Abstract
A series of mononuclear complexes, [Fe(L5)(bylim)](BPh4), where L5 represents a pentadentate Schiff base ligand, bylim is 1-benzyl-1-imidazole, and BPh4− is the tetraphenylborate anion, was synthesized. The determined crystal structures indicate the absence of significant cooperative interactions, which influence the properties of the eventual spin transition. Changes in magnetic behavior induced by substitution of the pentadentate ligand were investigated through magnetic susceptibility measurements. It was found that only complexes containing a non-substituted secondary amino group exhibit some form of spin crossover, whereas the majority of those with a methyl substituent remain in the high-spin state across the entire measured temperature range (2–300 K). The changes induced by the substitution of the secondary amine group were further explored through theoretical calculations at DFT and CASSCF/NEVPT2 levels of theory. The topology and energetics of electron density and atomic charges were investigated through QT-AIM calculations.
1. Introduction
Spin crossover (SCO) materials can be reversibly switched between reference electronic states of different spin multiplicities by external stimuli such as temperature, pressure, and light irradiation [1]. In hexacoordinate mononuclear complexes with a d5 electronic configuration of the central ion, there are two possible spin isomers: low spin (LS) with S = 1/2 and high spin (HS) with S = 5/2, corresponding to spin-only effective magnetic moments of μeff = 1.7 and 5.9 μB, respectively. For SCO to occur as an entropy-driven unimolecular reaction, the conditions ΔH > 0 and ΔS > 0 must be met. Chemical methods used to tune SCO primarily target the adjustment of ΔH, which can be influenced by factors such as a stronger ligand field, ligand denticity and rigidity, and non-covalent interactions [2,3]. These approaches can increase the critical temperature of the SCO (T1/2), where half of the molecules are in the high-spin state and the other half are in the low-spin state, according to the relationship T1/2 = ΔH/ΔS [4].
Herein, we are reporting about a series of mononuclear Fe(III) complexes possessing the {Fe(N2N′O2)N″} coordination environment with the general formula [Fe(L5)(bylim)](BPh4), where L5 is a pentadentate Schiff base ligand (Scheme 1), bylim is a monodentate 1-benzylimidazole ligand, and BPh4− stands for tetraphenylborate counter anion. Up to now, there are several examples of magnetic bistability in this class of mononuclear iron(III) complexes. Extensive research of changes driven by ligand substitution in a series of [Fe(L5)(L1)](BPh4) was carried out by Matsumoto et al. [5]. L1 is a monodentate ligand, which was altered through the series, and it was found that only pyridine-like and imidazole-like derivatives have suitable ligand field strength to invoke SCO behavior. This assumption was confirmed by further studies of mononuclear and binuclear [6,7,8,9,10,11,12,13], and this is also valid for polynuclear complexes, where sufficient ligand field strength for SCO is also provided by nitrogen atoms of cyanometallic bridging complexes [14,15]. Furthermore, by shortening one of the aliphatic chains to an ethyl group (group of the H2L’ ligands), we obtained systems that exhibit abrupt SCO, even with thermal hysteresis. For these systems, the crucial importance of the secondary amine N-H group was confirmed, revealing that hydrogen bonding involving this group can trigger or hinder SCO behavior [16,17]. Inspired by these findings, we decided to investigate this possibility of control over SCO behavior in the group of the [Fe(L5)(bylim)](BPh4) complexes. Due to the bulkiness of the tetraphenylborate anion, there is a lack of significant cooperative interactions aside from those related to the amine group. This allows us to study the SCO behavior free from other significant cooperative factors [18].
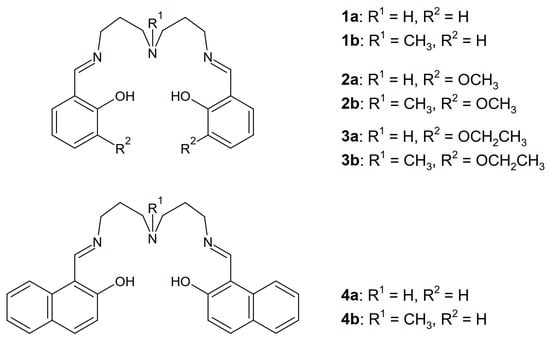
Scheme 1.
The pentadentate Schiff base ligands used in the present study.
2. Results
2.1. Synthesis and Crystal Structures
The synthesis of the studied compounds is not complicated and follows the protocol reported by N. Matsumoto et al. [5] The first step consists of preparing the chloride precursor, as we described previously [19]. In the second step, the chloride ligand was substituted with a heterocyclic ligand, which in the present series was bylim. The reaction was performed in a methanol solution, and after a brief reflux, the dark violet solution was filtered into NaBPh4 dissolved in a minimal amount of methanol. The resulting solutions were crystallized isothermally, and after a few days, microcrystalline or crystalline phases were obtained and isolated by filtration.
The composition of the studied compounds was first investigated using elemental analysis, which unequivocally confirmed the expected [Fe(L5)(bylim)](BPh4) composition. Infrared spectroscopy in the mid-infrared region was then employed to verify the presence of all ligands and BPh4⁻ anions. To achieve this, we compared the IR spectra of the pure ligands, NaBPh4, and the precursor [Fe(L5)Cl] complexes. The spectra of the precursor complexes contain numerous vibrational bands and were previously well described and analyzed [6,11,16,17,19,20]; therefore, rather than analyzing the full spectra, we focused on identifying differences between the precursor complexes and the corresponding [Fe(L5)(bylim)](BPh4) spectra (Figure S1 in Supplementary Materials). These differences were then compared to the spectra of bylim and NaBPh4. This approach allows for a convenient confirmation of each constituent’s presence. The bylim ligand is primarily identified by its C-H, C=C, and C=N vibrations, which also dominate the IR spectra of the precursor complexes. However, notable additional peaks appear at frequencies above 3100 cm⁻¹ in the bylim and also the NaBPh4 spectrum. Another important spectral region is that of the out-of-plane C–H bending vibrations. In this range, the bylim ligand exhibits a distinct vibration at approximately 660 cm⁻¹ that does not overlap with other bands. This region is also crucial for confirming the presence of the BPh4⁻ anion, which exhibits an intense vibrational band characteristic of a monosubstituted phenyl ring at approximately 705 cm⁻¹. Importantly, this spectral region does not overlap with bands originating from the pentadentate Schiff base ligand. The spectra of 1a–4a and 1b–4b contain these characteristic bands, thereby confirming the expected composition (Figures S1–S3 in Supplementary Materials).
