Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review
Abstract
1. Introduction
2. Characterization and Coordination Geometry of Cu(II) Complexes
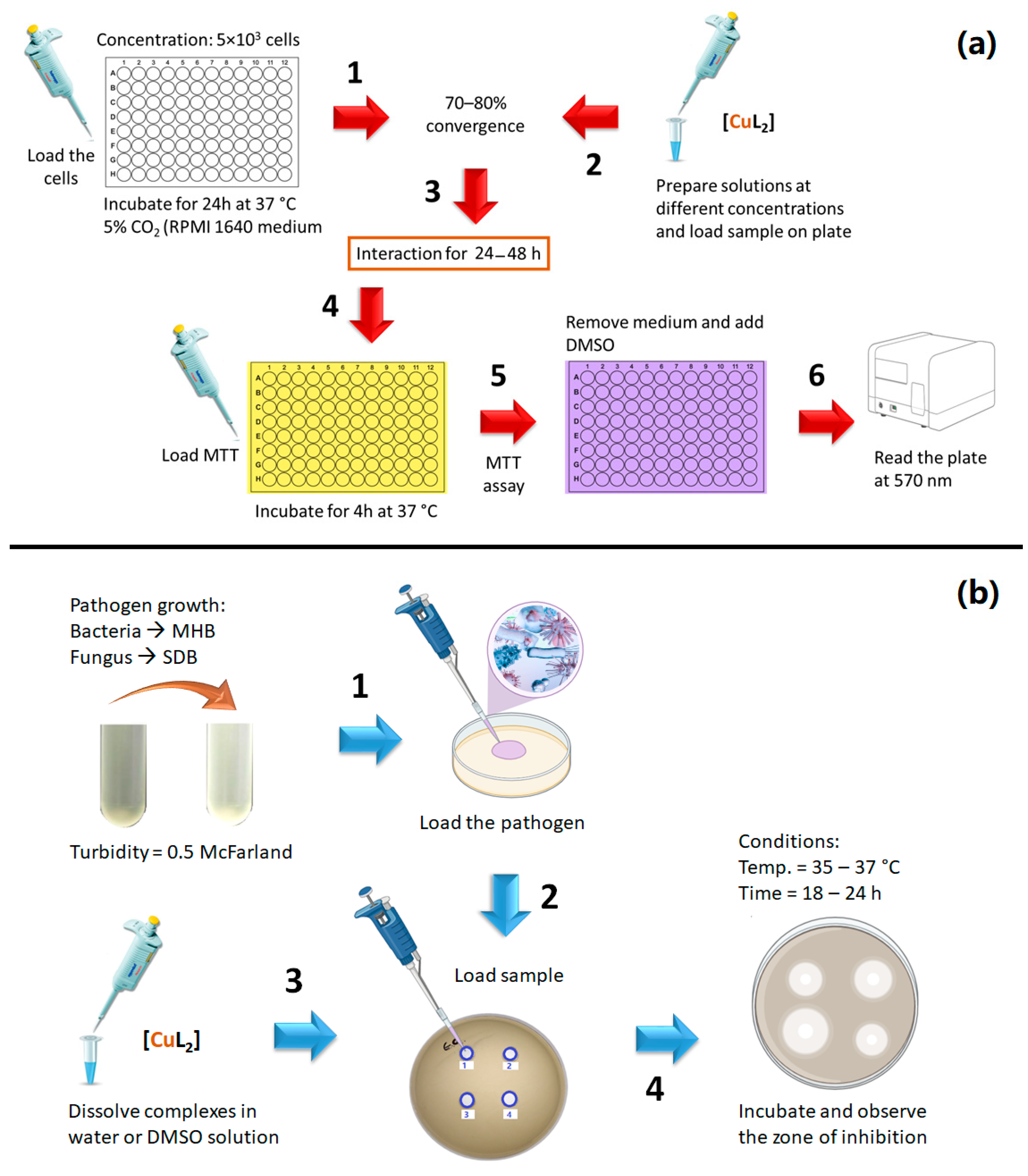
3. Anticancer Activity of Cu(II) Complexes
3.1. Mononuclear Complexes
3.2. Binuclear Complexes
3.3. Other Coordination Complexes
4. Antibacterial and Antifungal Activity of Square Planar Cu(II) Complexes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Noyon, M.R.O.K.; Hossain, M.A. Synthesis, Biological and Medicinal Impacts of Metallodrugs: A Study. Results Chem. 2023, 5, 100935. [Google Scholar] [CrossRef]
- Magner, L.; Kim, O. A History of Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Ehrlich, P.; Bertheim, A. Über Das Salzsaure 3.3′-Diamino-4.4′-dioxy-arsenobenzol Und Seine Nächsten Verwandten. Ber. Dtsch. Chem. Ges. 1912, 45, 756–766. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Freisinger, E.; Sigel, R.K.O. Metallo-Drugs: Development and Action of Anticancer Agents; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Gandin, V.; Hoeschele, J.D.; Margiotta, N. Special Issue “Cisplatin in Cancer Therapy: Molecular Mechanisms of Action 3.0”. Int. J. Mol. Sci. 2023, 24, 7917. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Zheng, W.; Luo, Q.; Han, J.; Liu, J.; Zhao, Y.; Jia, F.; Wu, K.; Wang, F. Discovery of Cisplatin Binding to Thymine and Cytosine on a Single-Stranded Oligodeoxynucleotide by High Resolution FT-ICR Mass Spectrometry. Molecules 2019, 24, 1852. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin Nephrotoxicity: New Insights and Therapeutic Implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Chattaraj, A.; Syed, M.P.; Low, C.A.; Owonikoko, T.K. Cisplatin-Induced Ototoxicity: A Concise Review of the Burden, Prevention, and Interception Strategies. JCO Oncol. Pract. 2023, 19, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Waters, J.E.; Stevens-Cullinane, L.; Siebenmann, L.; Hess, J. Recent advances in the development of metal complexes as antibacterial agents with metal-specific modes of action. Curr. Opin. Microbiol. 2023, 75, 102347. [Google Scholar] [CrossRef]
- Moreno-Ramirez, M.C.; Arias-Bravo, A.S.; Aragón-Muriel, A.; Godoy, C.A.; Liscano, Y.; Garzón, J.O.; Polo-Cerón, D. Design, Synthesis and Antimicrobial Potential of Conjugated Metallopeptides Targeting DNA. Sci. Pharm. 2024, 92, 21. [Google Scholar] [CrossRef]
- Hou, A.; Du, Y.; Su, Y.; Pang, Z.; Liu, S.; Xian, S.; Zhao, X.; Ma, L.; Liu, B.; Wu, H.; et al. CuS/Co-Ferrocene-MOF Nanocomposites for Photothermally Enhanced Chemodynamic Antibacterial Therapy. ACS Appl. Nano Mater. 2024, 7, 10998–11007. [Google Scholar] [CrossRef]
