Effect of Boron and Iron at Various Concentrations on the Catalytic Graphitization of the Polyacrylonitrile Derived from the Polymerization of Acrylonitrile
Abstract
1. Introduction
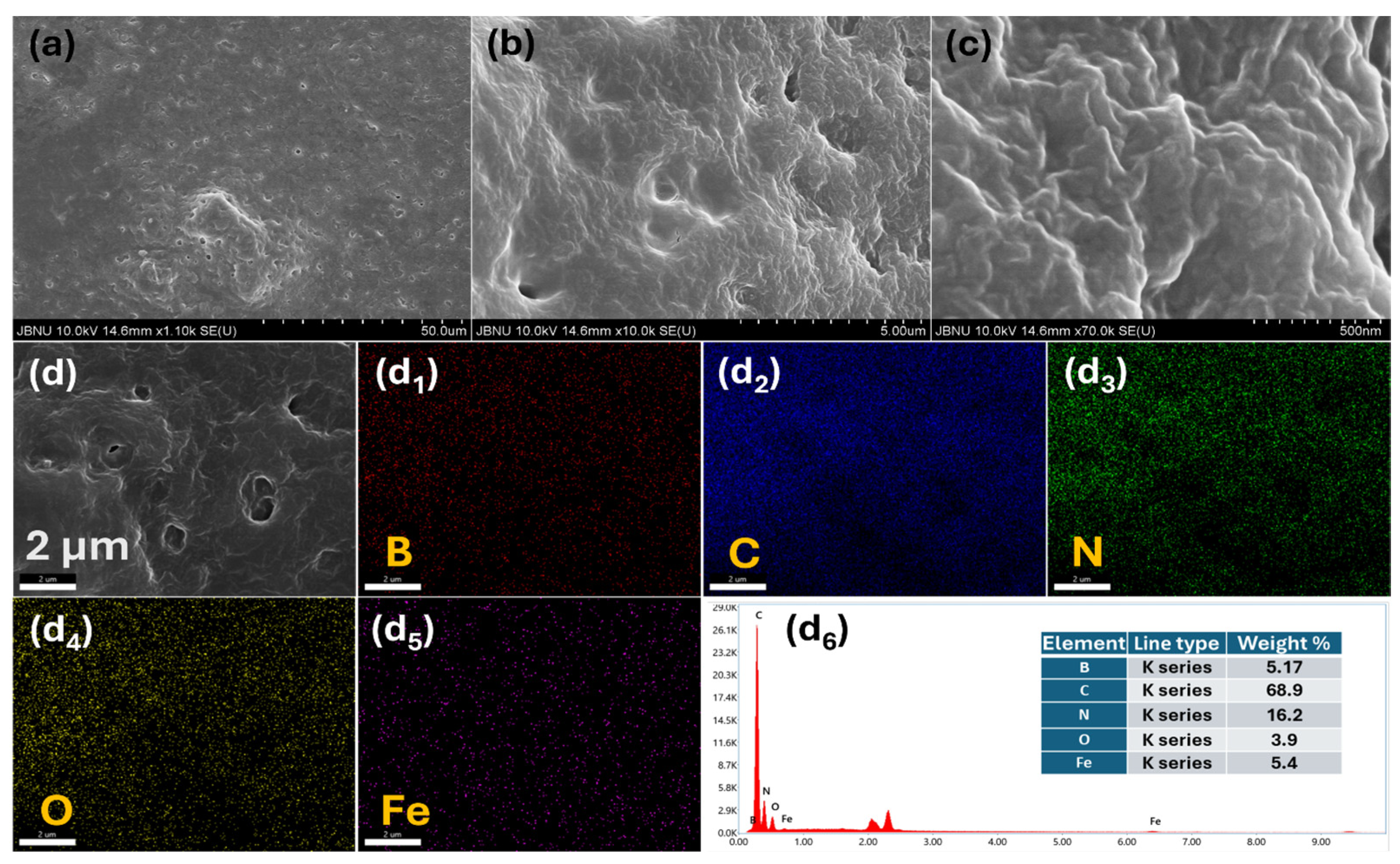
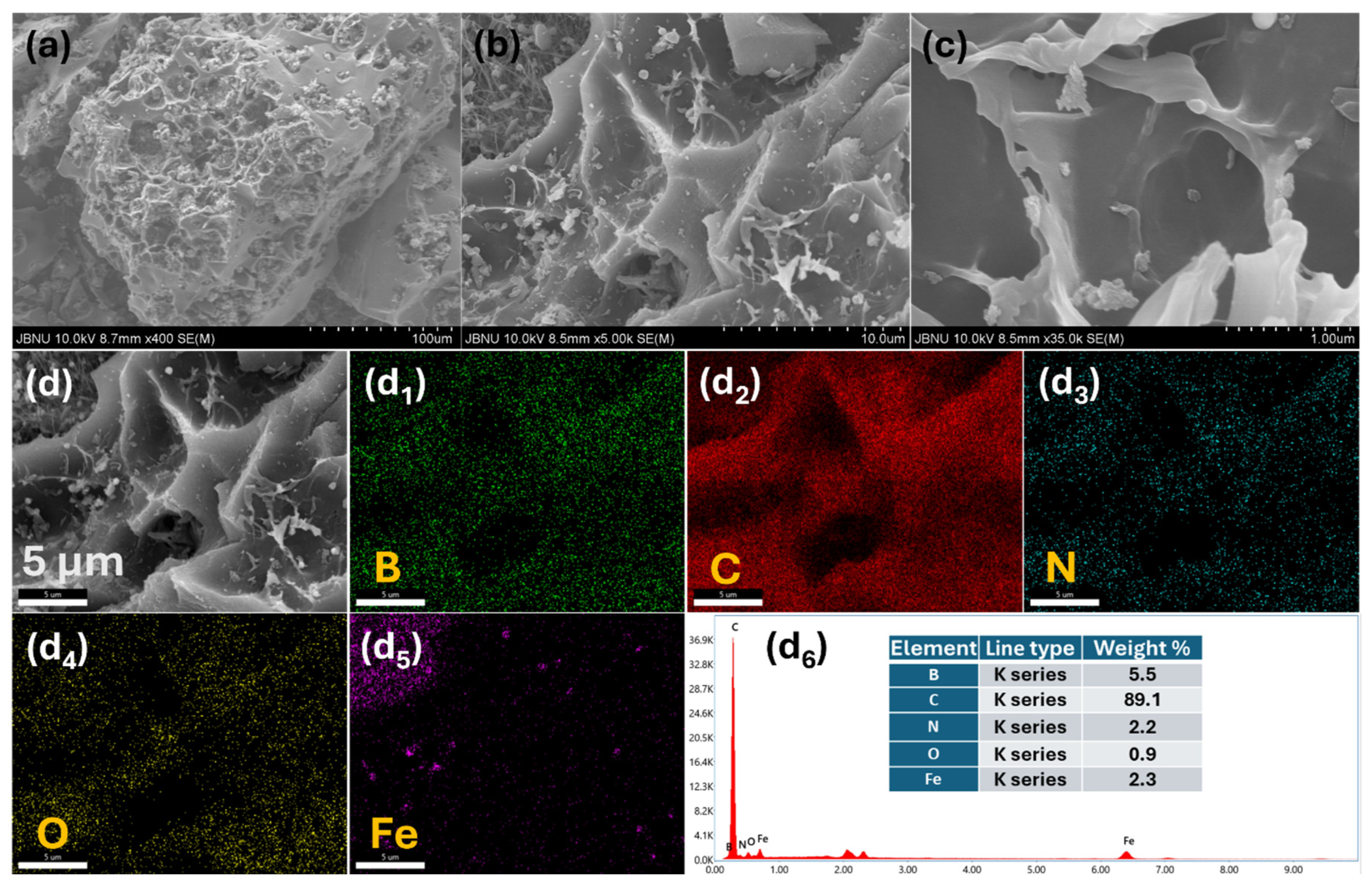
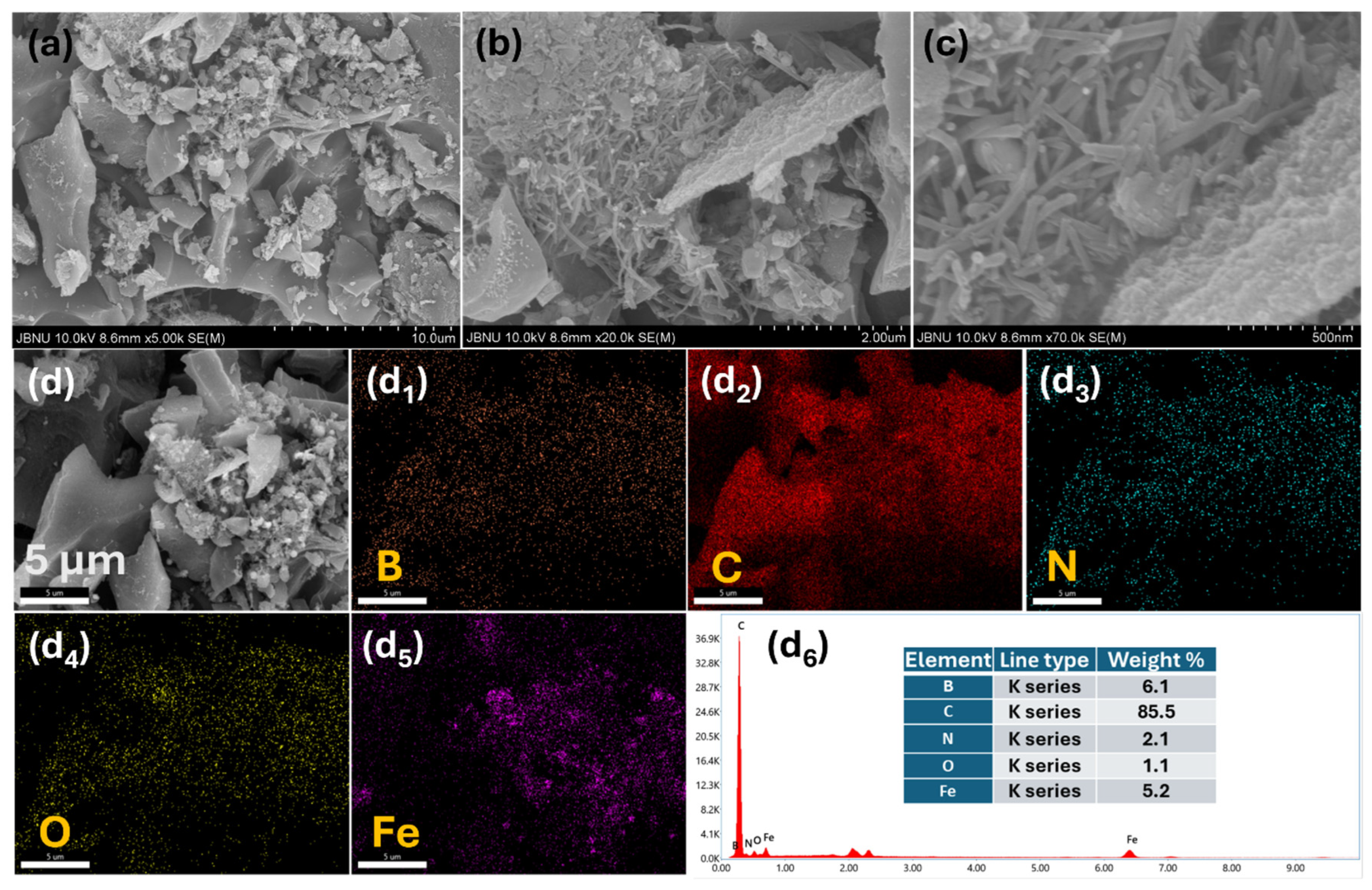
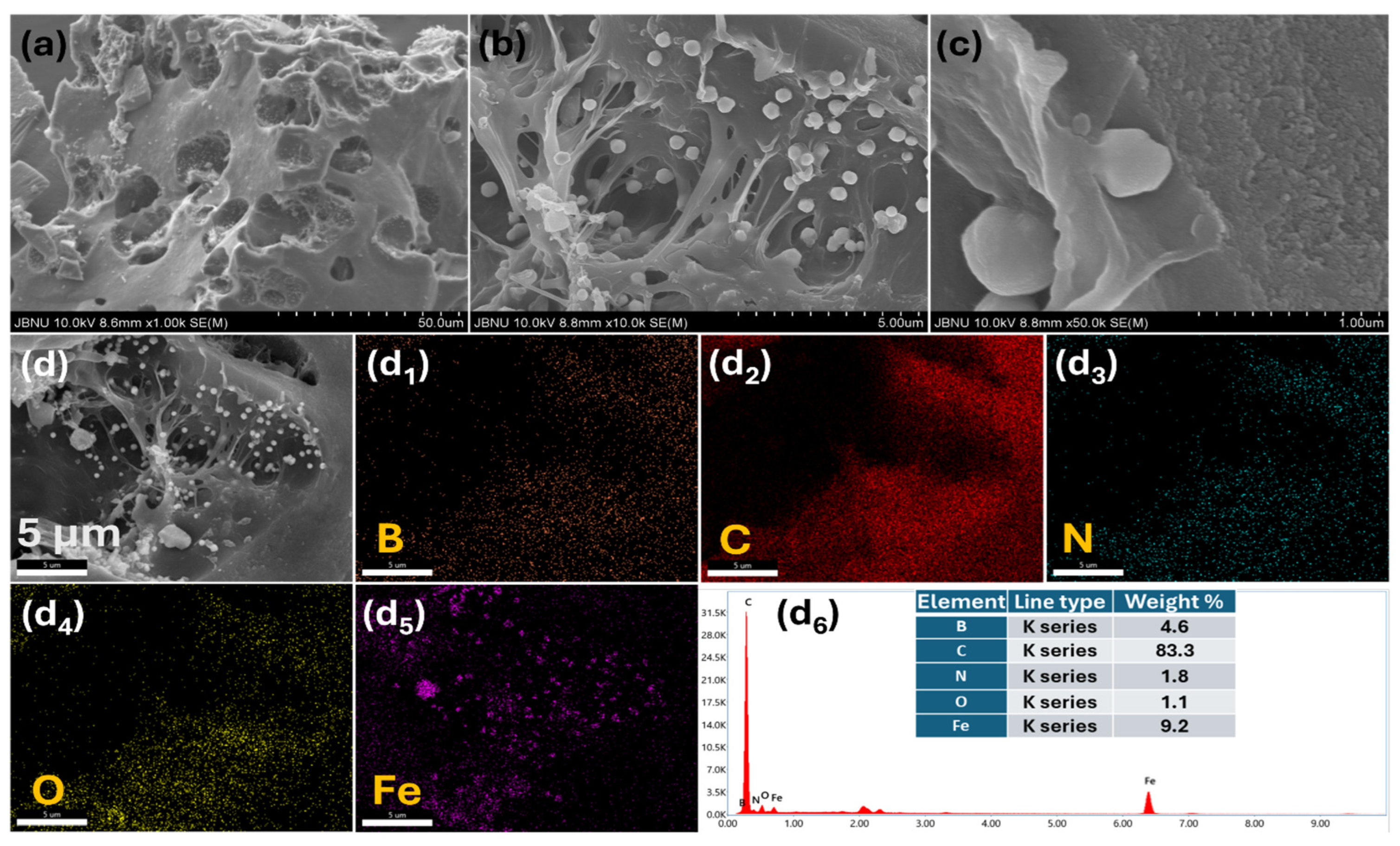
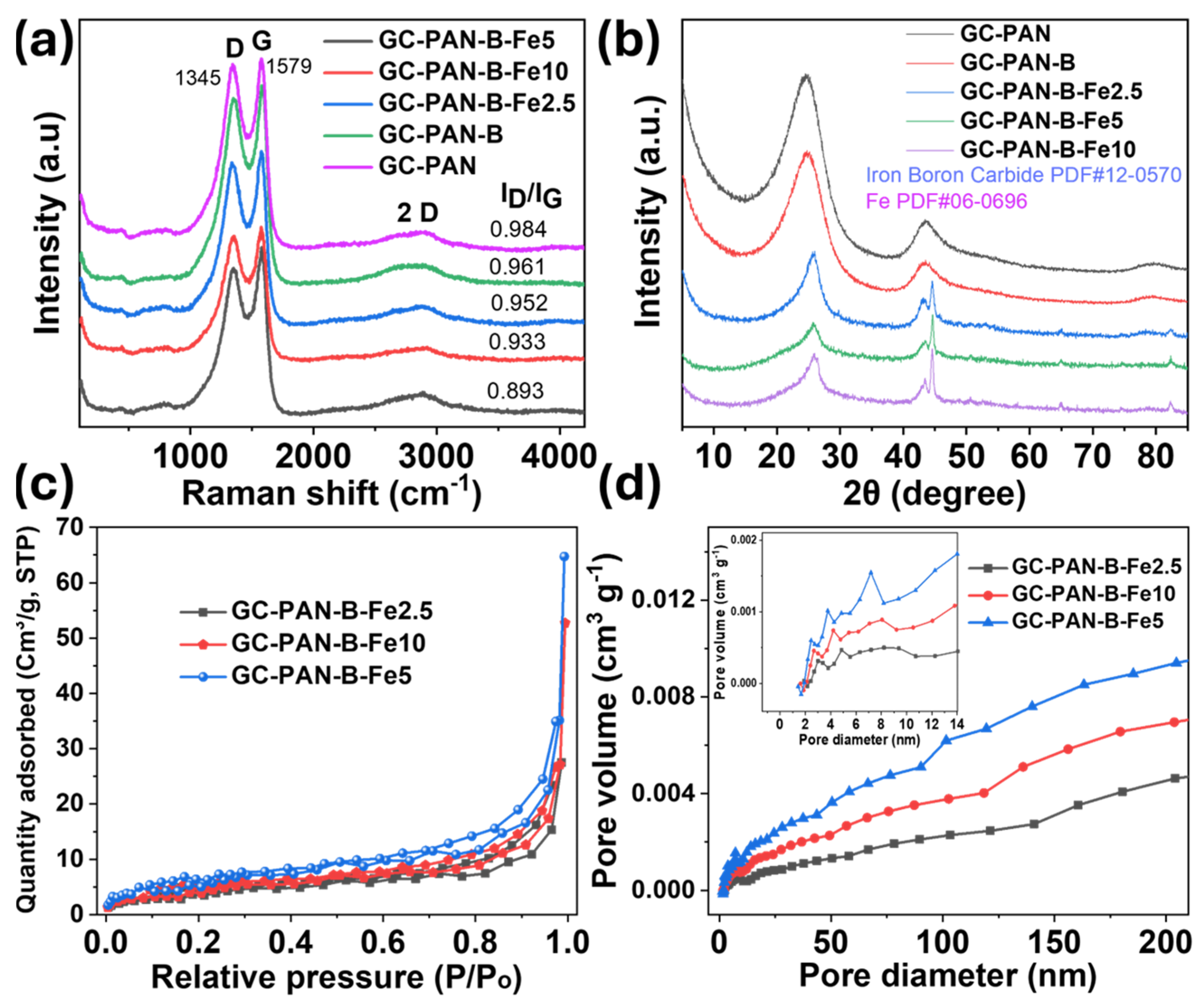
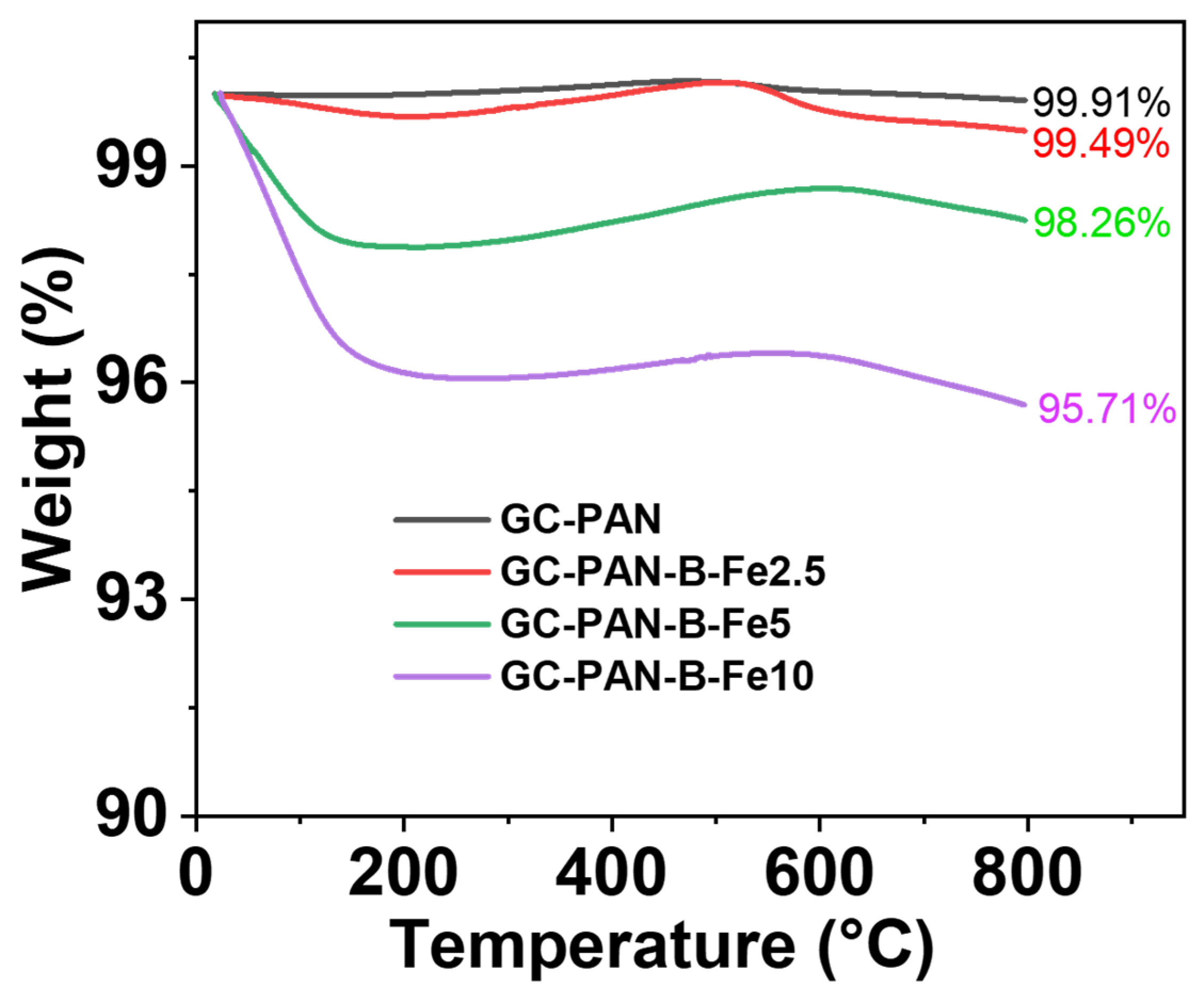
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
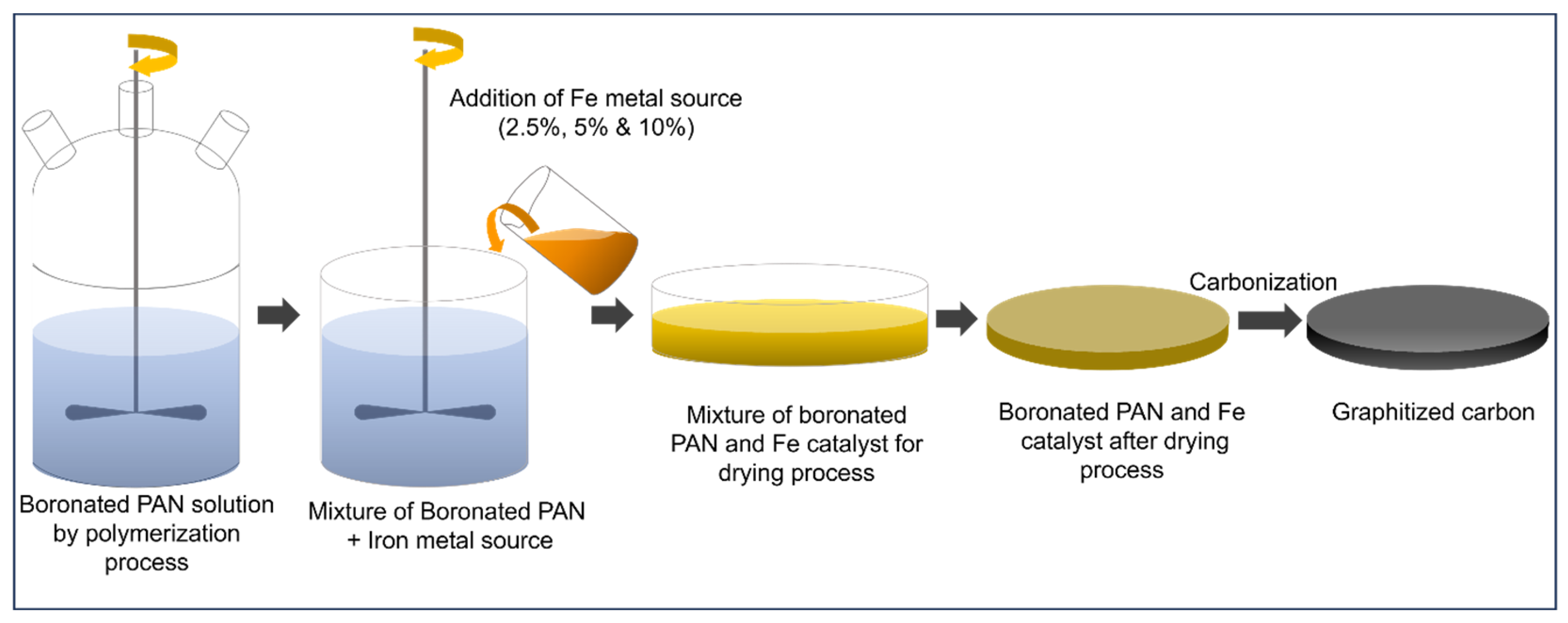
3.2. Preparation of Boron Iron Integrated PAN-Derived Graphitized Carbon (GC-PAN-B-Fe)
3.3. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kopeć, M.; Lamson, M.; Yuan, R.; Tang, C.; Kruk, M.; Zhong, M.; Matyjaszewski, K.; Kowalewski, T. Polyacrylonitrile-derived nanostructured carbon materials. Prog. Polym. Sci. 2019, 92, 89–134. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, Y.; Wang, H.; Zhang, B.; Hou, R.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Recent advances on energy and environmental application of graphitic carbon nitride (g-C3N4)-based photocatalysts: A review. J. Environ. Chem. Eng. 2023, 11, 110164. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, Y.-K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinning. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, R.; Zhang, P.; Wang, P.; Chen, N.; Qian, B.; Zhang, L.; Yu, J.; Dai, B. Functional Carbon from Nature: Biomass-Derived Carbon Materials and the Recent Progress of Their Applications. Adv. Sci. 2023, 10, 2205557. [Google Scholar] [CrossRef] [PubMed]
- Egbedina, A.O.; Bolade, O.P.; Ewuzie, U.; Lima, E.C. Emerging trends in the application of carbon-based materials: A review. J. Environ. Chem. Eng. 2022, 10, 107260. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Chung, Y.S.; Kim, J.; Moon, S.; Lee, S. Understanding the catalytic mechanism of calcium compounds for enhancing crystallinity in carbon fiber. Chem. Eng. J. 2024, 479, 147728. [Google Scholar] [CrossRef]
- Töngüç Yalçınkaya, T.; Çay, A.; Akduman, Ç.; Akçakoca Kumbasar, E.P.; Yanık, J. Polyacrylonitrile and polyacrylonitrile/cellulose-based activated carbon nanofibers obtained from acrylic textile wastes for carbon dioxide capture. J. Appl. Polym. Sci. 2024, 141, e55475. [Google Scholar] [CrossRef]
- Lee, S.; Cho, S.Y.; Chung, Y.S.; Choi, Y.C.; Lee, S. High electrical and thermal conductivities of a PAN-based carbon fiber via boron-assisted catalytic graphitization. Carbon 2022, 199, 70–79. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, J.; Wang, H.-L. Tuning of structural/functional feature of carbon fibers: New insights into the stabilization of polyacrylonitrile. Polymer 2023, 282, 126157. [Google Scholar] [CrossRef]