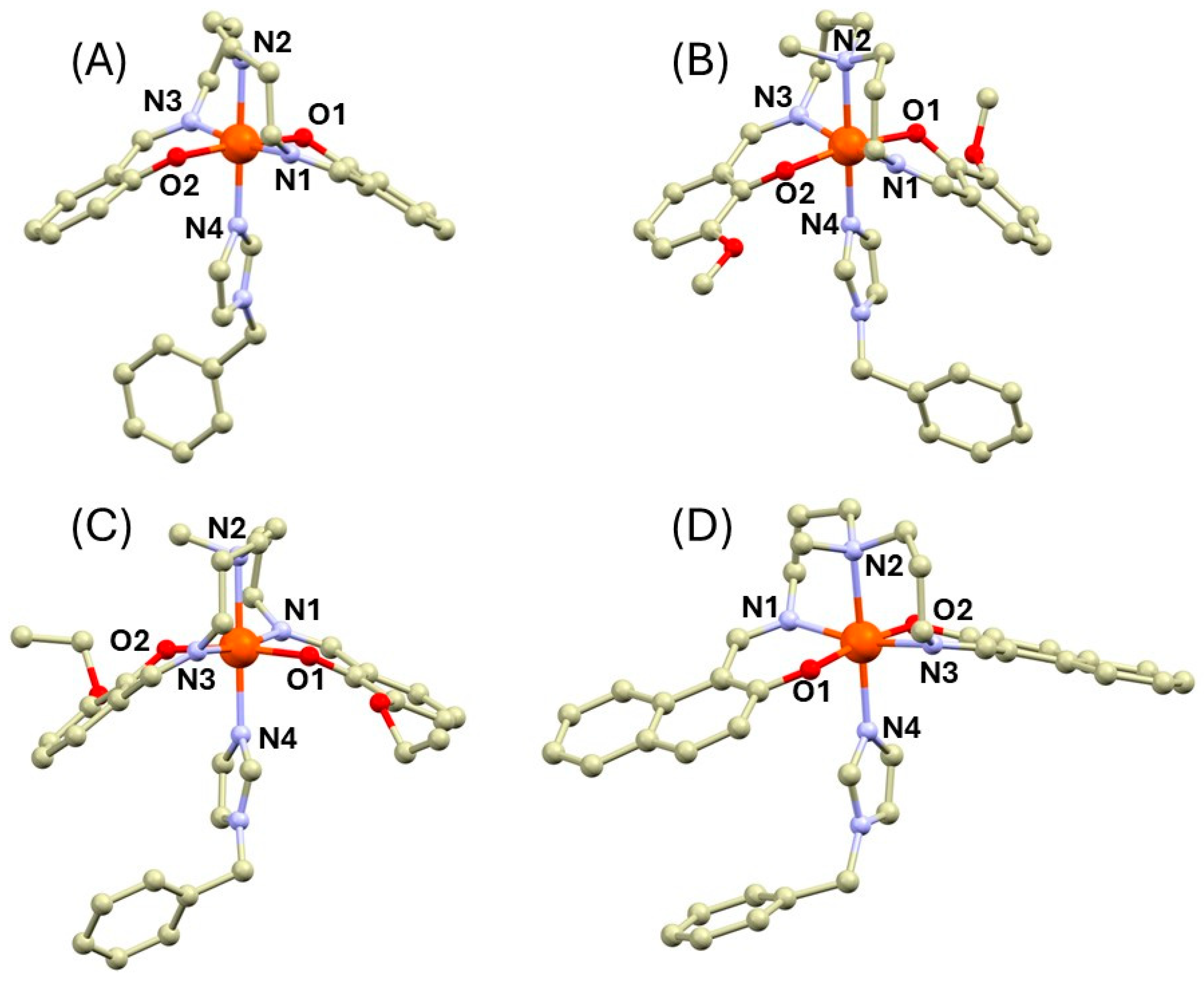
The suitable single crystals for single-crystal X-ray diffraction (SC-XRD) were obtained for compounds 1a, 2b, 3b, and 4b (Tables S1 and S2). Their crystal structures are composed of the [Fe(L5)(bylim)]+ cations and tetraphenylborate anions. In all the complexes, the pentadentate ligands wrap around the metal center with oxygen atoms in the trans position as is typical for the Fe(III) complexes with the L52− Schiff bases and heterocyclic co-ligands (Figure 1). The nitrogen atom of the bylim ligand is in the trans position to the secondary (1a) or tertiary (2b–4b) nitrogen atom. The crystal structures of the methylated complexes 2b–4b exhibit significantly longer metal–ligand (M-L) bond lengths than those in 1a and they are almost identical, as can be found in the previously published HS complexes of this group of compounds [16,20]. The longest M-L bonds were observed for bonds between the Fe(III) atom and tertiary amino nitrogen atom (2.22–2.29 Å), whereas the second longest bonds are those between the central atom and the nitrogen of the bylim ligand (Nbylim, 2.14–2.17 Å). The M-L bonds involving imino nitrogen atoms (Nim) and phenolic oxygen atoms are shorter (Fe-Nim = 2.04–2.09 Å, Fe-O = 1.91–1.95 Å).
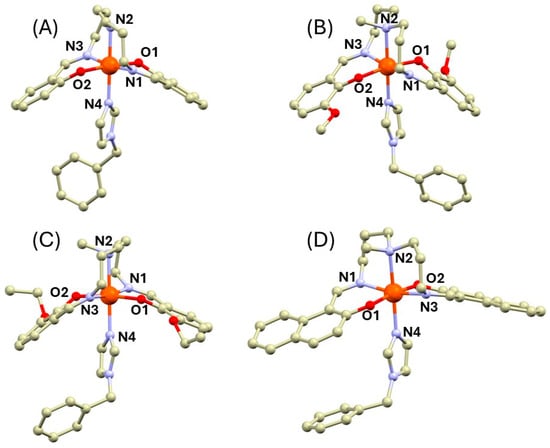
Figure 1.
Complex cations in the crystal structures of 1a (A), 2b (B), 3b (C), and 4b (D). The hydrogen atoms were omitted for clarity. Color code: carbon (light brown), iron (orange), nitrogen (light blue), and oxygen (red). The metal ligand bond lengths (in Å): 1a at 90K, Fe1-O1 = 1.9258(15), Fe1-O2 = 1.9074(16), Fe1-N1 = 2.0359(17), Fe1-N2 = 2.1347(18), Fe1-N3 = 2.0318(17), Fe1-N4 = 2.085(2); 1a at 298K, Fe1-O1 = 1.9382(19), Fe1-O2 = 1.9236(19), Fe1-N1 = 2.080(2), Fe1-N2 = 2.202(2), Fe1-N3 = 2.085(2), Fe1-N4 = 2.161(2); 2b, Fe1-N1 = 2.0635(14), Fe1-N2 = 2.2395(14), Fe1-N3 = 2.0870(14), Fe1-N4 = 2.1404(14), Fe1-O1 = 1.9179(11), Fe1-O2 = 1.9473(11); 3b, Fe1-O1 = 1.924(3), Fe1-O2 = 1.946(3), Fe1-N1 = 2.080(3), Fe1-N2 = 2.273(3), Fe1-N3 = 2.082(4), Fe1-N4 = 2.166(3); 4b, Fe1-N1 = 2.076(3), Fe1-N2 = 2.294(2), Fe1-N3 = 2.065(3), Fe1-N4 = 2.173(2), Fe1-O1 = 1.934(3), Fe1-O2 = 1.933(3).
In the case of the crystal structure of 1a, the diffraction data were collected at two different temperatures: 90 and 298K. Both measurements revealed the different metal–ligand bond lengths for each particular temperature. At 298 K, the M-L bond lengths correspond rather well to those in the structures of 2b–4b with the longest Fe-Nam bond (2.202(2) Å, Figure 1). At 90K, this bond becomes significantly shorter (2.1347(18) Å). Also, other M-L bond lengths become shorter upon cooling (Figure 1). This indicates that the SCO occurs in this compound between 90 and 298 K.
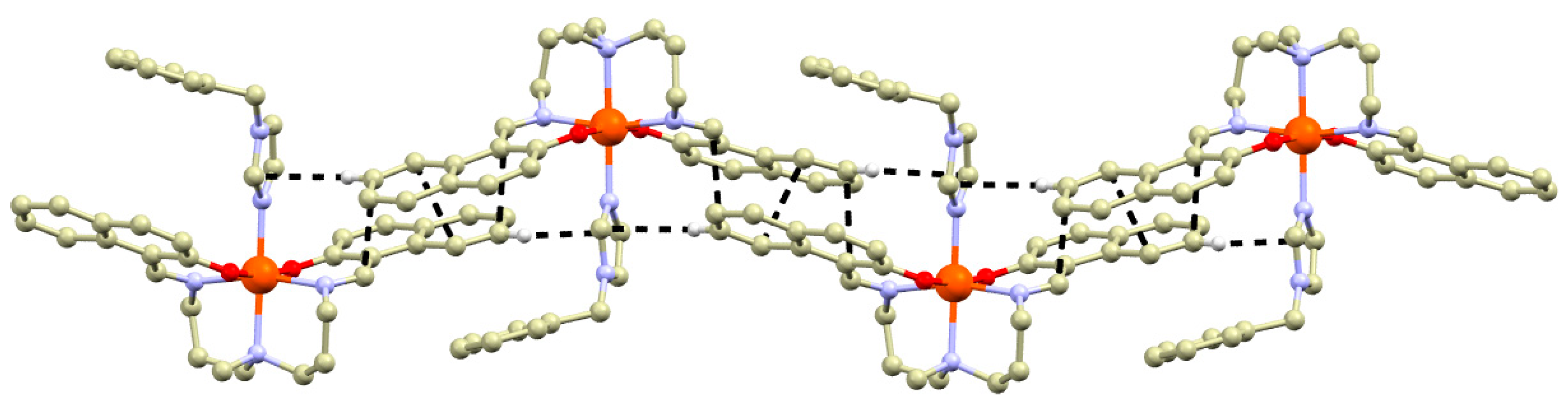
The non-covalent interactions stabilizing crystal structures of 1a, 2b–4b are mostly weak and without strong interactions interconnecting the complex cations. One exception is the crystal structure of 4b, in which the complex cations are interconnected via offset face-to-face π-π stacking between the naphthalene rings of the neighboring complex cations combined with face-to-edge interaction between naphthalene ring and bylim ligand (Figure 2). Such interactions could potentially increase SCO cooperativity, as has been shown in numerous examples previously [21].
