Supramolecular Assemblies and Anticancer Activities of Aminopyidine-Based Polynuclear and Mononuclear Co(II) Benzoates: Experimental and Theoretical Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses and General Aspects
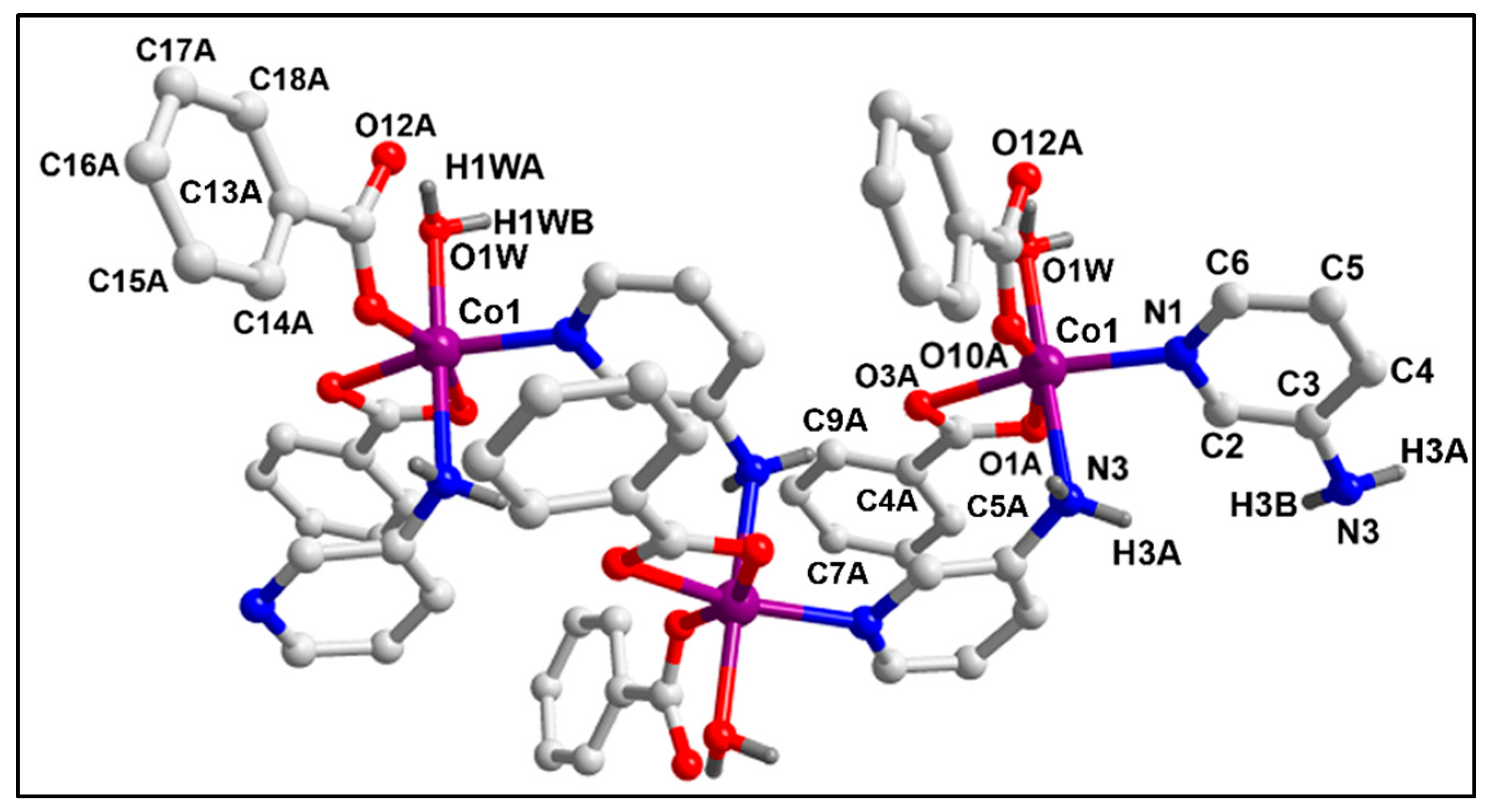
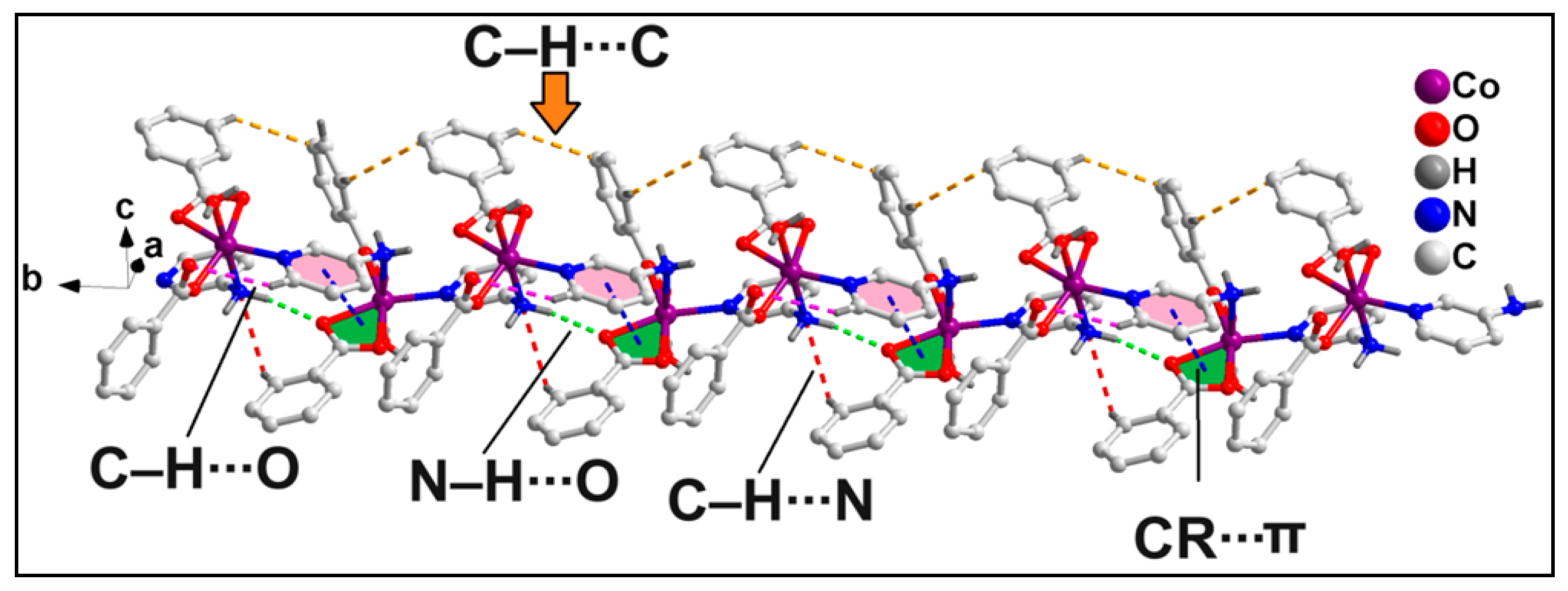
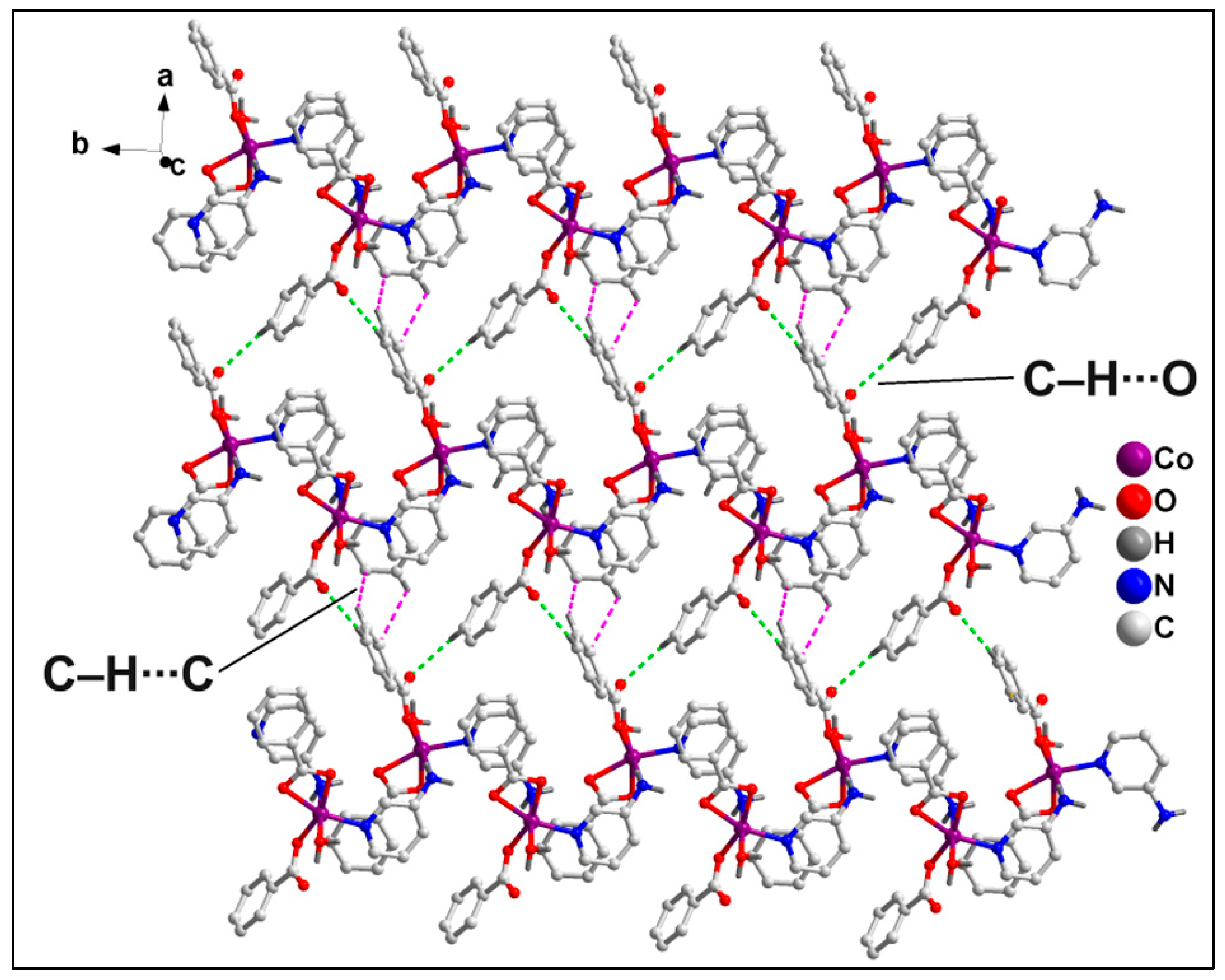
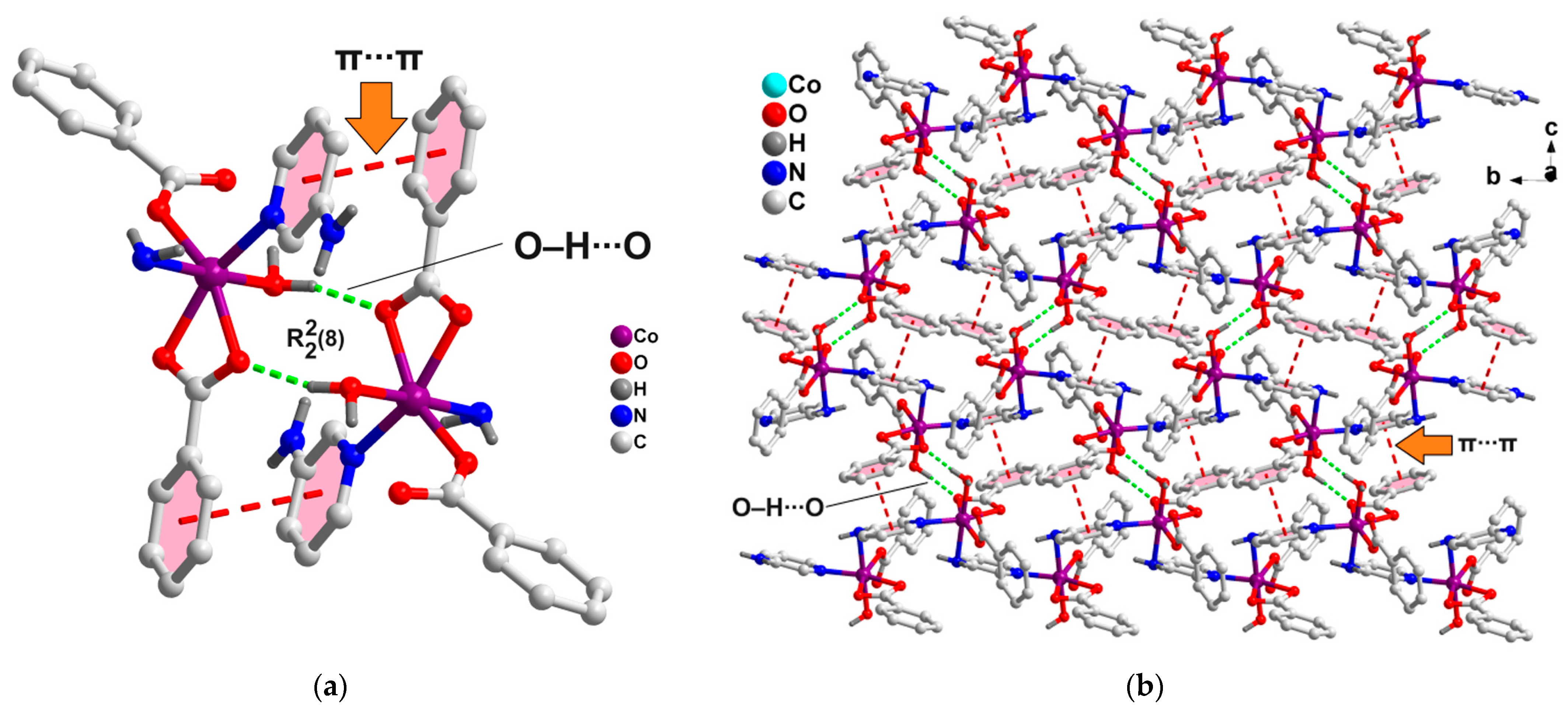
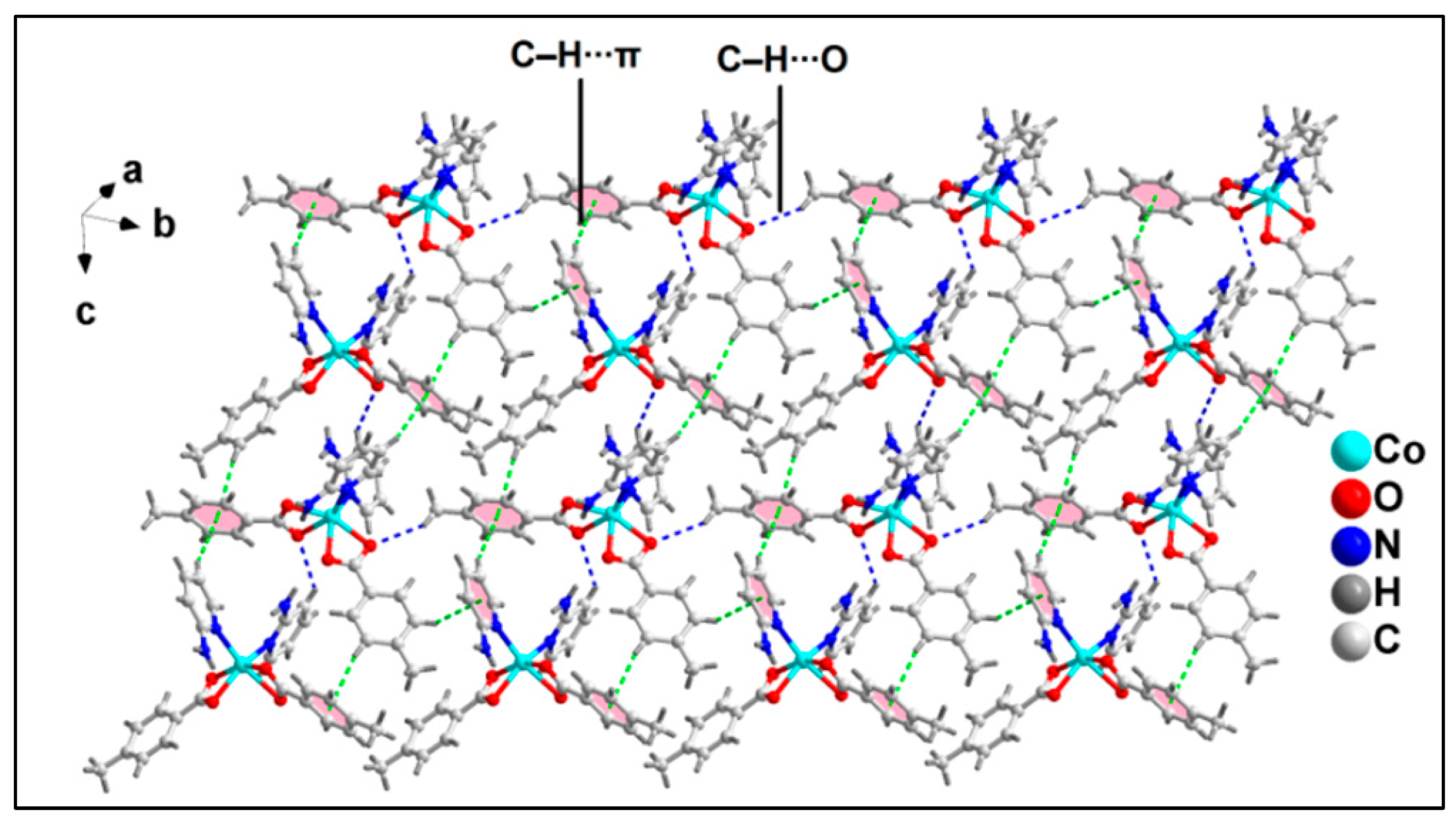
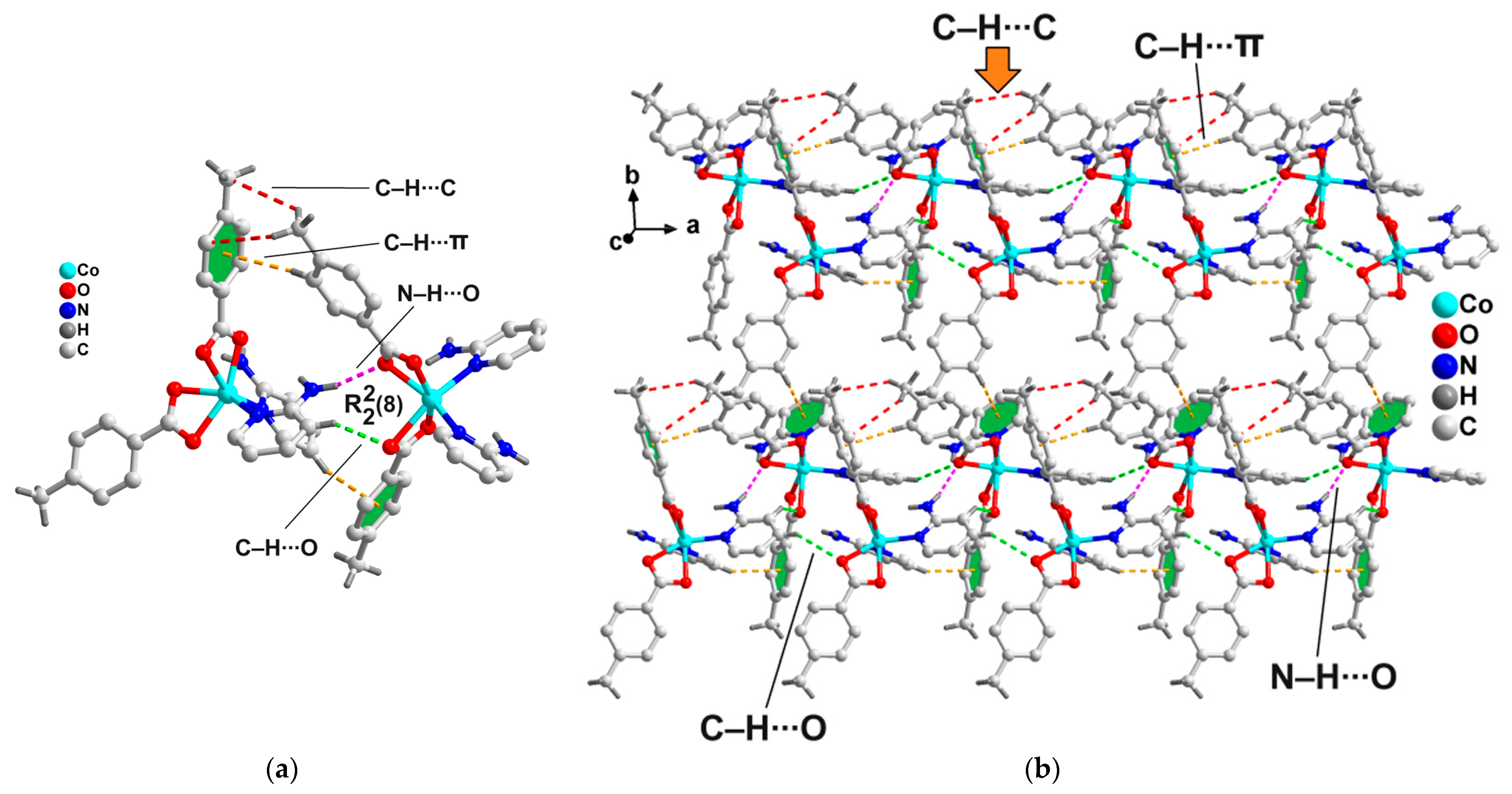
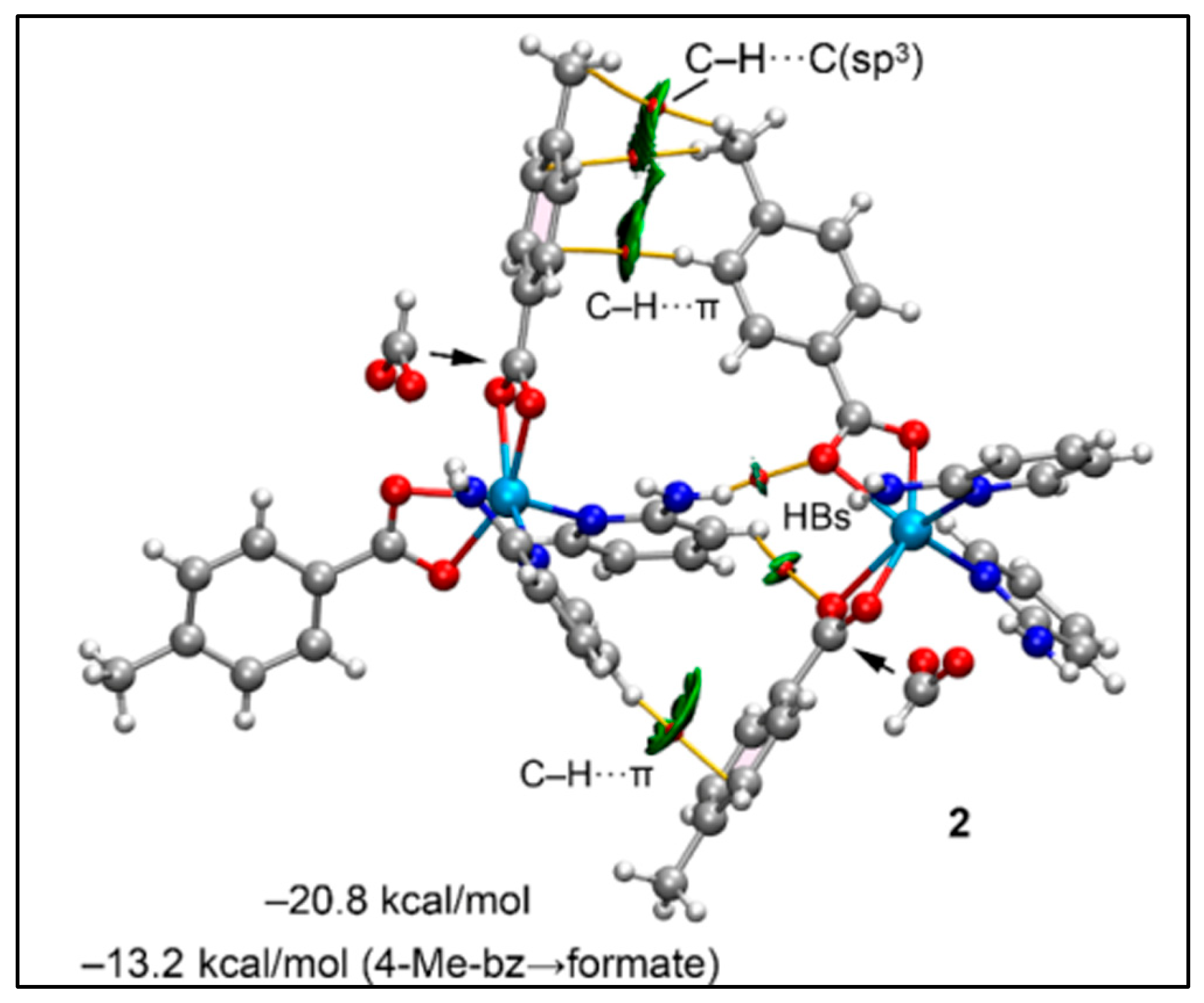
2.2. Crystal Structure Analysis
2.3. Spectral Studies
2.3.1. FT-IR Spectroscopy
2.3.2. Electronic Spectroscopy
2.4. Thermogravimetric Analysis
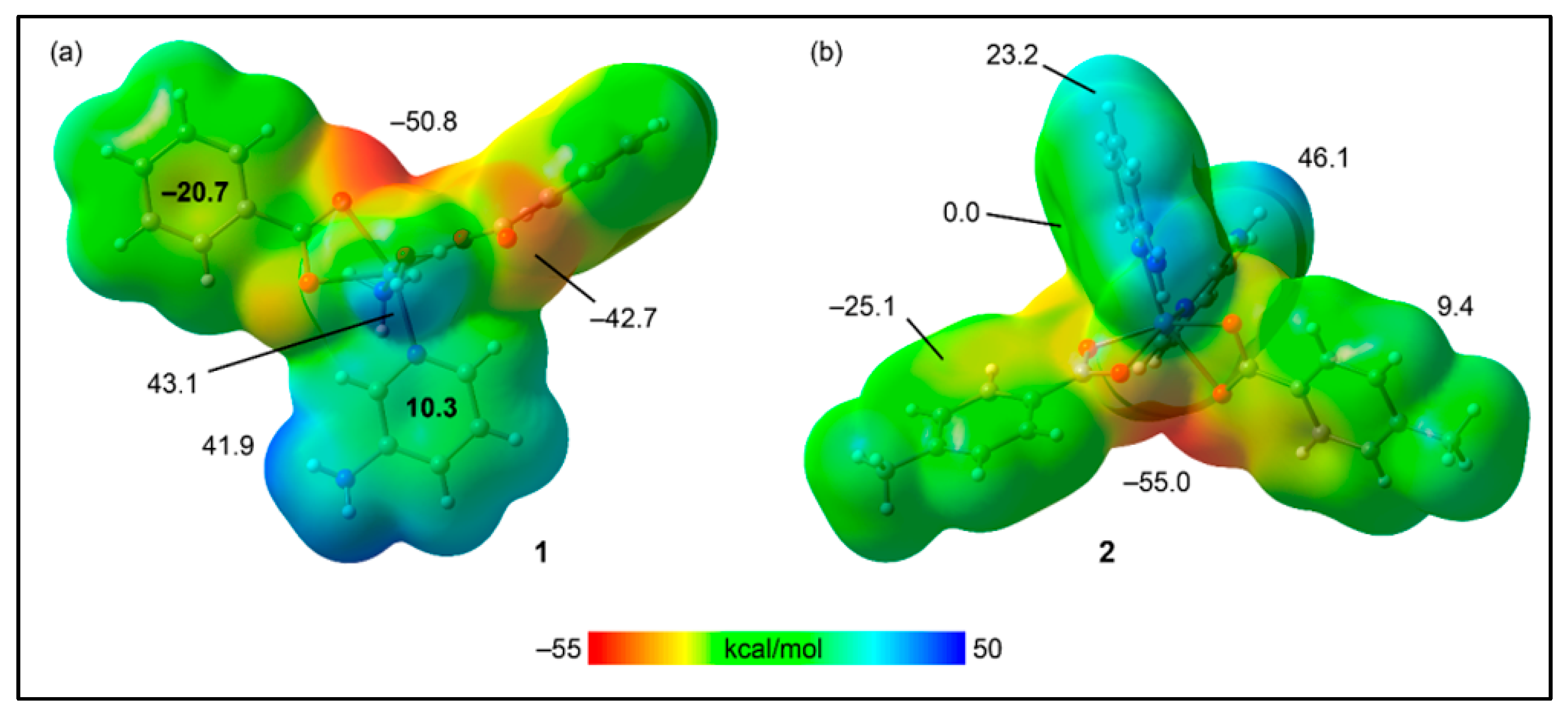
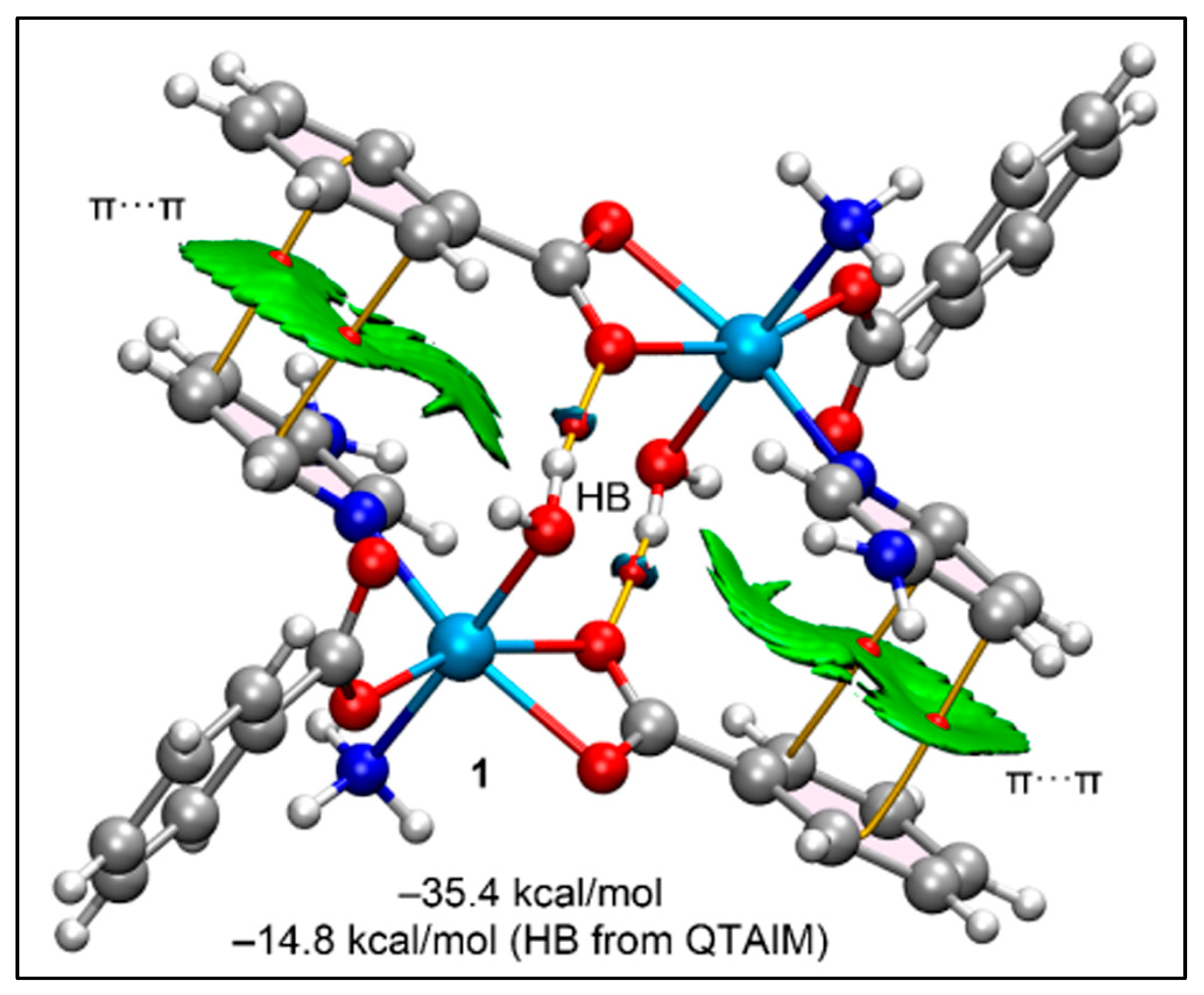
2.5. Theoretical Study
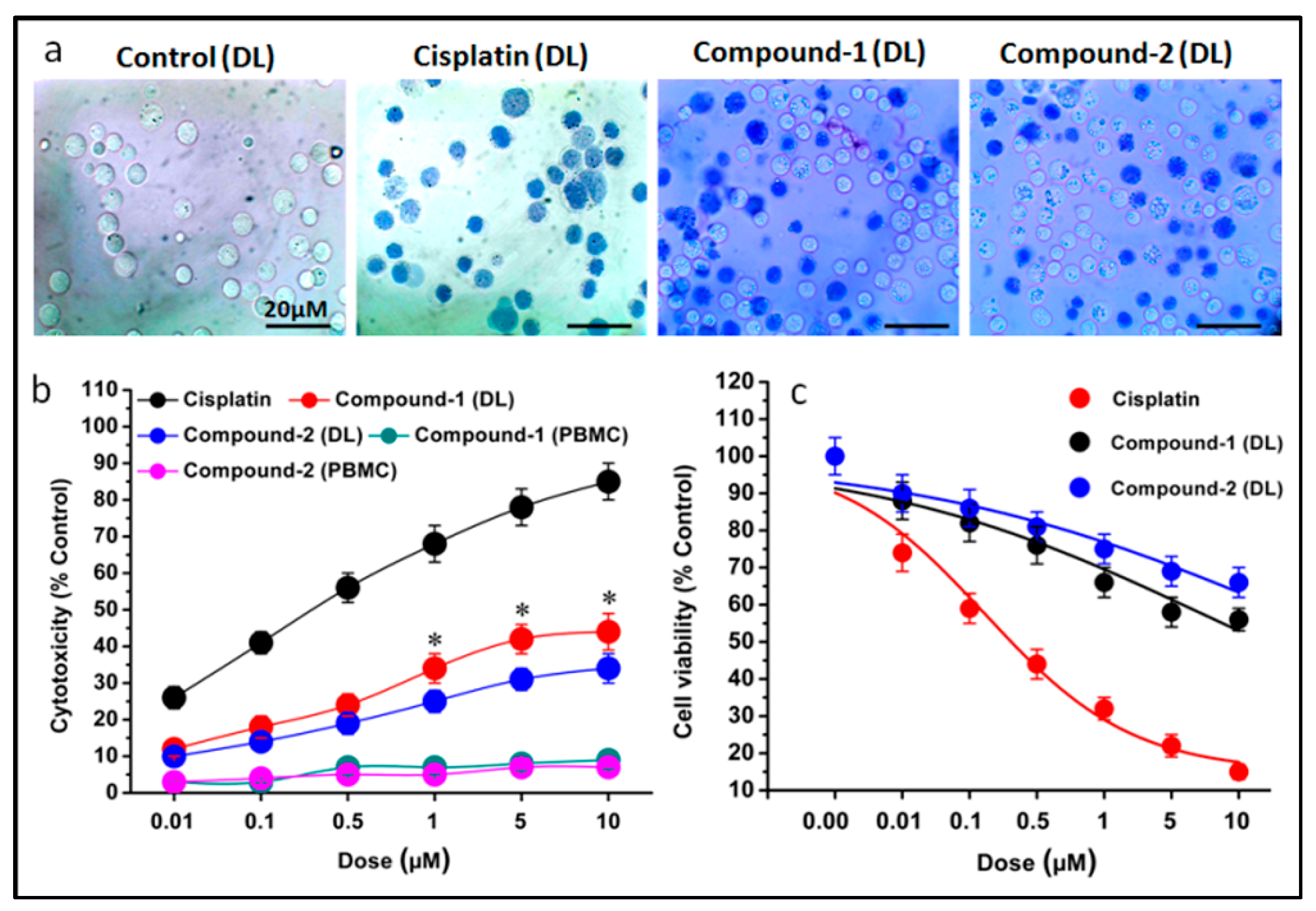
2.6. Trypan Blue Assay
2.7. Apoptosis Assay
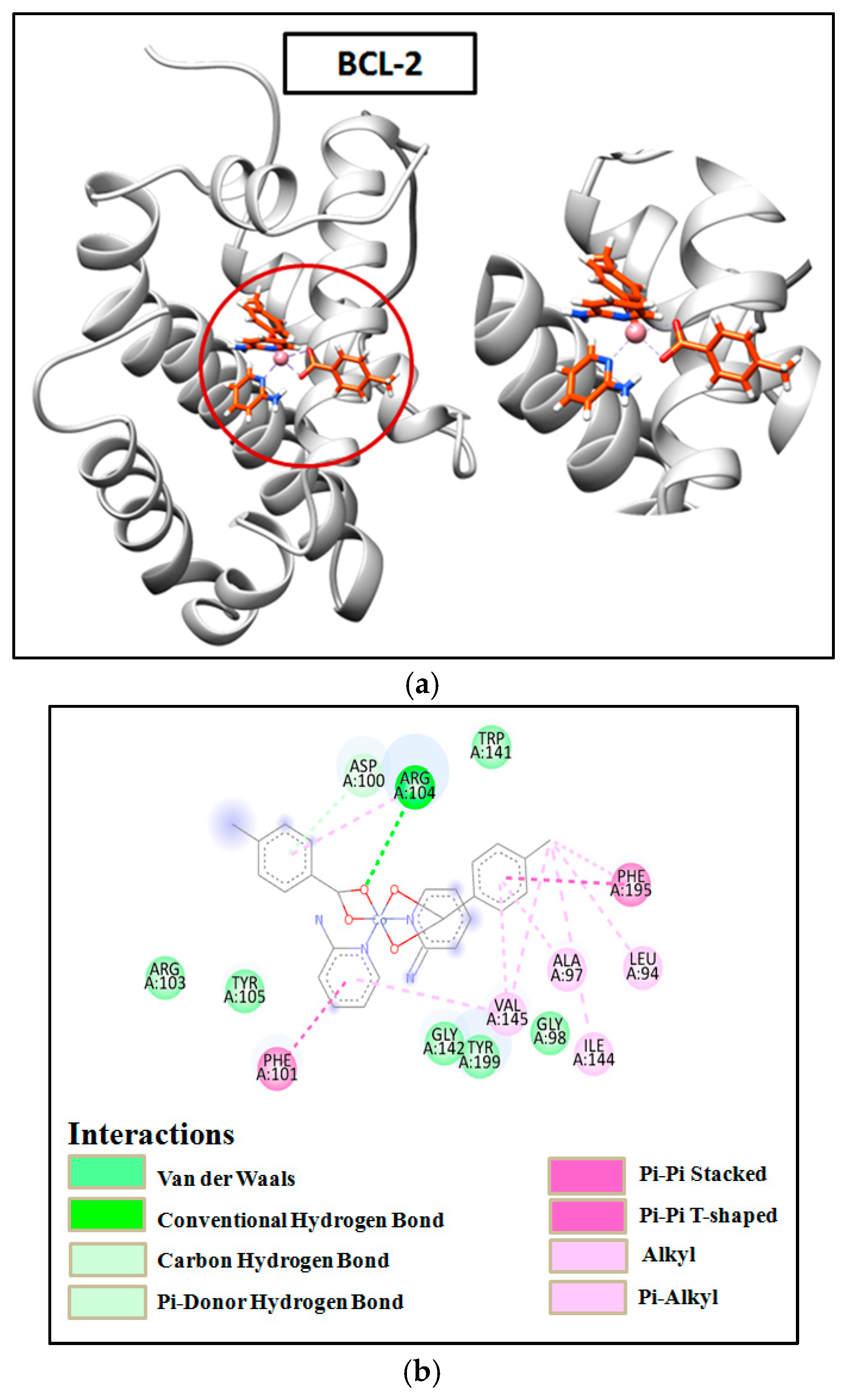
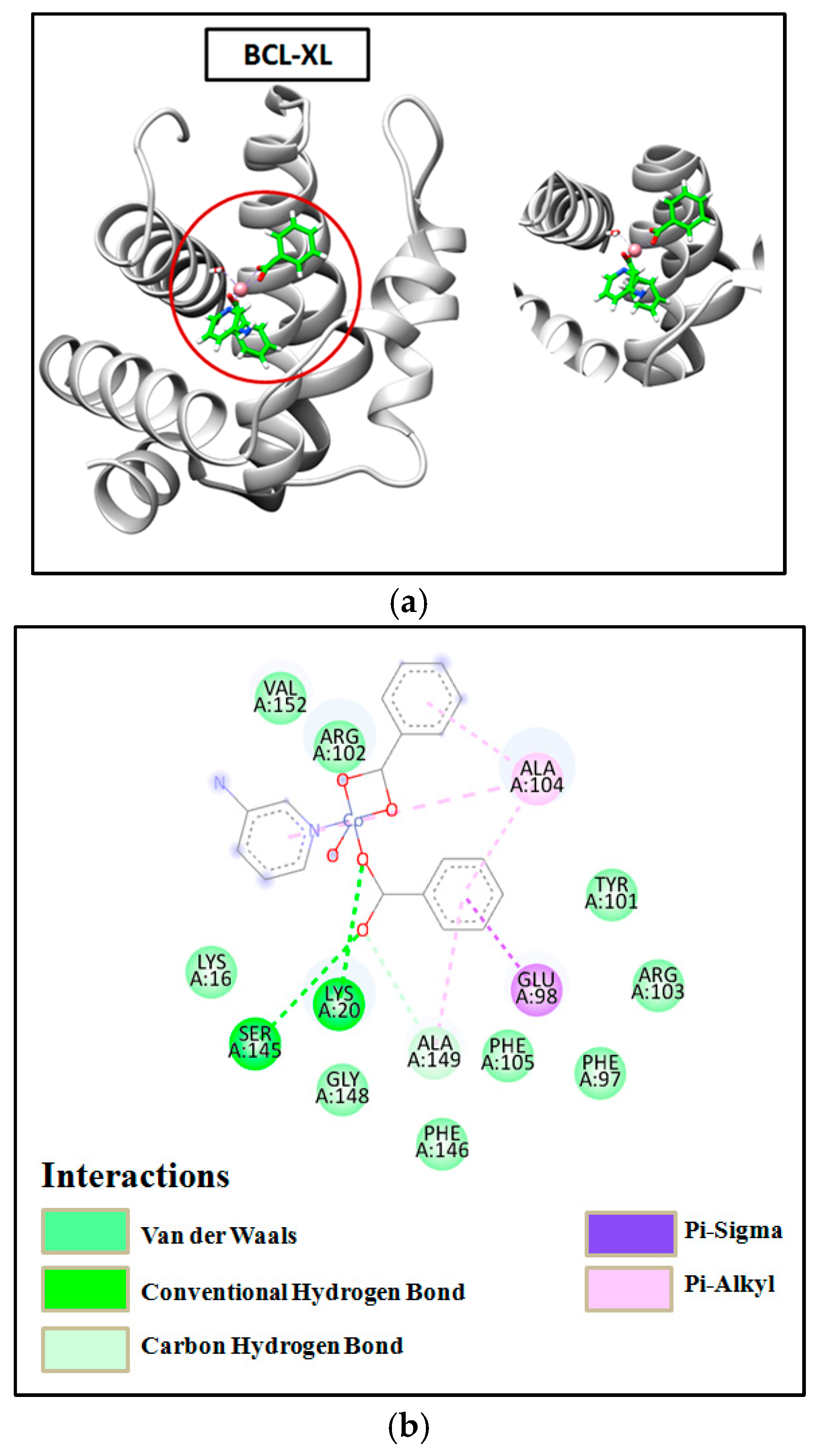
2.8. Molecular Docking
3. Materials and Methods
3.1. Syntheses
3.1.1. Synthesis of [Co(H2O)(bz)2(μ-3-Ampy)2]n (1)
3.1.2. Synthesis of [Co(4-Mebz)2(2-Ampy)2] (2)
3.2. Crystallographic Data Collection and Refinement
3.3. Computational Methods
3.4. Cell Line and Drug Preparation
3.5. Trypan Blue Cytotoxicity Assay
3.6. Apoptosis Study
3.7. Molecular Docking Simulation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Climova, A.; Pivovarova, E.; Rogalewicz, B.; Raducka, A.; Szczesio, M.; Korona-Głowniak, I.; Korga-Plewko, A.; Iwan, M.; Gobis, K.; Czylkowska, A. New Coordination Compounds Based on a Pyrazine Derivative: Design, Characterization, and Biological Study. Molecules 2022, 27, 3467. [Google Scholar] [CrossRef]
- Yang, D.-D.; Meng, F.-Q.; Shi, Y.-S.; Xiao, T.; Fang, Y.-H.; Tan, H.-W.; Zheng, X.-J. A Series of Zinc Coordination Compounds Showing Persistent Luminescence and Reversible Photochromic Properties via Charge Transfer. Chem. Eng. J. 2023, 466, 143202. [Google Scholar] [CrossRef]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc Coordination Complexes as Anticancer Agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Gul, Z.; Khan, S.; Ullah, S.; Ullah, H.; Khan, M.U.; Ullah, M.; Altaf, A.A. Recent Development in Coordination Compounds as a Sensor for Cyanide Ions in Biological and Environmental Segments. Crit. Rev. Anal. Chem. 2024, 54, 508. [Google Scholar] [CrossRef]
- Yıldırım, A.; Aksoy, M.S. Homoleptic Pyrazinoic Acid Transition Metal Complex as an Efficient Catalyst toward Elegant Three-Component Synthesis of 3,4-Dihydro-2H-1,3-Benzoxazines. Inorg. Chim. Acta 2023, 555, 121583. [Google Scholar] [CrossRef]
- Uvarova, M.A.; Lutsenko, I.A.; Babeshkin, K.A.; Sokolov, A.V.; Alexandrov, E.V.; Efimov, N.N.; Shmelev, M.A.; Khoroshilov, A.V.; Eremenko, I.L.; Kiskin, M.A. Solvent Effect in the Chemical Design of Coordination Polymers of Various Topologies with Co2+ and Ni2+ Ions and 2-Furoate Anions. CrystEngComm 2023, 25, 6786. [Google Scholar] [CrossRef]
- Kateshali, K.; Farahmand, A.; Gholizadeh Dogaheh, S.; Soleimannejad, J.; Blake, A.J. Structural Diversity and Applications of Ce(III)-Based Coordination Polymers. Coord. Chem. Rev. 2020, 419, 213392. [Google Scholar] [CrossRef]
- Amani, V.; Esmaeili, M.; Norouzi, F.; Khavasi, H.R. A Strategy for Obtaining Isostructurality Despite Structural Diversity in Coordination Compounds. CrystEngComm 2024, 26, 543. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Li, X.-X.; Du, M.-X.; Dong, W.-K.; Ding, Y.-J. Deep Insights into Novel Hexa-Coordinated Phenoxy-Bridged Trinuclear Ni(II) Asymmetric Salamo-like Complexes Affected by Different Types of Combined Solvents. J. Mol. Struct. 2024, 1298, 137071. [Google Scholar] [CrossRef]
- Danilescu, O.; Bulhac, I.; Shova, S.; Novitchi, G.; Bourosh, P. Coordination Compounds of Copper(II) with Schiff Bases Based on Aromatic Carbonyl Compounds and Hydrazides of Carboxylic Acids: Synthesis, Structures, and Properties. Russ. J. Coord. Chem. 2020, 46, 838. [Google Scholar] [CrossRef]