- Majid, S.A.; Mir, J.M.; Jan, G.; Shalla, A.H. Schiff Base Complexes, Cancer Cell Lines, and Anticancer Evaluation: A Review. J. Coord. Chem. 2022, 75, 2018–2038. [Google Scholar] [CrossRef]
- Kar, K.; Ghosh, D.; Kabi, B.; Chandra, A. A Concise Review on Cobalt Schiff Base Complexes as Anticancer Agents. Polyhedron 2022, 222, 115890. [Google Scholar] [CrossRef]
- Alorini, T.A.; Al-Hakimi, A.N.; Saeed, S.E.-S.; Alhamzi, E.H.L.; Albadri, A.E.A.E. Synthesis, Characterization, and Anticancer Activity of Some Metal Complexes with a New Schiff Base Ligand. Arab. J. Chem. 2022, 15, 103559. [Google Scholar] [CrossRef]
- Alfonso-Herrera, L.A.; Rosete-Luna, S.; Hernández-Romero, D.; Rivera-Villanueva, J.M.; Olivares-Romero, J.L.; Cruz-Navarro, J.A.; Soto-Contreras, A.; Arenaza-Corona, A.; Morales-Morales, D.; Colorado-Peralta, R. Transition Metal Complexes with Tridentate Schiff Bases (O N O and O N N) Derived from Salicylaldehyde: An Analysis of Their Potential Anticancer Activity. ChemMedChem 2022, 17, e202200367. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.H.V.; de Carvalho, A.B.; Silva, V.R.; Santos, L.D.S.; Soares, M.B.P.; Bezerra, D.P.; Oliveira, K.M.; Corrêa, R.S. Copper(II)/Diiminic Complexes Based on 2-Hydroxybenzophenones: DNA- and BSA-Binding Studies and Antitumor Activity against HCT116 and HepG2 Tumor Cells. Polyhedron 2023, 239, 116431. [Google Scholar] [CrossRef]
- McCalmont, A.S.; Ruiz, A.; Lagunas, M.C.; Al-Jamal, W.T.; Crawford, D.E. Cytotoxicity of Mechanochemically Prepared Cu(II) Complexes. ACS Sustain. Chem. Eng. 2020, 8, 15243–15249. [Google Scholar] [CrossRef]
- Sharma, H.; Pathak, M. Synthesis and Theoretical Evaluation of New Copper(II) Complexes Associated with a Crystalline Schiff Base: DNA/BSA Protein Interaction, Radical Scavenging and Cytotoxicity. Eur. J. Inorg. Chem. 2024, 27, e202400302. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A. Copper-Instigated Modulatory Cell Mortality Mechanisms and Progress in Oncological Treatment Investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Nunes, I.J.; Ferreira, W.V.; Tomasini, P.P.; Trindade, C.; Martins, C.C.; Wilhelm, E.A.; Oliboni, R.D.S.; Netz, P.A.; Stieler, R.; et al. Antioxidant and Anticancer Potential of the New Cu(II) Complexes Bearing Imine-Phenolate Ligands with Pendant Amine N-Donor Groups. Pharmaceutics 2023, 15, 376. [Google Scholar] [CrossRef]