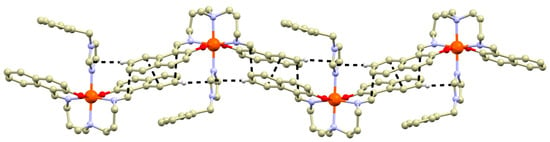
Figure 2.
A perspective view of the non-covalent interactions (black dashed lines) in the crystal structure of 4b.
2.2. Magnetic Properties
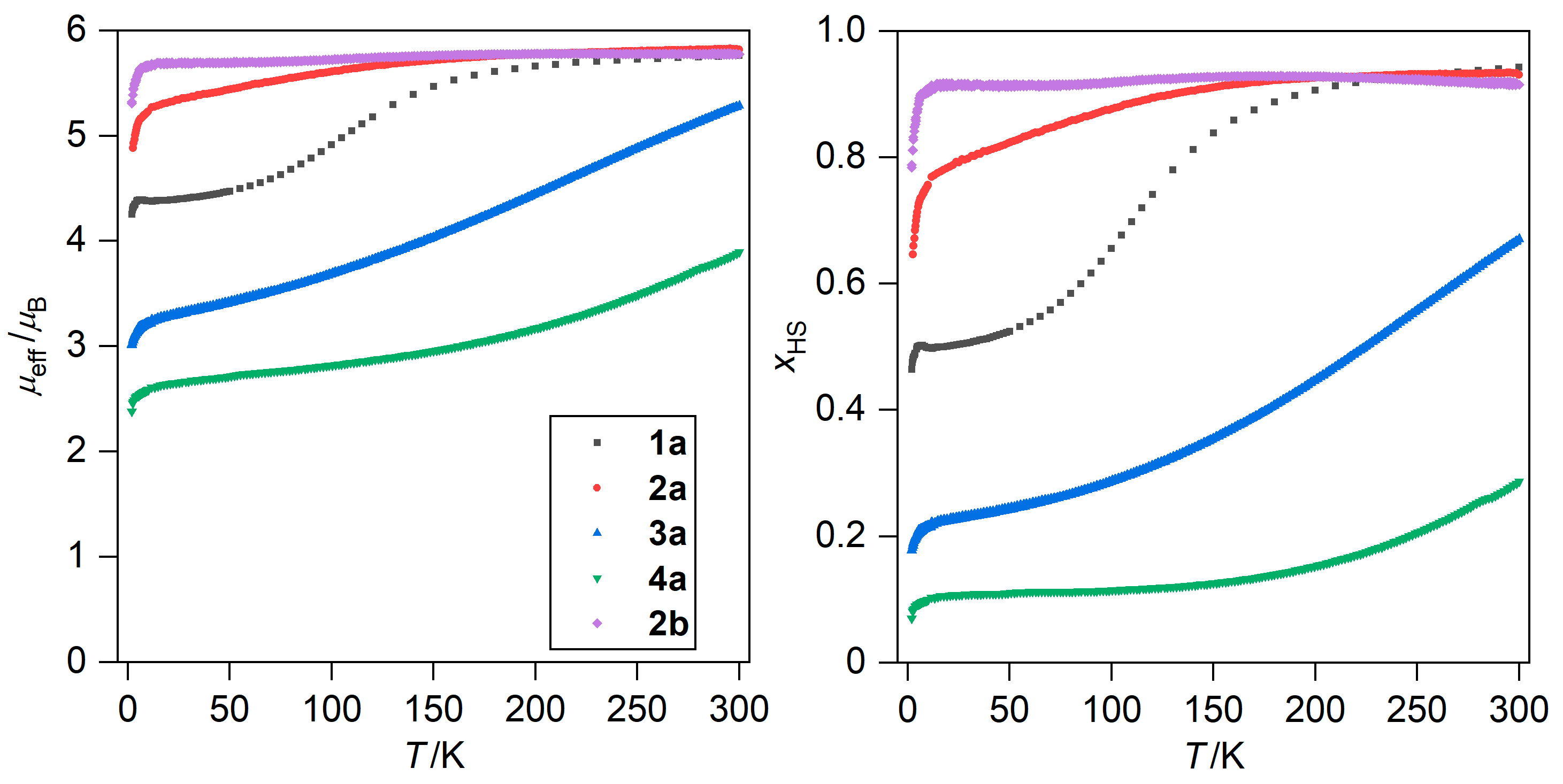
The magnetic properties of 1a–4a and 1b–4b were measured as the temperature dependence of magnetic moment (μeff/μB) over the range of 2–300 K, along with isothermal magnetization measurements (at 2 K, in the magnetic fields of 0–7 T, Figure 3 and Figures S4–S7 in Supplementary Materials). Two main types of magnetic behavior were identified: SCO for 1a–4a, and dominantly HS behavior in 1b–4b. Among the SCO compounds, the spin transitions were not complete within the measured temperature range (Figure 3). This is evident from a comparison of the measured magnetic data with the spin-only values calculated for giso = 2.023. The calculated values for each spin state are as follows: S = 1/2, (μeff/μB = 1.73) for the LS state, S = 5/2, (μeff/μB = 5.92) for the HS state. The LS state was most stabilized in 4a whereas the HS is the most pronounced in 2a (Figure 3). The only exception to the purely HS behavior in the 1b–4b compounds (Figures S3–S6) was 2b, in which a very gradual decrease of μeff/μB began at 130K upon further cooling (down to 5.7 μB at 15K, Figure 3). However, this decrease was minimal and no significant contraction in the metal–ligand bond lengths was observed in the crystal structure of 2b. The isothermal magnetization measurements (Figure S7) confirmed different HS fractions for 1a–4a at 2 K, as the values of molar magnetization (Mmol/(NAμB)) at a high field of 7 T decrease in the following order: 3.93 (2a) > 2.75 (1a) > 1.75 (3a) > 1.32 (4a). This is in agreement with the trend observed from temperature-dependent magnetic measurements. Compounds 1b, 3b, and 4b exhibited Mmol/(NAμB) values at 7 T ranging from 4.48 to 4.78, as expected for the HS Fe(III) complex with low magnetic anisotropy. Remarkably, the Mmol/(NAμB) value of 2b at 7 T is the lowest (4.31) among the 1b–4b compounds, further confirming the suspected partial SCO in this compound.
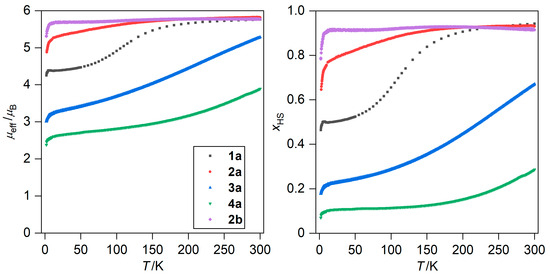
Figure 3.
Temperature dependence of μeff/μB for 1a–4a and 2b (left) and calculated dependence of the molar fraction of the high-spin state, xHS, assuming gHS = 2.0 and gLS = 2.15 (right).
2.3. Theoretical Calculations
The intriguing difference in the magnetic behavior between 1a–4a and 1b–4b motivated us to investigate the structures and energetics of these compounds through theoretical methods. First, we employed DFT calculations to optimize the structures of the complex cations of these compounds. Next, we analyzed the topology and energetics of electron density using QT-AIM calculations and evaluated the electronic structure and ligand field parameters using CASSCF/NEVPT2 calculations.
In order to select a suitable DFT functional, we performed initial screening with several DFT functionals: BP86 [22], B3LYP [23,24,25], BHANDHLYP [26], B97M-D4 [27], r2SCAN0 [28], TPSSh [29], wB97 [30], wB97M-D4rev [31], and CAM-B3LYP [32], and optimized the molecular structure of [Fe(L5)(bylim)]+ cation of 1a. Then, the root mean square deviation (RMSD) was calculated for the coordination polyhedra {FeN4O2} using the crystal structure data for the HS state [33]. The lowest RMSD was achieved with CAM-B3LYP functional (Table S3), which was then used to optimize the molecular structures for all cations of 1a–4a and 1b–4b. The optimization of the molecular structures was performed in water using the SMD solvation model, as the studied species in both spin states are charged (LS and HS). A similar procedure was applied recently in the study of tetranuclear Fe(II) SCO compound [34].
