Recent Advances in Formaldehyde Catalytic Oxidation Catalysts
Abstract
1. Introduction
2. Noble-Metal Catalysts
2.1. Metal Oxide Supports
2.2. Non-Metal Oxide Supports
3. Transition/Rare Earth Metal Catalysts
3.1. Single Metal Oxide Catalysts
3.2. Composite Metal Oxide Catalysts
4. Main Factors Affecting HCHO Oxidation Efficiency
4.1. Effect of Preparation Method
4.2. Effect of Reaction Conditions
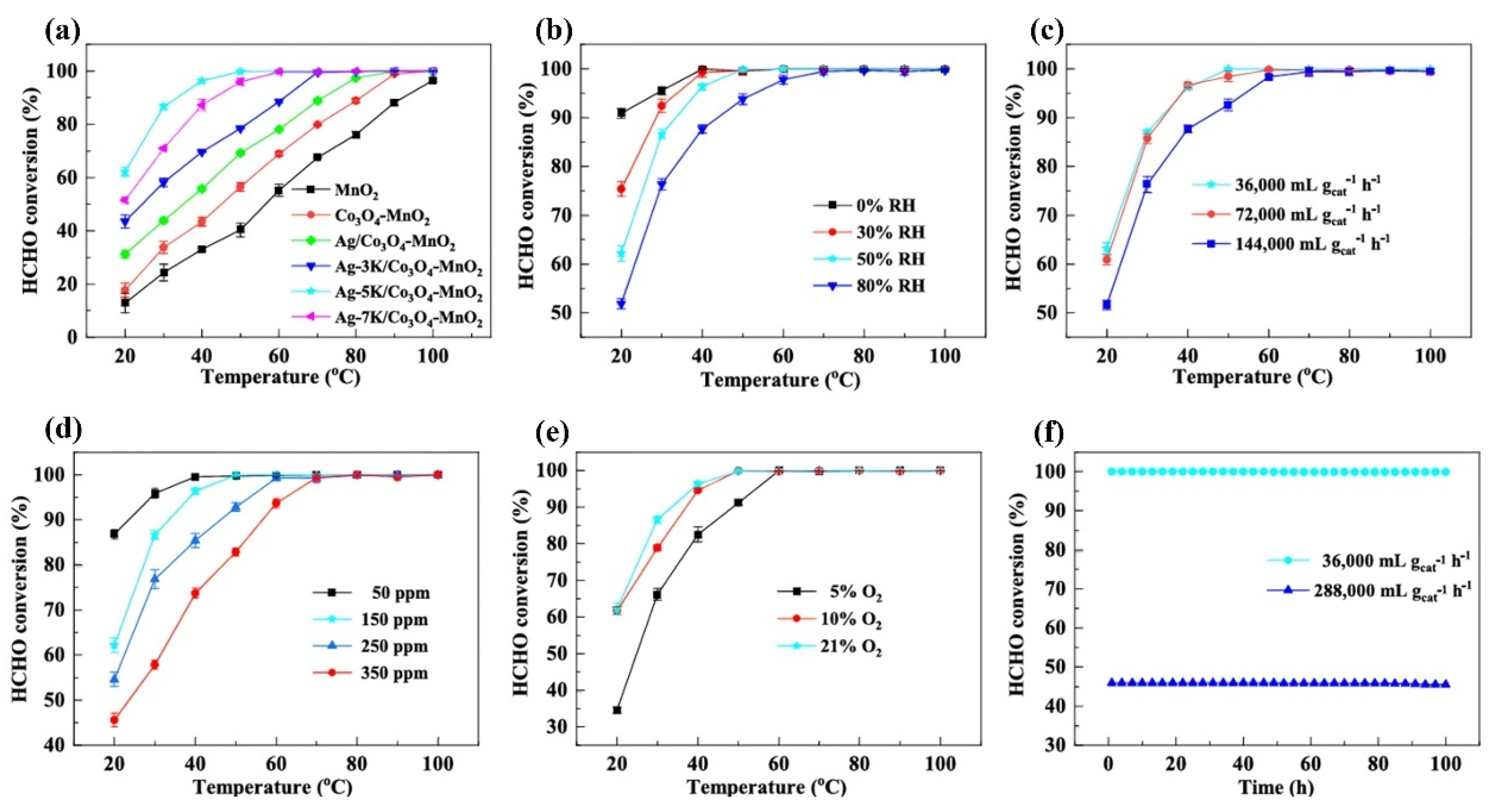
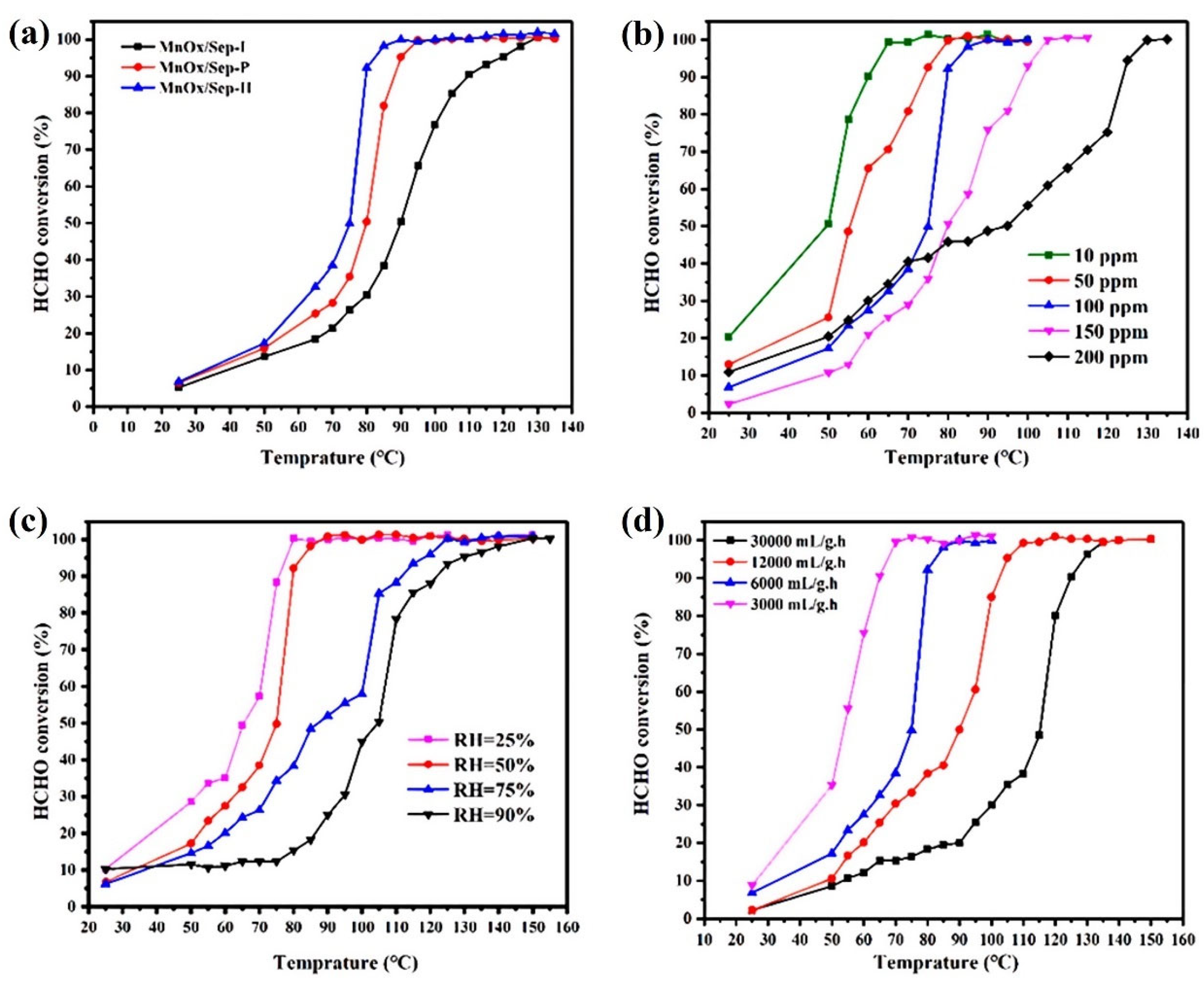
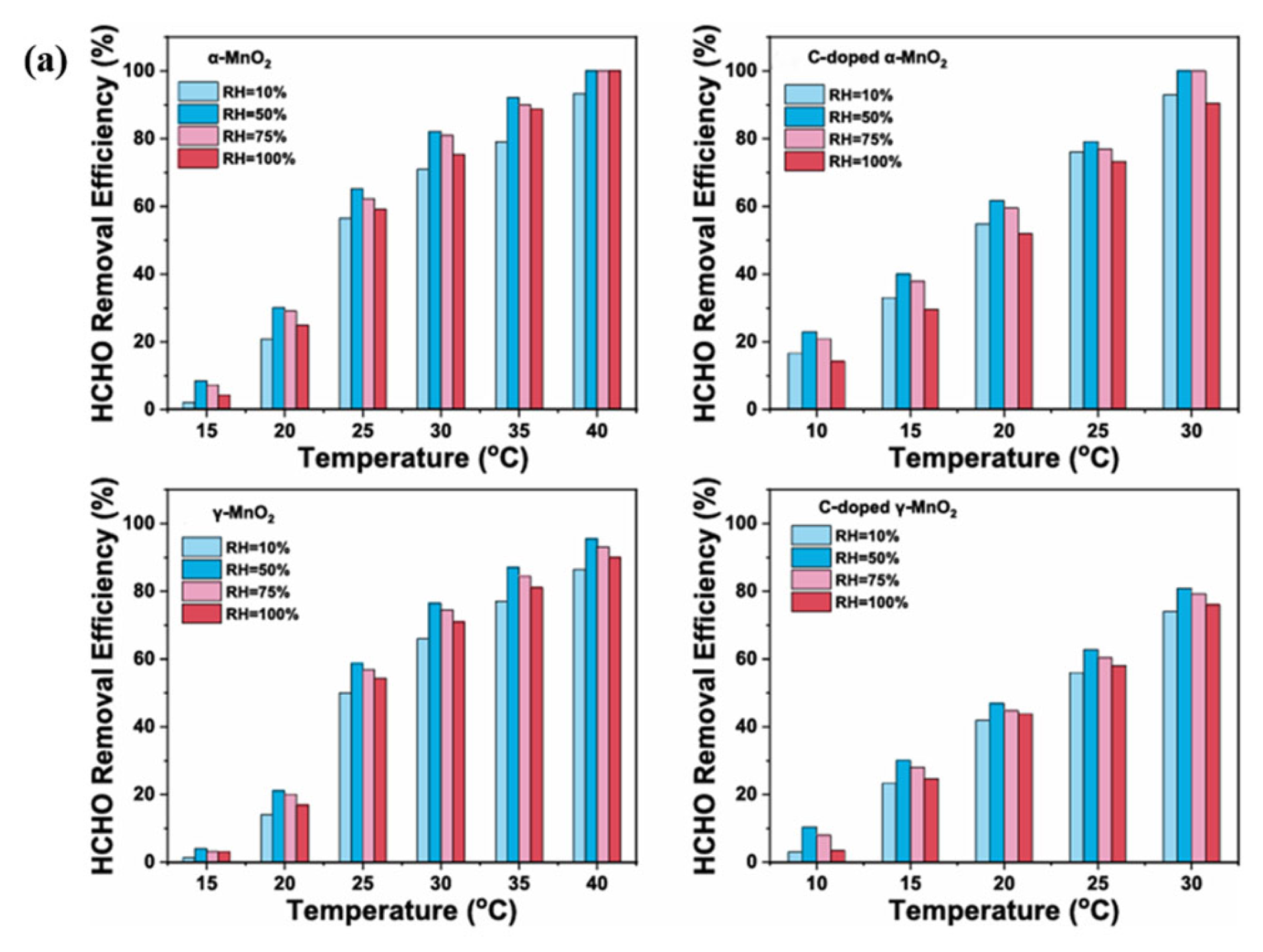
4.2.1. Temperature and Humidity
4.2.2. Space Velocity or Total Flow Rate
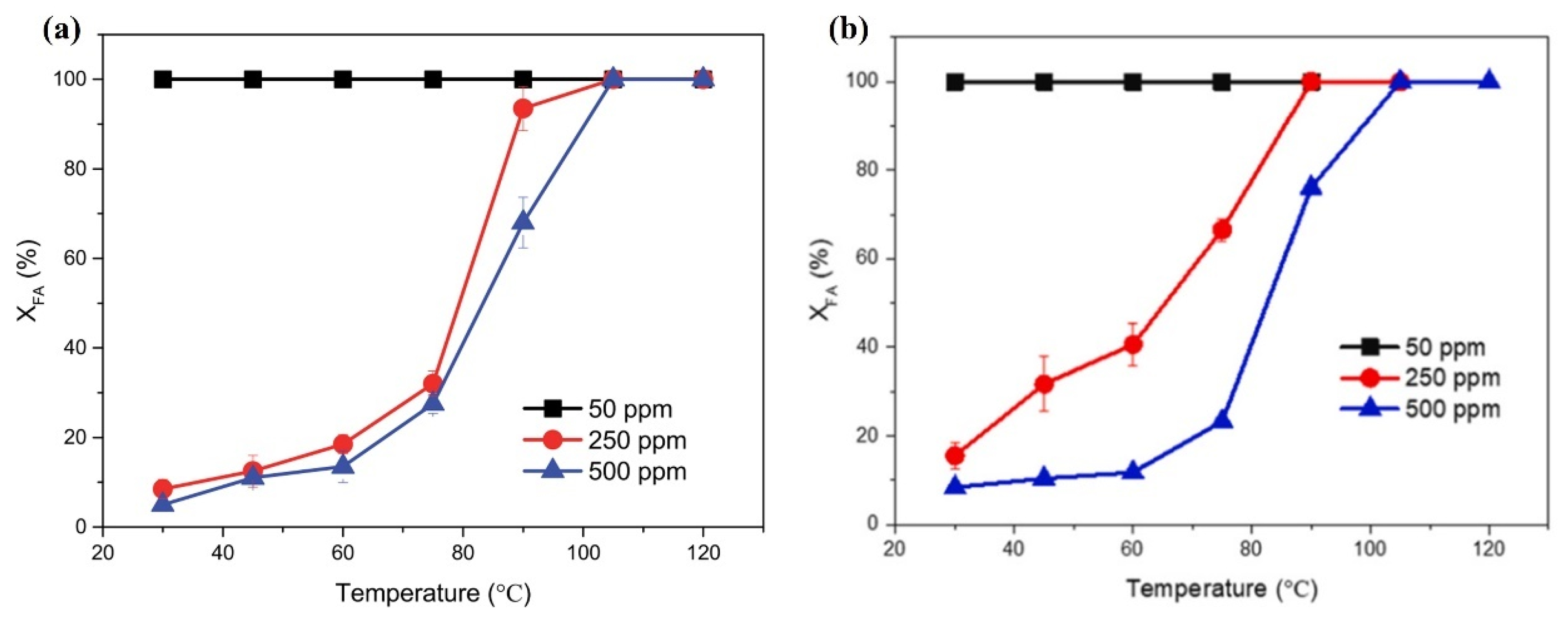
4.2.3. Initial HCHO Concentration
4.2.4. Catalyst Dosage
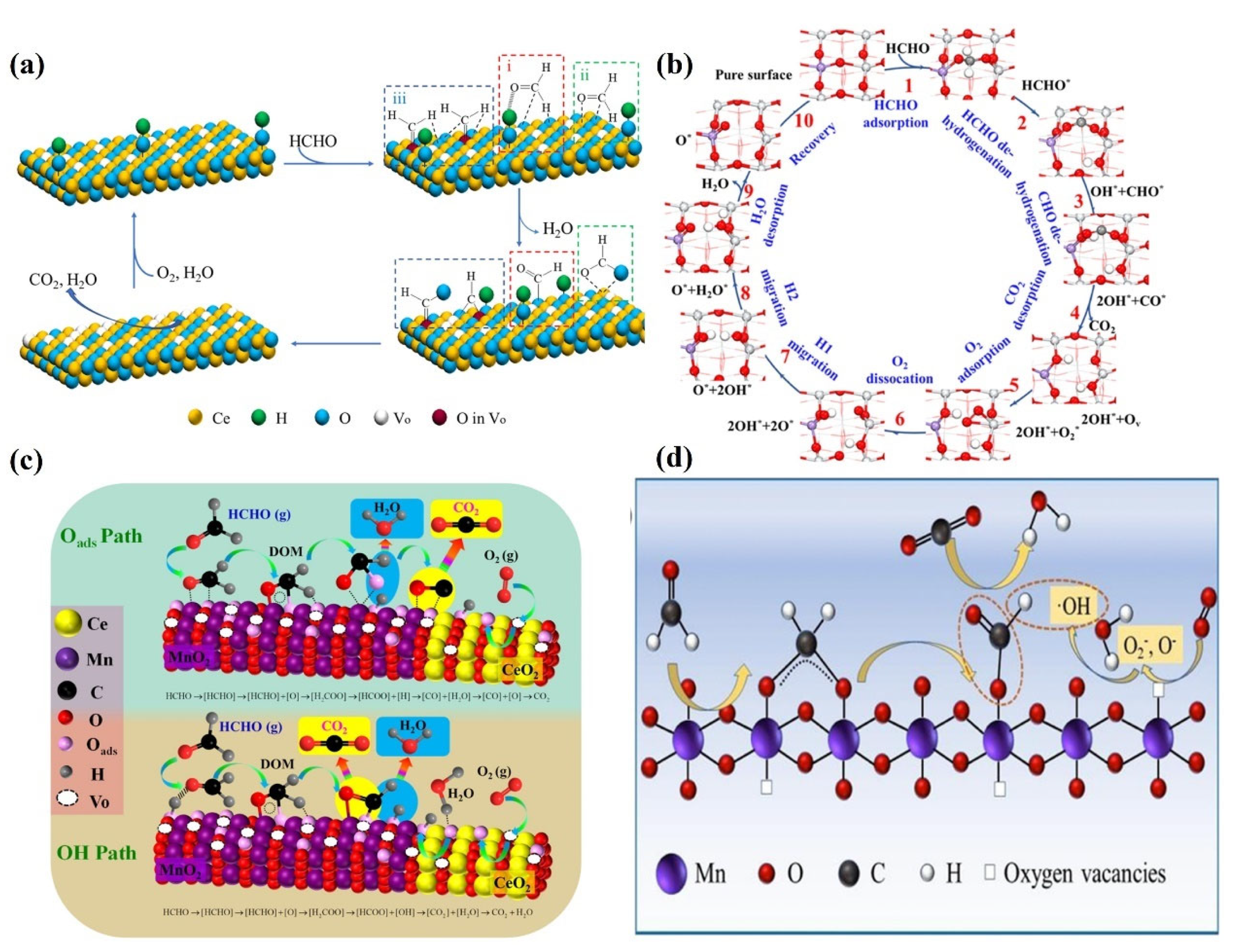
5. Reaction Mechanism
5.1. Reaction Mechanism on Noble Metal Catalysts
5.2. Reaction Mechanism on Transition/Rare Earth Metal Catalysts
6. Conclusions and Outlook
- Elevating the temperature is conducive to the catalytic oxidation of formaldehyde. This is not only beneficial for the single aspect of formaldehyde catalytic oxidation, but it can also be extended to the field of catalysis
- Regarding humidity, in the field of catalytic oxidation of formaldehyde, the optimal humidity range is between 30% and 75%. However, this conclusion cannot yet be drawn for other reactions
- Regarding the initial concentration of target pollutants, it is generally believed that the lower the initial concentration, the easier it is to remove. Space velocity is also a major factor affecting catalytic efficiency. When the space velocity is low, the contact time between reactants and catalysts is longer, which is conducive to a more thorough reaction
- The amount of catalyst used is generally appropriate at 100–200 mg, and an increase in dosage does not have a particularly significant impact on the catalytic oxidation efficiency of formaldehyde
- Surface modification and functionalization, the introduction of specific functional groups or protective layers on the surface of catalysts, can improve their resistance to corrosion and poisoning.