- Gu, J.; Wan, S.; Kirillova, M.V.; Kirillov, A.M. H-Bonded and Metal(II)–Organic Architectures Assembled from an Unexplored Aromatic Tricarboxylic Acid: Structural Variety and Functional Properties. Dalton Trans. 2020, 49, 7197. [Google Scholar] [CrossRef]
- Boruah, R.; Bhattacharjee, S.; Dutta Purkayastha, R.N.; Modak, S.; Aktar, T.; Maiti, D.; Sieroń, L.; Maniukiewicz, W.; Gomila, R.M.; Frontera, A. Insights into Synthesis, Crystal Structure, Bioactivity and Computational Studies of Cu(II) and Zn(II) Carboxylates Containing Aminopyridine. ChemistrySelect 2023, 8, e202204937. [Google Scholar] [CrossRef]
- Uddin, N.; Rashid, F.; Haider, A.; Tirmizi, S.A.; Raheel, A.; Imran, M.; Zaib, S.; Diaconescu, P.L.; Iqbal, J.; Ali, S. Triorganotin(IV) Carboxylates as Potential Anticancer Agents: Their Synthesis, Physiochemical Characterization, and Cytotoxic Activity against HeLa and MCF-7 Cancer Cells. Appl. Organomet. Chem. 2021, 35, e6165. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Ghadermazi, M.; Aliabadi, A. Study on Electrochemical Behavior and In Vitro Anticancer Effect of Co(II) and Zn(II) Complexes Containing Pyridine-2,6-Dicarboxylate. Inorg. Chim. Acta 2021, 527, 120549. [Google Scholar] [CrossRef]
- Štarha, P.; Křikavová, R. Platinum(IV) and Platinum(II) Anticancer Complexes with Biologically Active Releasable Ligands. Coord. Chem. Rev. 2024, 501, 215578. [Google Scholar] [CrossRef]
- Alhoshani, A.; Sulaiman, A.A.; Sobeai, H.M.A.; Qamar, W.; Alotaibi, M.; Alhazzani, K.; Monim-ul-Mehboob, M.; Ahmad, S.; Isab, A.A. Anticancer Activity and Apoptosis Induction of Gold(III) Complexes Containing 2,2′-Bipyridine-3,3′-Dicarboxylic Acid and Dithiocarbamates. Molecules 2021, 26, 3973. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Jantscher, P.V.; Fischer, R.C.; Torvisco, A.; Reichmann, K.; Massoud, S.S. Syntheses, Structural Characterization, and Thermal Behaviour of Metal Complexes with 3-Aminopyridine as Co-Ligands. Transit. Met. Chem. 2021, 46, 191. [Google Scholar] [CrossRef]
- Yurtcan, S.; Yolcu, Z. 4-Aminopyridine Containing Metal-2,6-Pyridine Dicarboxylates and Complex Embedded Hydrogels: Synthesis, Characterization and Antimicrobial Applications. Inorg. Chim. Acta 2024, 563, 121918. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Synthesis and Characterization of 1D Coordination Polymers of Metal(II)-Dicyanamido Complexes. Polyhedron 2019, 166, 36. [Google Scholar] [CrossRef]
- Yufanyi, D.M.; Nono, H.J.; Yuoh, A.C.B.; Tabong, C.D.; Judith, W.; Ondoh, A.M. Crystal Packing Studies, Thermal Properties, and Hirshfeld Surface Analysis in the Zn(II) Complex of 3-Aminopyridine with Thiocyanate as Co-Ligand. Open J. Inorg. Chem. 2021, 11, 63. [Google Scholar] [CrossRef]
- Kartal, Z.; Şahin, O.; Yavuz, A. The Synthesis of Two New Hofmann-Type M(3-Aminopyridine)2Ni(CN)4 [M = Zn(II) and Cd(II)] Complexes and the Characterization of Their Crystal Structure by Various Spectroscopic Methods. J. Mol. Struct. 2018, 1171, 578. [Google Scholar] [CrossRef]
- Beddar, K.; Bouchoucha, A.; Bourouai, M.A.; Zaater, S.; Djebbar, S. Transition Metal Complexes of 2-Aminopyridine Derivatives as Cyclooxygenase Inhibitors: Stability, Spectral, and Thermal Characterization, Electrochemical Behavior, DFT Calculations, Molecular Docking, and Biological Activities. Russ. J. Gen. Chem. 2023, 93, 2578. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mohamed, G.G.; El-Dessouky, M.M.I. Synthesis, Structural Characterization, In Vitro Antimicrobial and Anticancer Activity Studies of Ternary Metal Complexes Containing Glycine Amino Acid and the Anti-Inflammatory Drug Lornoxicam. J. Mol. Struct. 2015, 1082, 12. [Google Scholar] [CrossRef]
