Abstract
The development of biosensors for determining the most diverse biomolecules is a constant focus of many research groups. There is a latent need to propose sensors that combine portability, simple measurements, and good analytical performance. Here, we propose an electrochemical immunosensor that is fully portable and energy-independent for diagnosing antibodies against SARS-CoV-2 (the virus that causes COVID-19). Initially, disposable screen-printed carbon electrodes (SPEs) were covered by gold microblobs (AuMBs), which were synthesized amperometrically from Au3+ ions. Then, the SPE-AuMBs were coated with cysteamine, which allowed the N-hydroxysuccinimide-activated SARS-CoV-2 antigen (spike protein) to be immobilized. The antigen-activated electrode was used to detect COVID-19 antibodies from current measurements obtained by differential pulse voltammetry. The AuMBs synthesis time was optimized, and the presence of gold structures improved the electrochemical responses of the SPE. It was possible to quantitatively determine antibodies in the concentration range of 0.25 to 10 µg mL−1. This range includes concentrations found in biological fluids from patients at any stage of the disease. An analysis took approximately the same time as traditional rapid nasal tests (20 min) and costed less, considering all the steps necessary to prepare a disposable antigen-functionalized SPE.
1. Introduction
Electrochemical sensors and biosensors have emerged as up-and-coming contenders for point-of-care detection owing to their advantageous traits, such as reliability and the potential for sensitive and selective detection [1]. These devices are frequently preferred due to their high portability, user-friendliness, short analysis time, and easy sample preparation. They offer a convenient and versatile platform for detecting viral antigens, antibodies, or genetic material in various biological specimens, emerging as a noteworthy specimen for infectious disease testing due to their non-invasive nature and practicality [2,3]. The development of electrodes for application in various electrochemical devices is constantly proposed by many research groups. The demand for electrodes modified in increasingly sophisticated ways has increased, seeking very high analytical performances [4,5,6,7,8,9,10,11,12,13]. Furthermore, advanced analytical techniques (such as electrochemical impedance spectroscopy (EIS)) have been used where the response analysis is more complex and requires deeper technical knowledge [14,15,16]. Combining these factors has allowed the development of electrochemical sensing devices with incredible performance, and the determination of analytes on the picomole scale has been possible [13]. Despite this, the complexity of the experimental arrangement has distanced the electrochemical devices from real, practical applicability.
Very low detection limits and very high sensitivity are not always essential requirements. Sometimes, simplicity, speed, low cost, and no technical analyst are the most important aspects of an analysis. In this sense, using screen-printed electrodes (SPEs) associated with simpler electrochemical techniques, such as voltammetric ones, can be advantageous. Electrochemical devices that use SPEs without or with little preparation have been reported by many groups, and they have been useful for proposing devices capable of determining molecules and biomolecules with important applications in the medical field [15,16,17,18]. Gold structures, such as gold nanoparticles, have been used to optimize the response of SPEs used in biosensors [18,19,20]. They are relatively simple elements that can be produced directly in the electrochemical analysis cell. However, the steps for optimizing gold structure synthesis on the SPE surface and their activation to receive biorecognition elements are not normally presented.
Since its initial manifestation in late 2019, the coronavirus disease 2019 (COVID-19), attributed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has burgeoned into an international health emergency, inducing profound repercussions on public health and the global economy. An indispensable element in effectively managing an outbreak involves timely and expedient diagnostic modalities to curb infectious disease transmission [21]. To this end, SPE-based biosensors have been proposed and have become essential tools in general healthcare and analysis. For instance, Nascimento et al. [22] achieved a low detection limit in an electrochemical biosensor for the SARS-CoV-2 spike protein. The authors employed magnetic beads and gold nanoparticles conjugated to the human angiotensin-converting enzyme (ACE2) receptor on the surface of screen-printed carbon electrodes to recognize spike proteins in human samples using differential pulse voltammetry (DPV). In a sophisticated approach, Triastuti et al. [23] developed a SARS-CoV-2 aptasensor to detect the SARS-CoV-2 Spike Receptor Binding Domain (spike RBD). The authors functionalized a CeO2-modified screen-printed carbon electrode with an aptamer for spike RBD protein and achieved detection by DPV in human saliva and oropharyngeal swab samples. Brazaca et al. developed gold-modified screen-printed carbon electrodes functionalized with anti-spike protein to determine the SARS-CoV-2 spike protein. A more complex electrochemical analysis based on impedance spectroscopy was used to assess the protein in biological fluids [24].