- Optimization of carrier structure, by regulating the pore structure, surface defects, or morphology of the carrier, the interaction between the active component and the carrier can be enhanced, inhibiting its migration and agglomeration.
- Doping modification, introducing heteroatoms (such as transition metals or non-metallic elements) into the catalyst, can optimize the electronic structure of the active sites, inhibit sintering, and agglomeration.
- Core-shell structure design, constructing a structure with an active component as the core and an inert material as the shell (such as a carbon shell or an oxide shell) can effectively isolate the reactants from the active sites, preventing their loss or poisoning.
- Upgrade of catalyst system: noble metals moving towards single atomization: Au, Pt, Pd, etc., will be anchored on defective carriers such as TiO2, CeO2, MnO2, etc., in the form of single atoms or sub-nanometer clusters, capable of completely oxidizing formaldehyde at room temperature or even below 0 °C.
- Defect engineering of transition metal oxides: Utilizing oxygen vacancies, lattice distortions, and interfacial heterojunctions (Co3O4-CeO2, MnOx-Fe2O3) to enhance the concentration of active oxygen species, achieving noble metal-free or low noble metal formulations.
- MOF/COF-derived porous catalysts: By pyrolyzing precursors such as ZIF-8 and MIL-100, N-doped carbon-coated metal nanoparticles are prepared, featuring both high specific surface area and hierarchical pores, addressing the issue of mass transfer limitations.
- Photo-thermal synergistic catalysis: Coupling visible light-responsive Ag/TiO2-x with traditional thermal catalysis, utilizing surface plasmon localized heating to reduce the apparent activation energy, and achieving room temperature natural light-driven formaldehyde oxidation.
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Sun, Z.; Hardacre, C.; Tang, X.; Liu, Z. The current status of research on the catalytic oxidation of formaldehyde. Catal. Rev. 2024, 66, 1028–1083. [Google Scholar] [CrossRef]
- Chai, L.; Zhai, W.; Liu, X.; Xing, G.; Zhang, B.; Zang, J.; Yang, Y.K.; Ma, J.Z. Room Temperature Catalysts for High Effective Degradation of Formaldehyde: Research Progresses and Challenges. ChemistrySelect 2024, 9, e202304418. [Google Scholar] [CrossRef]
- Chen, D.; Zhai, M.; Zhang, J.; Miao, L.; Li, K.; Wang, Z.; Wang, X. Incorporation of NiO on attapulgite supported Au catalyst for HCHO removal at low temperature. Surf. Interfaces 2025, 60, 106083. [Google Scholar] [CrossRef]
- Faria, D.L.; Scatolino, M.V.; de Oliveira, J.E.; Gonçalves, F.G.; Soriano, J.; de Paula Protásio, T.; Lelis, R.C.C.; de Carvalho, L.M.H.; Mendes, L.M.; Junior, J.B.G. Cardanol-based adhesive with reduced formaldehyde emission to produce particleboards with waste from bean crops. Environ. Sci. Pollut. Res. 2023, 30, 48270–48287. [Google Scholar] [CrossRef]
- Justo Alonso, M.; Madsen, H.; Liu, P.; Jørgensen, R.B.; Jørgensen, T.B.; Christiansen, E.J.; Myrvang, O.A.; Bastien, D.; Mathisen, H.M. Evaluation of low-cost formaldehyde sensors calibration. Build. Environ. 2022, 222, 109380. [Google Scholar] [CrossRef]
- Han, Y.; Lee, J.; Haiping, G.; Kim, K.-H.; Peng, W.; Bhardwaj, N.; Oh, J.-M.; Brown, R.J.C. Plant-based remediation of air pollution: A review. J. Environ. Manag. 2022, 301, 113860. [Google Scholar] [CrossRef]
- Su, G.; Xiong, J.; Li, Q.; Luo, S.; Zhang, Y.; Zhong, T.; Harper, D.P.; Tang, Z.; Xie, L.; Chai, X.; et al. Gaseous formaldehyde adsorption by eco-friendly, porous bamboo carbon microfibers obtained by steam explosion, carbonization, and plasma activation. Chem. Eng. J. 2023, 455, 140686. [Google Scholar] [CrossRef]
- Becker, A.; Nizami, I.; Ehrstein, E.; Lara-Ibeas, I.; Jean-Marc, P.; Louis, B.; Stéphane, L.C. Adsorption of gaseous formaldehyde on Y zeolites and on metal-organic frameworks. Microporous Mesoporous Mater. 2022, 343, 112136. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Miao, Y.; Liu, H.; Huang, W.; Chen, Y.; Jia, L.; Zhang, W.; Huang, J. Preparation of CS@BAC composite aerogel with excellent flame-retardant performance, good filtration for PM2.5 and strong adsorption for formaldehyde. Process Saf. Environ. Prot. 2023, 173, 354–365. [Google Scholar] [CrossRef]
- Deng, B.; Chen, Z.; Yang, L.; Guo, J.; Cheng, C.; Li, X.; Zhang, S.; Luo, S. Converting formaldehyde in methanol with MoO2 under irradiation: A pollution-free strategy for cleaning air. J. Hazard. Mater. 2024, 466, 133606. [Google Scholar] [CrossRef]
- Kuk, S.K.; Ji, S.M.; Kang, S.; Dong, S.Y.; Hyuk, J.K.; Min, S.K.; Oh, S.; Hyun, C.L. Singlet-oxygen-driven photocatalytic degradation of gaseous formaldehyde and its mechanistic study. Appl. Catal. B Environ. 2023, 328, 122463. [Google Scholar] [CrossRef]
- Cho, M.-S.; Vikrant, K.; Boukhvalov, D.W.; Kim, K. The Photocatalytic Efficacy of Potassium Hydroxide–Based Modification of Titanium Dioxide in the Oxidative Destruction of Gaseous Formaldehyde. Small 2025, 21, 2501387. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Shen, Z.; Song, M. S-scheme heterojunction Bi2MoO6/WO3 toward efficient photocatalytic oxidation of indoor gaseous formaldehyde under indoor temperature, humidity and sunlight irradiation conditions. Build. Environ. 2025, 282, 113297. [Google Scholar] [CrossRef]