- Studer, V.; Anghel, N.; Desiatkina, O.; Felder, T.; Boubaker, G.; Amdouni, Y.; Ramseier, J.; Hungerbühler, M.; Kempf, C.; Heverhagen, J.T.; et al. Conjugates Containing Two and Three Trithiolato-Bridged Dinuclear Ruthenium(II)-Arene Units as In Vitro Antiparasitic and Anticancer Agents. Pharmaceuticals 2020, 13, 471. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M.; Saeed, H.; Owais, M.; Ashafaq, M. Tuning Biological Activity in Dinuclear Cu(II) Complexes Derived from Pyrazine Ligands: Structure, Magnetism, Catecholase, Antimicrobial, Antibiofilm, and Antibreast Cancer Activity. Appl. Organomet. Chem. 2021, 35, 6221. [Google Scholar] [CrossRef]
- Iqbal, M.; Ali, S.; Tahir, M.N.; Shah, N.A. Dihydroxo-Bridged Dimeric Cu(II) System Containing Sandwiched Non-Coordinating Phenylacetate Anion: Crystal Structure, Spectroscopic, Antibacterial, Antifungal, and DNA-Binding Studies of [(Phen)(H2O)Cu(OH)2Cu(H2O)(Phen)]2L·6H2O (HL = Phenylacetic Acid; Phen = 1,10-Phenanthroline). J. Mol. Struct. 2017, 1143, 23. [Google Scholar]
- Swiątkowski, M.; Lanka, S.; Czylkowska, A.; Gas, K.; Sawicki, M. Structural, Spectroscopic, Thermal, and Magnetic Properties of a New Dinuclear Copper Coordination Compound with Tiglic Acid. Materials 2021, 14, 2148. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Das, D.; Ray, P.P.; Banerjee, S.; Chattopadhyay, S. Phenoxo-Bridged Dinuclear Mixed Valence Cobalt(III/II) Complexes with Reduced Schiff Base Ligands: Synthesis, Characterization, Band Gap Measurements, and Fabrication of Schottky Barrier Diodes. Dalton Trans. 2021, 50, 1721. [Google Scholar] [CrossRef]
- Borkar, A.; Nnabuike, G.G.; Obaleye, J.A.; Harihar, S.; Patil, A.S.; Butcher, R.J.; Salunke-Gawali, S. Manganese(II)-Imidazole Complexes of the Non-Steroidal Anti-Inflammatory Drug Mefenamic Acid: Synthesis and Structural Studies. Inorg. Chim. Acta 2020, 512, 119878. [Google Scholar] [CrossRef]
- Sertcelik, M.; Ozbek, F.E.O.; Taslimi, P.; Necefoglu, H.; Hokelek, T. Supramolecular Complexes of Ni(II) and Co(II) 4-Aminobenzoate with 3-Cyanopyridine: Synthesis, Spectroscopic Characterization, Crystal Structure, and Enzyme Inhibitory Properties. Appl. Organomet. Chem. 2021, 35, 6182. [Google Scholar] [CrossRef]
- Kowol, C.R.; Trondl, R.; Arion, V.B.; Jakupec, M.A.; Lichtscheidl, I.; Keppler, B.K. Fluorescence Properties and Cellular Distribution of the Investigational Anticancer Drug Triapine (3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone) and Its Zinc(II) Complex. Dalton Trans. 2010, 39, 704. [Google Scholar] [CrossRef]
- Kahrovic, E.; Zahirović, A.; Pavelic, S.K.; Turkusic, E.; Harej, A. In Vitro Anticancer Activity of Binuclear Ru(II) Complexes with Schiff Bases Derived from 5-Substituted Salicylaldehyde and 2-Aminopyridine with Notably Low IC50 Values. J. Coord. Chem. 2017, 70, 1683. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S. Synthesis, Structural Analyses, and Antimicrobial Activity of the Water-Soluble 1D Coordination Polymer [Ag(3-Aminopyridine)]ClO4. J. Mol. Struct. 2017, 1149, 58. [Google Scholar] [CrossRef]
- Suksrichavalit, T.; Prachayasittikul, S.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Copper Complexes of Pyridine Derivatives with Superoxide Scavenging and Antimicrobial Activities. Eur. J. Med. Chem. 2009, 44, 3259. [Google Scholar] [CrossRef]
- Machura, B.; Jaworska, M.; Kruszynski, R. Synthesis, Crystal, Molecular and Electronic Structure of the [ReBr3(NO)(AsPh3)(pzH)] Complex. Polyhedron 2004, 23, 2523. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhao, B.; Cheng, P. Synthesis and Characterization of Unusual Heterotrinuclear Co(III)Ni(II)Co(III)Hydrotris(3,5-Dimethylpyrazolyl)Borate Complex with Mixed 1,1-Azide and Pyrazolate Bridges. Inorg. Chem. Commun. 2007, 10, 583. [Google Scholar] [CrossRef]