- Isanbor, C.; O’Hagan, D. Fluorine in Medicinal Chemistry: A Review of Anti-Cancer Agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Ojima, I. Use of Fluorine in the Medicinal Chemistry and Chemical Biology of Bioactive Compounds—A Case Study on Fluorinated Taxane Anticancer Agents. ChemBioChem 2004, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ahmed, H.E.A.; Ihmaid, S.; Omar, A.-S.M.; Abdelrahim, A.S. Synthesis and Screening of Some New Fluorinated Quinazolinone-Sulphonamide Hybrids as Anticancer Agents. J. Taibah Univ. Med. Sci. 2015, 10, 333–339. [Google Scholar] [CrossRef]
- Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O-Contreras, C.M.; Canseco-González, D.; Parra-Unda, J.R.; Avila-Sorrosa, A.; Enríquez, R.G.; Germán-Acacio, J.M.; Morales-Morales, D. Relevance of Fluorinated Ligands to the Design of Metallodrugs for Their Potential Use in Cancer Treatment. Pharmaceutics 2022, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic Fluorine Compounds: A Great Opportunity for Enhanced Materials Properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Daravath, S.; Rambabu, A.; Ganji, N.; Ramesh, G.; Lakshmi, P.V.A.; Shivaraj. Spectroscopic, Quantum Chemical Calculations, Antioxidant, Anticancer, Antimicrobial, DNA Binding and Photo Physical Properties of Bioactive Cu(II) Complexes Obtained from Trifluoromethoxy Aniline Schiff Bases. J. Mol. Struct. 2022, 1249, 131601. [Google Scholar] [CrossRef]
- Rambabu, A.; Daravath, S.; Shankar, D.S.; Shivaraj. DNA-Binding, Cleavage and Antimicrobial Investigation on Mononuclear Cu(II) Schiff Base Complexes Originated from Riluzole. J. Mol. Struct. 2021, 1244, 131002. [Google Scholar] [CrossRef]
- Sumalatha, V.; Rambabu, A.; Vamsikrishna, N.; Ganji, N.; Daravath, S.; Shivaraj. Synthesis, Characterization, DNA Binding Propensity, Nuclease Efficacy, Antioxidant and Antimicrobial Activities of Cu(II), Co(II) and Ni(II) Complexes Derived from 4-(Trifluoromethoxy)Aniline Schiff Bases. Chem. Data Collect. 2019, 20, 100213. [Google Scholar] [CrossRef]
- Rambabu, A.; Ganji, N.; Daravath, S.; Venkateswarlu, K.; Rangan, K.; Shivaraj. Mononuclear Co(II), Ni(II) and Cu(II) Complexes of the Schiff Base, 2-(((4-Trifluoromethoxy)Phenylimino)Methyl)-6-Tert-Butylphenol: Synthesis, Spectroscopic Characterization, X-Ray Study and Biological Evaluation. J. Mol. Struct. 2020, 1199, 127006. [Google Scholar] [CrossRef]
- Jyothi, N.; Ganji, N.; Daravath, S.; Shivaraj. Mononuclear Cobalt(II), Nickel(II) and Copper(II) Complexes: Synthesis, Spectral Characterization and Interaction Study with Nucleotide by in vitro Biochemical Analysis. J. Mol. Struct. 2020, 1207, 127799. [Google Scholar] [CrossRef]