- Tripathy, M.; Padhiari, S.; Ghosh, A.K.; Hota, G. Polyacrylonitrile support impregnated with amine-functionalized graphitic carbon nitride/magnetite composite nanofibers towards enhanced arsenic remediation: A mechanistic approach. J. Colloid Interface Sci. 2023, 640, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X. Thermal conductivity of carbon-based nanomaterials: Deep understanding of the structural effects. Green Carbon 2023, 1, 47–57. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Zhang, S.; Niu, D.; Guo, B. Study on the mechanical and electrical conductivity properties of waste short carbon fibers concrete and the establishment of conductivity models. J. Build. Eng. 2024, 95, 110296. [Google Scholar] [CrossRef]
- Pirabul, K.; Pan, Z.-Z.; Tang, R.; Sunahiro, S.; Liu, H.; Kanamaru, K.; Yoshii, T.; Nishihara, H. Structural Engineering of Nanocarbons Comprising Graphene Frameworks via High-Temperature Annealing. Bull. Chem. Soc. Jpn. 2023, 96, 510–518. [Google Scholar] [CrossRef]
- Kim, T.; Kim, B.-S.; Ko, T.H.; Kim, H.Y. Effect of Salt Variability on the Low-Temperature Metal-Catalyzed Graphitization of PAN/DMSO Solutions for the Synthesis of Nanostructured Graphitic Carbon. Inorganics 2024, 12, 212. [Google Scholar] [CrossRef]