- Pettinari, C.; Tabacaru, A.; Galli, S. Coordination Polymers and Metal–Organic Frameworks Based on Poly(pyrazole)-Containing Ligands. Coord. Chem. Rev. 2016, 307, 1. [Google Scholar] [CrossRef]
- Mondal, G.; Jana, H.; Acharjya, M.; Santra, A.; Bera, P.; Jana, A.; Panja, A.; Bera, P. Synthesis, In Vitro Evaluation of Antibacterial, Antifungal, and Larvicidal Activities of Pyrazole/Pyridine-Based Compounds and Their Nanocrystalline MS (M = Cu and Cd) Derivatives. Med. Chem. Res. 2017, 26, 3046. [Google Scholar] [CrossRef]
- Ajo, D.; Bencini, A.; Mani, F. Anisotropic Exchange in Dinuclear Complexes with Polyatomic Bridges. 2. Crystal and Molecular Structure and EPR Spectra of Tetraphenylphosphonium Bis(µ-pyrazolato)bisdihydrobis(1-pyrazolyl)boratodicuprate(II). Magneto-Structural Correlations in Bis(µ-pyrazolato)-Bridged Copper(II) Complexes. Inorg. Chem. 1988, 27, 2437. [Google Scholar]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Novel Schiff Base Ligand and Its Metal Complexes with Some Transition Elements: Synthesis, Spectroscopic, Thermal Analysis, Antimicrobial and In Vitro Anticancer Activity. Appl. Organomet. Chem. 2016, 30, 221. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rajput, L.D.; Desiraju, G.R. Designing Ternary Co-Crystals with Stacking Interactions and Weak Hydrogen Bonds: 4,4′-Bis-Hydroxyazobenzene. Cryst. Growth Des. 2014, 14, 2571. [Google Scholar] [CrossRef]
- Kong, Y.J.; Han, L.J. Synthesis, X-ray Structure Analysis, and Spectroscopic Characterization of trans-Aquabis(µ-benzoato-κ²O′)Bis[µ-N,N′-Bis(4-methoxyphenyl)formamidinato-κ²N′] Dimolybdenum(II). J. Chem. Crystallogr. 2017, 47, 208. [Google Scholar] [CrossRef]
- Moro, A.C.; Watanabe, F.W.; Ananias, S.R.; Mauro, A.E.; Netto, A.V.G.; Lima, A.P.R.; Ferreira, J.G.; Santos, R.H.A. Supramolecular Assemblies of cis-Palladium Complexes Dominated by C-H⋯Cl Interactions. Inorg. Chem. Commun. 2006, 9, 493. [Google Scholar] [CrossRef]
- Gogoi, A.; Dutta, D.; Verma, A.K.; Nath, H.; Frontera, A.; Guha, A.K.; Bhattacharyya, M.K. Energetically Favorable Anti-Electrostatic Hydrogen-Bonded Cationic Clusters in Ni(II) 3,5-Dimethylpyrazole Complexes: Anticancer Evaluation and Theoretical Studies. Polyhedron 2019, 168, 113. [Google Scholar] [CrossRef]
- Eom, G.H.; Park, H.M.; Hyun, M.Y.; Jang, S.P.; Kim, C.; Lee, J.H.; Lee, S.J.; Kim, S.J.; Kim, Y. Anion Effects on the Crystal Structures of ZnII Complexes Containing 2,2′-Bipyridine: Their Photoluminescence and Catalytic Activities. Polyhedron 2011, 30, 1555. [Google Scholar] [CrossRef]
- Gogoi, A.; Islam, S.M.N.; Frontera, A.; Bhattacharyya, M.K. Supramolecular Association in Cu(II) and Co(II) Coordination Complexes of 3,5-Dimethylpyrazole: Experimental and Theoretical Studies. Inorg. Chim. Acta 2019, 484, 133. [Google Scholar] [CrossRef]
- Dutta, D.; Chetry, S.; Gogoi, A.; Choudhury, B.; Guha, A.K.; Bhattacharyya, M.K. Supramolecular Association Involving Anion–π Interactions in Cu(II) Coordination Solids: Experimental and Theoretical Studies. Polyhedron 2018, 151, 381. [Google Scholar] [CrossRef]
- Sharma, P.; Gogoi, A.; Verma, A.K.; Frontera, A.; Bhattacharyya, M.K. Charge-Assisted Hydrogen Bond and Nitrile⋯Nitrile Interaction Directed Supramolecular Associations in Cu(II) and Mn(II) Coordination Complexes: Anticancer, Hematotoxicity, and Theoretical Studies. New J. Chem. 2020, 44, 5473. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; El-Bali, B.; Khan, A.; Dalvandi, K.; Marasini, B.P.; Noreen, S.; Malik, R.; Khan, S.; Choudhary, M.I. Synthesis and Enzyme Inhibitory Activities of Some New Pyrazole-Based Heterocyclic Compounds. Med. Chem. Res. 2012, 21, 2772. [Google Scholar] [CrossRef]