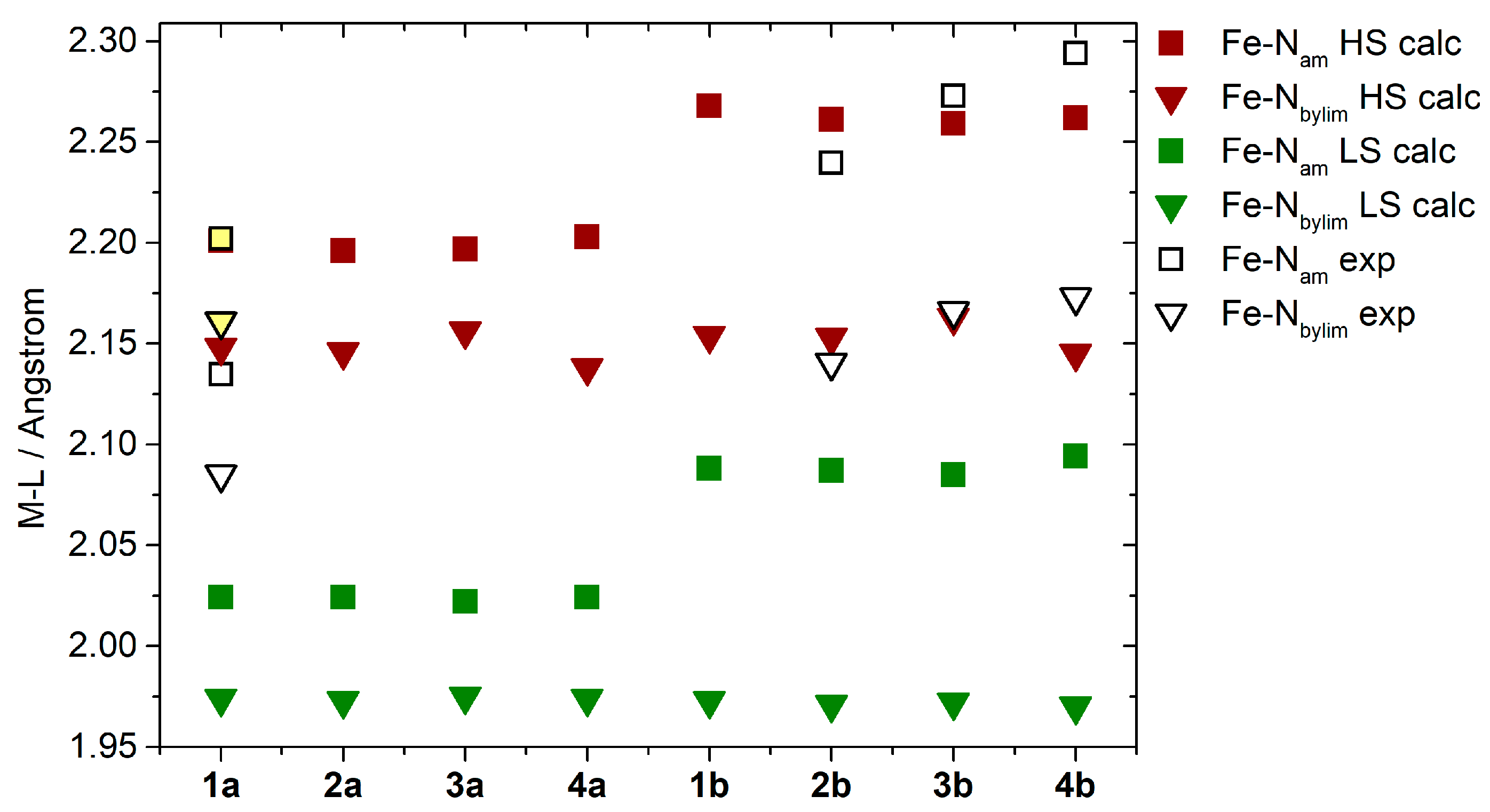
The calculated HS structures show reasonable agreement with the experimental structures, as demonstrated by a comparison of the metal–ligand bond lengths (Figure 4). The Fe–Nam bonds, which are the most sensitive to SCO effects in this group of compounds [8], show interesting features in both spin states. Their lengths are slightly longer in 1b–4b than in 1a–4a structures (≈2.25 vs. 2.20 Å in the HS state vs. ≈2.08 vs. 2.03 Å in the LS state, Figure 4). Obviously, the consequence of this prolongation should lead to a weaker ligand field in 1b–4b than in 1a–4a [35].
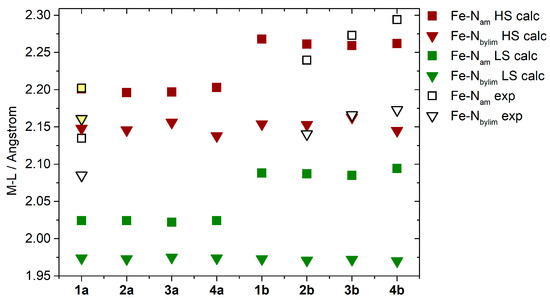
Figure 4.
A comparison of the calculated and experimentally determined Fe-Nam and Fe-Nbylim bond lengths. For 1a, yellow shading indicates measurements taken at 298 K, while unshaded bonds represent those measured at 90 K.
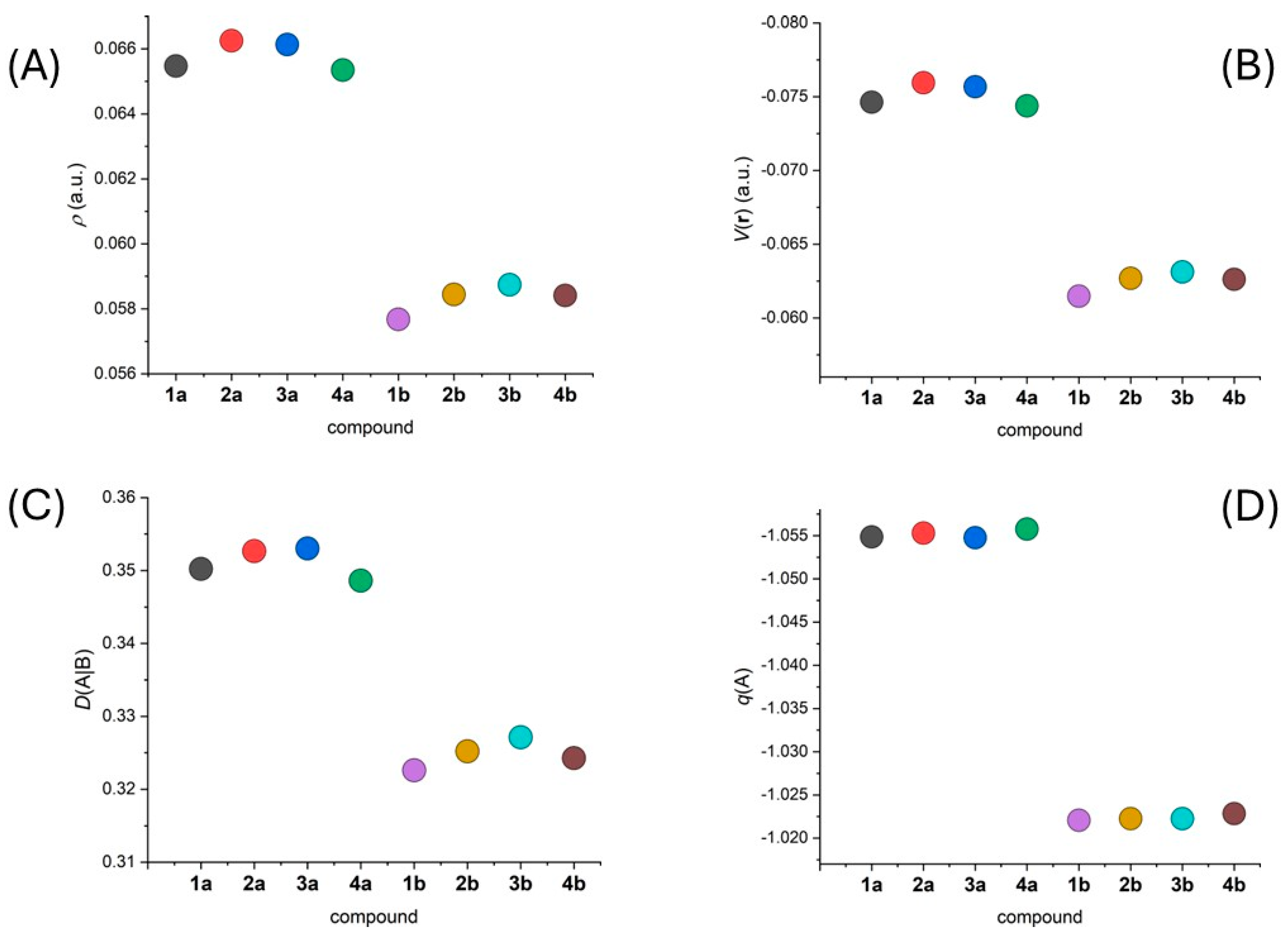
We utilized the DFT-calculated wavefunctions to analyze energetics and topology of electron density using QT-AIM calculations [36]. We identified (3,−1) bond critical points and we focused on the following parameters: electron density, r(r), and potential energy density, V(r), which are useful in determining the electron density and its localization. We also investigated the atomic charges, q(A), of the donor atoms and assessed the covalency of the M-L bonds through their delocalization indices, D(A|B).
