New Electromagnetic Shielding Materials Based on Viscose/Maghemite/Goethite/Polysiloxane
Abstract
1. Introduction
2. Results and Discussion
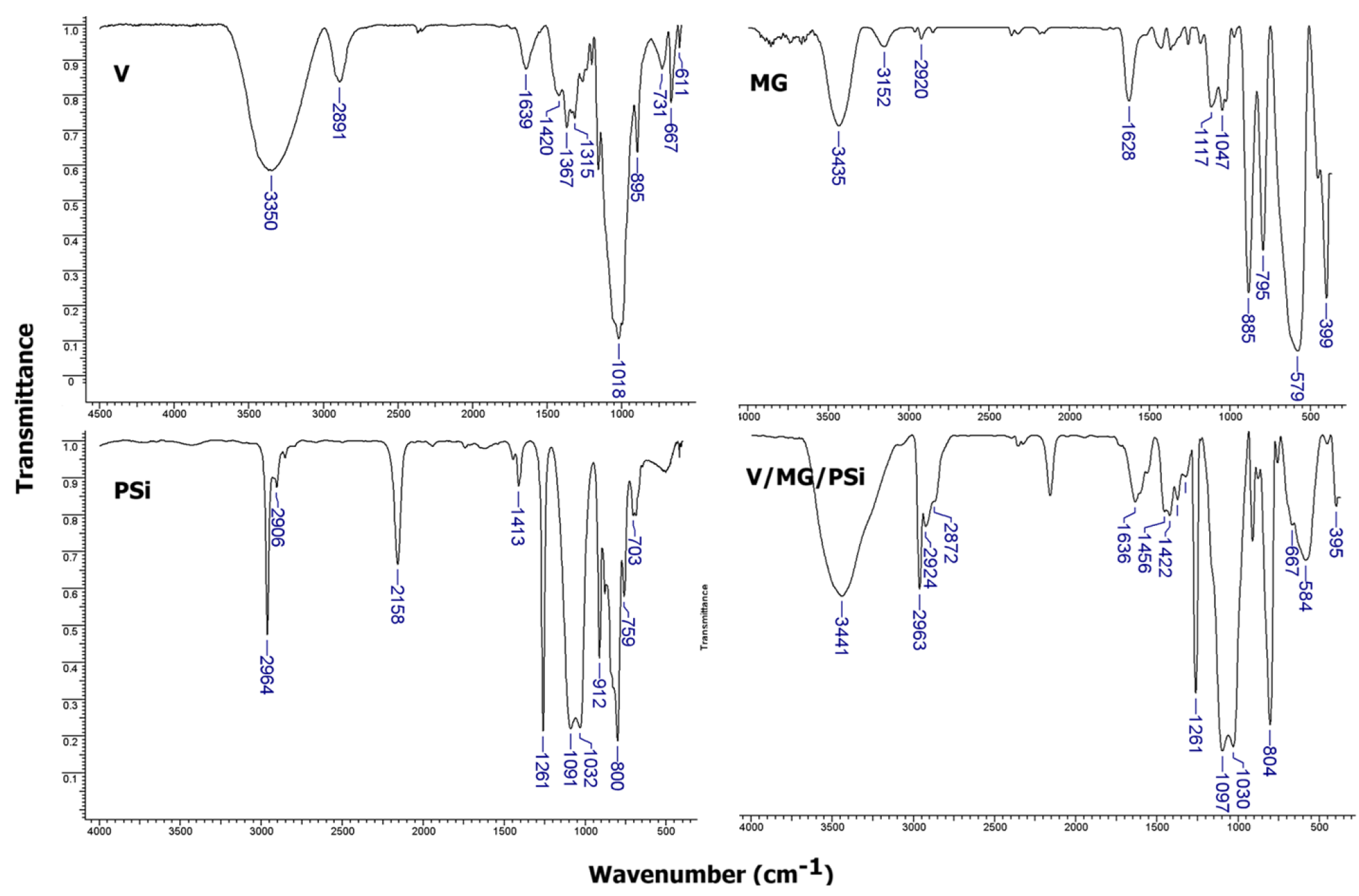
2.1. Structural Characterization
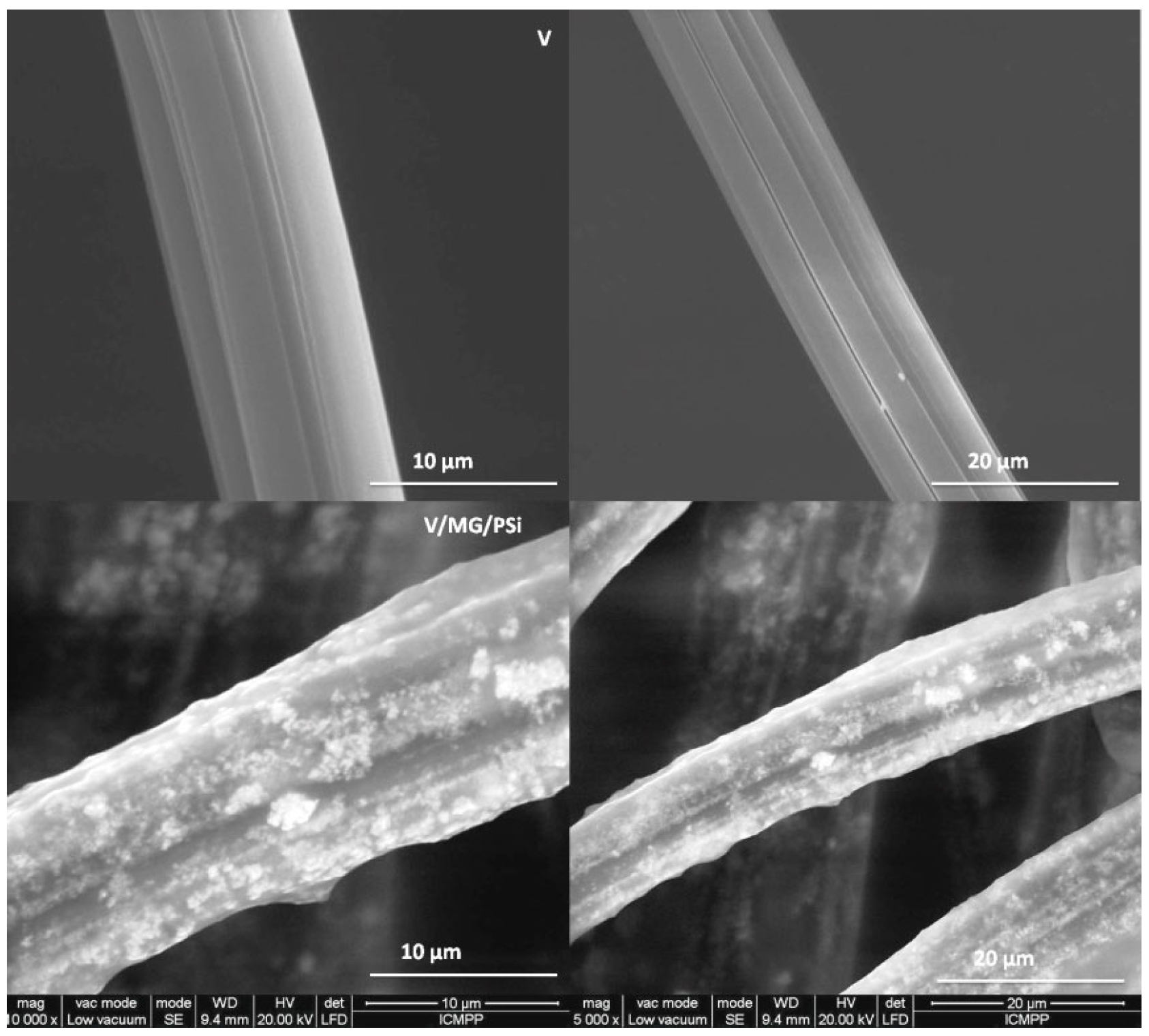
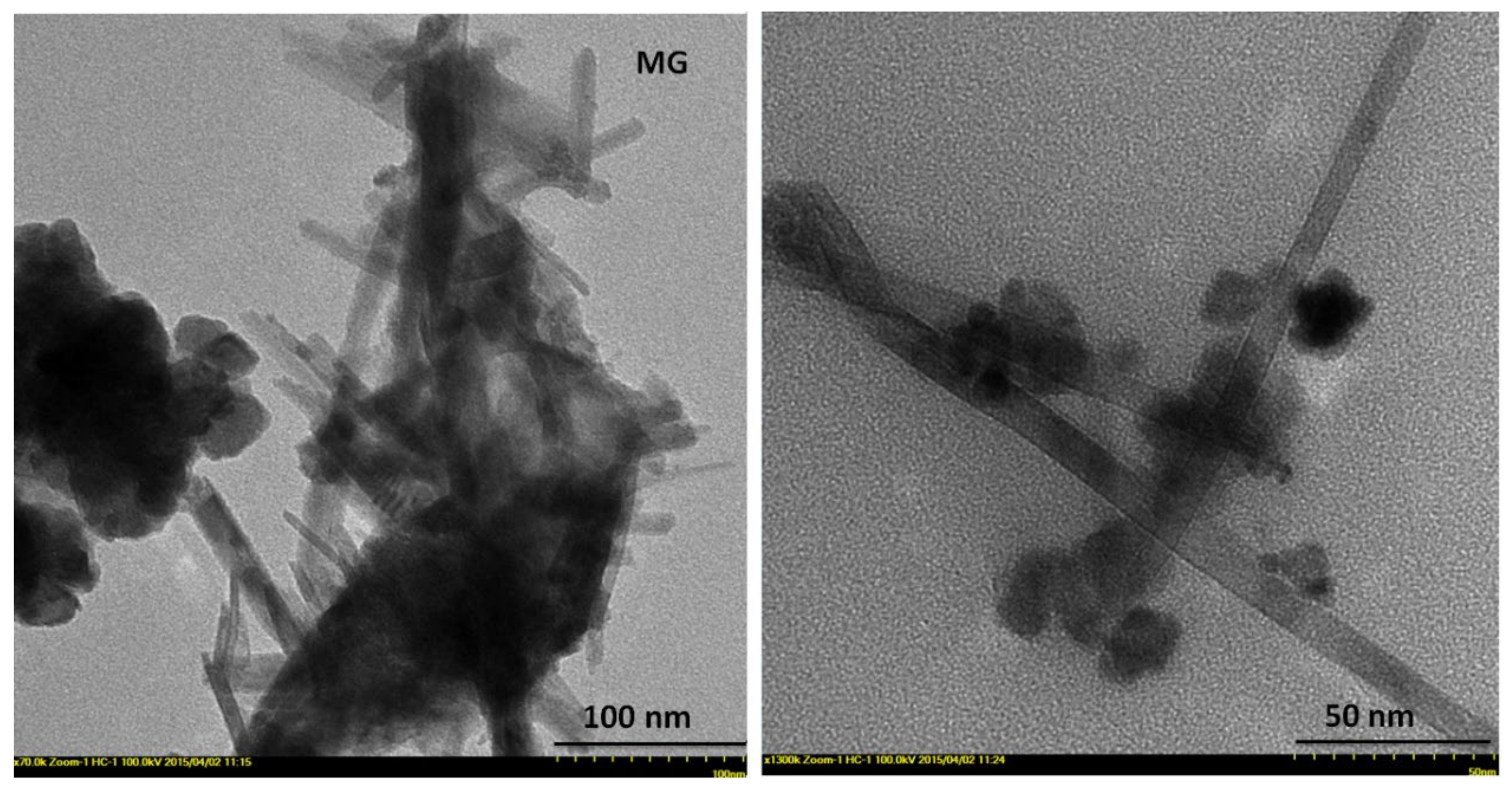
2.2. Morphology/Dimensional Characterization
2.3. Thermal Properties
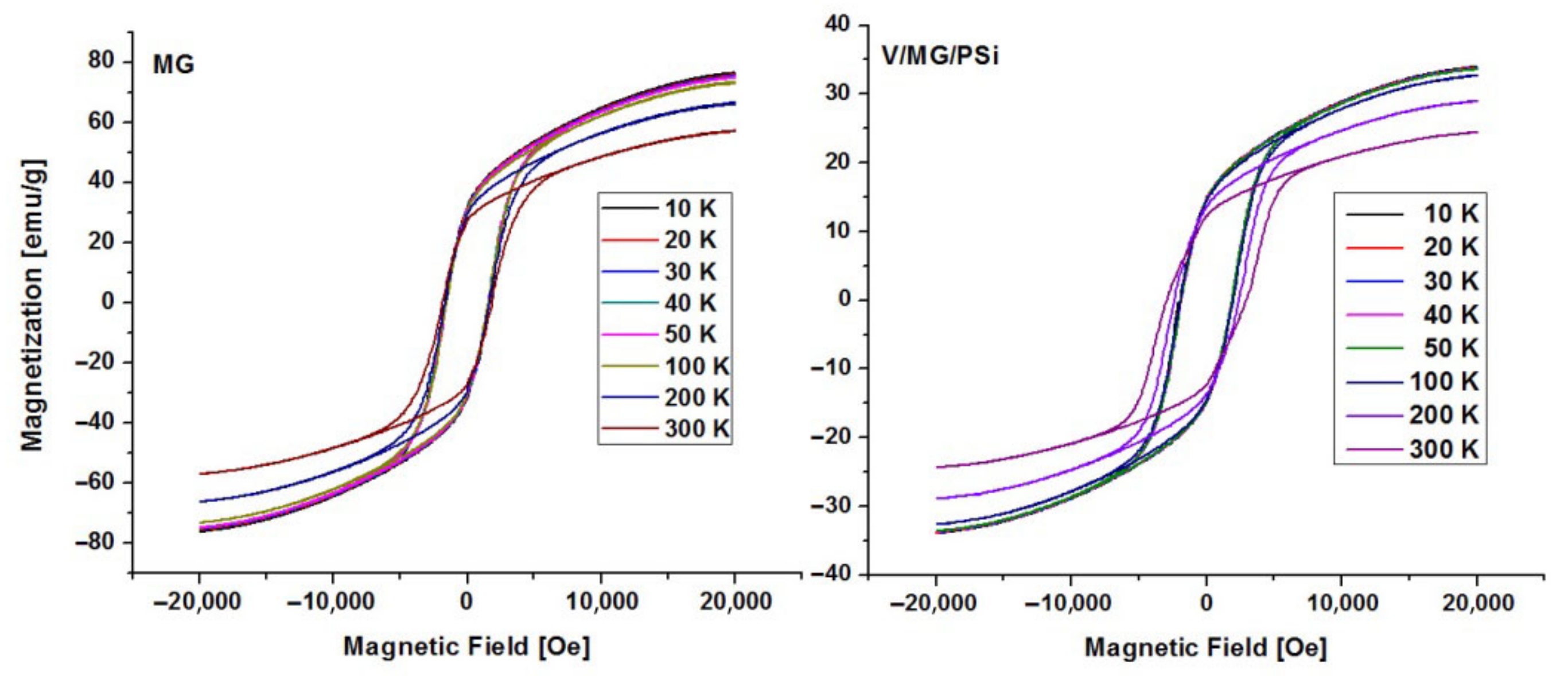
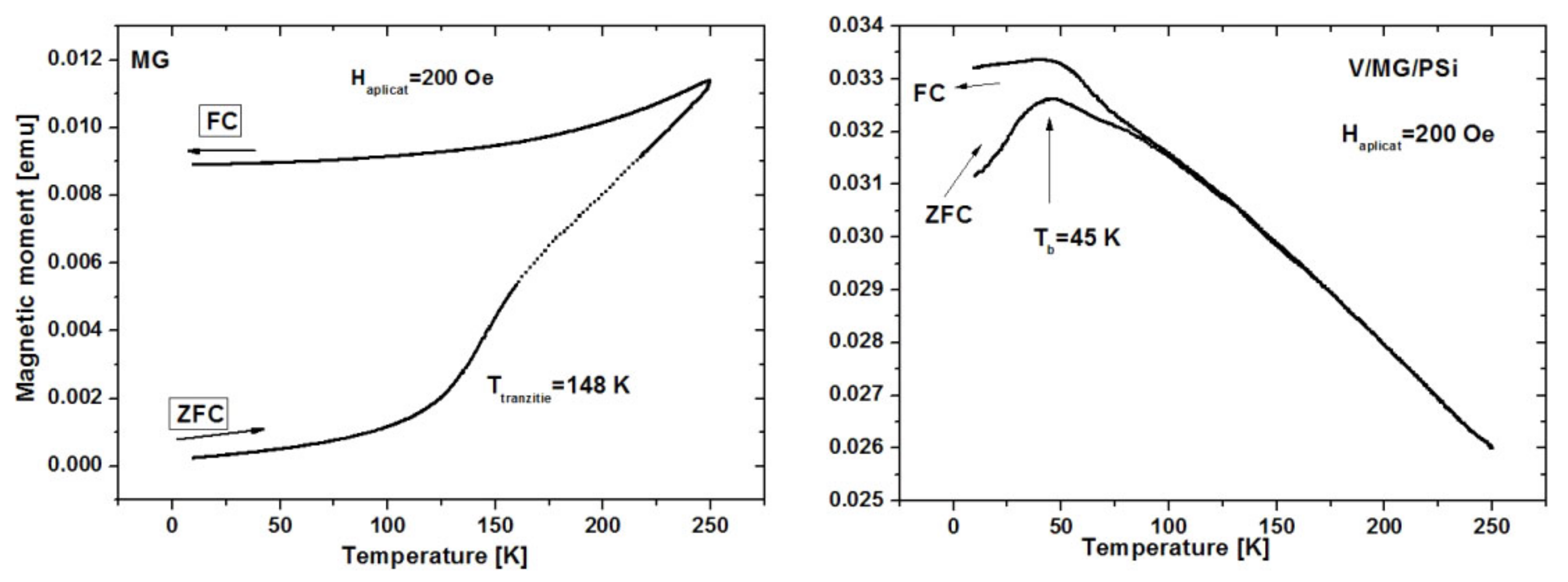
2.4. Magnetic Measurements
2.5. Wettability Study
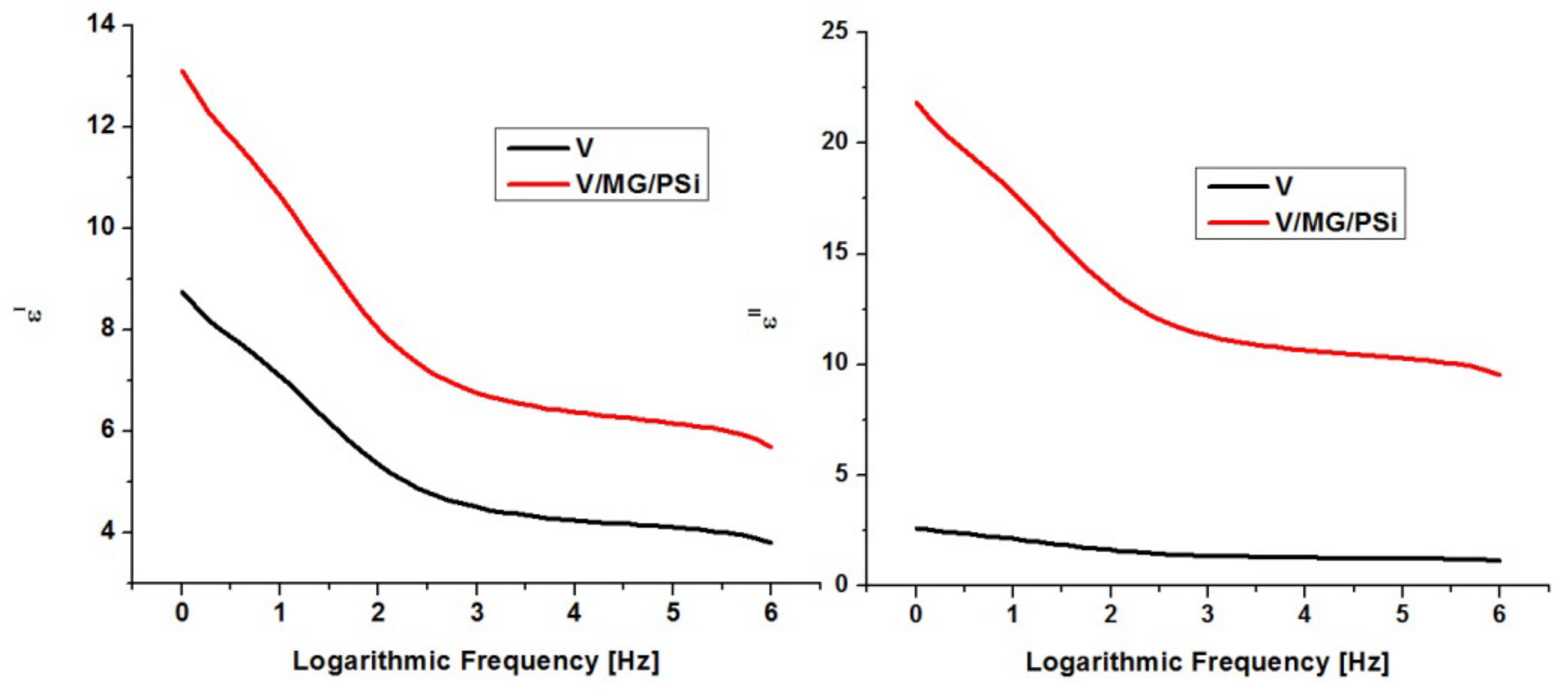
2.6. Dielectric Properties
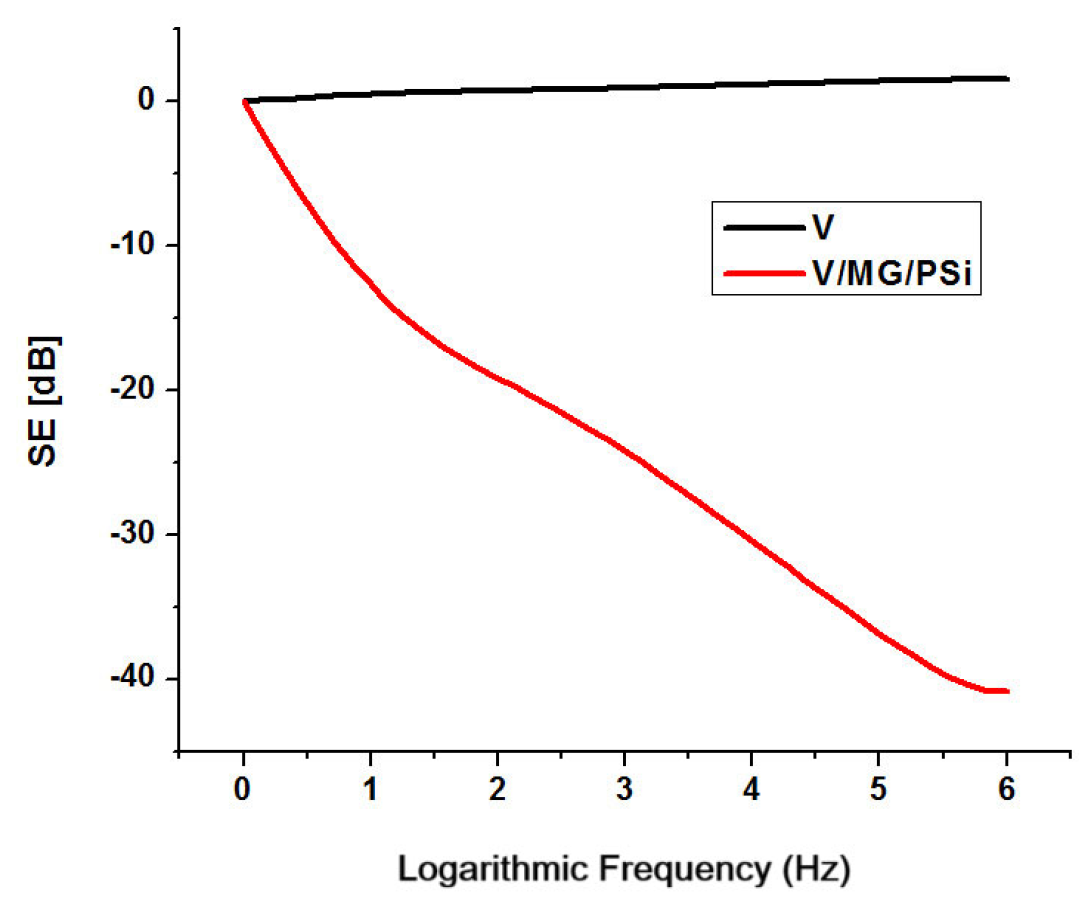
2.7. Shielding Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation
3.2.1. Synthesis of Maghemite/Goethite Ferromagnetic Nanoparticles (MG)
3.2.2. Preparation of Viscose/Maghemite/Goethite Composite (V/MG)
3.2.3. Preparation of Poly(methylhydro-dimethyl)siloxane Copolymers (PSi)
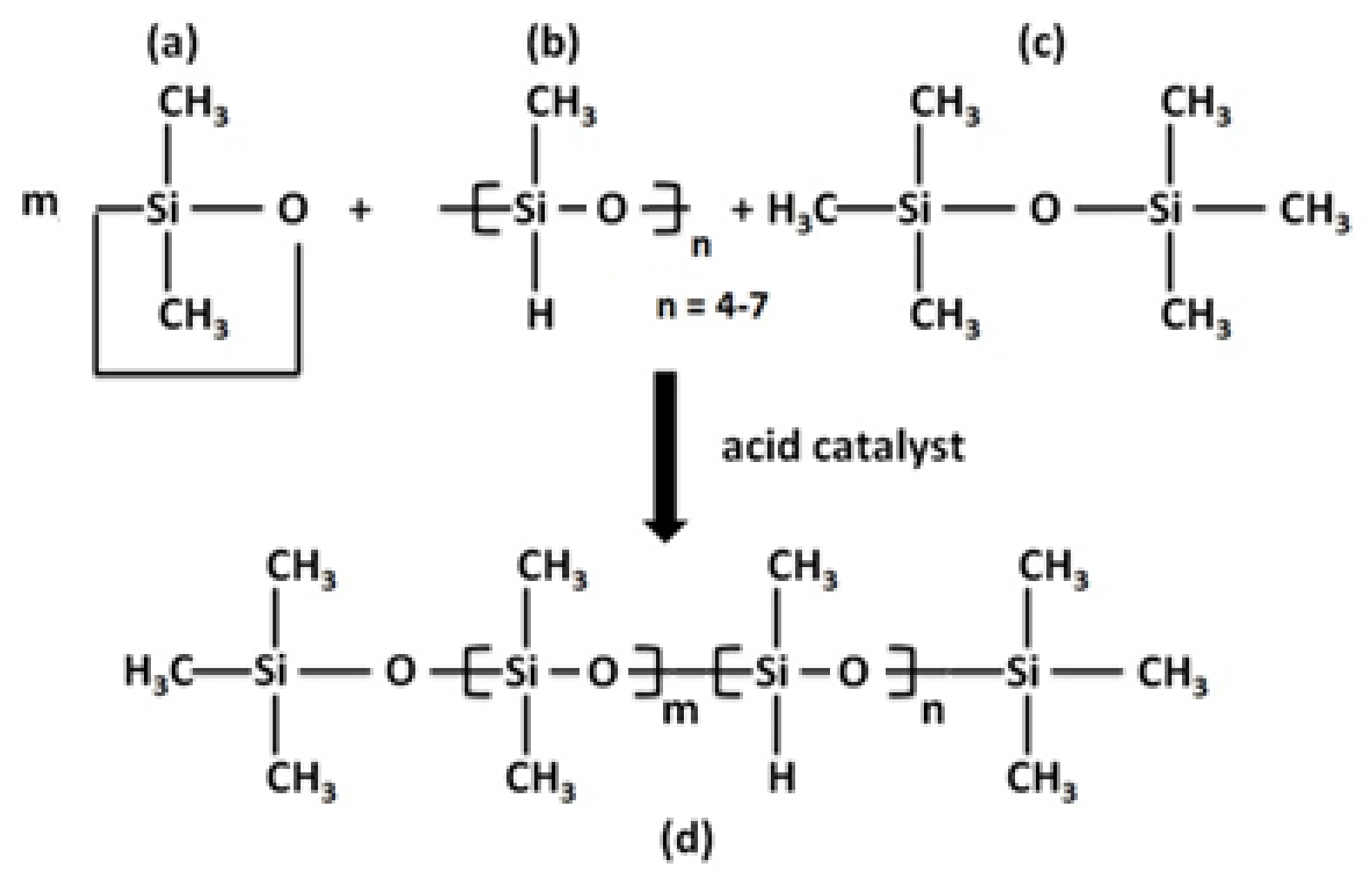
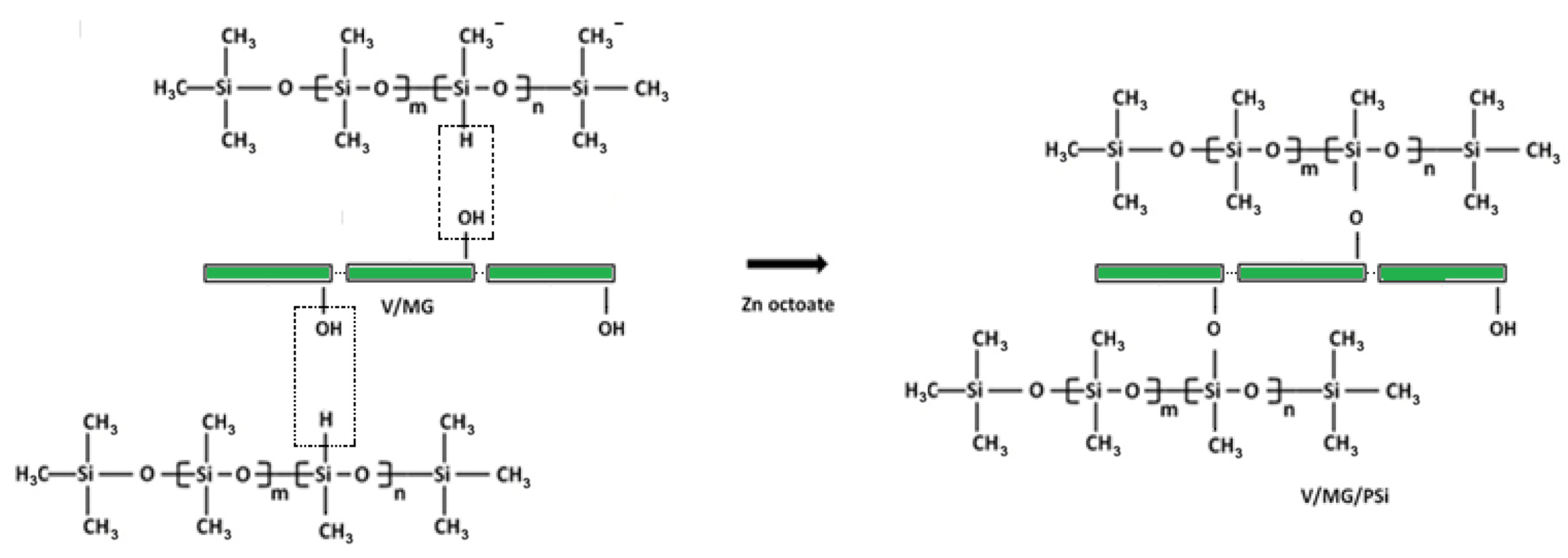
3.2.4. Preparation of Hydrophobic Composite (V/MG/PSi)
3.3. Equipment and Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Kapoor, N. Health implications of electromagnetic fields, mechanisms of action, and research needs. Adv. Biol. 2014, 24, 198609. [Google Scholar] [CrossRef]
- Yakymenko, I.; Sidorik, E. Risks of Carcinogenesis from electromagnetic radiation of mobile telephony devices. Exp. Oncol. 2010, 32, 54–60. [Google Scholar]