- Lunagariya, M.V.; Thakor, K.P.; Varma, R.R.; Waghela, B.N.; Pathak, C.; Patel, M.N. Synthesis, Characterization, and Biological Application of 5-Quinoline 1,3,5-Trisubstituted Pyrazole-Based Platinum(II) Complexes. MedChemComm 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Yousfi, E.B.; Sebbar, N.K.; Ramli, Y.; Taoufik, J.; Ouzidan, Y.; Ansar, M.; Mabkhot, Y.N.; Ghabbour, H.A.; Radi, S. New Pyrazole-Hydrazone Derivatives: X-ray Analysis, Molecular Structure Investigation via Density Functional Theory (DFT), and Their High In-Situ Catecholase Activity. Int. J. Mol. Sci. 2017, 18, 2215. [Google Scholar] [CrossRef] [PubMed]
- Mirdya, S.; Roy, S.; Chatterjee, S.; Bauza, A.; Frontera, A.; Chattopadhyay, S. Importance of π-Interactions Involving Chelate Rings in Addition to Tetrel Bonds in Crystal Engineering: A Combined Experimental and Theoretical Study on a Series of Hemi- and Holodirected Nickel(II)/Lead(II) Complexes. Cryst. Growth Des. 2019, 19, 5869. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Silva, M.F.C.G.; Pombeiro, A.J.L. Non-Covalent Interactions in the Synthesis of Coordination Compounds: Recent Advances. Coord. Chem. Rev. 2017, 345, 54. [Google Scholar] [CrossRef]
- Dutta, D.; Sharma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Baishya, T.; Bhattacharyyaa, M.K. Supramolecular Assemblies Involving Unconventional Non-Covalent Contacts in Pyrazole-Based Coordination Compounds of Co(II) and Cu(II) Pyridinedicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2022, 224, 116025. [Google Scholar] [CrossRef]
- El-ghamry, M.A.; Shebl, M.; Saleh, A.A.; Khalil, S.M.E.; Amir, M.D.; Ali, A.M. Spectroscopic Characterization of Cu(II), Ni(II), Co(II) Complexes, and Nano Copper Complex Bearing a New S, O, N-Donor Chelating Ligand. 3D Modeling Studies, Antimicrobial, Antitumor, and Catalytic Activities. J. Mol. Struct. 2022, 1249, 131587. [Google Scholar] [CrossRef]
- Fabiyi, F.S.; Olanrewaju, A.A. Synthesis, Characterization, Thermogravimetric and Antioxidant Studies of New Cu(II), Fe(II), Mn(II), Cu(II), Zn(II), Co(II), and Ni(II) Complexes with Benzoic Acid and 4,4,4-Trifluoro-1-(2-Naphthyl)-1,3-Butanedione. Int. J. Chem. 2019, 11, 1. [Google Scholar] [CrossRef]
- Rossin, A.; Credico, B.D.; Giambastiani, G.; Peruzzini, M.; Pescitelli, G.; Reginato, G.; Borfecchia, E.; Gianolio, D.; Lamberti, C.; Bordiga, S. Synthesis, Characterization, and CO2 Uptake of a Chiral Co(II) Metal–Organic Framework Containing a Thiazolidine-Based Spacer. J. Mater. Chem. 2012, 22, 10335. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc., Dalton Trans. 2000, 21, 3885. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Li, G.; Li, Y.; Zhang, P.; Xu, J.; Wang, Y. Synthesis, Crystal Structure, and Magnetic Properties of a New 1D Cobalt(II) Complex with 1,10-Phenanthroline and 3,5-Dinitrosalicylate Acid Ligands. Inorg. Chem. Commun. 2005, 8, 983. [Google Scholar] [CrossRef]
- Ali, H.A.; Shamma, A.A.; Kamel, S. New Mixed Ligand Cobalt (II/III) Complexes Based on the Drug Sodium Valproate and Bioactive Nitrogen-Donor Ligands: Synthesis, Structure and Biological Properties. J. Mol. Struct. 2017, 1142, 40. [Google Scholar] [CrossRef]
- Dojer, B.; Pevec, A.; Jagodic, M.; Kristl, M.; Drofenik, M. Three New Cobalt(II) Carboxylates with 2-, 3-, and 4-Aminopyridine: Syntheses, Structures, and Magnetic Properties. Inorg. Chim. Acta 2012, 383, 98. [Google Scholar] [CrossRef]
- Dojer, B.; Pevec, A.; Šegedin, P.; Jagličić, Z.; Stropnik, Č.; Kristl, M.; Drofenik, M. Cobalt (II) Coordination Compounds with Acetate and 2-Aminopyridine Ligands: Synthesis, Characterization, Structures and Magnetic Properties of Two Polymorphic Forms. Inorg. Chim. Acta 2010, 363, 1343. [Google Scholar] [CrossRef]
- Muthukkumar, M.; Karthikeyan, A.; Kamalesu, S.; Kadri, M.; Jennifer, S.J.; Razak, I.A.; Nehru, S. Synthesis, Crystal Structure, Optical and DFT Studies of a Novel Co(II) Complex with the Mixed Ligands 3-Bromothiophene-2-Carboxylate and 2-Aminopyridine. J. Mol. Struct. 2022, 1270, 133953. [Google Scholar] [CrossRef]