The results of the AIM calculations clearly confirmed a distinction between the properties of the secondary amine nitrogen atom in 1a–4a and its methylated analogue in 1b–4b. The shorter Fe-Nam bond lengths in 1a–4a compared to 1b–4b resulted in more negative values of q(r) and V(r), consistent with stronger Fe-Nam bonds and greater covalency in 1a–4a (Figure 5A,B). The greater covalency was further supported by the higher D(A|B) values calculated for the F-Nam bonds in 1a–4a (Figure 5C). Remarkably, the more negative atomic charges were calculated for the Nam atoms in 1a–4a, indicating a greater contribution to the ligand field compared to the analogues in 1b–4b (Figure 5D).
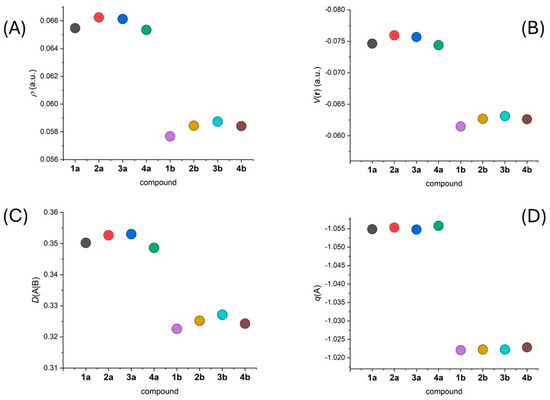
Figure 5.
Graphical depiction of the calculated values for the following parameters: (A) ρ(r), (B) V(r), and (C) D(A|B) at the (3,−1) bond critical point of the Fe–Nam bond in 1a–4b, and (D) q(A) over the basin of the Nam atom.
Moreover, the respective differences in CAM-B3LYP energies of HS and LS species were evaluated, also taking into account the zero-point vibrational energies (ΔEel.+ZPVE). The complexes under study can be ordered as follows (ΔEel.+ZPVE in kcal/mol): 1b (1.110) < 2b (1.176) < 3b (1.372) < 4b (2.729) < 2a (3.181) < 1a (3.305) < 3a (3.568) < 4a (5.439). It is well known that absolute energy differences calculated by DFT differ from functional to functional. However, the given order can be interpreted as the tendency of complexes on the left to stabilize the HS state and those on the right to stabilize the LS state. Indeed, complex 4a has the most pronounced SCO behavior, whereas complexes 1b–4b are all in the HS state.
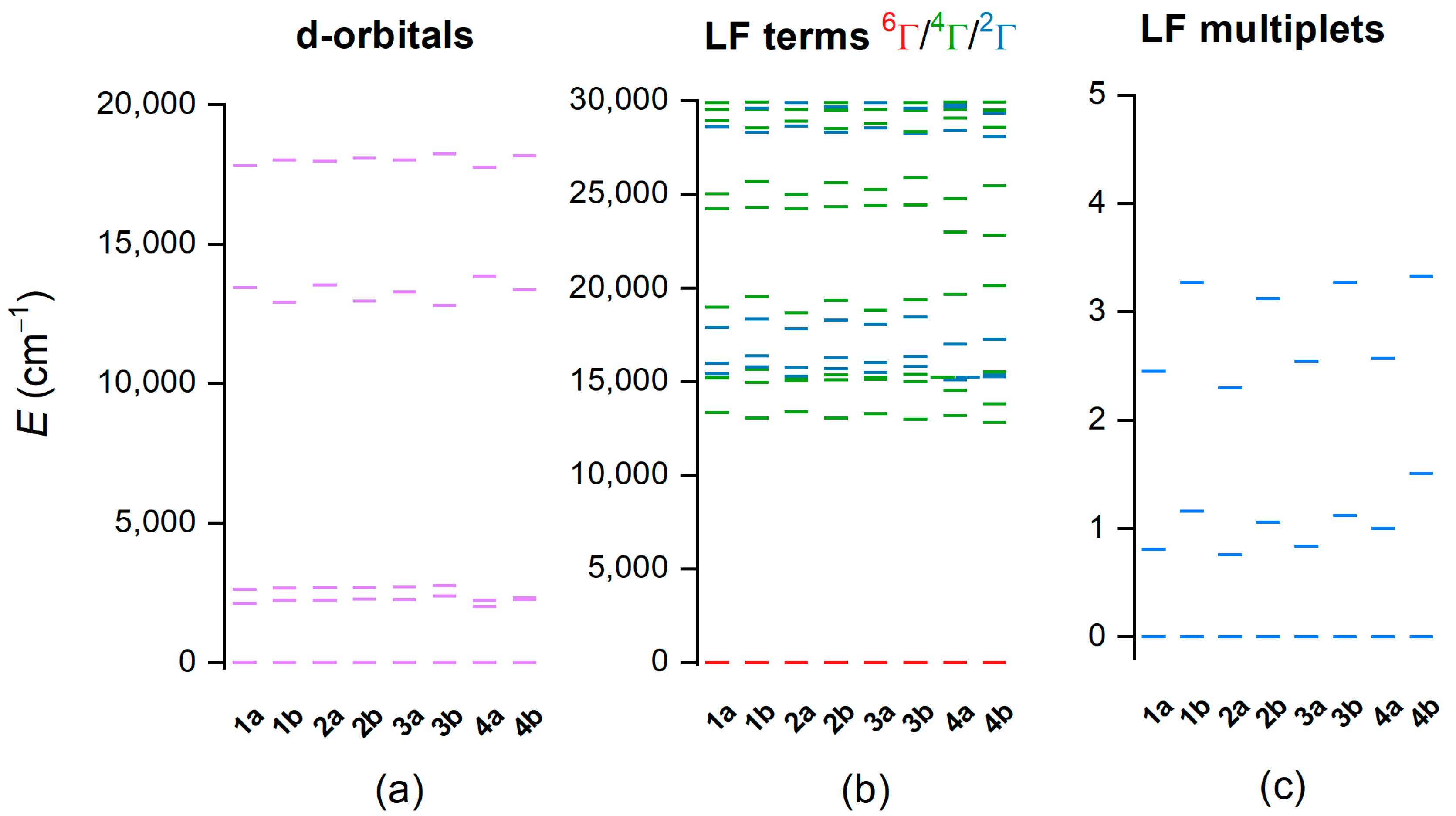
Furthermore, the post-Hartree–Fock method, CASSCF/NEVPT2, was employed to more deeply evaluate the electronic structure of these complexes. First, the respective calculations were carried out for 1a–4b complexes in the HS state using the active space defined by five electrons in five d-orbitals, CAS(5e,5o)—Figure 6. Ab initio ligand field theory [37,38] was used to calculate energies of d-orbitals (Figure 6a), which correspond to pseudo-octahedral geometry. The first three d-orbitals represent “t2g” orbitals (dxy, dxz, dyz), and the next two orbitals are “eg” orbitals (dx2-y2, dz2). There is an evident variation of energy of the fourth d-orbital; energies are slightly higher for 1b–4b complex (ca 13,450–13,830 cm−1) than for 1a–4a complexes (ca 12,800–13,350 cm−1). This orbital is oriented along Nam-Fe-Nbylim coordination bonds, and thus, the variation of the energy is related to the presence/absence of methyl group on the Nam atom—Figure S8. The calculations correctly found the sextet ligand-field term to be the ground state with excited terms of lower multiplicities located above ca 13,000 cm−1 (Figure 6b). As expected, the zero-field splitting of the ground state sextet due to the spin–orbit coupling is small, as shown in Figure 6c.
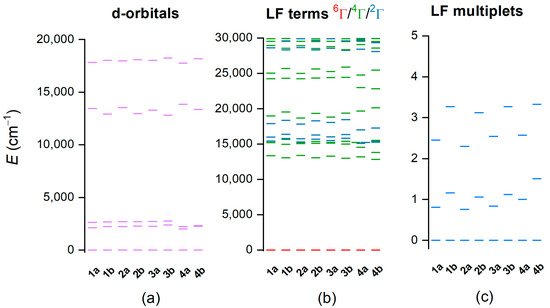
Figure 6.