- Mosii, P.; Jari, I.; Ursu, A.M.; Naum, A.G. The relationship between job strain and ischemic heart disease mediated by endothelial dysfunction markers and imaging. Medicina 2024, 60, 1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, W.; Liu, L.; Cheng, W.; Wang, W.; Liew, K.M.; Wang, B.; Hu, Y. Eco-friendly flame retardant and electromagnetic interference shielding cotton fabrics with multi-layered coatings. Chem. Eng. J. 2019, 372, 1077–1090. [Google Scholar] [CrossRef]
- Rotaru, R.; Peptu, C.; Harabagiu, V. Viscose-Barium Titanate Composites for electromagnetic shielding. Cell. Chem. Technol. 2016, 50, 621–628. [Google Scholar]
- Deng, S.; Xu, X.; Fan, C.; He, Q.; Wang, Y. Constructing pod-like ZnFe2O4@SiO2@C composite fibers network structure to enhance impedance matching for efficient microwave absorption. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138430. [Google Scholar] [CrossRef]
- Lei, Y.; He, Q.; Wang, Y.; Fan, C.; Yin, X.; Wang, C.; Liu, L. Sustainable CoFe-Doped Biomass Carbon Aerogels for Ultra-Broadband Electromagnetic Absorption and Thermal Management. ACS Sustain. Chem. Eng. 2025, 13, 16679–16693. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Singh, B.P.; Mathur, R.; Dhawan, S.K. Polyaniline–MWCNT nanocomposites for microwave absorption and EMI shielding. Mater. Chem. Phys. 2009, 113, 919. [Google Scholar] [CrossRef]