- Wang, F.; Xie, J.; Zhang, S.; Huang, C. Superior Performance of the δ-MnO2 Ultrathin Nanoflower for Photocatalytic HCHO Oxidation. Inorg. Chem. 2025, 64, 6275–6285. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, P.; Han, Q.; Feng, L.; Zhang, L. Enhancing visible light degradation of gaseous formaldehyde with CuOx/OVs-TiO2 photocatalyst loaded wallpaper: Preparation, efficacy and mechanism. Chemosphere 2025, 371, 144051. [Google Scholar] [CrossRef]
- Ma, Y.; Zhan, X.; Han, Y.; Cui, W.; Ye, X.; Li, J. The BiOBr/Bi2WO6/2K-g-C3N4 tri-heterojunction photocatalyst efficiently catalyzes the degradation of gaseous formaldehyde. Mater. Sci. Semicond. Process. 2025, 198, 109771. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, G.; Song, S.; Wen, M.; Li, G.; An, T. Enhanced pizeopotential-mediated catalytic oxidation mechanism of formaldehyde and anti-deactivation performance onto ZnO surface. Appl. Catal. B Environ. Energy 2024, 353, 124057. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Zhao, C.; Zhang, Y.; Mahmud, S.; Zhang, J. Ceramic Fiber Paper-Based Manganese Oxides Catalyst for Room Temperature Formaldehyde Oxidation. Catal. Lett. 2025, 155, 65. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Wang, Y.; Lv, B.; Huang, Z.; Wu, X.; Zhao, H.; Jing, G.; Shen, H. Y single atoms boost MnO2 for efficient ambient formaldehyde catalytic oxidation. Sep. Purif. Technol. 2025, 362, 131962. [Google Scholar] [CrossRef]
- Chen, M.; Huang, X.; Zhong, Z.; Wang, P. MnO2·MnO nanocrystalline as a highly efficient cooperative catalyst for formaldehyde oxidation. Appl. Surf. Sci. 2025, 705, 163530. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Shi, J.; Zhang, K. Facile Synthesis of Highly Active and Ultrastable Palladium Catalysts for Formaldehyde Oxidation. ChemistrySelect 2025, 10, e202405669. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, H.; Ding, Y.; Chang, Y.; Qu, Z. HPMo/SiO2||Ag/Al2O3 bifunctional catalyst for low-temperature formaldehyde oxidation via tandem catalysis. Chem. Eng. J. 2025, 519, 165479. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, M.; Huang, X.; Wang, P. Design and synthesis of Pt/TiO2 catalyst with abundant surface hydroxyl for formaldehyde oxidation. J. Hazard. Mater. 2025, 487, 137302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Guo, J.; Zhang, Y.; Wang, W.; Wu, F.; You, Z.; Hao, Z.; Zhang, Z. Porous graphitized carbon-supported Pt for catalytic oxidation of carbon monoxide and formaldehyde under ambient conditions. Sep. Purif. Technol. 2025, 361, 131512. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Lin, J. Enhanced efficient catalytic oxidation of formaldehyde using lignin-based fibers supported manganese dioxide. J. Mater. Sci. 2025, 60, 5765–5781. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.; Li, Z.; Shen, X.; Zhan, J.; Zhou, H.; Yi, X. Alpha-MnO2 catalyst with high formaldehyde oxidation and moisture resistance by joint cerium modification and phosphoric acid post-treatment. Environ. Res. 2025, 279, 121788. [Google Scholar] [CrossRef]
- Wang, R.; Yu, C.; Gong, J.; Su, Q.; Chen, H.; Yang, C.; Sun, S.; Huang, Z.; Shen, H.; Zhao, H.; et al. Identification of active sites for formaldehyde oxidation on mesoporous Pt/CeO2 catalyst at ambient temperature and low humidity. Fuel 2025, 392, 134905. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Kwon, E.E. Recent advances in the photothermocatalytic oxidation of formaldehyde in air. Adv. Colloid Interface Sci. 2025, 340, 103446. [Google Scholar] [CrossRef]
- Fan, J.; Wang, J.; Chang, B.; Xie, M.; Zhou, B.; Sun, P.; Dong, X. Nanostructured Cobalt Oxide Catalyst with a Three-Dimensional Flower-Like Structure for Photothermal Purification of Formaldehyde. ACS Appl. Nano Mater. 2025, 8, 6042–6051. [Google Scholar] [CrossRef]
- Ji, W.; Ren, M.; Jin, H.; Lou, Y.; Wang, S.; Fan, X.; Salma, T. Ball milling-assisted synthesis of attapulgite-rice husk biochar composites for efficient formaldehyde removal: Experimental and computational insights. Sep. Purif. Technol. 2025, 367, 132918. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, X.; Zhang, H.; Chen, H. Construction of Hierarchical Porous Composite Aerogel for High-Efficient Removal of Low-Level Gaseous Formaldehyde. Small 2025, 21, 2503361. [Google Scholar] [CrossRef]
- Li, F.; Zhu, T.; Yang, J.; Yuan, B.; Zhang, X.; Ju, Q.; Li, C.; Wang, M.; Zhang, X. Biodegradable porous adsorbent for efficient formaldehyde removal from indoor air. Environ. Res. 2025, 275, 121453. [Google Scholar] [CrossRef]
- Xu, J.; Guo, M.; Lin, M.; Ma, C.; Chen, G. Three-dimensional nitrogen-doped modified activated carbon/graphene composite aerogel for highly efficient removal of indoor formaldehyde. J. Environ. Chem. Eng. 2025, 13, 115576. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Liu, Y.; Bai, S.; Ji, J.; Tian, S.; Tang, Z. Formaldehyde and water adsorption in MFI zeolite Channels: A molecular simulation study. J. Mol. Liq. 2025, 431, 127804. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, Y.; Wu, Y.; Zhou, X.; Zhao, K.; Zhang, H.; Fang, L. Revealing the Mechanism of Pore Surface Structure in Adsorption of Trace Formaldehyde by Constructing Monolithic Carbon with Multiple Uniform Micropores and Surface Species. Environ. Sci. Technol. 2025, 59, 12397–12407. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-H.; Maitlo, H.A.; Younis, S.A.; Kim, K.-H. The practical utility of nitrogen doped TiO2 as a photocatalyst for the oxidative removal of gaseous formaldehyde. Mater. Today Nano 2024, 27, 100499. [Google Scholar] [CrossRef]