- Jyothi, N.; Daravath, S.; Swathi, M.; Jagadeshbabu, K.; Ganji, N.; Shivaraj. Synthesis, Geometry Optimization and Non-Isothermal Kinetic Parameters of Copper(II), Nickel(II) and Cobalt(II) Complexes of 5-(Trifluoromethyl)-2-Methoxybenzenamine: DNA Binding, Cytotoxicity, Antioxidant and Antimicrobial Activity. J. Mol. Struct. 2024, 1295 Pt 1, 136529. [Google Scholar] [CrossRef]
- Ommenya, F.K.; Nyawade, E.A.; Andala, D.M.; Kinyua, J. Synthesis, Characterization and Antibacterial Activity of Schiff Base, 4-Chloro-2-{(E)-[(4-Fluorophenyl)imino]methyl}phenol Metal (II) Complexes. J. Chem. 2020, 2020, 1745236. [Google Scholar] [CrossRef]
- Kanagavalli, C.; Kalanithi, M.; Gurusamy, S.; Asha, R.N.; Megtalin, N.M.; Sankarganesh, M. Computational, spectroscopic, sensor and biological studies of Cu(II) complex of Fluoro substituted Schiff base. Chem. Phys. Impact. 2024, 8, 100582. [Google Scholar] [CrossRef]
- Jiang, S.; Ni, H.; Liu, F.; Gu, S.; Yu, P.; Gou, Y. Binuclear Schiff Base Copper(II) Complexes: Syntheses, Crystal Structures, HSA Interaction and Anti-Cancer Properties. Inorg. Chim. Acta 2020, 499, 119186. [Google Scholar] [CrossRef]
- Savcı, A.; Turan, N.; Buldurun, K.; Eşref Alkış, M.; Alan, Y. Schiff Base Containing Fluorouracil and Its M(II) Complexes: Synthesis, Characterization, Cytotoxic and Antioxidant Activities. Inorg. Chem. Commun. 2022, 143, 109780. [Google Scholar] [CrossRef]
- Mamindla, A.; Varadhan, M.; Kartikeyan, R.; Amuthamozhi, A.; Akbarsha, M.A.; Rajendiran, V. Comparative DNA-/BSA-Binding, DNA Cleavage, and Cytotoxic Properties of Copper(II) Amino/Salicyl-Phenolate Schiff Bases (Phen) Complexes That Induce Generation of Phenoxyl Radicals. Polyhedron 2023, 243, 116534. [Google Scholar] [CrossRef]
- Jain, P.; Singh, V.; Ali, S.; Tripathi, V.; Saraswat, U. Synthesis, Characterization, Molecular Docking and Biological Activity of 5,6-Bis-(4-Fluoro-Phenyl)-3,4,7,8-Tetraaza-Bicyclo[8.3.1]Tetradeca-1(13),4,6,10(14),11-Pentaene-2,9-Dione and Its Transition Metal Complexes. J. Saudi Chem. Soc. 2018, 22, 546–557. [Google Scholar] [CrossRef]
- Narayanan, V.S.; Rajendran, A.; Mudradi, S.; Dhawa, S.; Rajendran, S.; Naidu Bobba, K.; Rajendran, J.; Ramani, P. Development of New Cobalt, Copper, and Zinc Complexes of Schiff-Base Ligands as Prospective Chemotherapeutic Agents. Polyhedron 2023, 245, 116622. [Google Scholar] [CrossRef]
- Kaştaş, G.; Albayrak, Ç.; Avşar, C. Structural, catalytic, antimicrobial and antioxidant properties of Cu(II)-complexes prepared by o-hydroxy Schiff bases with fluorine and ethoxy substituents. Mol. Cryst. Liq. Cryst. 2024, 768, 138–156. [Google Scholar] [CrossRef]