- Ott, H.W. Electromagnetic Compatibility Engineering; John Willey & Soons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Perumalraj, R.; Dasaradan, B.S. Electromagnetic shielding effectiveness of copper core yarn knitted fabrics. Indian J. Fibre Text. Res. 2009, 34, 149–154. [Google Scholar]
- Ortlek, H.G.; Kılıc, G.; Okyay, G.; Bilgin, S. Electromagnetic shielding characteristics of different fabrics knitted from yarns containing stainless steel wire. Ind. Textila 2011, 62, 304–308. [Google Scholar]
- Wang, Y.; Gordon, S.; Yu, W.; Wang, Z. A highly stretchable, easily processed and robust metal wire-containing woven fabric with strain-enhanced electromagnetic shielding effectiveness. Text. Res. J. 2021, 91, 2063–2073. [Google Scholar] [CrossRef]
- Culica, M.; Biliuta, G.; Rotaru, R.; Lisa, G.; Baron, R.; Coseri, S. New Electromagnetic Shielding Materials Based On Viscose-Carbon Nanotube Composites. Polym. Engeneering Sci. 2019, 59, 1499–1506. [Google Scholar] [CrossRef]
- Lan, C.; Zou, L.; Wang, N.; Qiu, Y.; Ma, Y. Multi-reflection-enhanced electromagnetic interference shielding performance of conductive nanocomposite coatings on fabrics. J. Colloid Interface Sci. 2021, 590, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F. Silicones: Beyond Softening in Garment Finishing. In Proceedings of the 1999 AATCC Garment Finishing Symposium, Charlotte, NC, USA, 4–5 May 1999. [Google Scholar]