- Liu, X.; Ling, C.; Chen, X.; Gu, H.; Zhan, G.; Liang, C.; Wei, K.; Wu, X.; Wang, K.; Wang, G. Single Mn atom modulated molecular oxygen activation over TiO2 for photocatalytic formaldehyde oxidation. J. Colloid Interface Sci. 2024, 666, 12–21. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, B.; Li, Q.; Tian, C.; Ma, L.; Li, Z. The Application of Bi-Doped TiO2 for the Photocatalytic Oxidation of Formaldehyde. Cryst. Res. Technol. 2022, 57, 202100231. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, Y.; Hu, J.; Geng, J.; Ma, S.; Liang, H.; Huang, G.; Tian, B.; Feng, J.; Lu, Y. Heterojunction photocatalyst MnO2-CsSnI3 for highly efficient formaldehyde oxidation at room temperature. Chem. Eng. J. 2024, 497, 154697. [Google Scholar] [CrossRef]
- Huang, X.; Wu, K.; Yang, F.; Fu, R.; Ji, Y.; Kong, W.; Su, C.; Xiao, B. Redox photocatalysis CuFe-BTC for nitrate reduction and formaldehyde oxidation. Chem. Eng. J. 2025, 504, 158995. [Google Scholar] [CrossRef]
- Shi, Y.; Qiao, Z.; Liu, Z.; Zuo, J. Cerium Doped Pt/TiO2 for Catalytic Oxidation of Low Concentration Formaldehyde at Room Temperature. Catal. Lett. 2019, 149, 1319–1325. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, C.; Li, J.; Fang, D.; Tan, P.; Fang, Q.; Chen, G. Efficient catalytic oxidation of formaldehyde by defective g–C3N4–anchored single-atom Pt: A DFT study. Chemosphere 2024, 361, 142517. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, K.; Ye, J.; Ming, L.; Xu, J.; Cao, S. Hierarchical Pt/NiCo2O4 nanosheets decorated carbon nanofibers for room-temperature catalytic formaldehyde oxidation. Appl. Surf. Sci. 2023, 623, 157012. [Google Scholar] [CrossRef]
- Duan, C.; Meng, M.; Huang, H.; Wang, H.; Ding, H.; Zhang, Q. Ag-promoted Cr/MnO2 catalyst for catalytic oxidation of low-concentration formaldehyde at room temperature. Phys. Chem. Chem. Phys. 2023, 25, 10155–10165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Su, Y.; Li, H.; Chen, Y.; Li, S.; Bu, Y.; Zhang, Z. Insight into the Ag-CeO2 interface and mechanism of catalytic oxidation of formaldehyde. Appl. Surf. Sci. 2021, 549, 149277. [Google Scholar] [CrossRef]
- Lu, S.; Su, C.; Wang, G.; Wang, H.; Xia, W.; Cui, J.; Zhu, Q. Metal–organic frameworks derived Ag/Co3O4–MnO2 for the catalytic oxidation of formaldehyde. React. Kinet. Mech. Catal. 2022, 135, 687–703. [Google Scholar] [CrossRef]
- Dong, N.; Fu, J.; Ye, Q.; Chen, M.; Fu, Z.; Dai, H. Effect of Preparation Method on Catalytic Performance of Ag/OMS-2 for the Oxidation of Ethyl Acetate and Formaldehyde. Catal. Surv. Asia 2020, 24, 259–268. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhang, C.; He, H. Formaldehyde Oxidation on Pd/TiO2 Catalysts at Room Temperature: The Effects of Surface Oxygen Vacancies. Top. Catal. 2020, 63, 810–816. [Google Scholar] [CrossRef]
- Tomar, S.; Bhadoria, B.S.; Jeong, H.; Choi, J.H.; Lee, S.-C.; Bhattacharjee, S. Single-Atom Pd Catalyst on a CeO2 (111) Surface for Methane Oxidation: Activation Barriers and Reaction Pathways. J. Phys. Chem. C 2024, 128, 8580–8589. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Y.; Jiang, G.; Hou, X.; Li, S.; Zhang, Z. Understanding of Au-CeO2 interface and its role in catalytic oxidation of formaldehyde. Appl. Catal. B Environ. Energy 2020, 260, 118138. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Shen, H. High-dispersed catalysts of core-shell structured Au@SiO2 for formaldehyde catalytic oxidation. Chem. Eng. J. 2020, 385, 123887. [Google Scholar] [CrossRef]
- He, T.; Rong, S.; Ding, D.; Zhou, Y.; Zhang, N.; He, W. Facet-Controlled Synthesis of Mn3O4 Nanorods for Photothermal Synergistic Catalytic Oxidation of Carcinogenic Airborne Formaldehyde. ACS Catal. 2023, 13, 8049–8062. [Google Scholar] [CrossRef]
- Kim, W.-K.; Verma, S.; Ahmadi, Y.; Cho, M.-S.; Kim, K.-H. The effects of metal-oxide content in MnO2-activated carbon composites on reactive adsorption and catalytic oxidation of formaldehyde and toluene in air. Sci. Total Environ. 2024, 926, 172137. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Wu, J.; Hu, Z.; Huang, L.; Zhou, J.; Ishihara, T.; Guo, L. Unveiling the Effects of Alkali Metal Ions Intercalated in Layered MnO2 for Formaldehyde Catalytic Oxidation. ACS Catal. 2020, 10, 10021–10031. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Song, D.; Feng, J.; Li, Z. Highly unsaturated oxygen promotes formaldehyde catalytic oxidation on the defective Co3O4 (111) surface: A DFT study. J. Catal. 2025, 448, 116172. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Z.; Fang, W.; Yao, X.; Zou, G.; Shangguan, W. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants. Chin. J. Catal. 2016, 37, 947–954. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Li, Y.; He, H. Acid pretreatment of support promotes Pd/SiO2 activity for formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2022, 12, 6540–6547. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Chi, Y.; Lin, C.; Chen, X.; Qin, Q. Synthesis of Pd/SiO2 catalyst with outstanding ambient-temperature formaldehyde decomposition via NH3 treatment of silica supports. J. Environ. Sci. 2025, 154, 774–783. [Google Scholar] [CrossRef]