- Zhong, D.-C.; Guo, G.-Q.; Zuo, X.-H.; Deng, J.-H.; Yuan, L.; Zhu, R.-H. Bis(2-Aminopyridine-κN1) Bis(Benzoato-κO) Cobalt(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m88. [Google Scholar] [CrossRef]
- Swiatkowski, M.; Sieranski, T.; Bogdan, M.; Kruszynski, R. Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System. Molecules 2019, 24, 3357. [Google Scholar] [CrossRef] [PubMed]
- Boro, M.; Banik, S.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Bhattacharyya, M.K. Supramolecular Assemblies in Mn(II) and Zn(II) Metal–Organic Compounds Involving Phenanthroline and Benzoate: Experimental and Theoretical Studies. Inorganics 2024, 12, 139. [Google Scholar] [CrossRef]
- Mahapatra, T.S.; Bauza, A.; Dutta, D.; Mishra, S.; Frontera, A.; Ray, D. Carboxylate Coordination Assisted Aggregation for Quasi-Tetrahedral and Partial-Dicubane [Cu4] Coordination Clusters. ChemistrySelect 2016, 1, 64. [Google Scholar] [CrossRef]
- Orhan, O.; Colak, A.T.; Emen, F.M.; Kismali, G.; Meral, O.; Sel, T.; Cilgi, G.K.; Tas, M. Syntheses of Crystal Structures and In Vitro Cytotoxic Activities of New Copper(II) Complexes of Pyridine-2,6-Dicarboxylate. J. Coord. Chem. 2015, 68, 4003. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistria, S.; Jana, A.D. A Rare Supramolecular Assembly Involving Ion Pairs of Coordination Complexes with a Host–Guest Relationship: Synthesis, Crystal Structure, Photoluminescence, and Thermal Study. CrystEngComm 2012, 14, 7415. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Dutta, D.; Islam, S.M.N.; Frontera, A.; Sharma, P.; Verma, A.K.; Das, A. Energetically Significant Antiparallel π-Stacking Contacts in Co(II), Ni(II), and Cu(II) Coordination Compounds of Pyridine-2,6-Dicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Inorg. Chim. Acta 2020, 501, 119233. [Google Scholar] [CrossRef]
- Aycan, T. Synthesis, Crystal Structure, Spectroscopic (FT-IR, UV–Vis, EPR) and Hirshfeld Surface Analysis Studies of Zn(II)-Benzoate Coordination Dimer. J. Mol. Struct. 2021, 1223, 128943. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Sharma, P.; Dutta, D.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Bhattacharyya, M.K. Benzoato Bridged Dinuclear Mn(II) and Cu(II) Compounds Involving Guest Chlorobenzoates and Dimeric Paddle Wheel Supramolecular Assemblies: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2021, 208, 115409. [Google Scholar] [CrossRef]
- Dutta, D.; Islam, S.M.N.; Saha, U.; Chetry, S.; Guha, A.K.; Bhattacharyya, M.K. Cu(II) and Co(II) Coordination Solids Involving Unconventional Parallel Nitrile(π)–Nitrile(π) and Energetically Significant Cooperative Hydrogen Bonding Interactions: Experimental and Theoretical Studies. J. Mol. Struct. 2019, 1195, 733. [Google Scholar] [CrossRef]
- Dutta, D.; Islam, S.M.N.; Saha, U.; Chetry, S.; Guha, A.K.; Bhattacharyya, M.K. Structural Topology of Weak Non-Covalent Interactions in a Layered Supramolecular Coordination Solid of Zinc Involving 3-Aminopyridine and Benzoate: Experimental and Theoretical Studies. J. Chem. Crystallogr. 2018, 48, 156. [Google Scholar] [CrossRef]
- Akyüz, S. The FT-IR Spectra of Transition Metal 3-Aminopyridine Tetracyanonickelate Complexes. J. Mol. Struct. 1998, 449, 23. [Google Scholar] [CrossRef]
- Bora, S.J.; Das, B.K. Synthesis and Properties of a Few 1-D Cobaltous Fumarates. J. Solid State Chem. 2012, 192, 93. [Google Scholar] [CrossRef]
- Batool, S.S.; Gilani, S.R.; Tahir, M.N.; Harrison, W.T.A.Z. Syntheses and Structures of Monomeric and Dimeric Ternary Complexes of Copper(II) with 2,2′-Bipyridyl and Carboxylate Ligands. Z. Anorg. Allg. Chem. 2016, 642, 1364. [Google Scholar] [CrossRef]