- Almeida, J.; Fortuna, M.E.; Pricop, L.; Lobiuc, A.; Leite, A.; Silva, A.M.N.; Monteiro, R.P.; Rangele, M.; Harabagiu, V.; Silva, A.M.G. (Aminophenyl)porphyrins as precursors for the synthesis of porphyrin-modified siloxanes. J. Porphyr. Phthalocyanines 2019, 23, 1001–1012. [Google Scholar] [CrossRef]
- Rotaru, R.; Fortuna, M.E.; Ungureanu, E.; Ungureanu, O.; Dascalu, A.; Harabagiu, V. In Situ and Partial In Situ Synthesis of Cellulose Magnetite/Maghemite Composites. Appl. Sci. 2025, 15, 492. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Cojocaru, C.; Pricop, L.; Samoilă, P.; Rotaru, R.; Harabagiu, V. Surface hydrophobization of polyester fibers with poly(methylhydro-dimethyl)siloxane copolymers and their application as nonwoven sorbents for oil spill cleanup. Polym. Test. 2017, 59, 377–389. [Google Scholar] [CrossRef]
- Montazer, M.; Harifi, T. Magnetic nanofinishes for textiles. In Nanofinishing of Textile Materials; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2018; pp. 225–240. [Google Scholar]
- Kommareddi, N.S.; Tata, M.; John, V.T.; McPherson, G.L.; Herman, M.F.; Lee, Y.S.; O’Connor, C.J.; Akkara, J.A.; Kaplan, D.L. Synthesis of Superparamagnetic Polymer−Ferrite Composites Using Surfactant Microstructures. Chem. Mater. 1996, 8, 801–809. [Google Scholar] [CrossRef]
- Fortuna, M.E.; Ignat, M.; Tudorachi, N.; Ungureanu, E.; Rotaru, R.; Harabagiu, V. Hybrid siloxane materials based on a mutually reactive epoxy–amine system: Synthesis, structure, and thermal stability investigations. Inorganics 2024, 12, 118. [Google Scholar] [CrossRef]