- Dong, H.; Yang, H.; Ning, Y.; Liu, F.; Bradley, R.; Zhao, B.; Wu, W. Large-scale facile green synthesis of porous silver nanocubes on monolithic activated carbon for room-temperature catalytic oxidation of formaldehyde. Appl. Phys. A 2022, 128, 976. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, F.; Liang, J.; Peng, L.; Li, C.; Sun, Q.; Sun, Z. Enhanced photo-assisted thermal catalytic oxidation of formaldehyde via abundant surface adsorbed oxygen in Co3O4 with the assistance of natural zeolite. Microporous Mesoporous Mater. 2024, 382, 113401. [Google Scholar] [CrossRef]
- Yu, L.; Ji, J.; Dong, T.; Liu, B.; Li, K.; Huang, H. Dual function catalysis of Pd/desilicated-ZSM-5 for formaldehyde oxidation at ambient temperature. Appl. Catal. B Environ. Energy 2025, 365, 124981. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Cheng, X.; Lu, B.; Liu, X.; Deng, B.; Peng, S.; Duan, Z. Preparation of MnO2@MPIA catalysts with a controlled oxygen vacancy for the efficient catalytic oxidation of formaldehyde at room temperature. J. Environ. Chem. Eng. 2024, 12, 112813. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Q.; Zhang, B.; Zhang, Y.; Wan, S.; Wang, S.; Lin, J.; Xiong, H.; Mei, D.; Wang, Y. Distinct Role of Surface Hydroxyls in Single-Atom Pt1/CeO2 Catalyst for Room-Temperature Formaldehyde Oxidation: Acid–Base Versus Redox. JACS Au 2022, 2, 1651–1660. [Google Scholar] [CrossRef]
- Bai, B.; Li, J. Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal. 2014, 4, 2753–2762. [Google Scholar] [CrossRef]
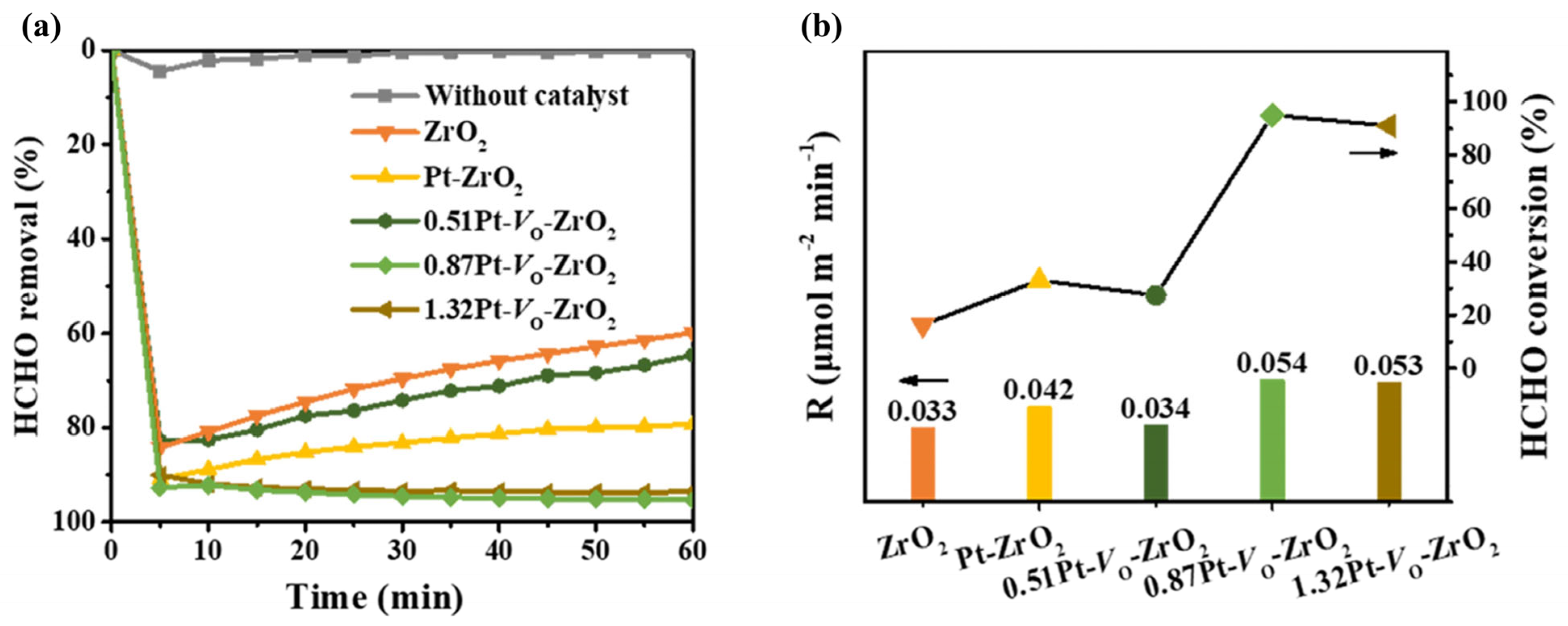
- Chen, Y.; Zhang, S.; Yang, X. Interfacial adsorption and catalysis of single-atom Pt-loaded ZrO2 surfaces for enhancing formaldehyde oxidation. Surf. Interfaces 2024, 52, 104871. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, G.; Wang, M.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt/MnO2 Nanoflowers Anchored to Boron Nitride Aerogels for Highly Efficient Enrichment and Catalytic Oxidation of Formaldehyde at Room Temperature. Angew. Chem. Int. Ed. 2020, 60, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, L.; Yang, J.; Tan, N. Effect of preparation method on the catalytic performance of formaldehyde oxidation over octahedral Fe3O4 microcrystals supported Pt catalysts. J. Dispers. Sci. Technol. 2019, 41, 1831–1838. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; He, H. Significant enhancement in activity of Pd/TiO2 catalyst for formaldehyde oxidation by Na addition. Catal. Today 2017, 281, 412–417. [Google Scholar] [CrossRef]
- Lu, S.; Sun, N.; He, N.; Zheng, F.; Jiang, Y.; Fu, H.; Liu, J.; Fang, Y. Mesoporous Ag-K-Al2O3 catalysts prepared via a one-pot evaporation induced self-assembly approach and their application in catalytic oxidation of formaldehyde. Appl. Surf. Sci. 2024, 679, 161203. [Google Scholar] [CrossRef]
- Guo, Y.; Di, Z.; Guo, X.; Wei, Y.; Zhang, R.; Jia, J. N/Ce doped graphene supported Pt nanoparticles for the catalytic oxidation of formaldehyde at room temperature. J. Environ. Sci. 2022, 125, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Tan, L.; Le Nguyen Quang, T.; Quang, L.N. Catalytic oxidation of formaldehyde over silver supported on ZSM-5: The role of Ag and mesopores. IOP Conf. Ser. Earth Environ. Sci. 2022, 964, 012026. [Google Scholar] [CrossRef]
- Li, N.; Dong, X.; Luo, J.; Wang, Y.; Wang, H.; Miao, D.; Pan, Y.; Jiao, F.; Xiao, J.; Qu, Z. Bifunctional zeolites-silver catalyst enabled tandem oxidation of formaldehyde at low temperatures. Nat. Commun. 2022, 13, 2209. [Google Scholar] [CrossRef]
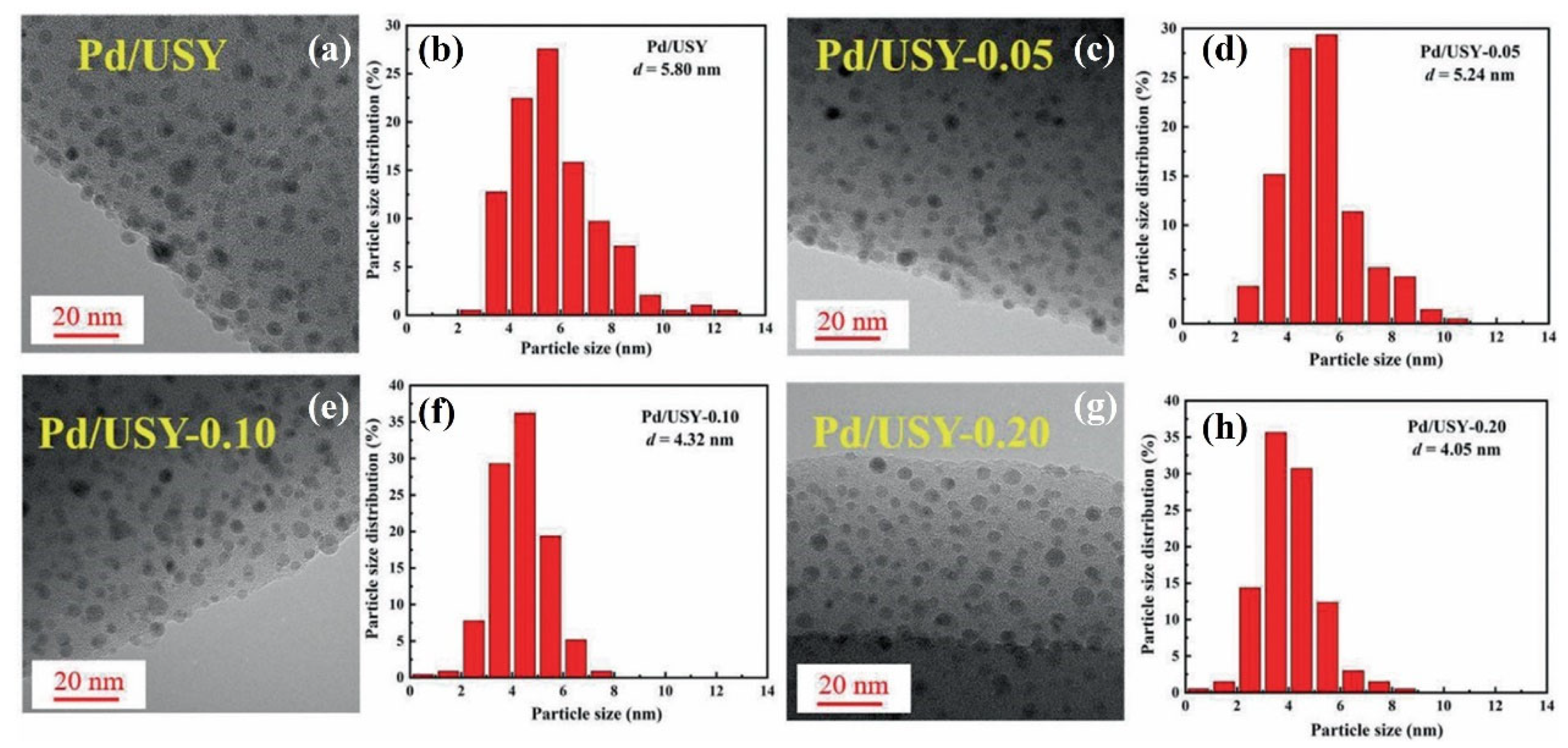
- Liu, X.; Wang, C.; Chen, Y.; Qin, Q.; Li, Y.; He, H. Formaldehyde oxidation on Pd/USY catalysts at room temperature: The effect of acid pretreatment on supports. J. Environ. Sci. 2022, 125, 811–822. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, F.; He, L.; Cai, T.; Wang, X.; Zhang, T.; Wang, N.; Sun, Q. Integration of Ultrasmall Pt Clusters With Silanol Groups in Pure Silica Zeolites for Robust Formaldehyde Oxidation. Chem.-A Eur. J. 2025, 31, e202500405. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Y.; Shi, X.; Li, R.; Wang, Z.; Peng, W.; Cao, J.; Lee, S. Enhancing molecular oxygen activation by nitrogen-doped carbon encapsulating FeNi alloys with ultra-low Pt loading. PNAS Nexus 2025, 4, 594. [Google Scholar] [CrossRef]
- Chen, X.; Qin, Q.; Wang, J.; Wen, W.; Liu, X.; Wang, C.; Zhou, L.; Deng, H.; Li, Y. Strong interaction between promoter and metal in Pd-Ba/TiO2 catalysts for formaldehyde oxidation. J. Colloid Interface Sci. 2025, 678, 520–531. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Purwanto, W.W.; Sudibandriyo, M.; Nasikin, M. Mesopore Catalytic Activated-Carbon to Reduce Harmful Gases Indoors: Adsorption, Catalytic Oxidation, and Prediction Mechanism. Environ. Qual. Manag. 2024, 34, 22294. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, C.; Xie, S.; Wang, J.; Li, Y.; Liu, F.; He, H. Diverse impacts of water on Pd/SiO2 catalysts for formaldehyde oxidation: Unraveling the crucial role of support defects. Appl. Catal. B Environ. Energy 2025, 364, 124843. [Google Scholar] [CrossRef]
- Ousji, R.; Bouabdellah, M.A.; Ksibi, Z.; Ghorbel, A.; Fontaine, C. Enhancing Low-Temperature HCHO Oxidation: Investigation selectivity and Catalytic Activity of Ag, Co, Mo, and Cr Catalysts Supported on γ-Al2O3. Chem. Afr. 2024, 7, 4005–4015. [Google Scholar] [CrossRef]
- Peng, S.; Li, R.; Huang, Y.; Zhang, Y.; Cao, J.; Lee, S. Interfacial dependent reactive oxygen species generation over Pt-ZrO2 nanoparticles for catalytic oxidation of formaldehyde at room temperature. Appl. Surf. Sci. 2022, 600, 154056. [Google Scholar] [CrossRef]