- Lower, L.; Dey, S.C.; Vook, T.; Nimlos, M.; Park, S.; Sagues, W.J. Catalytic Graphitization of Biocarbon for Lithium-Ion Anodes: A Minireview. ChemSusChem 2023, 16, e202300729. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.A.A.; Zheng, A.L.T.; Tsubota, T.; Andou, Y. Catalytic graphitization of biomass-derived ethanosolv lignin using Fe, Co, Ni, and Zn: Microstructural and chemical characterization. J. Anal. Appl. Pyrolysis 2023, 173, 106064. [Google Scholar] [CrossRef]
- Chen, L.; Fang, T.; Song, C.; Ju, W.; Li, H.; Hu, J. Boron-Catalytic Graphitization Boosting the Production of High-Performance Carbon Paper at a Moderate Temperature. Adv. Eng. Mater. 2021, 23, 2100305. [Google Scholar] [CrossRef]
- Khoshk Rish, S.; Tahmasebi, A.; Wang, R.; Dou, J.; Yu, J. Formation mechanism of nano graphitic structures during microwave catalytic graphitization of activated carbon. Diam. Relat. Mater. 2021, 120, 108699. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Gao, S.; Luo, G. Tuning microstructures of polyacrylonitrile-based carbon fibers by catalytic influence of boron for enhancement of mechanical properties and oxidation resistance. Diam. Relat. Mater. 2024, 142, 110758. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jia, X.; Chen, Y.; Tang, Y.; Wan, P.; Pan, J. Boron, oxygen co-doped porous carbon derived from waste tires with enhanced hydrophilic interface as sustainably high-performance material for supercapacitors. J. Energy Storage 2024, 80, 110320. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, S.; Yuan, M.; Wang, H.; Jung, J.C.-Y.; Zhang, J. Advanced boron-doped carbon papers with excellent electrical conductivity and low graphitization temperature for PEM fuel cells. Int. J. Hydrog. Energy 2024, 50, 1279–1293. [Google Scholar] [CrossRef]
- Ōya, A.; Yamashita, R.; Ōtani, S. Catalytic graphitization of carbons by borons. Fuel 1979, 58, 495–500. [Google Scholar] [CrossRef]
- Hong, S.; Nam, J.; Park, S.; Lee, D.; Park, M.; Lee, D.S.; Kim, N.D.; Kim, D.-Y.; Ku, B.-C.; Kim, Y.A.; et al. Carbon nanotube fibers with high specific electrical conductivity: Synergistic effect of heteroatom doping and densification. Carbon 2021, 184, 207–213. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Lu, W.; Cai, N.; Xiao, H.; Chen, X.; Chen, Y.; Wang, X.; Wang, S.; Wu, P.; et al. Fe–Co based synergistic catalytic graphitization of biomass: Influence of the catalyst type and the pyrolytic temperature. Energy 2022, 239, 122262. [Google Scholar] [CrossRef]
- Hunter, R.D.; Takeguchi, M.; Hashimoto, A.; Ridings, K.M.; Hendy, S.C.; Zakharov, D.; Warnken, N.; Isaacs, J.; Fernandez-Muñoz, S.; Ramirez-Rico, J.; et al. Elucidating the Mechanism of Iron-Catalyzed Graphitization: The First Observation of Homogeneous Solid-State Catalysis. Adv. Mater. 2024, 36, 2404170. [Google Scholar] [CrossRef] [PubMed]