- Kleinschek, K.S.; Ribitsch, V.; Kreže, T.; Smole, M.S.; Peršin, Z. Correlation of regenerated cellulose fibres morphology and surface free energy components. Lenzing. Berichte 2003, 82, 83–95. [Google Scholar]
- Munaro, M.; Moreno, L.; Scarpa, P.C.N.; Das-Gupta, D.K. Polarization behaviour in polymers. In Proceedings of the 1999 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (Cat. No.99CH36319), Austin, TX, USA, 17–20 October 1999; Volume 1, pp. 19–22. [Google Scholar]
- Shuvayev, V.P. Ionic migrational polarization of polymers. Polym. Sci. USSR 1985, 27, 2350–2356. [Google Scholar] [CrossRef]
- Ying, S.X.; Young, J.; Lee, J. 3D Orientation imaging of polymer chains with polarization-controlled coherent Raman microscopy. J. Am. Chem. Soc. 2022, 144, 23030–23043. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Randall, C.A.; Manias, E. Polarization mechanism underlying strongly enhanced dielectric permittivity in polymer composites with conductive fillers. J. Phys. Chem. C 2022, 126, 7596–7604. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Zhao, Z.; Zhang, P.; Sun, N.; Cheng, X. The luminescence enhancement of NaLaSiO4:Tb3+ green phosphor by co-substituting Si4+-O2−/La3+-O2− with B3+-F−/Ba2+-F− for white light-emitting diodes. Ceram. Int. 2025, 51, 12831–12841. [Google Scholar] [CrossRef]