Although antigens (such as spike protein) have been used to diagnose infectious diseases in the early stages, human antibodies against SARS-CoV-2 spike protein have mainly been utilized to determine immune status or disease stage in COVID-19 diagnosis [25,26,27,28]. For example, the group of Drobysh et al. used gold nanostructure-activated SPEs to detect antibodies against recombinant SARS-CoV-2 spike protein (anti-rSpike). For this, the electrode received covalent immobilization of rSpike to recognize anti-rSpike in serum [29]. A study reported by Nature showed that the concentration of IgM antibody against SARS-CoV-2 spike (related to the disease’s active phase) in the serum is 1.5 μg mL−1 between 4 and 10 days after the disease and reaches a maximum value around 8.0 μg mL−1 after 16–20 days of infection [30]. The relatively high biomarker levels in biological fluid make the use of SPE-based electrochemical biosensors interesting for COVID-19 diagnosis. The use of SPE-based electrochemical biosensors to determine antibodies against the SARS-CoV-2 virus is not a novelty, since numerous reports have been published over the last few years [22,23,24,31]. However, we wanted to combine the best elements to enable a novel electrode configuration to obtain a ready-for-use, simple, practical, and fully portable electrochemical device. This study introduces a gold microblob (AuMB)-modified SPE for electrochemical biosensing obtention using an antibody against SARS-CoV-2 as a detection model. An optimization study of electrochemical AuMB synthesis and SPE surface amination (surface activation by L-cysteine) is also presented. Using disposable SPE electrodes and a compact and energy-autonomous electrochemical apparatus enables obtaining a portable, straightforward analysis, quick response, and cost-effective platform. The biosensor evaluated the presence of antibodies against SARS-CoV-2-Spike both qualitatively and quantitatively using antigen-functionalized SPEs and simple DPV measurements.
2. Results and Discussion
2.1. Optimization of the AuMB Synthesis
The synthesis of AuMBs on carbon-based electrodes has already been proposed, and the electrode electrochemical signal has improved [31]. The synthesis of AuMBs on SPEs was optimized with respect to the electrochemical synthesis time. The potential was fixed at 1.2 V, which is the minimum to reduce gold to AuMBs in a controlled manner. Scanning electron microscopy (SEM) images of the AuMBs for each synthesis condition are shown in Figure 1. The AuMBs had a spherical shape and a rough surface. The average diameter of the particles was clearly larger, as the synthesis time increased up to 50 s. This is an expected result since applying a longer growth time results in larger particles. The average diameters were 5.5 ± 0.6 µm and 9.2 ± 0.9 µm (average of 50 particles) for times of 30 and 40 s, respectively. It was impossible to obtain the diameter accurately for 50 s as the particles are clearly agglomerated, without precisely defined contours and overlapping (formation of particle overlayers). A film of agglomerated particles without defined boundaries was observed in the delayed condition of 300 s.
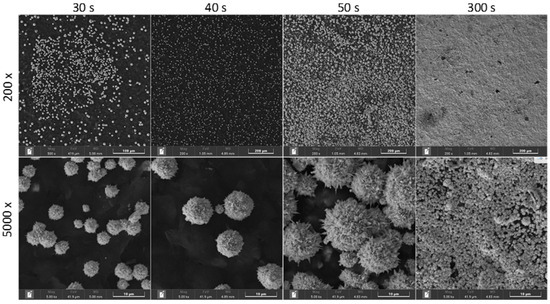
Figure 1.
SEM images for SPE-AuMBs were obtained at different times for AuMB electrochemical synthesis.
The SPE-AuMBs produced were subjected to cyclic voltammetry (CV) measurements in the potassium ferrocyanide medium to evaluate the signal produced by the electrodes. The voltammograms are shown in Figure 2, and the SPE signals are compared before and after using different AuMBs synthesis times. The cyclic voltammograms show that the anodic peak moved to lower potential values for SPE-AuMBs produced between 30 and 50 s compared to the bare SPE. In this way, the voltammogram profile for potassium ferrocyanide better adjusted the reversible behavior of potassium ferrocyanide electrochemical reactions (mirrored anodic (Iap) and cathodic (Icp) current peaks). Furthermore, the Iap increased in these electrodes compared to that obtained for the bare SPE. The Iap increased by 10.2, 5.77, and 4.70 µA for 30, 40, and 50 s, respectively. For the SPE produced in an extrapolated time of 300 s, the voltammogram profile changed abruptly, indicating the occurrence of more complex reactions. The electrode produced at the 30 s condition presented an optimized electrochemical signal and was chosen to proceed with subsequent analyses. The presence of smaller AuMBs (which showed a greater surface area in relation to volume) played a fundamental role in the better electroactive performance.