- Oboňová, B.; Valentová, J.; Litecká, M.; Pašková, Ľ.; Hricovíniová, J.; Bilková, A.; Bilka, F.; Horváth, B.; Habala, L. Novel Copper (II) Complexes with Fluorine-Containing Reduced Schiff Base Ligands Showing Marked Cytotoxicity in the HepG2 Cancer Cell Line. Int. J. Mol. Sci. 2024, 25, 9166. [Google Scholar] [CrossRef]
- Malik, M.A.; Raza, M.K.; Mohammed, A.; Wani, M.Y.; Al-Bogami, A.S.; Hashmi, A.A. Unravelling the Anticancer Potential of a Square Planar Copper Complex: Toward Non-Platinum Chemotherapy. RSC Adv. 2021, 11, 39349–39361. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-Mello, M.V.; Caballero, A.B.; Lopez-Espinar, A.; Guedes, G.P.; Caubet, A.; de Souza, A.M.T.; Lanznaster, M.; Gamez, P. DNA-Interacting Properties of Two Analogous Square-Planar Cis-Chlorido Complexes: Copper versus Palladium. J. Biol. Inorg. Chem. 2021, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Barilli, A.; Atzeri, C.; Bassanetti, I.; Ingoglia, F.; Dall’Asta, V.; Bussolati, O.; Maffini, M.; Mucchino, C.; Marchiò, L. Oxidative Stress Induced by Copper and Iron Complexes with 8-Hydroxyquinoline Derivatives Causes Paraptotic Death of HeLa Cancer Cells. Mol. Pharm. 2014, 11, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, C.; Martoriati, A.; Pelinski, L.; Cailliau, K. Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef] [PubMed]
- Skos, L.; Borutzki, Y.; Gerner, C.; Meier-Menches, S.M. Methods to Identify Protein Targets of Metal-Based Drugs. Curr. Opin. Chem. Biol. 2023, 73, 102257. [Google Scholar] [CrossRef]
- Annunziata, A.; Liberti, D.; Bedini, E.; Cucciolito, M.E.; Loreto, D.; Monti, D.M.; Merlino, A.; Ruffo, F. Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 8704. [Google Scholar] [CrossRef]
- Arjmand, F.; Khursheed, S.; Roisnel, T.; Siddique, H.R. Copper (II)-Based Halogen-Substituted Chromone Antitumor Drug Entities: Studying Biomolecular Interactions with ct-DNA Mediated by Sigma Hole Formation and Cytotoxicity Activity. Bioorg. Chem. 2020, 104, 104327. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 194. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, S.; Rattan, R.; Fatima, M.; Goel, M.; Bhat, M.; Dutta, S.; Ranjan, R.K.; Sharma, M. Antimicrobial Agents Based on Metal Complexes: Present Situation and Future Prospects. Int. J. Biomater. 2022, 2022, 6819080. [Google Scholar] [CrossRef]
- Rzycki, M.; Gładysiewicz-Kudrawiec, M.; Kraszewski, S. Molecular Guidelines for Promising Antimicrobial Agents. Sci. Rep. 2024, 14, 4641. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]





| Complex | IC50 (µM) | General Mechanism of Action | Ref. |
| a6 | HeLa: 15.99; A549: 19.44 | DNA intercalation binding | [35] |