Results of the CASSCF/NEVPT2 with CAS(5e,5o) computation for CAM-B3LYP-optimized geometries of [Fe(L5)(bylim)]+ cations of 1a–4b in high-spin (HS) state. Plot of the d-orbital splitting calculated by ab initio ligand field theory (AILFT) (a), low-lying ligand-field terms (LFT) (b), and ligand-field multiplets (LFM) (c). Note: different multiplicities of LFT are shown in different colors.
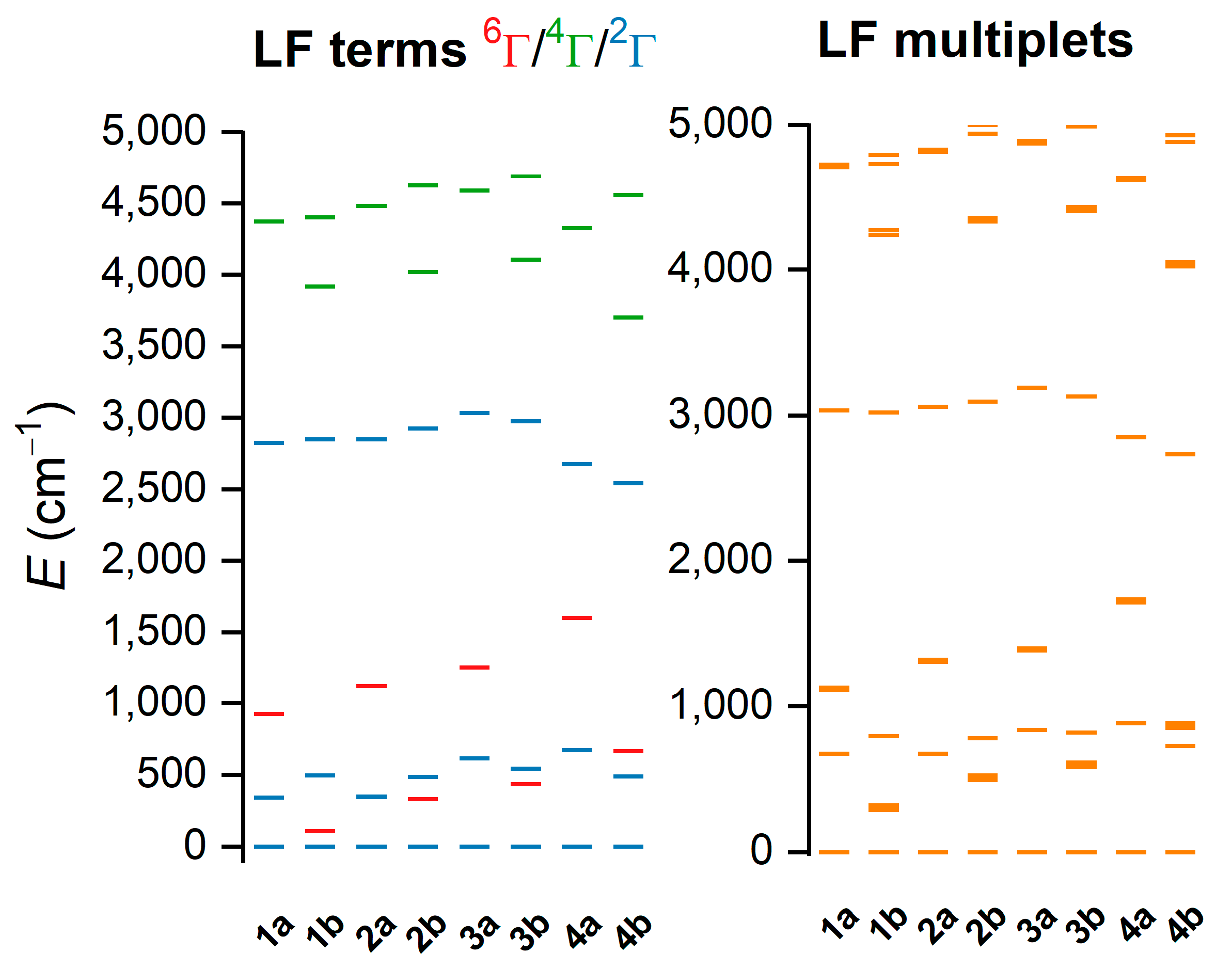
Analogous calculations were also performed for LS-optimized geometries. However, it was found that CAS(5e,5o)-active space led to the sextet ground state—Figure S9. Therefore, the two additional ligand-based orbitals were added to the active space, balancing bonding and antibonding orbitals, resulting in CAS(9e,7o)—Figure S10. Now, the singlet was in the ground state, as expected for LS species—Figure 7. Please note that the AILFT procedure is not possible for this active space, so d-orbitals are not shown in Figure 7. It is obvious that for the 1a–4a LS complexes, there is a much larger energy separation between the ground doublet state and first excited sextet LFT state (Δ2→6) than for 1b–4b complexes. The LS complexes can be ordered according to Δ2→6 (cm−1) as follows: 1b (108) < 2b (331) < 3b (435) < 4b (667) < 1a (925) < 2a (1123) < 3a (1253) < 4a (1600). This order is very similar to those found by CAM-B3LYP calculations, and again it shows that the substitution of the hydrogen atom to the methyl group on Nam in L5 leads to the stabilization of the HS state.
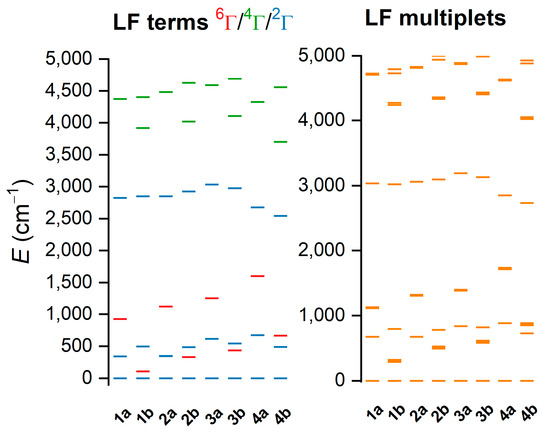
Figure 7.
Results of the CASSCF/NEVPT2 with CAS(9e,7o) computation for CAM-B3LYP-optimized geometries of [Fe(L5)(bylim)]+ cations of 1a–4b in low-spin (LS) state. Plot of low-lying ligand-field terms (LFT) (left), and ligand-field multiplets (LFM) (right). Note: different multiplicities of LFT are shown in different colors.
3. Materials and Methods
3.1. General Considerations and Instrumentation
Chemicals were purchased from commercial sources and used as received. Elemental analysis was carried out on a Flash 2000 (ThermoFisher Scientific, Waltham, MA, USA). Infrared spectra were collected on a FT/IR-4700 spectrometer (Jasco, Heckmondwike, UK) using the ATR technique on a diamond plate in the range of 400–4000 cm−1.
Magnetic susceptibility and magnetization measurements were performed using a SQUID magnetometer (Quantum Design Inc., San Diego, CA, USA) from T = 2 K at B = 0.1 T. The magnetization data were taken at T = 2.0 K. Raw susceptibility was corrected, and diamagnetic corrections of the constituent atoms were estimated from Pascal constants. The effective magnetic moment was calculated as usual: μeff/μB = 798(χT)1/2 when SI units are employed.
3.2. X-Ray Diffraction Analysis
A suitable single crystal of compounds 1a (at 298K), 3b, and 4b was used for the X-ray diffraction experiment using a Rigaku XtaLAB Synergy-I diffractometer (Rigaku, Tokyo, Japan) with a microfocused RTG-source PhotonJet-i (Cu Kα radiation, λ = 1.54184 Å) and a HyPix Bantam detector. The multi-scan absorption corrections were applied using the program CrysAlisPro 1.171.40.82a [39]. The X-ray diffraction data for 1a at 90 K were collected using a Bruker SMART APEX-II diffractometer (Bruker Co., Billerica, MA, USA) (Mo Kα radiation, λ = 0.71073 Å). Data collection, reduction, and absorption correction were performed by Bruker AXS software (Version 6.01) [40]. Single-crystal X-ray diffraction data for 2b were collected on an Oxford diffractometer Xcalibur2 (Oxford Diffraction Ltd., Oxford, UK) with a Sapphire CCD detector and fine-focused sealed tube (Mo Kα radiation, λ = 0.71073 Å).