- Pintilie, L.; Boni, G.A.; Chirila, C.F.; Stancu, V.; Trupina, L.; Istrate, C.M.; Radu, C.; Pintilie, I. Homogeneous versus inhomogeneous polarization switching in PZT thin films: Impact of the structural quality and correlation to the negative capacitance effect. Nanomaterials 2021, 11, 2124. [Google Scholar] [CrossRef]
- Sarkar, K.; Das, D.; Chattopadhyay, S. Smart and Economic Conductive Textile for Electromagnetic Interference Shielding. Procedia Eng. 2017, 216, 93–100. [Google Scholar] [CrossRef]
- Kardarian, K.; Busani, T.; Osório, I.; Domingos, H.; Igreja, R.; Franco, R.; Cortez, J. Sintering of nanoscale silver coated textiles, a new approach to attain conductive fabrics for electromagnetic shielding. Mater. Chem. Phys. 2014, 147, 815–822. [Google Scholar] [CrossRef]
- Onar, N.; Akşit, A.C.; Ebeoglugil, M.F.; Birlik, I.; Celik, E.; Ozdemir, I. Structural, electrical, and electromagnetic properties of cotton fabrics coated with polyaniline and polypyrrole. J. Appl. Polym. Sci. 2009, 4, 2003–2010. [Google Scholar] [CrossRef]
- Neruda, M.; Vojtech, L. Electromagnetic Shielding Effectiveness of Woven Fabrics with High Electrical Conductivity: Complete Derivation and Verification of Analytical Model. Materials 2018, 11, 1657. [Google Scholar] [CrossRef]
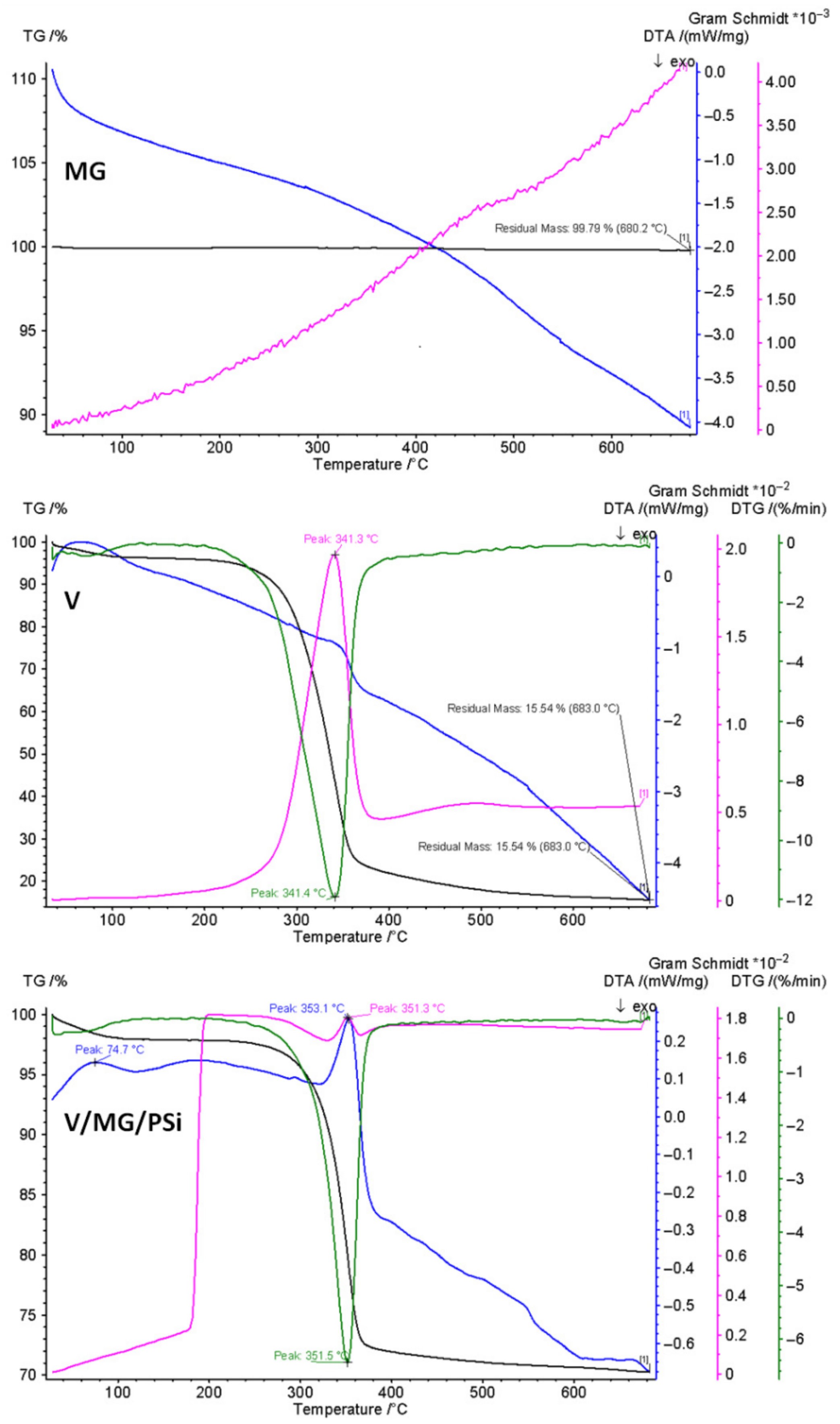
| Sample | Degradation Stage | Tonset °C | Tpeak DTG °C | W wt.% | T10 °C | T20 °C | Tpeak DTA °C |