| a7 | HeLa: 18.47; A549: 21.04 | [35] | |
| a10 | A549: 38.09; MCF-7: 34.07 | DNA intercalation binding | [31] |
| a11 | A549: 42.05; MCF-7: 39.04 | [31] | |
| a12 | A549: 48.03; MCF-7: 45.01 | [31] | |
| a13 | HeLa: 25.78; A549: 26.62 | DNA intercalation binding | [36] |
| a14 | HeLa: 28.63; A549: 30.58 | [36] | |
| b1 | Bell-7402: 2.7; HeLa: 0.5; MCF-7: 0.8; MGC-803: 3.0; WI-38: 3.2 | HSA interaction | [39] |
| b2 | Caco-2: 31.8; L-929: 285 | Not studied | [40] |
| c1 | A549: 3.52 | DNA cleavage | [41] |
| c3 | SCC4: >100 | EGFR Kinase receptor binding | [42] |
| c4 | SCC4: 74.9 | [42] | |
| c5 | SCC4: 54.3 | [42] | |
| c6 | HeLa: 16.66; MCF-7: 8.9; TIB-71: 85.43 | DNA intercalation binding | [43] |
| c7 | HeLa: 13.39; MCF-7: 5.28; TIB-71: 95.33 | [43] | |
| c10 | HepG2: 61.3 | DNA intercalation binding | [45] |
| c11 | HepG2: 28.7 | [45] | |
| c12 | HepG2: 64.4 | [45] | |
| c13 | HepG2: 189.1 | [45] |
| Complex | Kb (M−1) | KSV (M−1) | Ref. |
|---|---|---|---|
| a1 | 1.41 ± 0.02 × 105 | --- | [33] |
| a2 | 1.34 ± 0.02 × 105 | --- | [33] |
| a3 | 2.33 ± 0.02 × 105 | 2.47 ± 0.02 × 104 | [33] |
| a4 | 1.41 ± 0.02 × 105 | 2.01 ± 0.01 × 104 | [33] |
| a5 | 4.92 ± 0.04 × 105 | 2.30 ± 0.01 × 104 | [34] |
| a6 | 5.13 ± 0.01 × 105 | 4.26 ± 0.02 × 104 | [35] |
| a7 | 4.62 ± 0.01 × 105 | 4.09 ± 0.03 × 104 | [35] |
| a8 | 5.93 ± 0.01 × 105 | 6.85 ± 0.02 × 105 | [32] |
| a10 | 7.25 ± 0.01 × 105 | 6.73 ± 0.02 × 104 | [31] |
| a11 | 6.85 ± 0.02 × 105 | 5.89 ± 0.02 × 104 | [31] |
| a12 | 6.25 ± 0.03 × 105 | 5.50 ± 0.01 × 104 | [31] |
| a13 | 5.23 ± 0.01 × 105 | 5.36 ± 0.02 × 104 | [36] |
| a14 | 4.13 ± 0.01 × 105 | 4.99 ± 0.03 × 104 | [36] |
| Ethidium bromide | 7 × 107 | --- |
| Inhibition Zone (mm) | Inhibition Zone (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria Strains | Fungi Strains | ||||||||||
| Gram-Positive | Gram-Negative | ||||||||||
| Compound | B. amyloliquefaciens | B. subtilis | S. aureus | E. coli | P. aeruginosa | K. pneumoniae | A. niger | C. albicans | M. phaseolina | S. rolfsii | Ref. |
| a1 | - | 10 ± 0.1 | 8 ± 0.2 | 10 ± 0.1 | 9 ± 0.1 | - | 10 ± 0.1 | 11 ± 0.2 | - | - | [33] |
| a2 | - | 8 ± 0.3 | 11 ± 0.1 | 12 ± 0.2 | 11 ± 0.1 | - | 12 ± 0.2 | 11 ± 0.2 | - | - | [33] |
| a3 | 22 ± 0.3 | - | 24 ± 0.2 | 23 ± 0.1 | 21 ± 0.3 | - | - | - | 22 ± 0.1 | 23 ± 0.2 | [33] |
| a4 | 20 ± 0.2 | - | 22 ± 0.2 | 21 ± 0.3 | 20 ± 0.1 | - | - | - | 20 ± 0.1 | 22 ± 0.1 | [33] |
| a5 | 9 ± 0.38 | - | - | 10 ± 0.22 | - | - | - | - | 8 ± 0.26 | 3 ± 0.22 | [34] |
| a6 | 26 ± 0.2 | - | 23 ± 0.4 | 21 ± 0.3 | - | 22 ± 0.2 | - | - | 21 ± 0.2 | 20 ± 0.2 | [35] |