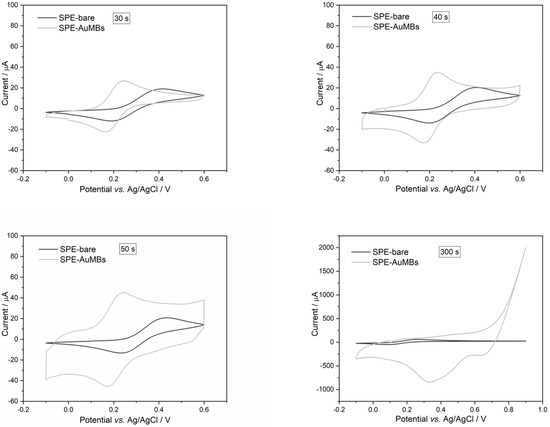
Figure 2.
Cyclic voltammograms for bare SPE and SPE-AuMBs obtained at different electrochemical synthesis times. Measurements were performed for potassium ferrocyanide in PBS (pH = 7.43) using 25 mV s−1 and 10 mV step.
The electroactive area for SPE-AuMBs (with and without AuMBs) at the optimized synthesis time condition (30 s) was calculated by the Randles–Sevcik equation (Equation (1)) [32]. For this, cyclic voltammograms were obtained for the electrodes under different scanning speeds, as shown in Figure 3.
where Iap is the anodic peak current (A), A is the electroactive area (cm2), C0 is the concentration of the electroactive species (mol cm−3), D is the diffusion coefficient of the electroactive species (6.39 × 10−06 cm2 s−1 for potassium ferrocyanide in KCl 0.5 M), and ν is the scan rate (V s−1).
Iap = 2.686 × 105An3/2C0D1/2ν1/2,
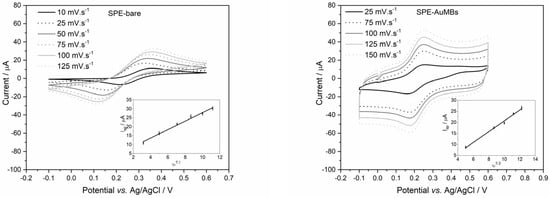
Figure 3.
SPE and SPE-AuMBs cyclic voltammograms obtained in potassium ferrocyanide 5 mM in KCl 0.5 M at different scan rates (10 to 150 mV s−1) for SPE with and without AuMBs. The insets in the figures are plots of Iap versus ν1/2.
Using data from Figure 3, Iap versus ν1/2 were plotted (inserts in Figure 3). The A for each electrode was determined from the slope of the straight line, and the values were 0.690 and 0.711 cm2 for the SPE and SPE-AuMBs, respectively. This confirms that the presence of AuMBs on the electrode surface contributes to an increase in the electroactive area and justifies the improvement in the electroactive performance of the electrode.
2.2. Cysteamine-Activation of SPE-AuMBs
The immobilization of an antigen (Ant) on the electrode is necessary to obtain the immunosensor for the COVID-19 diagnosis proposed here. To achieve this, creating a good Ant immobilization strategy is important. Amination of the electrode surface is typically the strategy used to immobilize N-hydroxysuccinimide(NHS)-activated Ant. Typically, alkaneaminothiols (such as cysteamine) are used for the amination of gold-containing surfaces. Thiols show a good chemical affinity for gold and are useful for the amination of this surface type. Amination is crucial as it links the inorganic (electrode) and organic (Ant) parts and directly affects the quality of the immunosensor’s performance. Sometimes, researchers do not pay due attention to this stage. Therefore, the immobilization of cysteamine on the electrode was evaluated, and two strategies were tested: drop coating and electrochemistry coating. Cyclic voltammograms (Figure 4) for potassium ferrocyanide were used to evaluate the aminated SPE-AuMBs.