- Basumatary, D.; Lal, R.A.; Kumar, A. Synthesis and Characterization of Low- and High-Spin Manganese(II) Complexes of Polyfunctional Adipoyldihydrazone: Effect of Coordination of N-Donor Ligands on Stereo-Redox Chemistry. J. Mol. Struct. 2015, 1092, 122. [Google Scholar] [CrossRef]
- Islam, S.M.N.; Dutta, D.; Guha, A.K.; Bhattacharyya, M.K. An Unusual Werner-Type Clathrate of Mn(II) Benzoate Involving Energetically Significant Weak C–H⋯C Contacts: A Combined Experimental and Theoretical Study. J. Mol. Struct. 2019, 1175, 130. [Google Scholar] [CrossRef]
- Goher, M.A.; Hafez, A.K.; Abu-Youssef, M.A.M.; Badr, A.M.A.; Gspan, C.; Mautner, F.A. New Metal(II) Complexes Containing Monodentate and Bridging 3-Aminopyridine and Azido Ligands. Polyhedron 2004, 23, 2349. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Shi, S.; Zang, J. Crystal Structure and Properties of a Terbium m-Methylbenzoate Complex with 1,10-Phenanthroline. J. Coord. Chem. 2002, 55, 215. [Google Scholar] [CrossRef]
- Piccinini, F.; Tesei, A.; Arienti, C.; Bevilacqua, A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol. Proced. Online 2017, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, P.F.; Chen, J.; Byun, J.H.; Platko, K.; Austin, R.C. The Trypan Blue Cellular Debris Assay: A Novel Low-Cost Method for the Rapid Quantification of Cell Death. MethodsX 2019, 6, 1174. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Manfredi, A. Chromatin and Cell Death. Biochim. Biophys. Acta Gene Struct. Expr. 2004, 1677, 181. [Google Scholar] [CrossRef]
- Wyllie, A.H.; Beattie, G.J.; Hargreaves, A.D. Chromatin Changes in Apoptosis. Histochem. J. 1981, 13, 681. [Google Scholar] [CrossRef]
- Verma, A.K.; Prasad, S.B. Antitumor Effect of Blister Beetles: An Ethno-Medicinal Practice in Karbi Community and Its Experimental Evaluation Against a Murine Malignant Tumor Model. J. Ethnopharmacol. 2013, 148, 869. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Das, A.; Frontera, A.; Verma, A.K.; Bhattacharyya, M.K. Energetically Significant Unconventional π–π Contacts Involving Fumarate in a Novel Coordination Polymer of Zn(II): In-Vitro Anticancer Evaluation and Theoretical Studies. Inorg. Chim. Acta 2019, 493, 1. [Google Scholar] [CrossRef]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Tsukamoto, H.; Kamiya, T.; Kataoka, H.; Joh, T. Mechanisms of Cisplatin-Induced Apoptosis and of Cisplatin Sensitivity: Potential of BIN1 to Act as a Potent Predictor of Cisplatin Sensitivity in Gastric Cancer Treatment. Int. J. Surg. Oncol. 2012, 2012, 862879. [Google Scholar] [CrossRef]
- Lima, A.P.; Pereira, F.C.; Almeida, M.A.P.; Mello, F.M.S.; Pires, W.C.; Pinto, T.M.; Delella, F.K.; Felisbino, S.L.; Moreno, V.; Batista, A.A.; et al. Cytoxicity and Apoptotic Mechanism of Ruthenium(II) Amino Acid Complexes in Sarcoma-180 Tumor Cells. PLoS ONE 2014, 9, 105865. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Cho, S.G. Correlation Between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, V.; Singh, S.; Goswami, B.C.; Camps, I.; Sekar, A.; Yoon, S.; Lee, S. Repurposing Potential of Ayurvedic Medicinal Plants Derived Active Principles Against SARS-CoV-2 Associated Target Proteins Revealed by Molecular Docking, Molecular Dynamics, and MM-PBSA Studies. Biomed. Pharmacother. 2021, 137, 1. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315. [Google Scholar] [CrossRef]
- Verma, A.K.; Aggarwal, R. Repurposing Potential of FDA-Approved and Investigational Drugs for COVID-19 Targeting SARS-CoV-2 Spike and Main Protease and Validation by Machine Learning Algorithm. Chem. Biol. Drug Des. 2020, 97, 836. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From Basic Apoptosis Discoveries to Advanced Selective BCL-2 Family Inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- SADABS, V2.05; Bruker AXS: Madison, WI, USA, 1999.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, 3.1f; Crystal Impact GbR: Bonn, Germany, 2008.