| a7 | 25 ± 0.3 | - | 23 ± 0.1 | 20 ± 0.2 | - | 21 ± 0.3 | - | - | 22 ± 0.2 | 20 ± 0.2 | [35] |
| a8 | 7 ± 0.28 | - | 7 ± 0.18 | 9 ± 0.20 | 6 ± 0.18 | - | - | - | 8 ± 0.28 | 8 ± 0.26 | [32] |
| a9 | 6 ± 0.30 | - | 7 ± 0.20 | 8 ± 0.20 | 6 ± 0.22 | - | - | - | 6 ± 0.24 | 7 ± 0.22 | [32] |
| a10 | 20 ± 0.21 | - | - | 19 ± 0.16 | - | - | - | - | 18 ± 0.15 | 18 ± 0.18 | [31] |
| a11 | 17 ± 0.14 | - | - | 16 ± 0.21 | - | - | - | - | 16 ± 0.16 | 15 ± 0.24 | [31] |
| a12 | 16 ± 0.18 | - | - | 16 ± 0.15 | - | - | - | - | 15 ± 0.19 | 14 ± 0.15 | [31] |
| a13 | 26 ± 0.2 | - | 24 ± 0.4 | 25 ± 0.3 | - | 22 ± 0.2 | - | - | 20 ± 0.2 | 21 ± 0.2 | [36] |
| a14 | 24 ± 0.3 | - | 22 ± 0.3 | 23 ± 0.4 | - | 22 ± 0.2 | - | - | 20 ± 0.2 | 21 ± 0.2 | [36] |
| a15 | - | 110.7 ± 0.5 | - | 8.9 ± 0.9 | 11.7 ± 1.2 | - | - | - | - | - | [37] |
| a16 | - | - | 17 | 10 | 15 | - | - | 17 | - | - | [38] |
| Ampicillin | 30 ± 0.2 | - | 31 ± 0.2 | 30 ± 0.2 | - | 30 ± 0.2 | - | - | - | - | |
| Streptomycin | 15 ± 0.18 | - | 11.0 ± 0.14 | 10.0 ± 0.22 | 10.0 ± 0.26 | - | - | - | - | - | |
| Ketoconazole | - | - | - | - | - | - | 15 ± 0.2 | 16 ± 0.3 | - | - | |
| Mancozeb | - | - | - | - | - | - | - | - | 31 ± 0.2 | 30 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Narváez, M.E.; González-Sebastián, L.; Colorado-Peralta, R.; Reyes-Márquez, V.; Franco-Sandoval, L.O.; Romo-Pérez, A.; Cruz-Navarro, J.A.; Mañozca-Dosman, I.V.; Aragón-Muriel, A.; Morales-Morales, D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics 2025, 13, 38. https://doi.org/10.3390/inorganics13020038
Moreno-Narváez ME, González-Sebastián L, Colorado-Peralta R, Reyes-Márquez V, Franco-Sandoval LO, Romo-Pérez A, Cruz-Navarro JA, Mañozca-Dosman IV, Aragón-Muriel A, Morales-Morales D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics. 2025; 13(2):38. https://doi.org/10.3390/inorganics13020038
Chicago/Turabian StyleMoreno-Narváez, María Esther, Lucero González-Sebastián, Raúl Colorado-Peralta, Viviana Reyes-Márquez, Luz Ofelia Franco-Sandoval, Adriana Romo-Pérez, Jesús Antonio Cruz-Navarro, Ivone Vanessa Mañozca-Dosman, Alberto Aragón-Muriel, and David Morales-Morales. 2025. "Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review" Inorganics 13, no. 2: 38. https://doi.org/10.3390/inorganics13020038
APA StyleMoreno-Narváez, M. E., González-Sebastián, L., Colorado-Peralta, R., Reyes-Márquez, V., Franco-Sandoval, L. O., Romo-Pérez, A., Cruz-Navarro, J. A., Mañozca-Dosman, I. V., Aragón-Muriel, A., & Morales-Morales, D. (2025). Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics, 13(2), 38. https://doi.org/10.3390/inorganics13020038