The structures were solved using the SHELXT [41] program and refined via the full matrix least-squares procedure with SHELXL [42] in OLEX2 (version 1.5) [43]. Non-routine aspects of refinement:
1a@90K: The crystal structure could be modeled as a whole molecule disorder of two LS and HS sites; however, such a model does not provide realistic metal–ligand bond lengths. Therefore, we restricted modeling the disorder only to the aliphatic chain of the pentadentate ligand, phenyl rings of the bylim ligand, and BPh4− anion. Due to this approach, a B-alert on Hirshfeld test results occurred.
1a@298K: The phenyl ring of the bylim ligand was modeled as a positional disorder over two sites.
2b: The aliphatic part of the pentadentate ligand was modeled as a positional disorder over two sites.
3b: The measured crystal was an inversion twin; therefore, twin and BASF refinement was performed. The aliphatic part of the pentadentate ligand was modeled as a positional disorder over two sites.
4b: The aliphatic part of the pentadentate ligand was modeled as a positional disorder over two sites.
3.3. Synthesis
The synthesis of the precursor chloride complexes was performed according to previously reported procedures [5]. The 1a–4b complexes were prepared using the same method and, therefore, only a synthesis of 1a will be described in detail.
To a solution of the chloride precursor complex (86 mg, 0.2 mmol, 25 mL CH3OH), a portion of the bylim ligand (31.6 mg, 0.2 mmol) was added. The violet solution was refluxed for 30 min and filtered through a paper filter into a solution of NaBPh4 (68 mg in 5 mL of CH3OH, 0.2 mmol). Violet crystals precipitated overnight. The crystals were collected, washed with methanol and diethyl ether, and dried. The single crystals suitable for X-ray diffraction were obtained for 1a, 2b, 3b, and 4b.
The results of elemental analysis:
1a: calcd (%) for C54H53BFeN5O2, Mw = 870.7 g·mol−1, C, 74.5; H, 6.1; N, 8.0. Found: C, 74.1; H, 6.0; N, 7.9.
1b: calcd (%) for C55H55BFeN5O2, Mw = 884.7 g·mol−1, C, 74.7; H, 6.3; N, 7.9. Found: C, 74.5; H, 6.2; N, 7.9.
2a: calcd (%) for C56H57BFeN5O4, Mw = 930.7 g·mol−1, C, 72.3; H, 6.2; N, 7.5. Found: C, 71.9; H, 6.2; N, 7.4.
2b: calcd (%) for C57H59BFeN5O4, Mw = 944.8 g·mol−1, C, 72.5; H, 6.3; N, 7.4. Found: C, 72.3; H, 6.4; N, 7.1.
3a: calcd (%) for C58H61BFeN5O4, Mw = 958.8 g·mol−1, C, 72.7; H, 6.4; N, 7.3. Found: C, 72.9; H, 6.7; N, 7.1.
3b: calcd (%) for C59H63BFeN5O4, Mw = 972.8 g·mol−1, C, 72.8; H, 6.5; N, 7.2. Found: C, 72.9; H, 6.7; N, 7.0.
4a: calcd (%) for C62H57BFeN5O2, Mw = 970.8 g·mol−1, C, 76.7; H, 5.9; N, 7.2. Found: C, 76.4; H, 6.1; N, 7.0.
4b: calcd (%) for C63H59BFeN5O2, Mw = 984.8 g·mol−1, C, 76.8; H, 6.0; N, 7.1. Found: C, 76.3; H, 6.0; N, 7.0.
3.4. Theoretical Methods
The quantum chemical calculations at DFT and CASSSCF levels of theory were performed with ORCA 6.0 [44,45]. The def2-TZVP Ahlrich vase was used for all atoms [46]. The calculations were sped up using the def2/J Coulomb fitting basis set [47] and RIJCOSX approximation [48,49,50]. The DFT calculations were performed with atom-pairwise dispersion correction (D4) [51] and using the SMD solvation model for water [52]. The largest integration grid (DefGrid3) was used in all calculations together with tightSCF criteria. The vibrational analyses confirmed proper convergence for complexes at the local energy minimum, as no imaginary frequencies were found. The thermochemistry data were calculated as implemented in ORCA. The calculated electron density was analyzed with AIMAll software, version 19.10.12 [53]. Next, the state average complete active space self-consistent field (SA-CASSCF) wave function method [54] was employed, and these calculations were complemented by N-electron valence second-order perturbation theory (NEVPT2) [55,56]. The 1 sextet, 24 quartet, and 75 doublets states were involved in these calculations.
4. Conclusions
In this article, we report on the crystal structures and magnetic properties of a series of [Fe(L5)(bylim)](BPh4) complexes. The prepared compounds can be divided into two subseries based on the substitution of the secondary amine group. Magnetic measurements revealed that compounds with a non-substituted secondary amine (Nam) group (1a–4a) exhibit some form of spin crossover or spin equilibrium, whereas those with a methylated secondary amine group (1b–4b) favor the high spin state. This intriguing feature was further investigated through QT-AIM calculations, which revealed that the 1a–4a complexes have stronger bonds with higher covalence of the Fe-Nam bonds compared to those in 1b–4b. The atomic charges of the Nam atom were more negative in 1a–4a than in 1b–4b, which is consistent with a larger ligand field contribution. Furthermore, both DFT and CASSCF/NEVPT2 calculations showed that methyl substitution on the Nam atom leads to enhancing the stability of the high-spin state, which is in accordance with the experimental magnetic data.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics13020057/s1, Table S1: Crystal data and details of structure determination of complexes 1a and 2b; Table S2: Crystal data and details of structure determination of complexes 3b and 4b; Table S3: The root-mean-square deviation (RMSD) calculated for the coordination polyhedral; Figures S1–S3: FT-IR spectra of studied compounds; Figure S4: Temperature dependence of the magnetic moment measured between 2–300 K for 1b; Figure S5: Temperature dependence of the magnetic moment measured between 2–300 K for 3b; Figure S6: Temperature dependence of the magnetic moment measured between 2–300 K for 4b; Figure S7: Field dependence of the isothermal magnetization measured at T = 2 K for 1a–4a and 1b–4b; Figure S8: AILFT d-orbitals resulting from the CASSCF/NEVPT2 calculations; Figure S9: Results of the CASSCF/NEVPT2 with CAS(5e,5o) computation; Figure S10: Active orbitals used for CASSCF/NEVPT2 calculations with CAS(9e,7o).