|---|---|---|---|---|---|---|---|
| V | I Residue | 312 | 341.3 | 95.5 4.5 | 314 | 325 | 341.4 |
| MG | I Residue | - | - | 0.2 99.8 | - | - | - |
| V/MG/PSi | I Residue | 301 | 351.3 | 72.8 27.2 | 320 | 332 | 351.5 |
| Sample | CAw [Deg] | CAeg [Deg] | Standard Deviation [Deg] |
|---|---|---|---|
| V | 83.2 | 75.7 | 0.15–0.18 |
| V/MG/PSi | 122.5 | 117.8 | 0.14–0.19 |
| Composite | Thickness [mm] | Shielding Type | Shielding Value [dB] | Frequency [Hz] | Reference |
|---|---|---|---|---|---|
| Viscose/barium titanate (felt) | 3 | Absorption | 18–22 | 104–105 | [5] |
| Viscose/barium titanate (felt) | 3 | Absorption | 4–9 | 50–55 | [5] |
| Viscose/carbon nanotube (felt) | 1–1.5 | Multiple reflection | 60–110 | 50–55 | [13] |
| Viscose/carbon nanotube (felt) | 1–1.5 | Multiple reflection | 25 | 106 | [13] |
| Oxidized viscose/carbon nanotube (felt) | 1–1.5 | Multiple reflection | 70–80 | 50–55 | [13] |
| Oxidized viscose/carbon nanotube (felt) | 1–1.5 | Multiple reflection | 20 | 106 | [13] |
| Cotton/carbon/rubber, polyvinyl alcohol/glutaraldehyde (woven textile) | 4–6 | Reflection and absorption | 18 | 109 | [30] |
| Cotton/silver nanoparticles (woven textile) | 5 | Reflection | 14–19 | 105–109 | [31] |
| Cotton/polyaniline/polypyrrole (woven textile) | 4 | Reflection | 3.8–6 | 109 | [32] |
| Wool/polyester/gold (woven textile) | 3–6 | Reflection, absorption | 25–50 | 107 | [33] |
