. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, X.; Yang, X.; Jia, S.; Zhong, H. Competing Deprotonation and Electron Capture Dissociation in MALDI Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 322–329. [Google Scholar] [CrossRef]
- Patchkovskii, S.; Thiel, W. C60 Dimers: A Route to Endohedral Fullerene Compounds? J. Am. Chem. Soc. 1998, 120, 556–563. [Google Scholar] [CrossRef][Green Version]
- Han, S.; Yoon, M.; Berber, S.; Park, N.; Osawa, E.; Ihm, J.; Tománek, D. Microscopic mechanism of fullerene fusion. Phys. Rev. B 2004, 70, 113402. [Google Scholar] [CrossRef]
- Wang, Y.; Zettergren, H.; Rousseau, P.; Chen, T.; Gatchell, M.; Stockett, M.H.; Domaracka, A.; Adoui, L.; Huber, B.A.; Cederquist, H.; et al. Formation dynamics of fullerene dimers C 118+, C 119+, and C 120+. Phys. Rev. A 2014, 89, 062708. [Google Scholar] [CrossRef]
- Verkhovtsev, A.; Korol, A.V.; Solovyov, A.V. Classical molecular dynamics simulations of fusion and fragmentation in fullerene-fullerene collisions. Eur. Phys. J. D 2017, 71, 1–9. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.F.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Xu, H.; Wu, B.; Gan, L.H. Molecular dynamics simulation of the coalescence behavior of small carbon clusters at high temperature. Chem. Phys. 2020, 539, 110931. [Google Scholar] [CrossRef]
- Shen, W.; Hu, S.; Lu, X. Endohedral metallofullerenes: New structures and unseen phenomena. Chem. Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bao, X. Formation of Endohedral Metallofullerene (EMF) ions of MnC2m+ (M = La, Y, n ≤ 6, 50 ≤ 2m ≤ 194) in the Laser Ablation Process with Graphene as Precursor. Rapid Comm. Mass. Spectrom. 2017, 31, 865–872. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Ren, J.; Mu, L.; Kong, X.L. A systematic study on the generation of multimetallic lanthanide fullerene ions by laser ablation mass spectrometry. Rapid Comm. Mass. Spectrom. 2018, 32, 1396–1402. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Shi, Y.Y.; Fan, X.T.; Ren, J.; Kong, X.L. Encapsulation of an Ionic Bond in Fullerenes: What is the Difference? Inorg. Chem. 2019, 58, 3601–3605. [Google Scholar] [CrossRef]
- Hou, Y.M.; Mu, L.; Zhou, S.Z.; Xu, Y.; Kong, X. Structure and bonding properties of the platinum-mediated tetrametallic endohedral fullerene La3Pt@C98. Dalton. Trans. 2023, 52, 7021–7030. [Google Scholar] [CrossRef]
- Hou, Y.; Kong, X. Endometallofullerenes in the Gas-Phase: Progress and Prospect. Inorganics 2024, 12, 68. [Google Scholar] [CrossRef]
- Martin, T.P.; Malinowski, N.; Zimmermann, U.; Näher, U.; Schaber, H. Metal coated fullerene molecules and clusters. J. Chem. Phys. 1993, 99, 4210–4212. [Google Scholar] [CrossRef]
- Gauthier, J.W.; Trautman, T.R.; Jacobson, D.B. Sustained off-resonance irradiation for collision-activated dissociation involving Fourier transform mass spectrometry. Collision-activated dissociation technique that emulates infrared multiphoton dissociation. Anal. Chim. Acta 1991, 246, 211–225. [Google Scholar] [CrossRef]
- Hansen, K.; Zettergren, H. Clusters of fullerenes: Structures and dynamics. J. Phys. Chem. A 2022, 126, 8173–8187. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Thompson, C.J.; Van Orden, S.L.; O’connor, P.B. Phase correction of Fourier transform ion cyclotron resonance mass spectra using MatLab. J. Am. Soc. Mass Spectrom. 2011, 22, 138–147. [Google Scholar] [CrossRef]
- Qi, Y.; O’Connor, P.B. Data processing in Fourier transform ion cyclotron resonance mass spectrometry. Mass Spectrum. Rev. 2014, 33, 333–352. [Google Scholar] [CrossRef]
- Cody, R.B.; Hein, R.E.; Goodman, S.D.; Marshall, A.G. Stored waveform inverse fourier transform excitation for obtaining increased parent ion selectivity in collisionally activated dissociation: Preliminary results. Rapid Commun. Mass Spectrom. 1987, 1, 99–102. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).