- Zhang, S.; Zhan, J.; Zhou, H.; Niu, M.; Yang, H.; Zhou, X.; Yi, X.; Liu, Y. Lithium promotes Ag-CoOx composite for formaldehyde oxidation at ambient temperature: Chemically adsorbed oxidative oxygen formed by the interaction between AgCoO2 and catalyst parent. J. Environ. Chem. Eng. 2022, 10, 108844. [Google Scholar] [CrossRef]
- Tang, J.; He, H.; Yang, X.; Chen, J.; Wang, R. One-step synthesis of efficient silver-manganese catalyst with tunable dual active site content for formaldehyde removal at room temperature. Mol. Catal. 2025, 582, 115207. [Google Scholar] [CrossRef]
- Badshah, M.; Mehdi, S.; Alam, K.; Khan, K.I.; Abbas, I.; Iezzi, L.; Segneri, V.; Stoller, M. Room temperature oxidation of gaseous formaldehyde over silver-doped manganese oxide catalyst. Chem. Pap. 2024, 78, 4383–4393. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; W, H.; Dong, X.; Li, J.; Yang, F.; Yuan, A.; Ji, H. MnCo-Layered double hydroxides nanosheets supported Pd nanoparticles for complete catalytic oxidation of formaldehyde at room temperature. Appl. Surf. Sci. 2022, 606, 154702. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Wen, M.; Guo, Y.; Wei, Y.; Li, G.; An, T. Catalytic oxidation of formaldehyde over a Au@Co3O4 nanocomposite catalyst enhanced by visible light: Moisture indispensability and reaction mechanism. Environ. Sci. Nano 2022, 9, 4162–4176. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, M.; Li, J.; Wang, X.; Zhou, Y.; Yang, C.; Hua, Y.; Wang, C.; Yuan, A. Lattice-confined Ag over MnCoLDH accelerated catalytic oxidation of formaldehyde at ambient temperature: The novel pathway with different oxygen species. Appl. Surf. Sci. 2024, 669, 160513. [Google Scholar] [CrossRef]
- Lu, S.; Sun, N.; Gong, L.; Xu, C.; Guo, R.; Li, Y.; Wang, S.; Liu, J.; Fang, Y.; Liu, G. Constructing hollow urchin-like Ag-K/Co3O4-MnO2 spheres with low Ag loading as a highly efficient catalyst for formaldehyde oxidation. Fuel 2025, 398, 135522. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Mamba, B.B.; Nkambule, T.T.I. Fabrication of palladium and platinum nanocatalysts stabilized by polyvinylpyrrolidone and their use in the hydrogenolysis of methyl orange. React. Kinet. Mech. Catal. 2020, 129, 991–1005. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Xue, T.; Yang, Y.; Gong, Z.; Dong, F. Efficient and Durable Oxidation Removal of Formaldehyde over Layered Double Hydroxide Catalysts at Room Temperature. Environ. Sci. Technol. 2024, 58, 10378–10387. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, J.; Zhang, H.; Liu, F.; Ran, W.; Xu, W.; Yu, T.; Chen, S.; Rong, S. Sodium alginate-mediated engineering of 3D-MnO2 architectures for efficient room-temperature formaldehyde catalytic decomposition. Sep. Purif. Technol. 2025, 370, 133200. [Google Scholar] [CrossRef]
- Gao, E.; Jin, Q.; Zhang, T.; Han, L.; Li, N.; Xu, J.; Yao, S.; Wu, Z.; Li, J.; Zhu, J.; et al. Unraveling the promotional effects of K-doping on the mobility of surface oxygen species of CoCr2O4 for improved formaldehyde catalytic oxidation: The weakened metal-oxygen bond strength. Chem. Eng. J. 2023, 474, 145618. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, A.; Min, J.; Yu, C. Effects of Doping First Main Group Elements on the Structure and Catalytic Performance of δ-MnO2. Catal. Lett. 2023, 154, 1173–1183. [Google Scholar] [CrossRef]
- Han, Z.; Zou, X.; Wang, C.; Liu, H.; Chen, T.; Zhang, P.; Chen, D.; Wang, Q.; Chen, J.; Huang, A.; et al. Frenkel defects facilitate oriented 1O2 formation in birnessite for enhanced catalytic oxidation of formaldehyde. Appl. Catal. B Environ. Energy 2025, 362, 124744. [Google Scholar] [CrossRef]
- He, H.; Chen, Q.; Deng, X.; Tang, J.; Zhang, J.; Wang, G.; Wang, R.; Chen, J. Formaldehyde Adsorption and Degradation at Room Temperature Boosted by Increasing the Specific Surface Area and Surface-Active Oxygen Content of Manganese Oxides. Ind. Eng. Chem. Res. 2024, 63, 10501–10511. [Google Scholar] [CrossRef]
- Hua, Y.; Vikrant, K.; Kim, K.-H.; Heynderickx, P.M.; Boukhvalov, D.W. The catalytic efficacy of modified manganese-cobalt oxides for room-temperature oxidation of formaldehyde in air. J. Hazard. Mater. 2024, 476, 135016. [Google Scholar] [CrossRef]
- Hua, Y.; Vikrant, K.; Kim, K.-H.; Heynderickx, P.M.; Boukhvalov, D.W. Alkali-modified copper manganite spinel for room temperature catalytic oxidation of formaldehyde in air. Chin. J. Catal. 2024, 60, 337–350. [Google Scholar] [CrossRef]
- Jiao, Y.; Jing, C.; Wang, Y.; Yao, F.; Ye, G.; Wang, X.; Zhao, G.; Peng, W.; Huang, H.; Ye, D. Electrospinning synthesis of Co3O4 porous nanofiber monolithic catalysts for the room-temperature indoor catalytic oxidation of formaldehyde at low concentrations. Appl. Surf. Sci. 2023, 639, 158215. [Google Scholar] [CrossRef]
- Kim, W.-K.; Vikrant, K.; Younis, S.A.; Kim, K.-H.; Heynderickx, P.M. Metal oxide/activated carbon composites for the reactive adsorption and catalytic oxidation of formaldehyde and toluene in air. J. Clean. Prod. 2023, 387, 135925. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, C.; Liu, X.; Du, X.; Zhu, Y.; Zhang, Y.; Zhao, J.; Huang, L.; Zhang, Z.; Hu, M. Mixed-phase MnOx on modified hydroxyapatite for synergistic removal of toluene and formaldehyde: From aquaculture waste to catalysts. J. Environ. Chem. Eng. 2025, 13, 117066. [Google Scholar] [CrossRef]
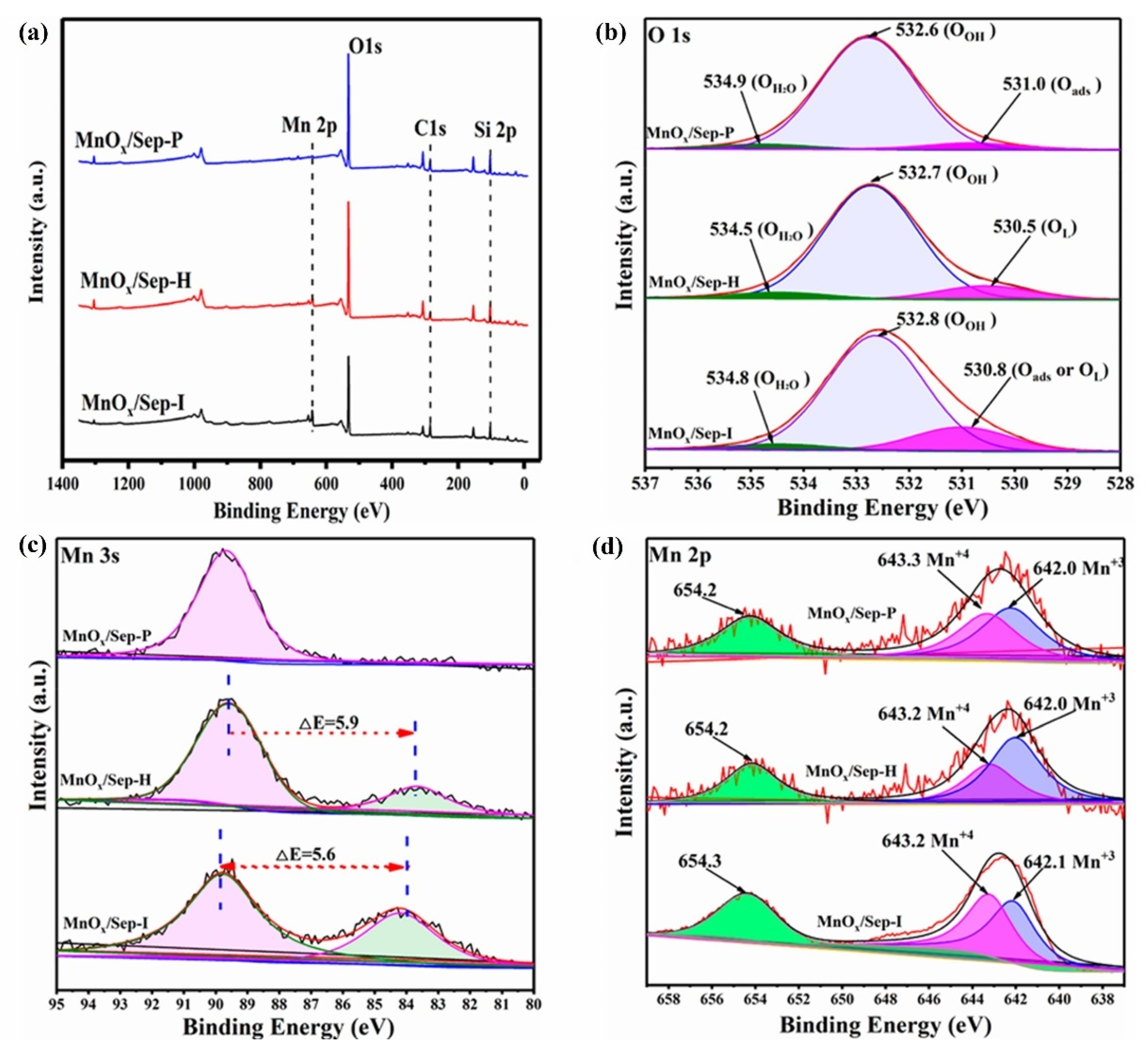