- Ahlrichs, R.; Bär, M.; Hacer, M.; Horn, H.; Kömel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Mewes, J.-M.; Ehlert, S.; Grimme, S. Extension and Evaluation of the D4 London-Dispersion Model for Periodic Systems. Phys. Chem. Chem. Phys. 2020, 22, 8499. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graphics 1996, 14, 33. [Google Scholar] [CrossRef]
- Dutta, D.; Singh, N.S.; Aggarwal, R.; Verma, A.K. Cordyceps militaris: A Comprehensive Study on Laboratory Cultivation and Anticancer Potential in Dalton’s Ascites Lymphoma Tumor Model. Anti-Cancer Agents Med. Chem. 2024, 24, 668. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3-B. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Fatmi, M.Q.; Devi, M.; Singh, N.S.; Verma, A.K. Antitumor Activity of Cordycepin in Murine Malignant Tumor Cell Line: An In Vitro and In Silico Study. J. Mol. Struct. 2023, 1297, 136946. [Google Scholar] [CrossRef]
- Verma, A.K.; Prasad, S.B. Bioactive Component, Cantharidin from Mylabris cichorii and Its Antitumor Activity Against Ehrlich Ascites Carcinoma. Cell Biol. Toxicol. 2012, 28, 133. [Google Scholar] [CrossRef] [PubMed]








| Bond lengths and angles of 1 (Å, °) | |||
| Co1–O1A | 2.1414(12) | O10A–Co1–O1W | 94.82(5) |
| Co1–O3A | 2.1787(12) | O10A–Co1–N1 | 100.64(6) |
| Co1–O10A | 2.0093(12) | O10A–Co1–N3 | 83.53(5) |
| Co1–O1W | 2.1235(13) | ||
| Co1–N1 | 2.0992(15) | O1W–Co1–O1A | 91.95(5) |
| Co1–N3 | 2.2608(15) | O1W–Co1–O3A | 88.64(5) |
| O1W–Co1–N3 | 177.88(5) | ||
| O1A–Co1–O3A | 60.88(5) | ||
| O1A–Co1–N3 | 90.05(3) | N1–Co1–O1A | 98.53(5) |
| N1–Co1–O3A | 159.38(6) | ||
| O3A–Co1–N3 | 92.96(5) | N1–Co1–O1W | 93.47(5) |
| N1–Co1–N3 | 85.53(6) | ||
| Bond lengths and angles of 2 (Å, °) | |||
| Co1–O1 | 2.160(3) | O3–Co1–N3 | 107.77(13) |
| Co1–O4 | 2.328(3) | O3–Co1–N1 | 95.64(13) |
| Co1–O3 | 2.063(3) | N3–Co1–N1 | 100.36(14) |
| Co1–N1 | 2.099(3) | O3–Co1–O2 | 156.82(13) |
| Co1–N3 | 2.102(4) | N3–Co1–O2 | 90.82(13) |
| Co1–O2 | 2.160(3) | N1–Co1–O2 | 94.44(15) |
| O3–Co1–O1 | 96.69(12) | N1–Co1–O4 | 154.89(12) |
| N3–Co1–O1 | 145.05(14) | O2–Co1–O4 | 109.92(13) |
| N1–Co1–O1 | 101.69(13) | O1–Co1–O4 | 85.73(11) |
| O2–Co1–O1 | 60.78(12) | ||
| O3–Co1–O4 | 59.48(11) | ||
| N3–Co1–O4 | 85.69(13) | ||
| D–H⋯A | d(D–H) | d(D⋯A) | d(H⋯A) | ∠(DHA) |
|---|---|---|---|---|
| Compound 1 | ||||
| C6–H6⋯O12A | 0.95 | 3.61 | 2.83 | 139.8 |
| N3–H3A⋯O3A | 0.89 | 2.96 | 2.08 | 169.6 |
| C9–H9A⋯N3 | 0.95 | 3.43 | 2.87 | 119.1 |
| C16A–H16A⋯O12A | 0.95 | 3.42 | 2.56 | 151.8 |
| O1W–H1WB⋯O1A | 0.87 | 2.74 | 1.89 | 163.6 |
| Compound 2 | ||||
| C12–H12⋯O4 | 0.95 | 3.26 | 2.58 | 129.2 |
| C24–H24B⋯O2 | 0.98 | 3.48 | 2.64 | 144.2 |
| N4–H4B⋯O1 | 0.88 | 2.95 | 2.09 | 164.0 |
| Parameters | 1 | 2 |
|---|---|---|
| Formula | C19H18CoN2O5 | C26H26CoN4O4 |
| Formula weight | 413.28 | 517.44 |
| Temp, [K] | 100.0 | 100.0 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | Cc |
| a, [Å] | 13.5185(5) | 9.8795(3) |
| b, [Å] | 9.3996(4) | 23.5377(8) |
| c, [Å] | 14.2297(5) | 10.5489(3) |
| α, [°] | 90 | 90 |
| β, [°] | 91.846(2) | 93.877(2) |
| γ, [°] | 90 | 90 |
| V, [Å3] | 1807.21(12) | 2447.43(1) |
| Z | 4 | 4 |
| Absorption coefficient (mm−1) | 7.744 | 5.829 |
| F(0 0 0) | 852.0 | 1076.0 |
| ρcalc g/cm3 | 1.519 | 1.404 |
| index ranges | −16 ≤ h ≤ 16, −11 ≤ k ≤ 11, | −10 ≤ h ≤ 11, −28 ≤ k ≤ 28, |
| −17 ≤ l ≤ 17 | −12 ≤ l ≤ 12 | |
| Crystal size, [mm3] | 0.13 × 0.12 × 0.08 | 0.21 × 0.14 × 0.12 |
| 2θ range, [°] | 11.46 to 137.48 | 9.728 to 136.576 |
| Independent Reflections | 3297 | 3595 |
| Reflections collected | 31,819 | 28,791 |
| Refinement method | Full-matrix | Full-matrix |
| least-squares on F2 | least-squares on F2 | |
| Data/restraints/parameters | 3297/4/261 | 3595/6/335 |
| Goodness-of-fit on F2 | 1.073 | 1.035 |
| Final R indices [I > 2σ(I)] | R1 = 0.0317, wR2 =0.0778 | R1 = 0.0384, wR2 =0.0924 |
| R indices (all data) | R1 = 0.0327, wR2 = 0.0790 | R1 = 0.0395, wR2 = 0.0931 |
| Largest hole and peak [e·Å−3] | −0.55 and 0.358 | −0.418 and 0.344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, K.K.; Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. Supramolecular Assemblies and Anticancer Activities of Aminopyidine-Based Polynuclear and Mononuclear Co(II) Benzoates: Experimental and Theoretical Studies. Inorganics 2025, 13, 51. https://doi.org/10.3390/inorganics13020051
Dutta KK, Baishya T, Gomila RM, Frontera A, Barcelo-Oliver M, Verma AK, Das J, Bhattacharyya MK. Supramolecular Assemblies and Anticancer Activities of Aminopyidine-Based Polynuclear and Mononuclear Co(II) Benzoates: Experimental and Theoretical Studies. Inorganics. 2025; 13(2):51. https://doi.org/10.3390/inorganics13020051
Chicago/Turabian StyleDutta, Kamal K., Trishnajyoti Baishya, Rosa M. Gomila, Antonio Frontera, Miquel Barcelo-Oliver, Akalesh Kumar Verma, Jumi Das, and Manjit K. Bhattacharyya. 2025. "Supramolecular Assemblies and Anticancer Activities of Aminopyidine-Based Polynuclear and Mononuclear Co(II) Benzoates: Experimental and Theoretical Studies" Inorganics 13, no. 2: 51. https://doi.org/10.3390/inorganics13020051
APA StyleDutta, K. K., Baishya, T., Gomila, R. M., Frontera, A., Barcelo-Oliver, M., Verma, A. K., Das, J., & Bhattacharyya, M. K. (2025). Supramolecular Assemblies and Anticancer Activities of Aminopyidine-Based Polynuclear and Mononuclear Co(II) Benzoates: Experimental and Theoretical Studies. Inorganics, 13(2), 51. https://doi.org/10.3390/inorganics13020051