Author Contributions
Conceptualization, I.N. and R.H.; Methodology, I.N. and R.H.; Formal analysis, I.N. and R.H.; Investigation, I.N. and R.H.; Writing—original draft, I.N. and R.H.; Writing—review & editing, I.N. and R.H. Both authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the institutional sources of the Department of Inorganic Chemistry, Palacký University Olomouc, Czech Republic.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors would like to thank P. Richterova for the measurement of the elemental analysis and Peter Antal for the measurement of the IR spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Koningsbruggen, P.J.; Maeda, Y.; Oshio, H. Iron(III) spin crossover compounds. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin, Germany, 2004; pp. 259–324. [Google Scholar]
- Boonprab, T.; Thammasangwan, W.; Chastanet, G.; Gonidec, M.; Harding, P.; Harding, D.J. Halide Anion Effects and Magnetostructural Relationships in Iron(III) Spin Crossover Complexes. Cryst. Growth Des. 2024, 24, 8145–8152. [Google Scholar] [CrossRef]
- Díaz-Torres, R.; Gómez-Coca, S.; Ruiz, E.; Harding, P.; Harding, D.J. Improving spin crossover characteristics in heteroleptic [FeIII(qsal-5-I)(qsal-5-OMe)]A complexes. Dalton Trans. 2023, 52, 18148–18157. [Google Scholar] [CrossRef]
- Harding, D.J.; Harding, P.; Phonsri, W. Spin crossover in iron(III) complexes. Coord. Chem. Rev. 2016, 313, 38–61. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ohta, S.; Yoshimura, C.; Ohyoshi, A.; Kohata, S.; Okawa, H.; Maeda, Y. Studies on spin-equilibrium iron(III) complexes. Part 1. Syntheses and magnetic properties of a new family of spin cross-over iron(III) complexes with a unidentate ligand over a wide range of the spectrochemical series and a quinquedentate ligand derived. J. Chem. Soc. Dalton Trans. 1985, 12, 2575. [Google Scholar] [CrossRef]
- Boča, R.; Fukuda, Y.; Gembický, M.; Herchel, R.; Jaroščiak, R.; Linert, W.; Renz, F.; Yuzurihara, J. Spin crossover in mononuclear and binuclear iron(III) complexes with pentadentate Schiff-base ligands. Chem. Phys. Lett. 2000, 325, 411–419. [Google Scholar] [CrossRef]
- Tanimura, K.; Kitashima, R.; Bréfuel, N.; Nakamura, M.; Matsumoto, N.; Shova, S.; Tuchagues, J.-P. Infinite chain structure and steep spin crossover of a Fe III Complex with a N3O2 pentadentate schiff-base ligand and 4-aminopyridine. Bull. Chem. Soc. Jpn. 2005, 78, 1279–1282. [Google Scholar] [CrossRef]
- Nemec, I.; Svoboda, I.; Herchel, R. Spin Crossover in Three Mononuclear Iron (III) Schiff Base Complexes. Metals 2019, 9, 849. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; De Bonneval, B.G.; Molnár, G.; Bousseksou, A.; Gomez-Garcia, C.J.; Coronado, E.; Valade, L. Towards molecular conductors with a spin-crossover phenomenon: Crystal structures, magnetic properties and Mössbauer spectra of [Fe(salten)mepepy][M(dmit)2] complexes. Eur. J. Inorg. Chem. 2005, 2005, 3261–3270. [Google Scholar] [CrossRef]
- Bannwarth, A.; Schmidt, S.O.; Peters, G.; Sönnichsen, F.D.; Thimm, W.; Herges, R.; Tuczek, F. FeIII spin-crossover complexes with photoisomerizable ligands: Experimental and theoretical studies on the ligand-driven light-induced spin change effect. Eur. J. Inorg. Chem. 2012, 2012, 2776–2783. [Google Scholar] [CrossRef]
- Nemec, I.; Boča, R.; Herchel, R.; Trávníček, Z.; Gembický, M.; Linert, W. Dinuclear Fe(III) complexes with spin crossover. Monatshefte Chem. Chem. Mon. 2009, 140, 815–828. [Google Scholar] [CrossRef]
- Hayami, S.; Hiki, K.; Kawahara, T.; Maeda, Y.; Urakami, D.; Inoue, K.; Ohama, M.; Kawata, S.; Sato, O. Photo-Induced Spin Transition of Iron(III) Compounds with π-π Intermolecular Interactions. Chem.-A Eur. J. 2009, 15, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Si-Guo, W.; Najbul, H.M.; Jie-Yu, Z.; Guo-Zhang, H.; Vu, H.A.N.; Liviu, U.; Wei-Xiong, Z.; Zhao-Ping, N.; Ming-Liang, T. Multiresponsive Spin Crossover Driven by Rotation of Tetraphenylborate Anion in an Iron(III) Complex. CCS Chem. 2020, 3, 453–459. [Google Scholar] [CrossRef]
- Pavlik, J.; Masárová, P.; Nemec, I.; Fuhr, O.; Ruben, M.; Šalitroš, I. Heteronuclear Iron(III)-Schiff Base Complexes with the Hexacyanidocobaltate(III) Anion: On the Quest to Understand the Governing Factors of Spin Crossover. Inorg. Chem. 2020, 59, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Herchel, R.; Boča, R.; Gembický, M.; Kožísek, J.; Renz, F. Spin Crossover in a Tetranuclear Cr(III)−Fe(III)3 Complex. Inorg. Chem. 2004, 43, 4103–4105. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Boča, R.; Trávníček, Z.; Svoboda, I.; Fuess, H.; Linert, W. Tuning of spin crossover behaviour in iron(III) complexes involving pentadentate Schiff bases and pseudohalides. Dalton Trans. 2011, 40, 10090–10099. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Trávníček, Z. The relationship between the strength of hydrogen bonding and spin crossover behaviour in a series of iron(iii) Schiff base complexes. Dalton Trans. 2015, 44, 4474–4484. [Google Scholar] [CrossRef]
- Vela, S.; Paulsen, H. Cooperativity in Spin Crossover Systems. An Atomistic Perspective on the Devil’s Staircase. Inorg. Chem. 2018, 57, 9478–9488. [Google Scholar] [CrossRef] [PubMed]
- Nemec, I.; Boča, R.; Gembický, M.; Dlháň, L.; Herchel, R.; Renz, F. High-spin Schiff-base dinuclear iron(III) complexes bridged by N-oxide ligands. Inorg. Chim. Acta 2009, 362, 4754–4759. [Google Scholar] [CrossRef]
- Pogány, L.; Brachňaková, B.; Masárová, P.; Moncol, J.; Pavlik, J.; Gál, M.; Mazúr, M.; Herchel, R.; Nemec, I.; Šalitroš, I. Impact of the Schiff base ligand substituents on the solid state and solution properties of eleven iron(III) complexes. New J. Chem. 2019, 43, 13916–13928. [Google Scholar] [CrossRef]
- Milocco, F.; De Vries, F.; Siebe, H.S.; Engbers, S.; Demeshko, S.; Meyer, F.; Otten, E. Widening the Window of Spin-Crossover Temperatures in Bis(formazanate)iron(II) Complexes via Steric and Noncovalent Interactions. Inorg. Chem. 2021, 60, 2045–2055. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Najibi, A.; Goerigk, L. DFT-D4 counterparts of leading meta-generalized-gradient approximation and hybrid density functionals for energetics and geometries. J. Comput. Chem. 2020, 41, 2562–2572. [Google Scholar] [CrossRef]
- Bursch, M.; Neugebauer, H.; Ehlert, S.; Grimme, S. Dispersion corrected r2SCAN based global hybrid functionals: r2SCANh, r2SCAN0, and r2SCAN50. J. Chem. Phys. 2022, 156, 134105. [Google Scholar] [CrossRef] [PubMed]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Rajchel, Ł.; Żuchowski, P.S.; Hapka, M.; Modrzejewski, M.; Szczęśniak, M.M.; Chałasiński, G. A density functional theory approach to noncovalent interactions via interacting monomer densities. Phys. Chem. Chem. Phys. 2010, 12, 14686–14692. [Google Scholar] [CrossRef]
- Friede, M.; Ehlert, S.; Grimme, S.; Mewes, J.-M. Do Optimally Tuned Range-Separated Hybrid Functionals Require a Reparametrization of the Dispersion Correction? It Depends. J. Chem. Theory Comput. 2023, 19, 8097–8107. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Calculate Root-Mean-Square Deviation (RMSD) of Two Molecules Using Rotation, GitHub. Version rmsd-1.5.1. Available online: https://github.com/charnley/rmsd (accessed on 11 November 2024).
- Li, W.; Li, X.; Robeyns, K.; Wolff, M.; Kfoury, J.; Oláh, J.; Herchel, R.; Demeshko, S.; Meyer, F.; Garcia, Y. Spin-state versatility in FeII4L6 supramolecular cages with a pyridyl-hydrazone ligand scaffold modulated by solvents and counter anions. Dalton Trans. 2024, 53, 1449–1459. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Applications; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-31776-0. [Google Scholar]
- Bader, R. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Atanasov, M.; Ganyushin, D.; Sivalingam, K.; Neese, F. A Modern First-Principles View on Ligand Field Theory Through the Eyes of Correlated Multireference Wavefunctions; Springer: Berlin/Heidelberg, Germany, 2011; pp. 149–220. ISBN 978-3-642-27378-0. [Google Scholar]
- Singh, S.K.; Eng, J.; Atanasov, M.; Neese, F. Covalency and chemical bonding in transition metal complexes: An ab initio based ligand field perspective. Coord. Chem. Rev. 2017, 344, 2–25. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.40.82a, Rigaku Oxford Diffraction: Oxford, UK, 2020.
- Bruker AXS Inc. APEX2, SMART-Plus and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shedrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F. A perspective on the future of quantum chemical software: The example of the ORCA program package. Faraday Discuss. 2024, 254, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A “chain-of-spheres” algorithm for the Hartree-Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef] [PubMed]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An improved chain of spheres for exchange algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef]
- AIMAll (Version 19.10.12), Todd A. Keith, TK Gristmill Software: Overland Park, KS, USA, 2019. Available online: https://aim.tkgristmill.com (accessed on 16 March 2023).
- Malmqvist, P.-Å.; Roos, B.O. The CASSCF state interaction method. Chem. Phys. Lett. 1989, 155, 189–194. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.-P. N-electron valence state perturbation theory: A fast implementation of the strongly contracted variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.-P. Introduction of n -electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).