- Li, D.; Liu, H.; He, X.; Yao, Y.; Liu, H.; Chen, J.; Deng, B.; Lan, X. Sepiolite-Supported Manganese Oxide as an Efficient Catalyst for Formaldehyde Oxidation: Performance and Mechanism. Molecules 2024, 29, 2826. [Google Scholar] [CrossRef] [PubMed]
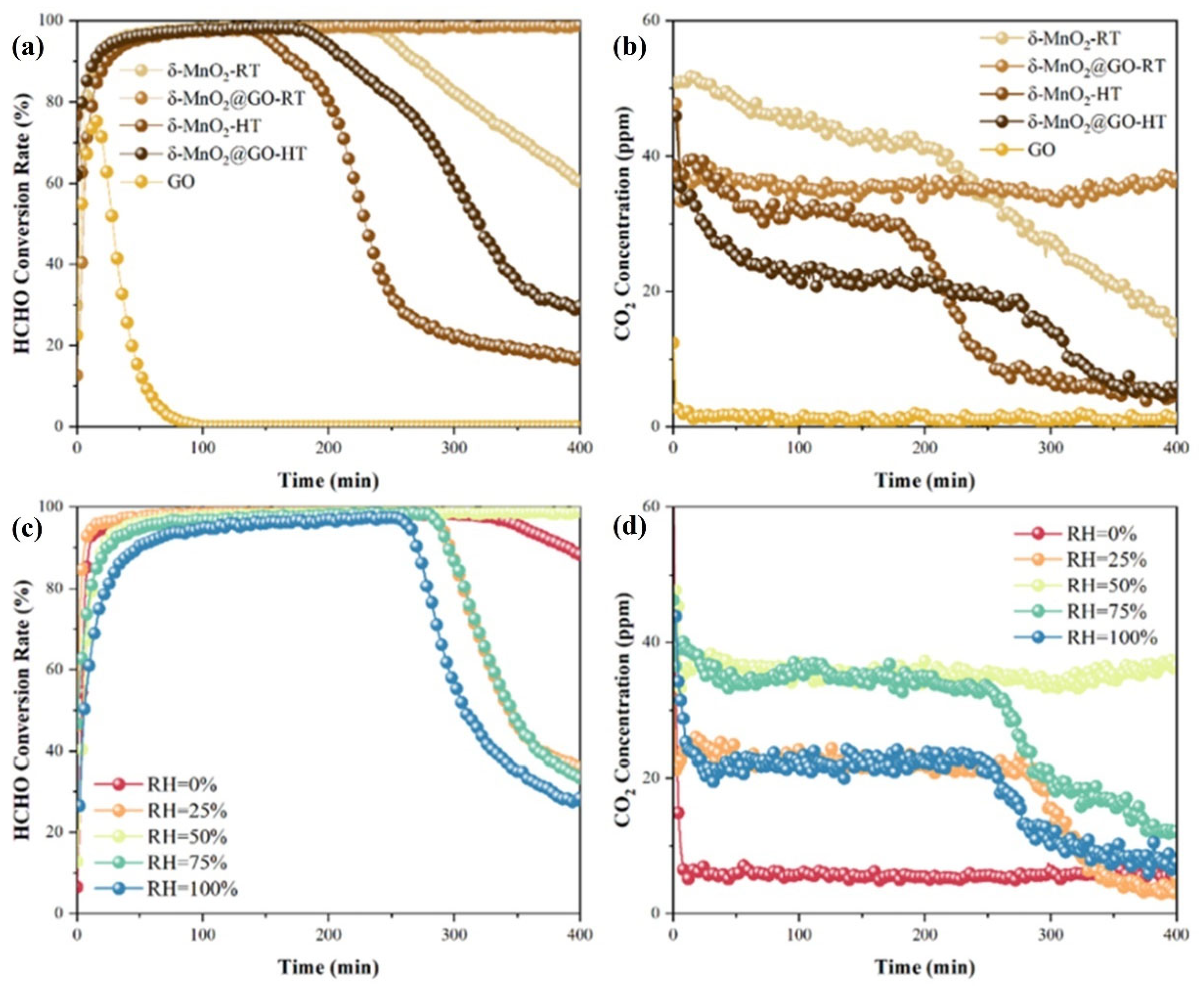
- Li, Y.; Dong, T.; Huang, P.; Ji, J.; Huang, H. Efficient HCHO oxidation at room temperature via maximizing catalytic sites in 2D coralloid δ-MnO2@GO. Appl. Catal. B Environ. Energy 2024, 341, 123322. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Zhou, H.; Yi, X.; Liu, Y. Effective formaldehyde elimination over pyrolusite-manganite hybrid catalysts promoted by Keggin acid decoration: Tungsten doping and chemically adsorbed active oxygen. J. Environ. Chem. Eng. 2024, 12, 112541. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, F.; Wang, H.; Wei, J.; Wang, X.; Liu, Y.; Liu, J.; Fang, Y. Engineering MnO2 Nanotubes@Co3O4 Polyhedron Composite with Cross-linked Network Structure for Efficient Catalytic Oxidation of Formaldehyde. Catal. Lett. 2023, 154, 2949–2962. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Sun, K.; Ahmed, J.; Tian, W.; Xu, J. Insights into the roles of superficial lattice oxygen in formaldehyde oxidation on birnessite. Nanoscale 2024, 16, 12541–12549. [Google Scholar] [CrossRef]
- Nie, L.; Xin, S.; Fang, C.; Chen, H.; Yang, Y. Fabrication of cobalt (II, III) oxide @nitrogen doped carbon/honeycomb ceramics with porous structure for room-temperature formaldehyde oxidation. Chem. Eng. J. 2024, 479, 147830. [Google Scholar] [CrossRef]
- Hua, Y.; Vikrant, K.; Kim, K.-H.; Weon, S.; Boukhvalov, D.W. Thermocatalytic oxidation potential of cobalt (II, III) oxide against gaseous formaldehyde in relation to hydrothermal synthesis temperature. Chem. Eng. J. 2024, 494, 152778. [Google Scholar] [CrossRef]
- Chang, T.; Wang, Z.; An, H.; Li, F.; Xue, W.; Wang, Y. Morphology effects of CeO2 for catalytic oxidation of formaldehyde. J. Environ. Chem. Eng. 2022, 10, 108053. [Google Scholar] [CrossRef]
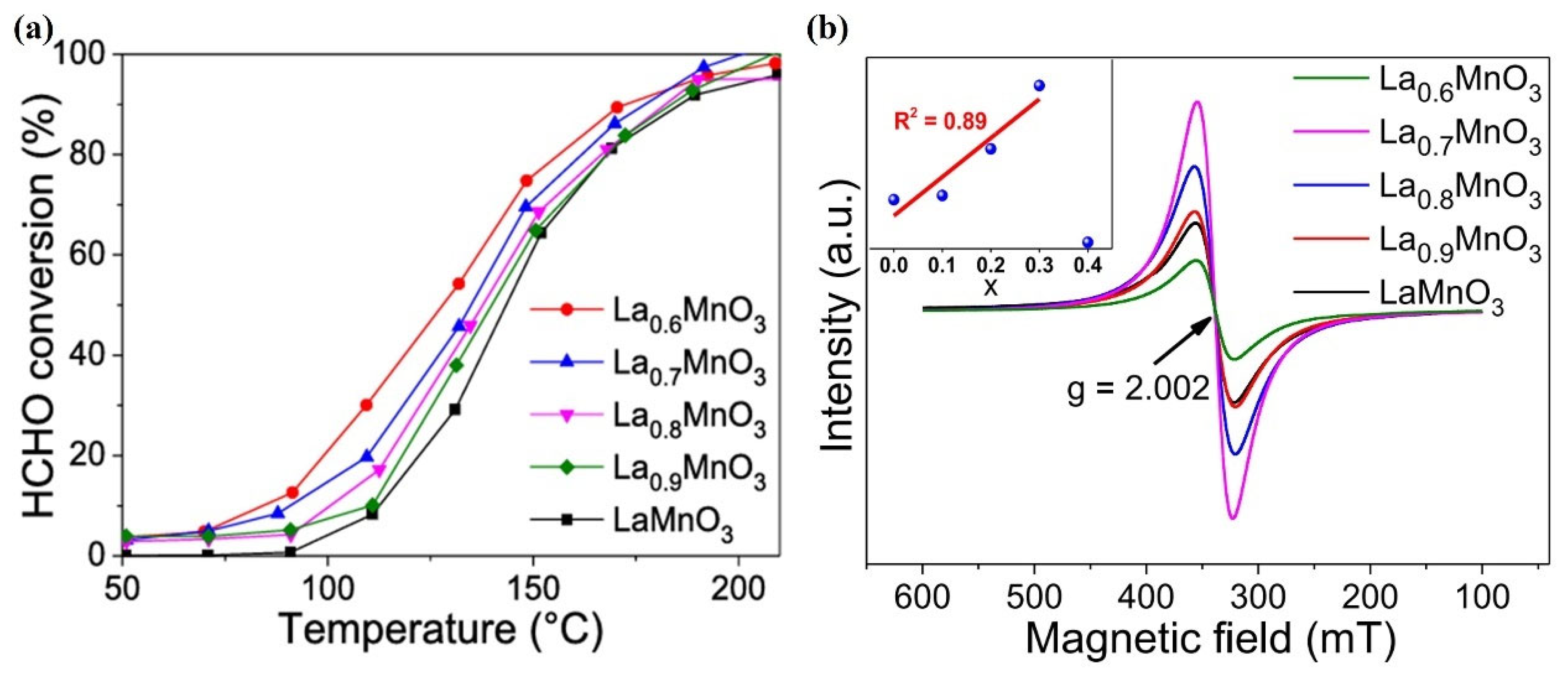
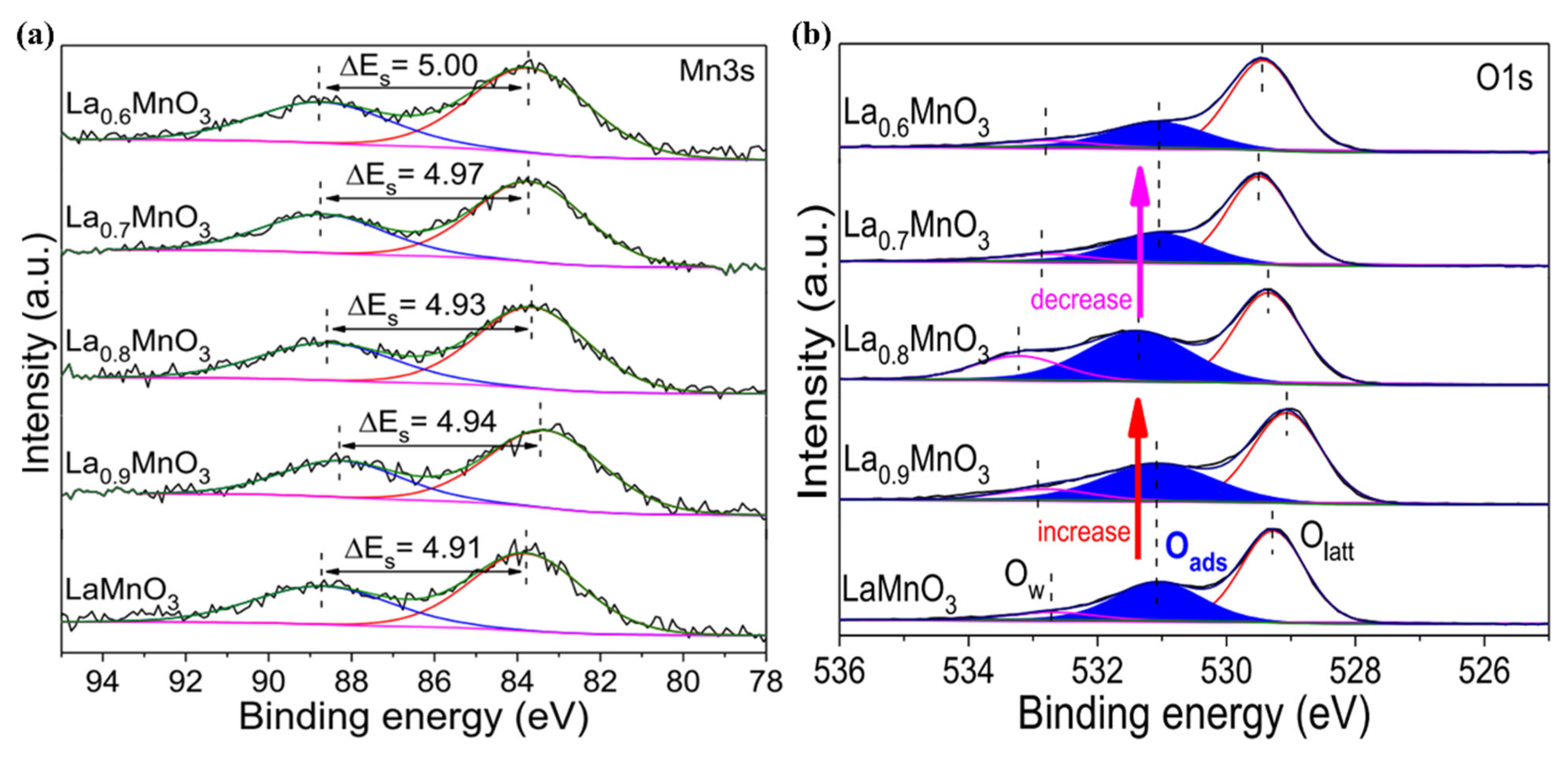
- Xu, Y.; Dhainaut, J.; Dacquin, J.-P.; Lamonier, J.-F.; Zhang, H.; Royer, S. On the role of cationic defects over the surface reactivity of manganite-based perovskites for low temperature catalytic oxidation of formaldehyde. Appl. Catal. B Environ. Energy 2023, 342, 123400. [Google Scholar] [CrossRef]
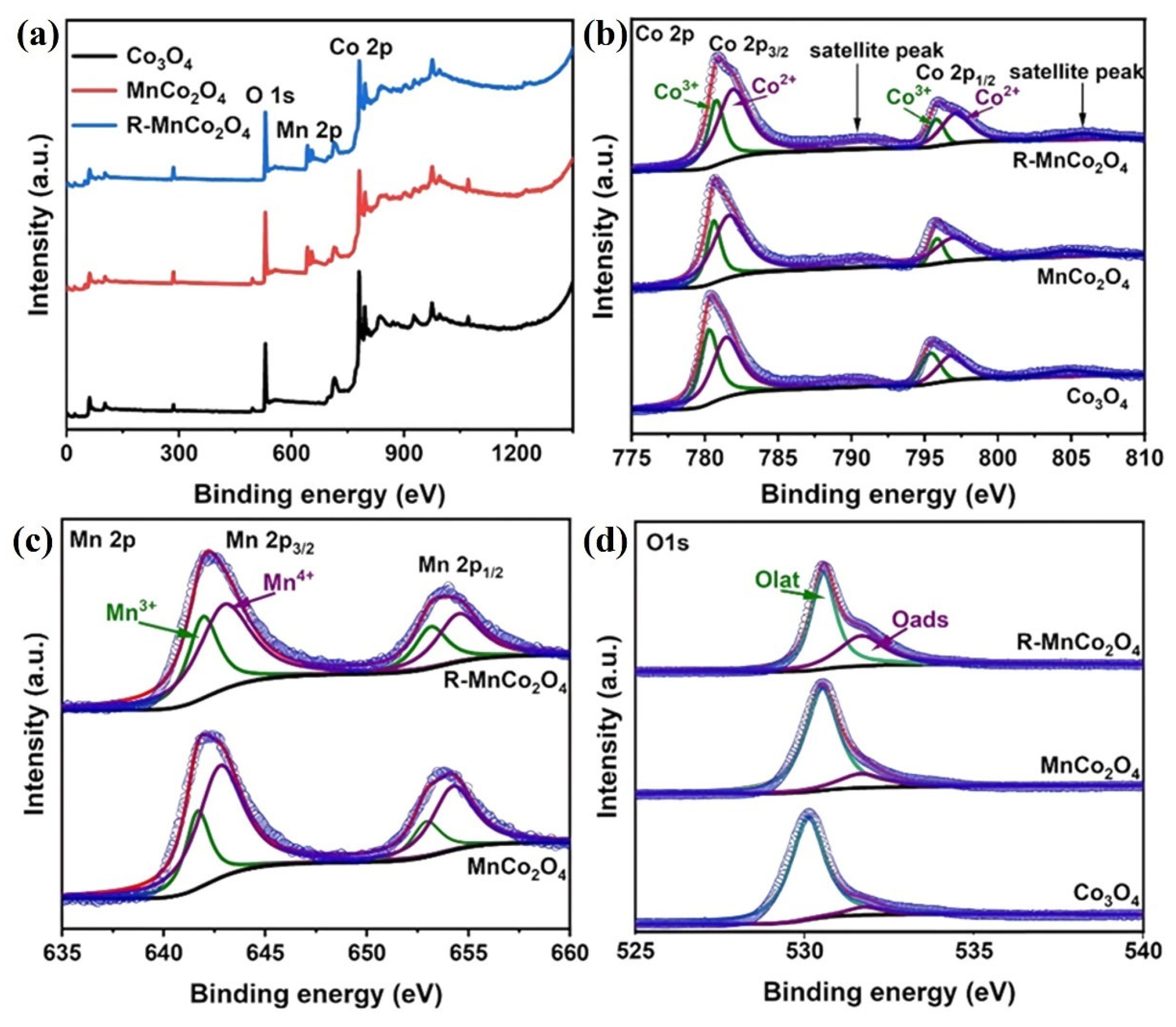
- Yan, G.; Du, X.; Guo, X.; Cao, X.; Shi, H. NaBH4 reduced Mn-doped cobalt tetroxide R-MnxCo3−xO4 catalysts with plentiful oxygen vacancies for HCHO oxidation at low temperature. New J. Chem. 2024, 48, 8314–8323. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Q.; Tian, J.; Wan, J.; Liu, Y.; Wang, X.; Kan, J.; Yang, X.; Wu, G. Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal. Catalysts 2023, 13, 1283. [Google Scholar] [CrossRef]
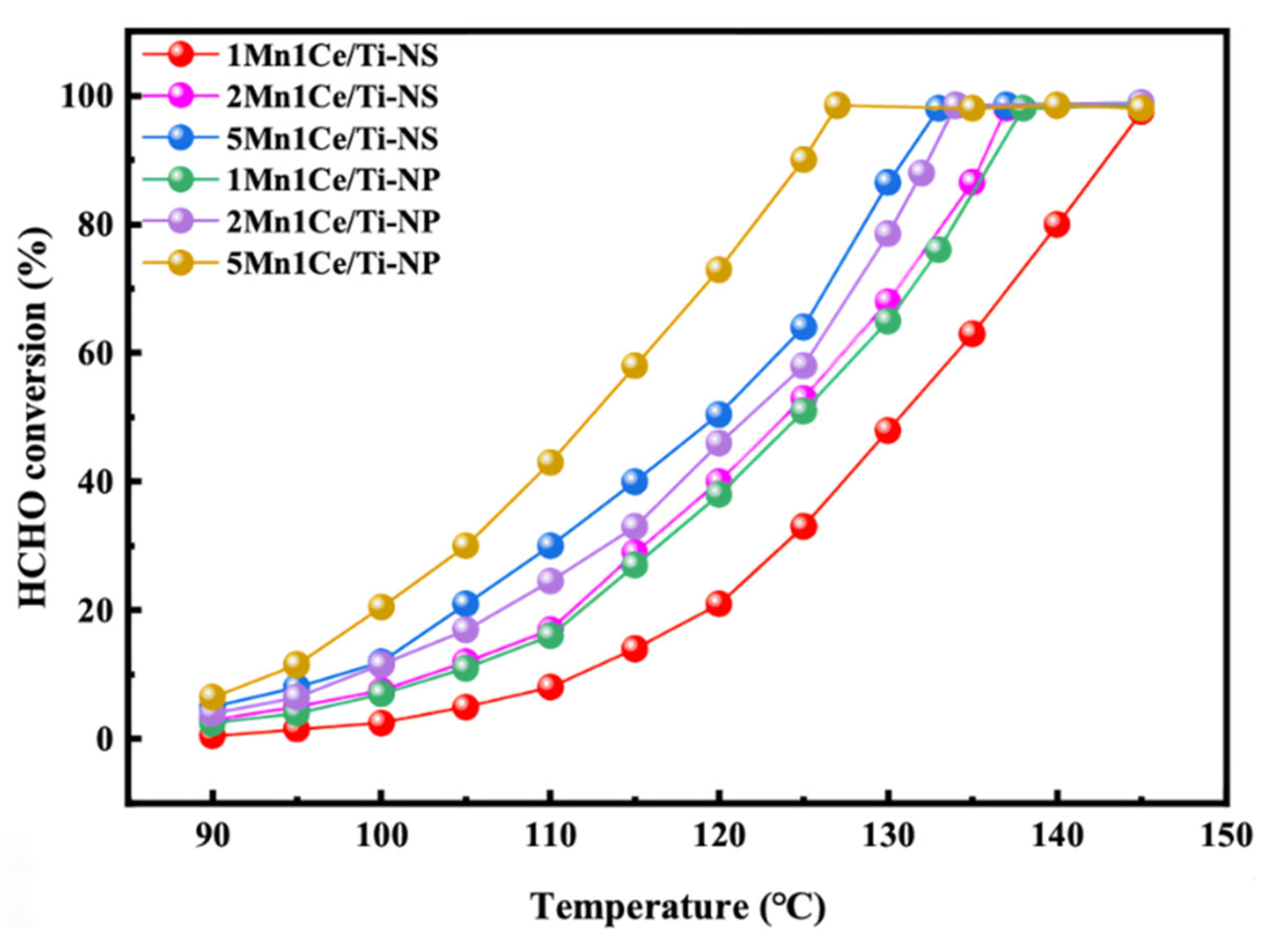
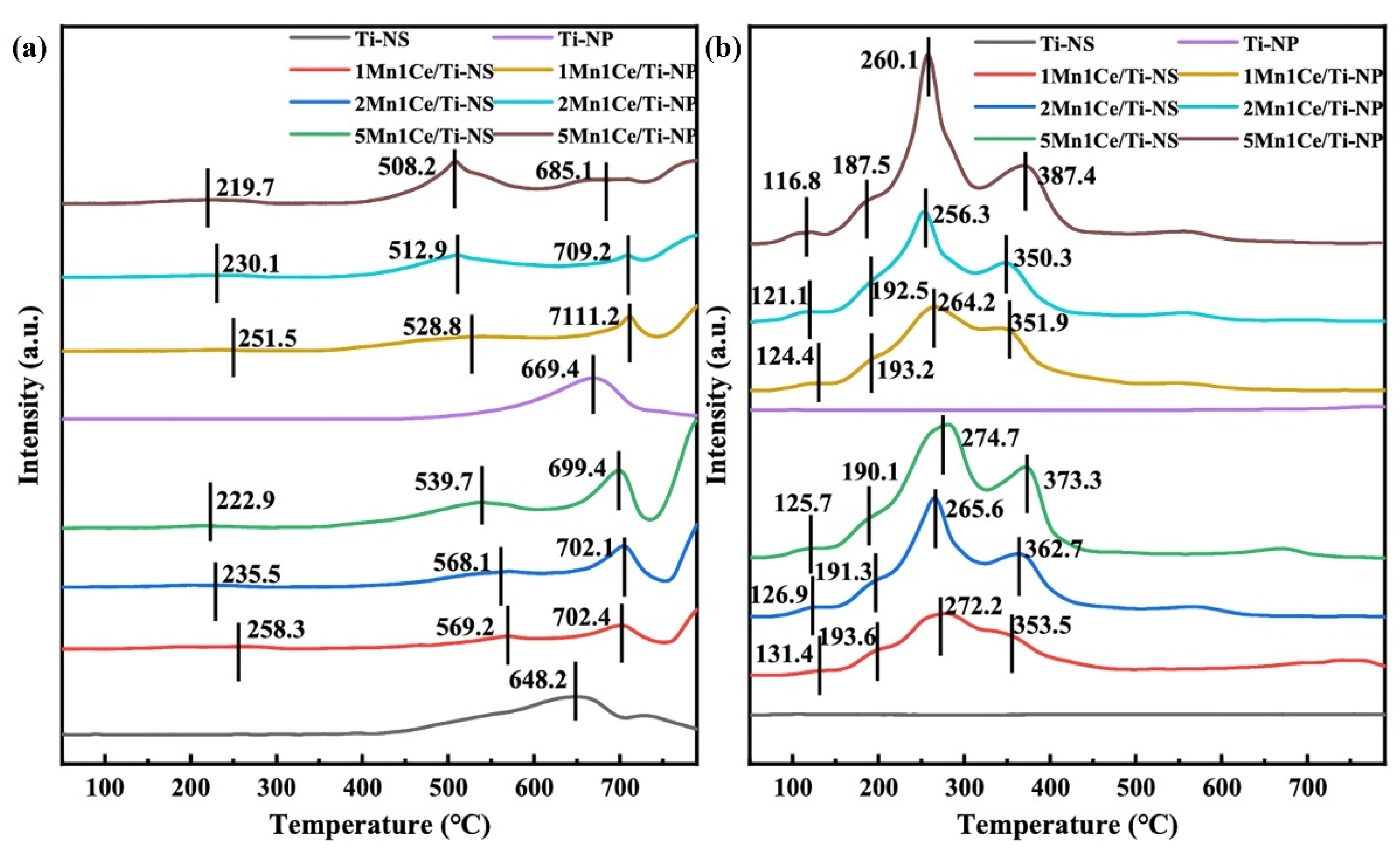
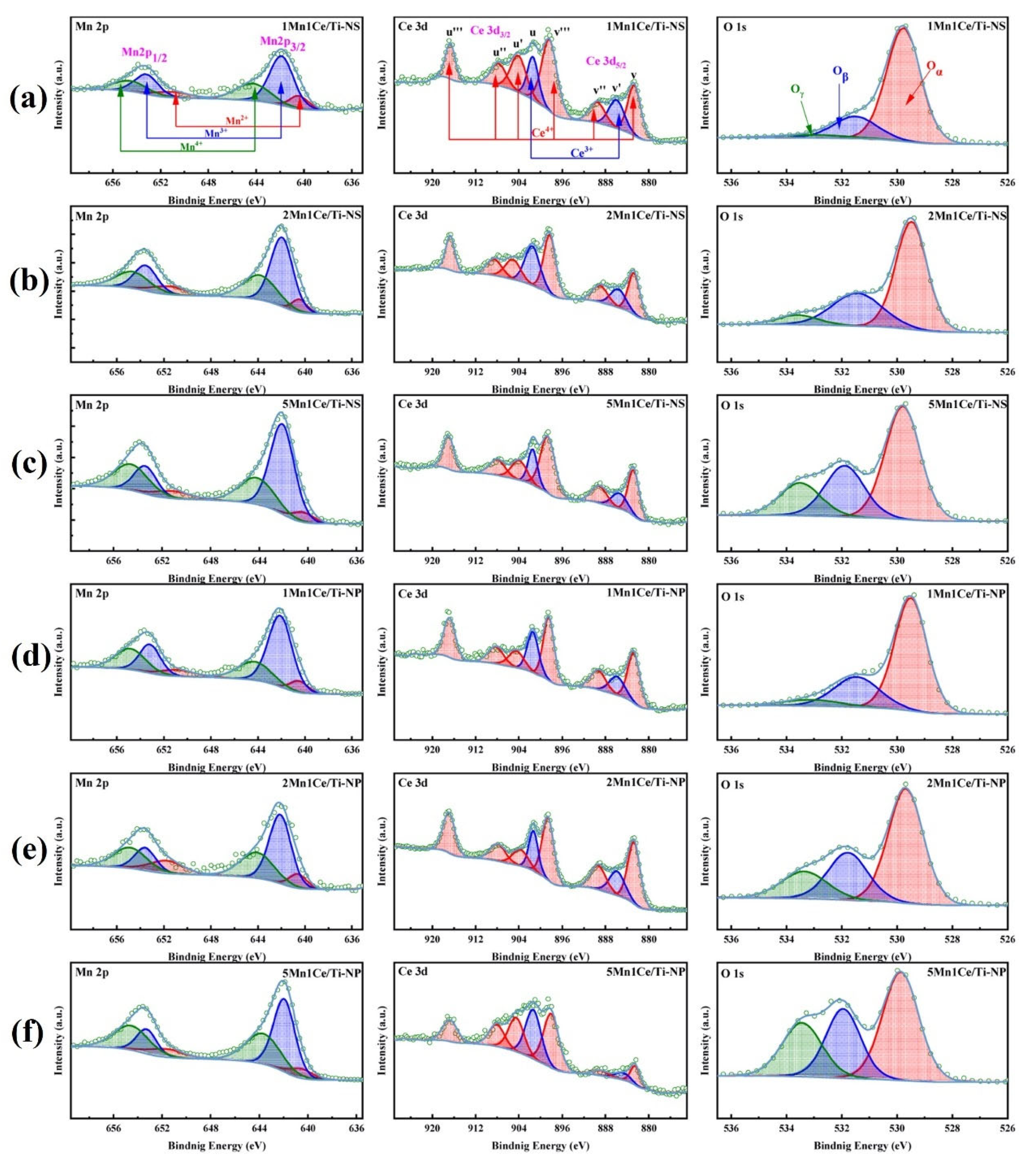
- Zheng, Z.; Zhang, C.; Li, J.; Fang, D.; Tan, P.; Fang, Q.; Chen, G. Insight into the effect of exposed crystal facets of anatase TiO2 on HCHO catalytic oxidation of Mn-Ce/TiO2. J. Hazard. Mater. 2024, 474, 134710. [Google Scholar] [CrossRef]
- Wang, M.; Hong, X.; Chen, J.; Li, J.; Chen, X.; Mi, J.; Liu, Z.; Xiong, S. Two-step hydrothermal synthesis of highly active MnOx-CeO2 for complete oxidation of formaldehyde. Chem. Eng. J. 2022, 440, 135854. [Google Scholar] [CrossRef]
- Xie, J.; Liu, Q.; Chen, C.; Shen, T.; Tian, X.; Shi, W.; Wang, F. Promoting active oxygen species on MnO2 by doping Fe for performance enhancement of formaldehyde oxidation. Fuel 2025, 379, 133136. [Google Scholar] [CrossRef]
- Xie, J.; Shen, T.; Fan, W.; Shi, W.; Wang, F. Efficient oxidative removal of formaldehyde over C-doped MnO2 catalysts at low temperatures. Chem. Eng. Sci. 2025, 304, 121002. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Hou, B.; He, Y.; Weng, W.; Zhu, Y.; Wang, Z. Catalytic Ozonation of Formaldehyde with an Oxygen-Vacancy-Rich MnOx/γ-Al2O3 Catalyst at Room Temperature. J. Catal. 2024, 14, 885. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, L.; Xiao, W.; Zeng, C.; Li, N.; Lv, T.; Zhu, H. Oriented growth of layered-MnO2 nanosheets over α-MnO2 nanotubes for enhanced room-temperature HCHO oxidation. Appl. Catal. B Environ. Energy 2017, 207, 233–243. [Google Scholar] [CrossRef]
- Zeng, X.; Zeng, Z.; Hu, Q.; Liu, K.; Ming, L.; Cheng, B.; Wang, W.; Luo, G.; Cao, S. Hierarchical Pt/NiO hollow nanofibers for catalytic oxidation of HCHO at room temperature. Chin. J. Struct. Chem. 2025, 44, 100575. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Cheng, B.; Jiang, C.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. Synergy between Platinum and Gold Nanoparticles in Oxygen Activation for Enhanced Room-Temperature Formaldehyde Oxidation. Adv. Funct. Mater. 2022, 32, 2110423. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, C.; Li, J.; Zhao, Y.; Fang, D.; Han, A.; Tan, P.; Fang, Q.; Chen, G. Catalytic oxidation of formaldehyde by MnOx-decorated anatase TiO2 {001}: Investigation of the effect of calcination temperature on catalytic activity and reaction mechanism. J. Environ. Chem. Eng. 2024, 12, 112578. [Google Scholar] [CrossRef]
- Lu, S.; He, N.; Xu, C.; Sun, N.; Jiang, Y.; Fu, H.; Wang, H.; Liu, J.; Fang, Y.; Liu, G. Controlled growth of villiform MnO2 over CeO2 nanorods for the efficient catalytic oxidation of formaldehyde. J. Environ. Chem. Eng. 2025, 13, 116984. [Google Scholar] [CrossRef]