| Cotton/graphene oxide/polypyrrole (woven fabrics) | 5 | Multiple reflection, absorption | 39 | 109 | [14] |
| Viscose/maghemite/goethite/poly(methylhydro-dimethyl)siloxane | 3 | Absorption | 1.8–40.8 | 10−2–106 | This work |
| Viscose/maghemite/goethite/poly(methylhydro-dimethyl)siloxane | 3 | Absorption | 16.9 | 50–55 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotaru, R.; Ungureanu, E.; Tofănică, B.M.; Ungureanu, O.C.; Fortună, M.E. New Electromagnetic Shielding Materials Based on Viscose/Maghemite/Goethite/Polysiloxane. Inorganics 2025, 13, 388. https://doi.org/10.3390/inorganics13120388
Rotaru R, Ungureanu E, Tofănică BM, Ungureanu OC, Fortună ME. New Electromagnetic Shielding Materials Based on Viscose/Maghemite/Goethite/Polysiloxane. Inorganics. 2025; 13(12):388. https://doi.org/10.3390/inorganics13120388
Chicago/Turabian StyleRotaru, Razvan, Elena Ungureanu, Bogdan M. Tofănică, Ovidiu C. Ungureanu, and Maria E. Fortună. 2025. "New Electromagnetic Shielding Materials Based on Viscose/Maghemite/Goethite/Polysiloxane" Inorganics 13, no. 12: 388. https://doi.org/10.3390/inorganics13120388
APA StyleRotaru, R., Ungureanu, E., Tofănică, B. M., Ungureanu, O. C., & Fortună, M. E. (2025). New Electromagnetic Shielding Materials Based on Viscose/Maghemite/Goethite/Polysiloxane. Inorganics, 13(12), 388. https://doi.org/10.3390/inorganics13120388