- Dyjak, S.; Wyrębska, I.; Błachowski, A.; Kaszuwara, W.; Sobczak, K.; Polański, M.; Gratzke, M.; Kiciński, W. The role of heteroatoms in iron-assisted graphitization of hard carbons derived from synthetic polymers: The special case of sulfur-doping. Carbon 2024, 218, 118717. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Influence of iron (III) acetylacetonate on structure and electrical conductivity of Fe3O4/carbon composite nanofibers. Polymer 2012, 53, 6000–6007. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Z.-Q.; Wang, C.-Y. The structure and electrochemical properties of carbonized polyacrylonitrile microspheres. Solid State Ion. 2014, 261, 5–10. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jung, S. Template-free synthesis of polyacrylonitrile-derived porous carbon nanoballs on graphene for efficient oxygen reduction in zinc–air batteries. J. Mater. Chem. A 2021, 9, 9644–9654. [Google Scholar] [CrossRef]
- Pathak, I.; Dahal, B.; Acharya, D.; Chhetri, K.; Muthurasu, A.; Raj Rosyara, Y.; Kim, T.; Saidin, S.; Hoon Ko, T.; Yong Kim, H. Integrating V-doped CoP on Ti3C2Tx MXene-incorporated hollow carbon nanofibers as a freestanding positrode and MOF-derived carbon nanotube negatrode for flexible supercapacitors. Chem. Eng. J. 2023, 475, 146351. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Huang, Y.; Zhang, S.; Zhang, S.; Zou, J. Rational design of N-doped porous biomass carbon nanofiber electrodes for flexible asymmetric supercapacitors with high-performance. Appl. Surf. Sci. 2023, 638, 158137. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Lei, S.; Lu, W.; Cai, N.; Xiao, H.; Chen, Y.; Chen, H. Iron salt catalytic pyrolysis of biomass: Influence of iron salt type. Energy 2023, 262, 125415. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Bystrzejewski, M.; Zhu, Y. Synthesis, properties, and applications of carbon-encapsulated metal nanoparticles. Coord. Chem. Rev. 2024, 520, 216125. [Google Scholar] [CrossRef]
- Bisheh, H.; Abdin, Y. Carbon Fibers: From PAN to Asphaltene Precursors; A State-of-Art Review. C 2023, 9, 19. [Google Scholar] [CrossRef]
- Kwon, T.-G.; Park, H.; Jo, O.H.; Chun, J.; Kang, B.-G. Facile Preparation of Magnetite-Incorporated Polyacrylonitrile-Derived Carbons for Li-Ion Battery Anodes. ACS Appl. Energy Mater. 2022, 5, 1262–1270. [Google Scholar] [CrossRef]
- Gao, Z.; Sanjuán, I.; Hagemann, U.; Wittmar, A.S.M.; Andronescu, C.; Ulbricht, M. Polyacrylonitrile-based porous polymer spheres and their conversion to N-doped carbon materials for adsorption and electrocatalysis. J. Appl. Polym. Sci. 2024, 141, e55438. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Mondal, K.; Sharma, A.; Aminabhavi, T.M. Versatile Graphitized Carbon Nanofibers in Energy Applications. ACS Sustain. Chem. Eng. 2022, 10, 1334–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Kim, B.-S.; Ko, T.H.; Kim, H.Y. Effect of Boron and Iron at Various Concentrations on the Catalytic Graphitization of the Polyacrylonitrile Derived from the Polymerization of Acrylonitrile. Inorganics 2025, 13, 52. https://doi.org/10.3390/inorganics13020052
Kim T, Kim B-S, Ko TH, Kim HY. Effect of Boron and Iron at Various Concentrations on the Catalytic Graphitization of the Polyacrylonitrile Derived from the Polymerization of Acrylonitrile. Inorganics. 2025; 13(2):52. https://doi.org/10.3390/inorganics13020052
Chicago/Turabian StyleKim, Taewoo, Byoung-Suhk Kim, Tae Hoon Ko, and Hak Yong Kim. 2025. "Effect of Boron and Iron at Various Concentrations on the Catalytic Graphitization of the Polyacrylonitrile Derived from the Polymerization of Acrylonitrile" Inorganics 13, no. 2: 52. https://doi.org/10.3390/inorganics13020052
APA StyleKim, T., Kim, B.-S., Ko, T. H., & Kim, H. Y. (2025). Effect of Boron and Iron at Various Concentrations on the Catalytic Graphitization of the Polyacrylonitrile Derived from the Polymerization of Acrylonitrile. Inorganics, 13(2), 52. https://doi.org/10.3390/inorganics13020052