- Fang, R.; Huang, X.; Luo, X.; Sun, Y.; Liu, Z.; Ao, L.; Dong, F.; Huang, H. Excellent stability for catalytic oxidation formaldehyde over defective δ-MnO2 nanoparticles at room temperature. J. Environ. Chem. Eng. 2023, 11, 109064. [Google Scholar] [CrossRef]

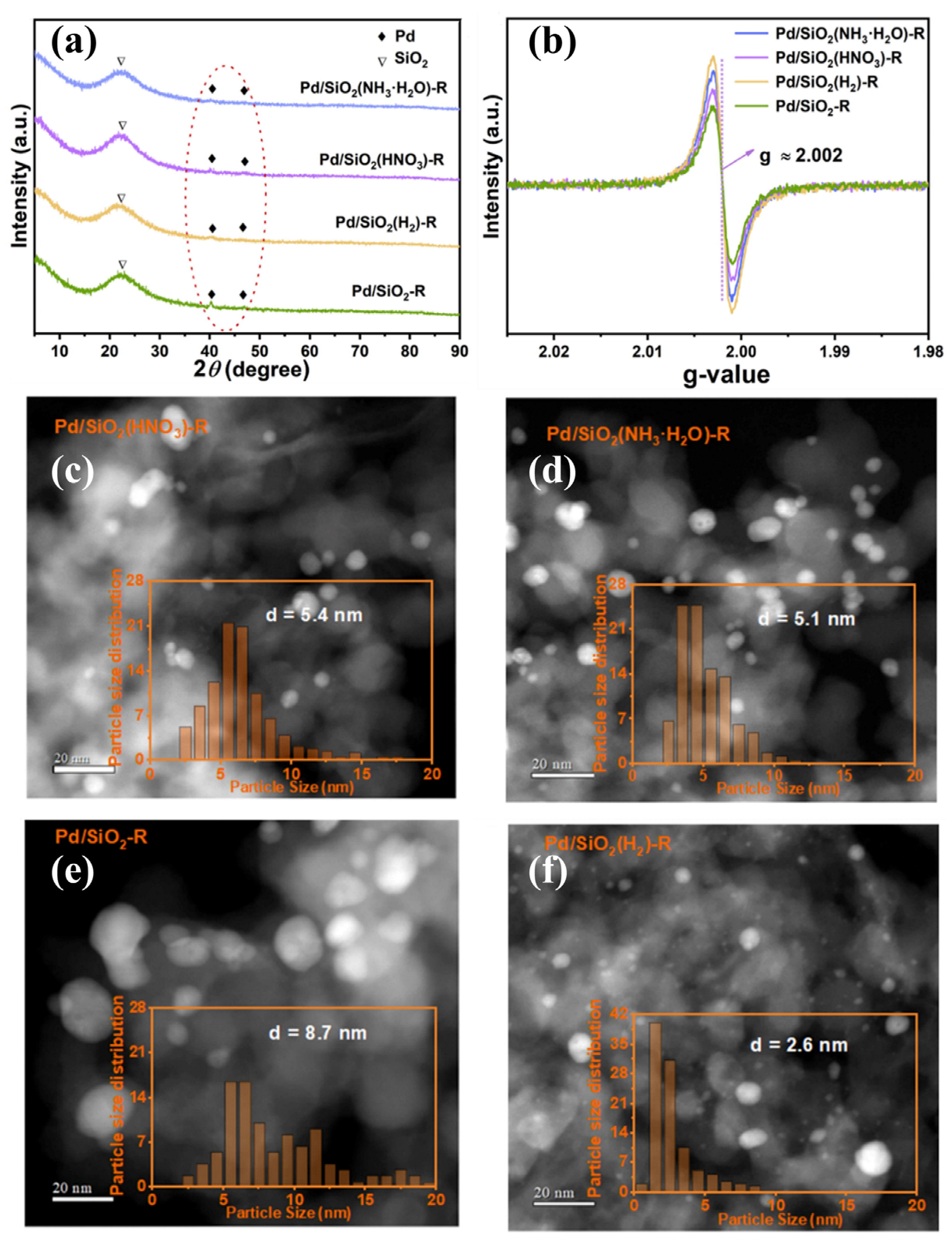

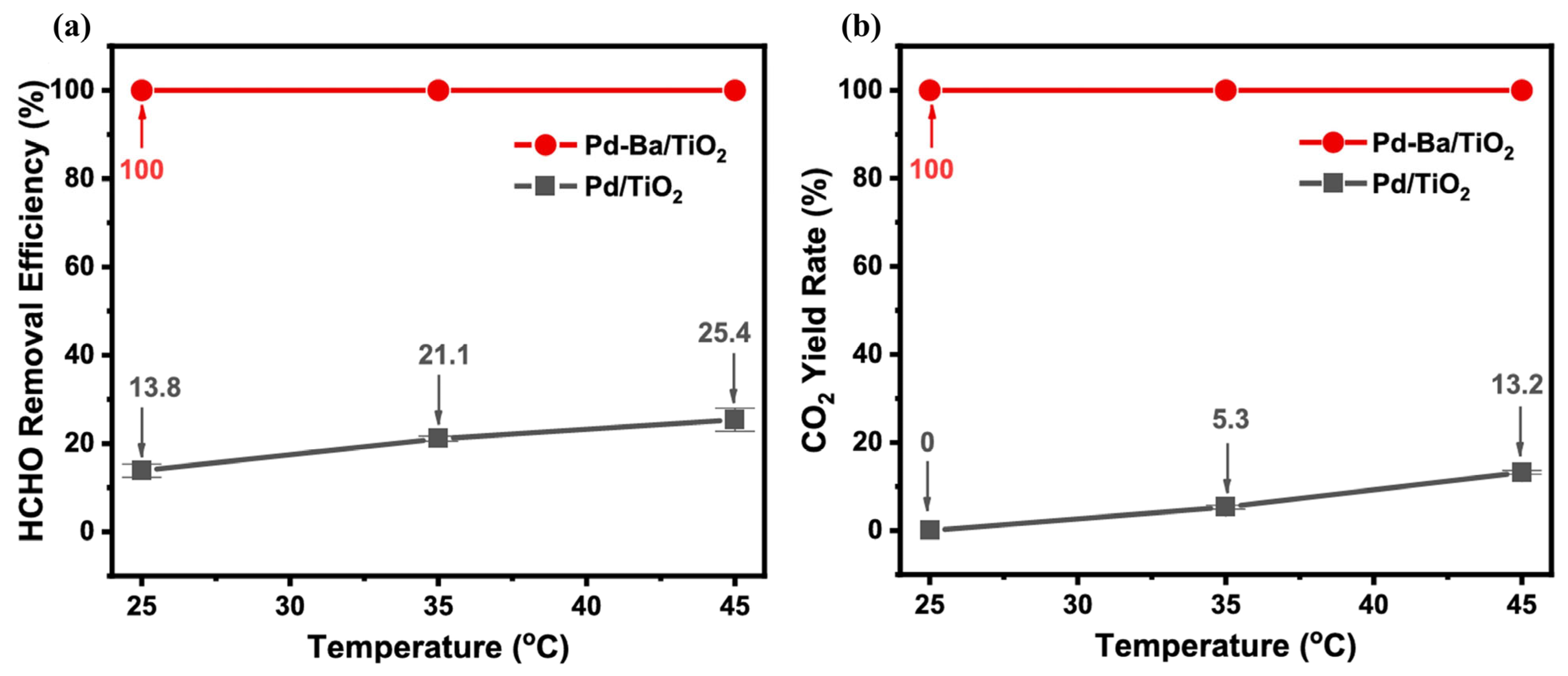
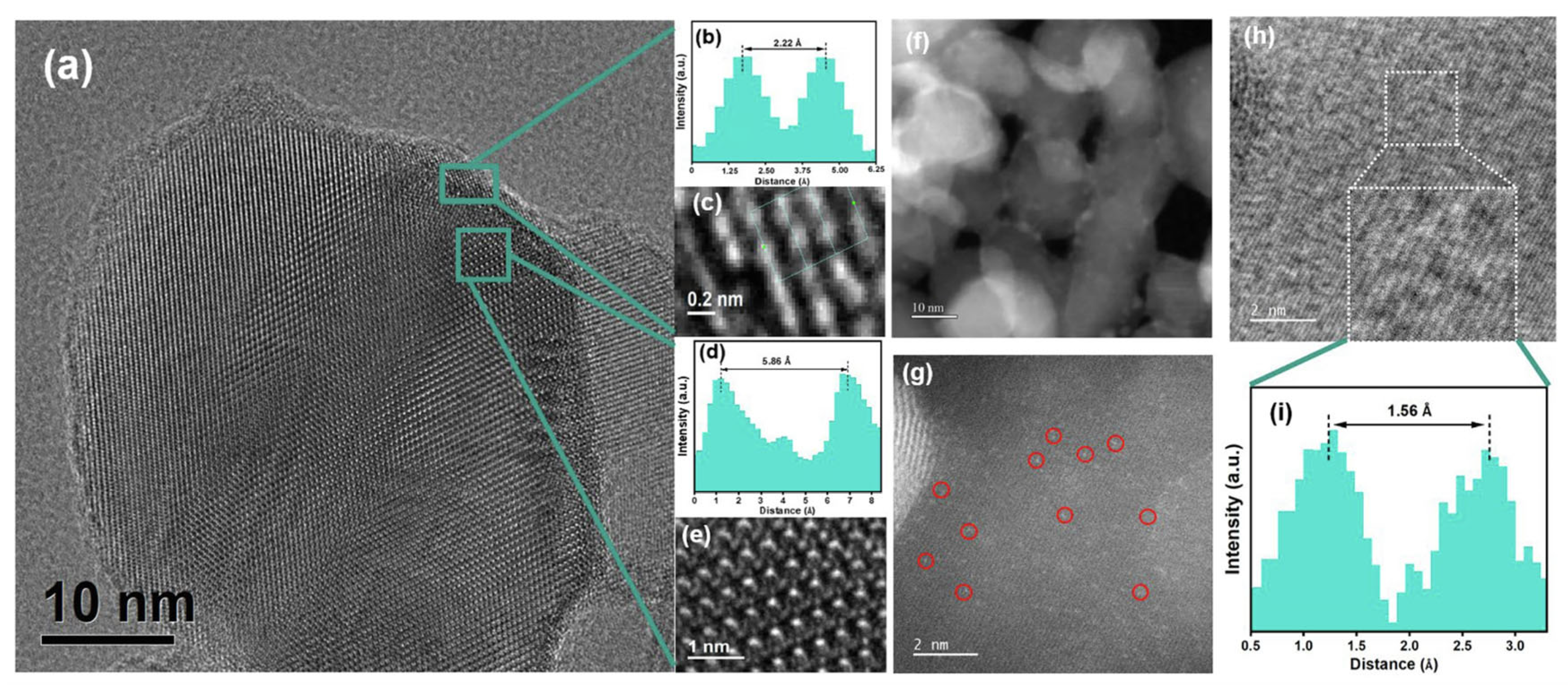
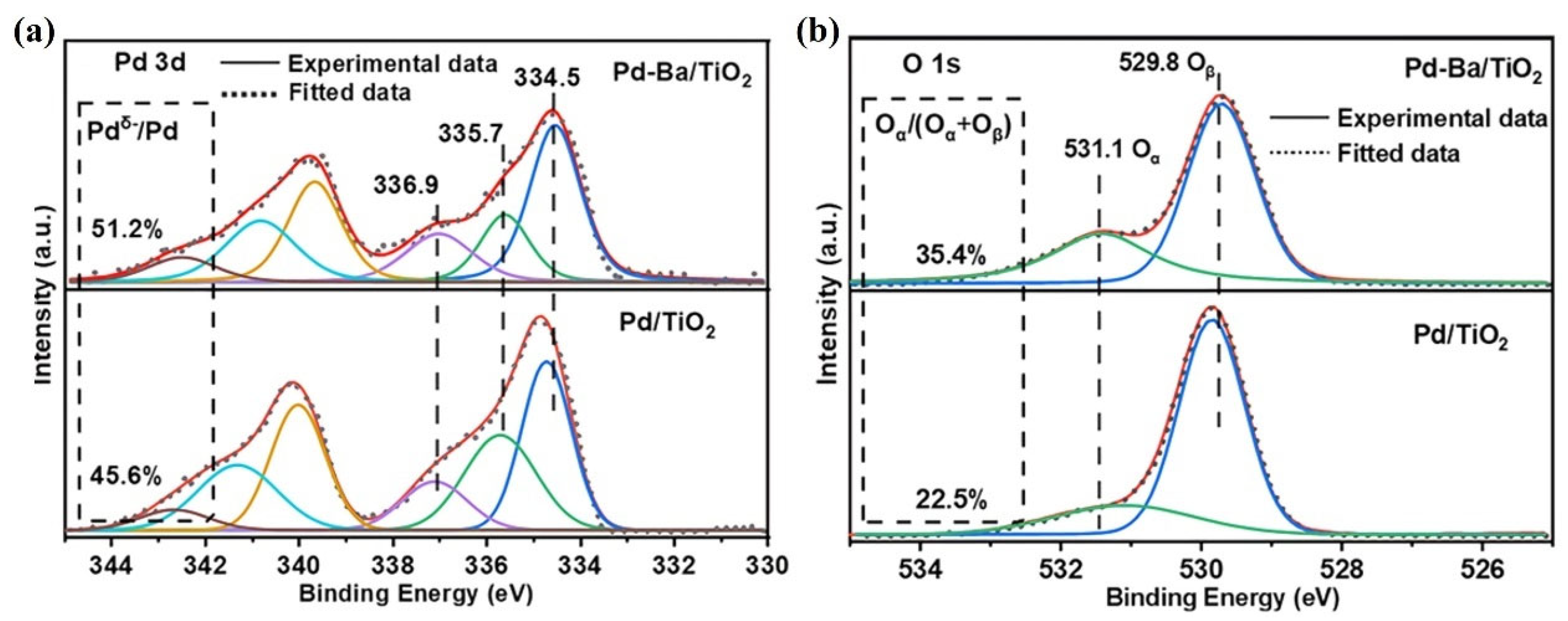








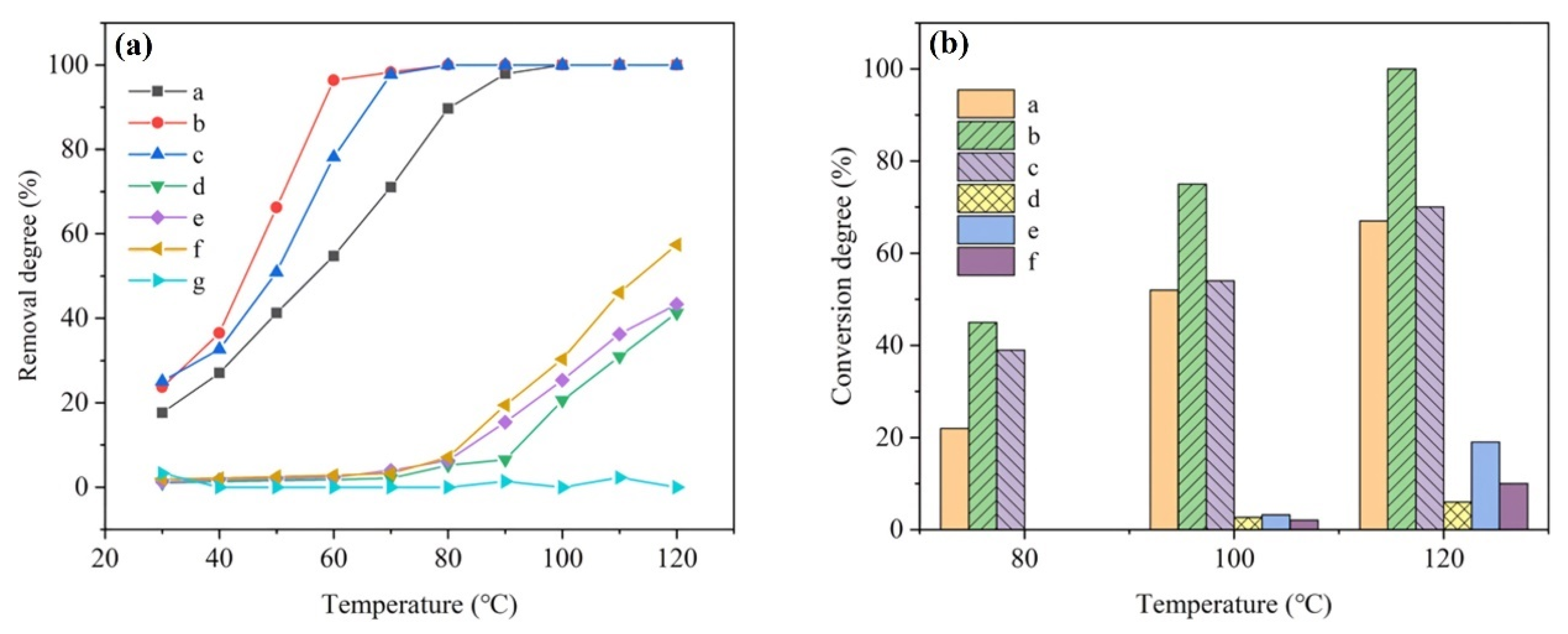

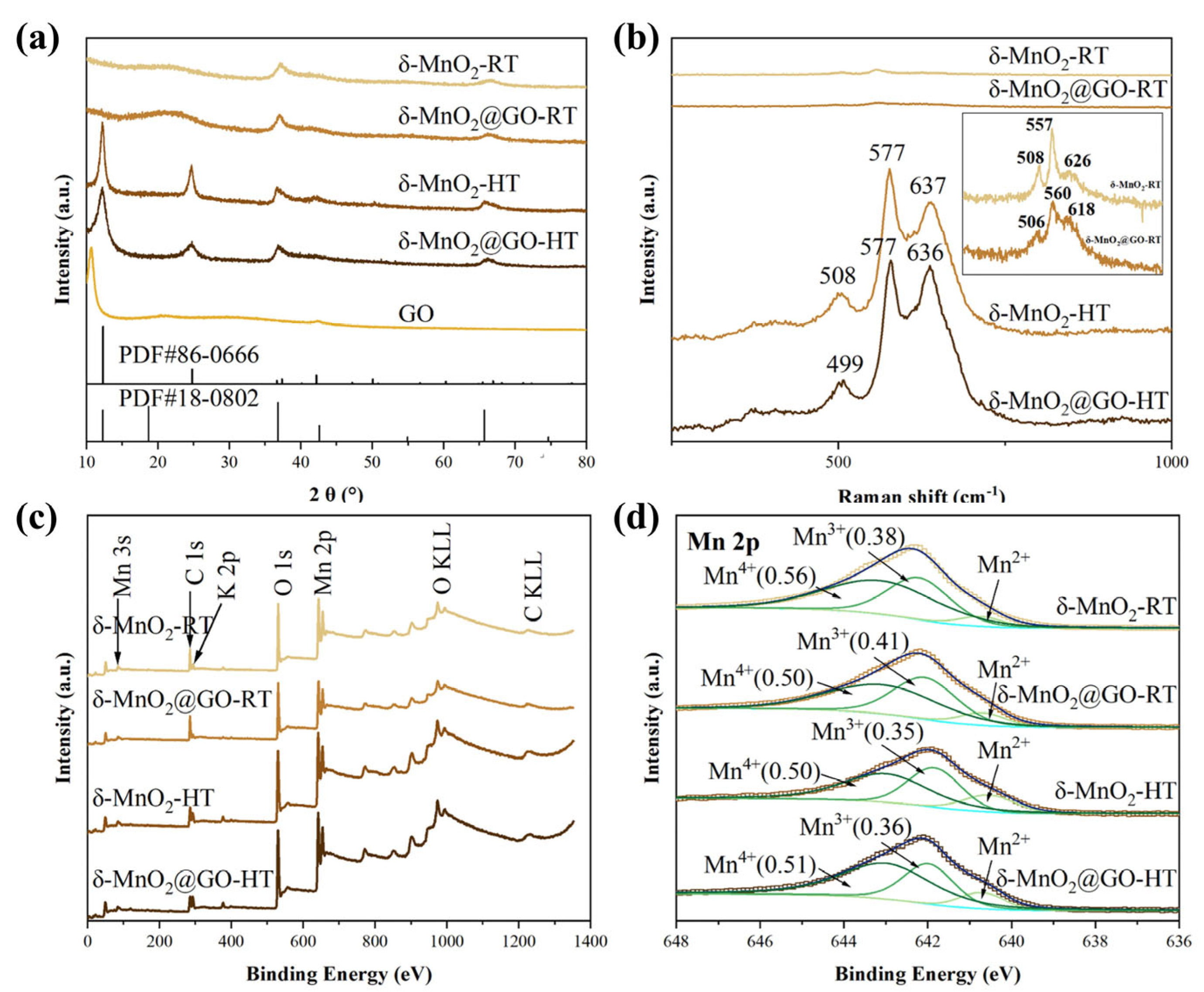
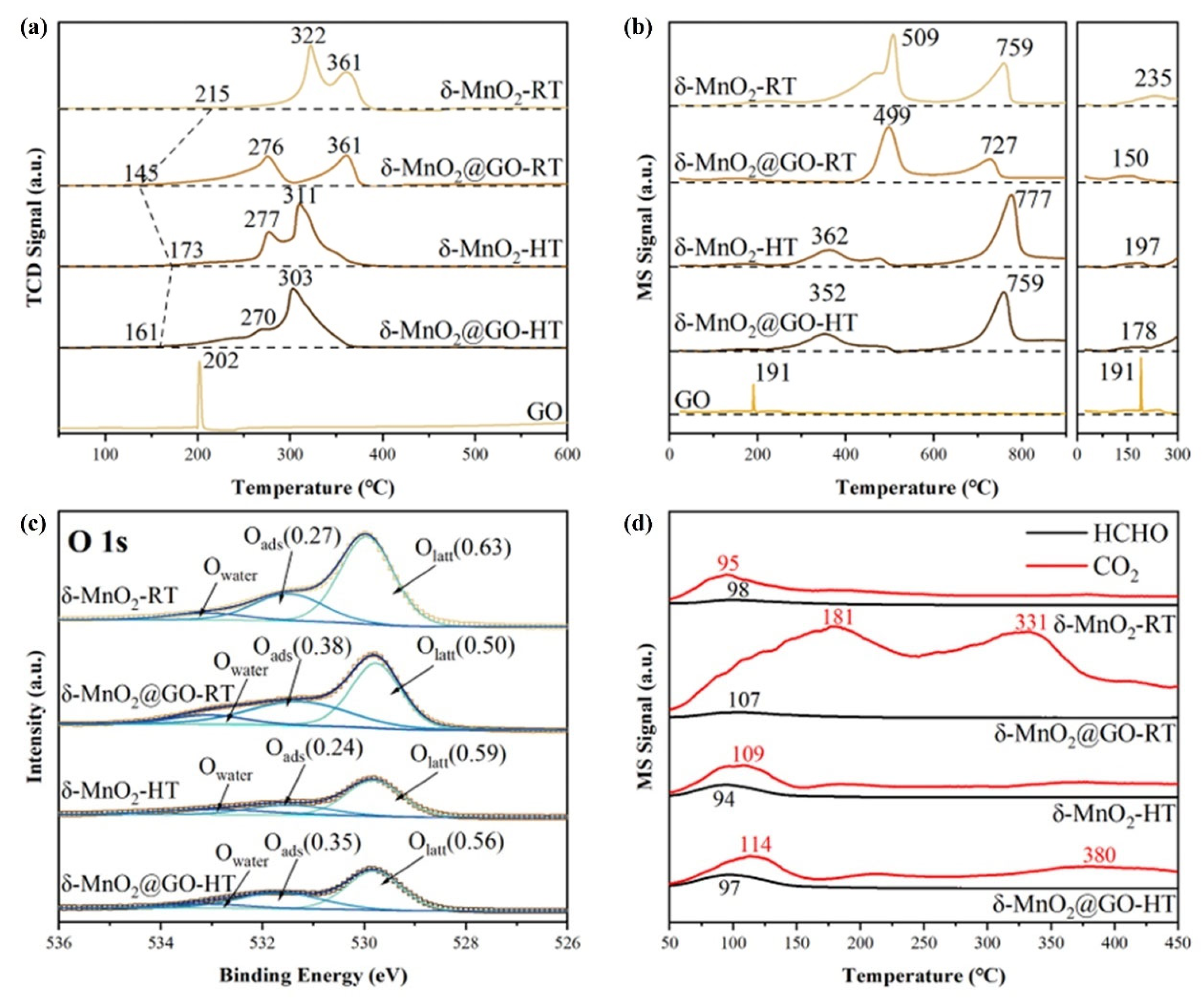

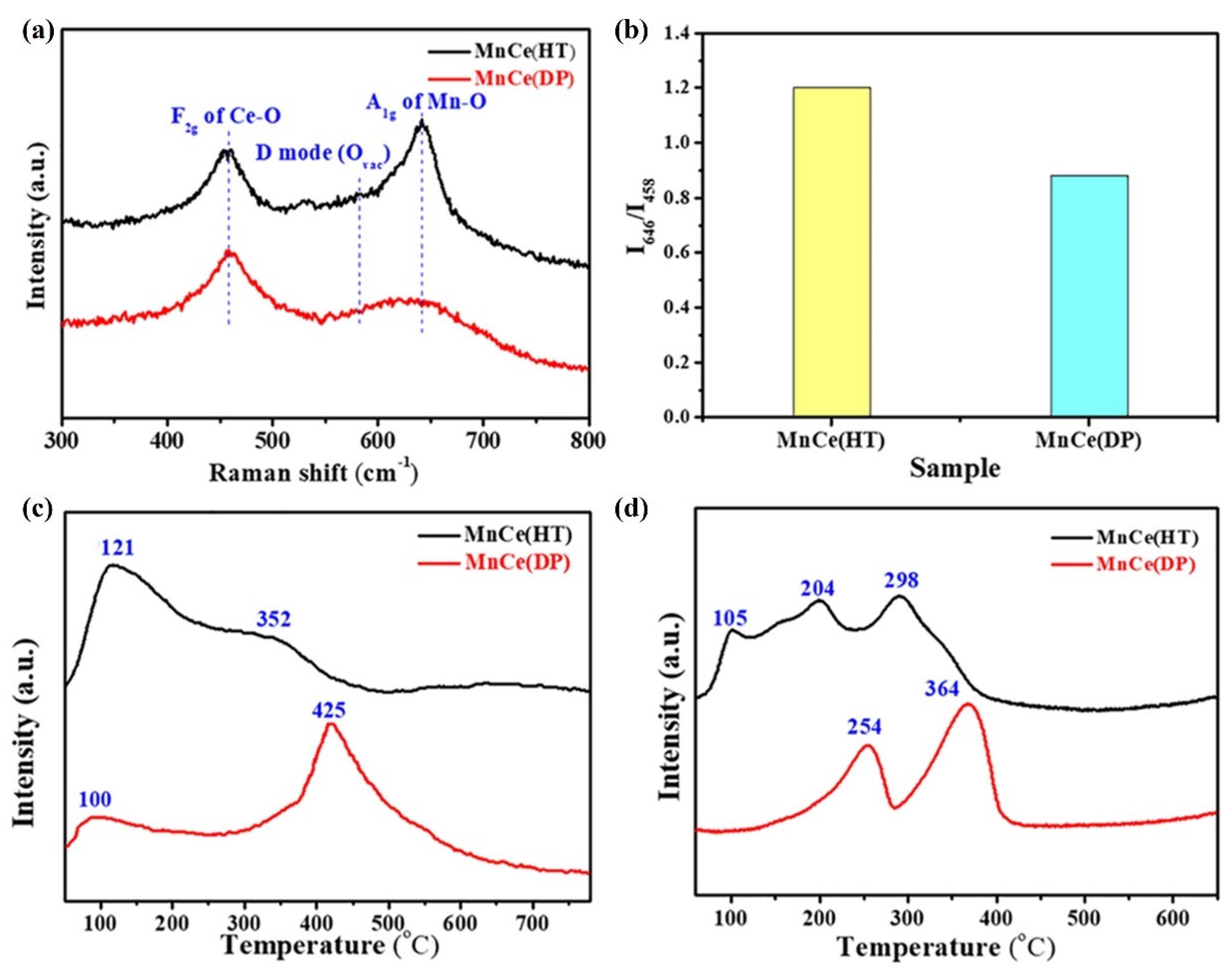
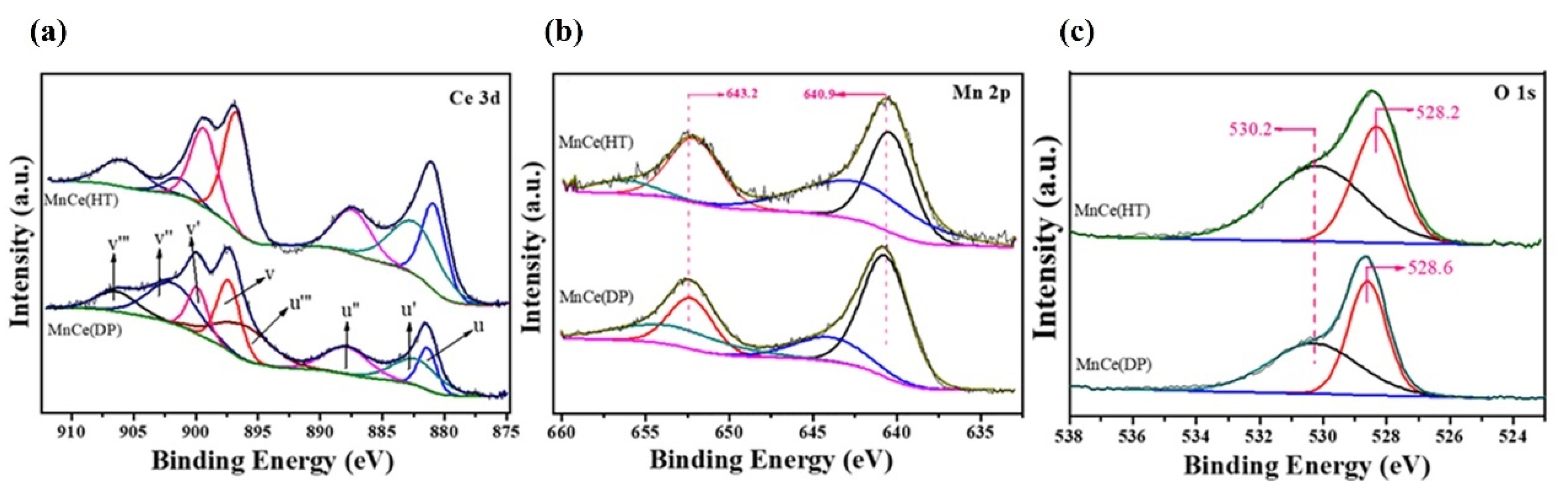




| Catalysts | Reaction Conditions | Conversion | Ref. |
|---|---|---|---|
| Supports with large specific surface area | |||
| Pt-CeO2/N-rGO | 100 ppm HCHO, GHSV = 80,000 mL/(g·h), RH = 35% | 100% at R.T. | [71] |
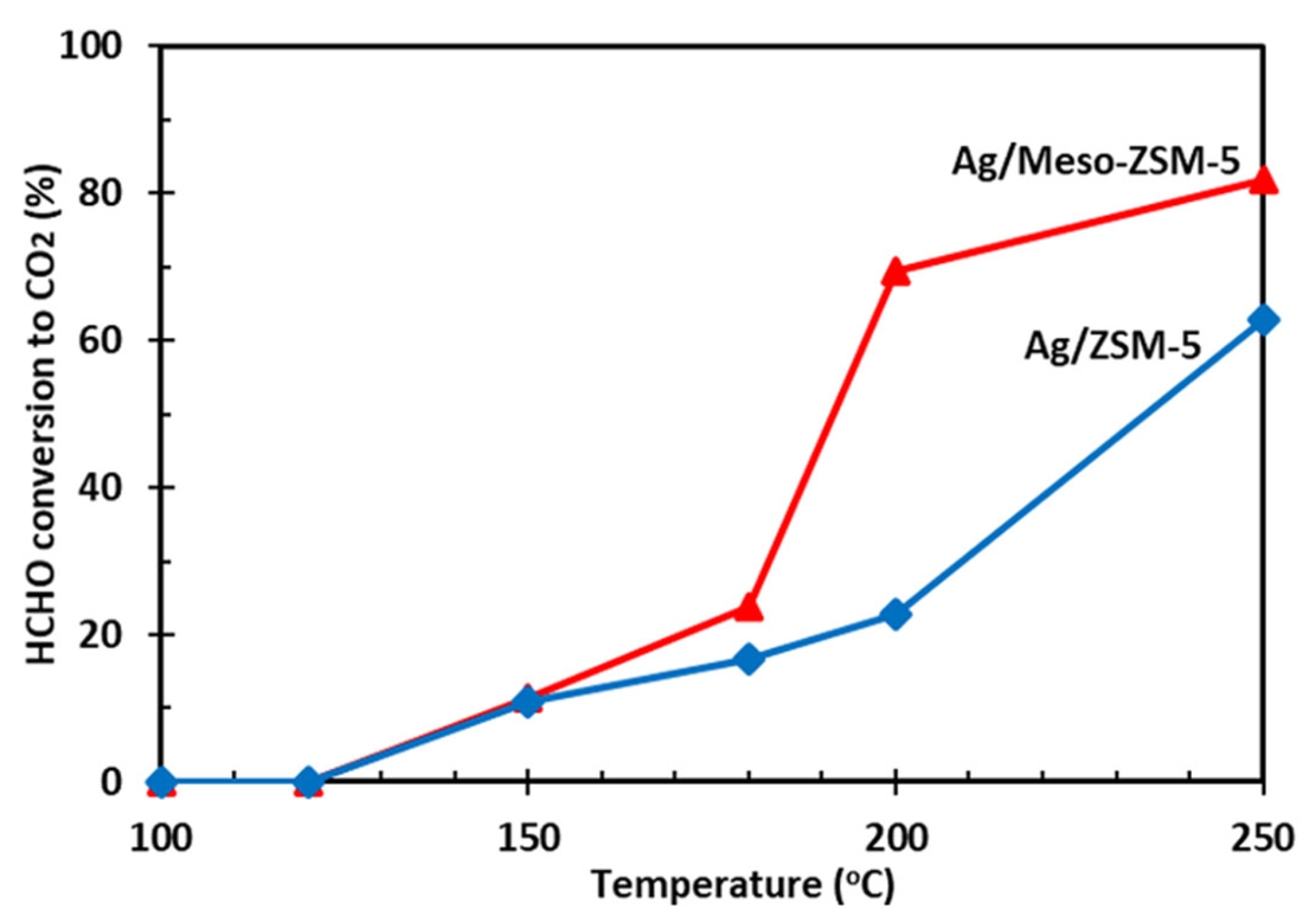
| Ag/Meso-ZSM-5 | 90 ppm HCHO, 20 vol.% O2, total flow rate = 50 mL/min RH = 65% | 80% at 250 | [72] |
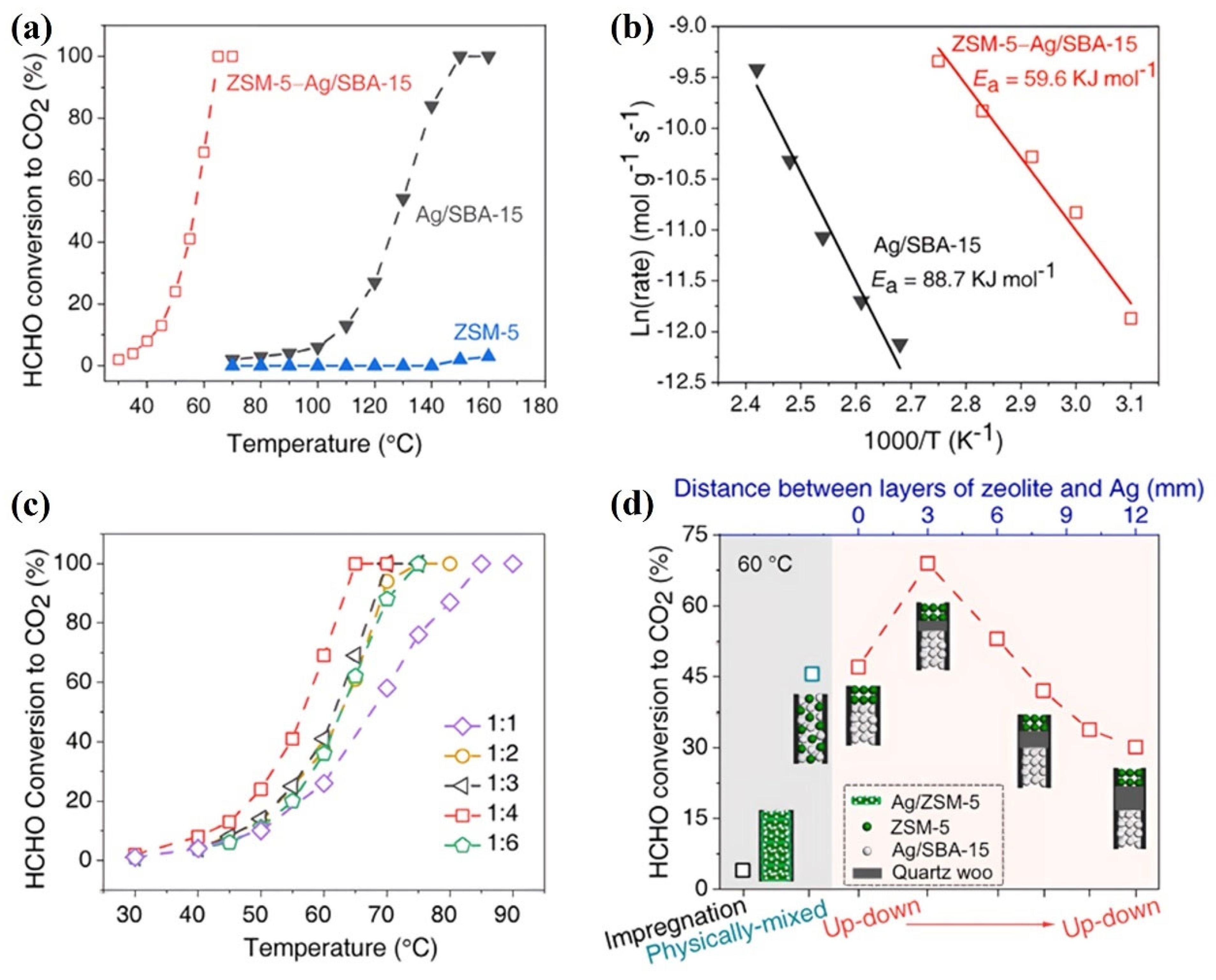
| ZSM-5-Ag/SBA-15 | 100 ppm HCHO, 20 vol.% O2, GHSV = 36,000 mL/(g·h) | 100% at 65 | [73] |
| Pd/USY | 150 ppm HCHO, 20 vol.% O2, WHSV = 150,000 mL/(g·h), RH = 35% | 100% at 25 | [74] |
| Ag-K-Al2O3 | 100 ppm HCHO, 30 mL/min 21% O2, WHSV = 36,000 mL/(g·h), RH = 10/30/50% | 100% at 40 | [70] |
| Pt@S-1 | 120 ppm HCHO, 20 vol.% O2, GHSV = 180,000 mL/(g·h), RH = 0–98% | 100% at R.T. | [75] |
| FeNi@NC/Pt | 100 ppm HCHO, 20 vol.% O2, GHSV = 60,000 mL/(g·h), RH = 30% | 100% at R.T. | [76] |
| Pd-Ba/TiO2 | 150 ppm HCHO, 20 vol.% O2, total flow rate = 100 mL/min RH = 35% | 100% at R.T. | [77] |
| Ag-AC | 100 ppm HCHO, total flow rate = 500 mL/min | 100% at RT | [78] |
| Pd/DZ200 | 80 ppm HCHO, Air, WHSV = 120,000 mL/(g·h), RH = 50% | 100% at RT | [61] |
| Pd/SiO2(H2)-R | 150 ppm HCHO, 20 vol.% O2, total flow rate = 100 mL/min RH = 35% | 100% at 25 | [79] |
| Ag/γ-Al2O3 | 100 ppm HCHO, 19.5 vol.% O2, GHSV = 84,000 mL/(g·h) | 100% at 125 | [80] |
| Metal oxide supports with high-temperature oxidation activities | |||
| Pt/ZrO2 | 100 ppm HCHO, 21 vol.% O2, GHSV = 60,000 mL/(g·h), RH = 30% | 95.3% at 20 | [81] |
| Ag5-LCO-I | 320–350 mg/m3 HCHO, Air | 52% at 30 | [82] |
| Ag/MnOx-0.5 | 1 ppm HCHO, 21 vol.% O2, GHSV = 150 L/(g·h), RH = 55% | >80% at 25 | [83] |
| Pt/kit-CeO2 | 20 ppm HCHO, 20 vol.% O2, WHSV = 200,000 mL/(g·h), RH = 30% | ~95% at 30 | [27] |
| 4-Ag/MnOX | 20 ppm HCHO, Air, WHSV = 200,000 mL/(g·h) | 94% at R.T. | [84] |
| Metal oxide supports with special morphologies | |||
| 0.3PdLN | 50 ppm HCHO, GHSV = 24,000 mL/(g·h) | 100% at R.T. | [85] |
| Au@Co3O4 | 75 ppm HCHO, GHSV = 300,000 mL/(g·h), RH = 4/45/76% | ~95% at 30 | [86] |
| 8AgMCL-H | 35 ppm HCHO, RH = 50%, GSHV = 22,200 mL/(g·h) | 100% at 30 | [87] |
| 3Ag-5K/Co3O4-MnO2 spheres | 150 ppm HCHO, 21 vol% O2, RH = 30%, WHSV = 36,000 mL/(g·h) | 100% at 50 | [88] |
| Catalysts | Reaction Conditions | Conversion | Ref. |
|---|---|---|---|
| NiTi-LDH | 10 ppm HCHO, 20 vol.% O2, WHSV = 75,000 mL/(g·h), RH = 35% | 99.2% at RT | [90] |
| MnO2·MnO | 100 ppm HCHO, 21 vol% O2, GHSV =120 L/(g·h), RH = 50% | 100% at 70 | [20] |
| Ce0.2Mn-P | 310–325 mg/m3 HCHO, RH = 20–23% | ~83% at RT | [26] |
| 3D-δ-MnO2 | 100 ppm HCHO, RH = 55%, 21% O2, GHSV = 90 L/(g·h) | 100% at RT | [91] |
| K0.02Co0.98Cr2O4 | 200 ppm HCHO, 20 vol% O2, WHSV = 60,000 mL/(g·h) | 100% at 150 | [92] |
| Rb-MnO2 | ~200 ppm HCHO | ~97% at 36 | [93] |
| R-Bir | 10 ppm HCHO, GHSV = 60 L/(g·h) | 100% at RT | [94] |
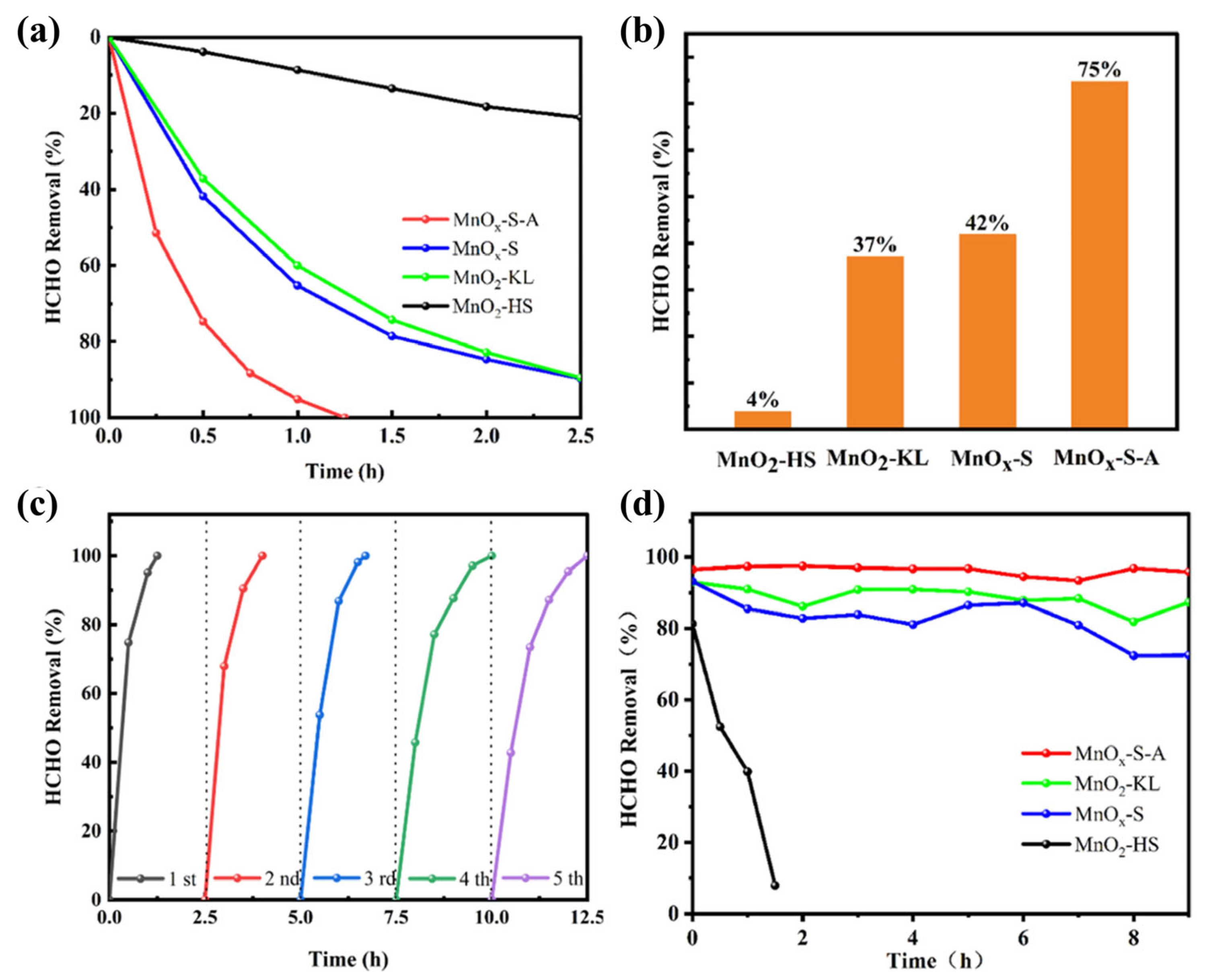
| MnOx-S-A | ∼1 ppm HCHO, GHSV = 150 L/(g·h), RH = 55% | >95% at RT | [95] |
| 1-MnCo2O4 | 50 ppm HCHO, flow rate = 50 mL/min, 21 vol. % O2 | 100% at 90 | [96] |
| 1-CuMn2O4 | 50 ppm HCHO, flow rate = 50 mL/min, | 100% at RT | [97] |
| Co3O4 | 10–12 ppm HCHO, GHSV = 60,000 mL/(g·h) | >99% at RT | [98] |
| Am-MnO2-AC | 100 ppm HCHO | 100% at 100 | [99] |
| MnOx/Sep-H | 100 ppm HCHO, RH = 50%, GHSV = 6000 mL/(g·h) | 100% at 85 | [100] |
| δ-MnO2@GO-RT | 100 ppm HCHO, RH =50%, GHSV =72 L/(g·h) | 100% at RT | [101] |
| Co-N/C-1000 | 100 ppm HCHO, GHSV = 72,000 mL/(g·h) | 92.8% at RT | [102] |
| CeO2@MnO2-5 | 300 ppm HCHO, 21% O2, RH = 30%, WHSV = 36,000 mL/(g·h) | 100% at 40 | [103] |
| MnO2@Co3O4-45 | 200 ppm HCHO, 21 vol% O2, RH = 30%, WHSV = 36,000 mL/(g·min) | 100% at 60 | [104] |
| MnO2-3K | 500 ppm HCHO, 20 vol% O2, RH = 70%, GHSV =60,000 mL/(g·h) | 100% at 90 | [105] |
| 1.0Co3O4@NC-HC | 150 ppm HCHO, RH = 50% | 96.5% at RT | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Gao, Y.; Luo, X.; Lian, L.; He, J.; Xie, S.; Su, J.; Liu, T.; Xu, L. Recent Advances in Formaldehyde Catalytic Oxidation Catalysts. Inorganics 2025, 13, 345. https://doi.org/10.3390/inorganics13110345
Sun G, Gao Y, Luo X, Lian L, He J, Xie S, Su J, Liu T, Xu L. Recent Advances in Formaldehyde Catalytic Oxidation Catalysts. Inorganics. 2025; 13(11):345. https://doi.org/10.3390/inorganics13110345
Chicago/Turabian StyleSun, Gaoxin, Yike Gao, Xue Luo, Linshui Lian, Jing He, Shuwen Xie, Jiayi Su, Tiancheng Liu, and Leilei Xu. 2025. "Recent Advances in Formaldehyde Catalytic Oxidation Catalysts" Inorganics 13, no. 11: 345. https://doi.org/10.3390/inorganics13110345
APA StyleSun, G., Gao, Y., Luo, X., Lian, L., He, J., Xie, S., Su, J., Liu, T., & Xu, L. (2025). Recent Advances in Formaldehyde Catalytic Oxidation Catalysts. Inorganics, 13(11), 345. https://doi.org/10.3390/inorganics13110345