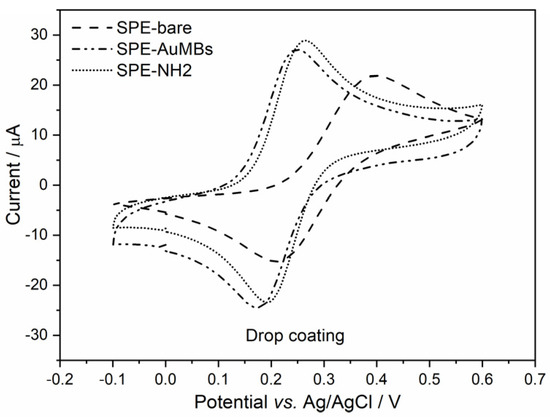
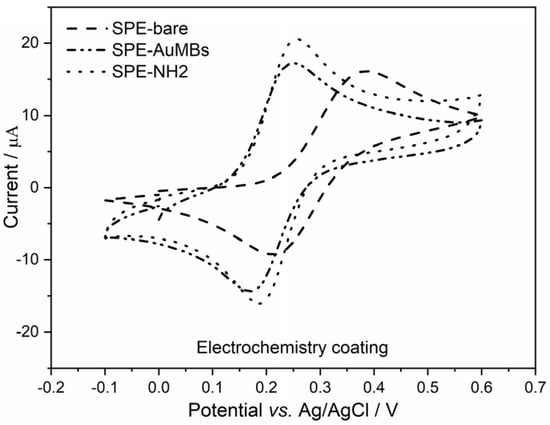
Figure 4.
Cyclic voltammograms for NH2-activated SPE-AuMBs that were aminated by drop coating and electrochemistry coating. Measurements were performed for potassium ferrocyanide in PBS (pH = 7.43) using 25 mV s−1 and 10 mV step.
The Iap variation in SPE-AuMBs/-NH2 with respect to SPE-AuMBs was 4.6 µA for drop coating and 4.9 µA for electrochemistry coating. A slightly greater advantage for electrochemical amination was observed. Therefore, this was the strategy chosen for the next steps.
The presence of cysteamine on the amino electrode through electrochemistry coating was evaluated by energy-dispersive X-ray spectroscopy (EDS). The elemental spectra obtained in the working electrode region in the SPE-AuMBs and SPE-AuMBs/-NH2 are shown in Figure 5.
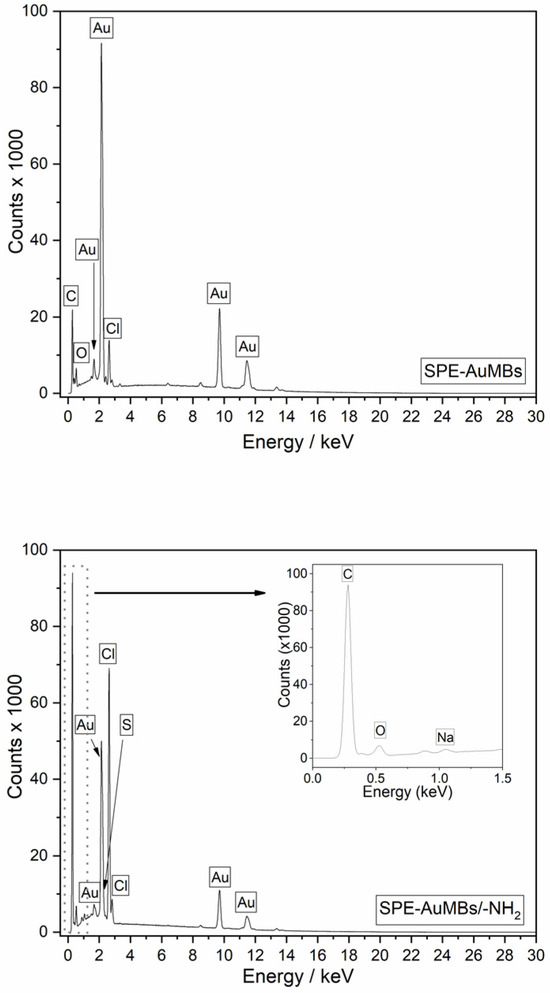
Figure 5.
EDS spectrum for SPE-AuMBs (top) and SPE-AuMBs/-NH2 (bottom).
The nitrogen and sulfur signals that characterize cysteamine’s presence occur at energies close to the expressive signals for carbon and gold, respectively. Furthermore, S and N should have a low occurrence because, theoretically, only a monolayer of cysteamine is adsorbed on the surface. This makes it difficult to identify signals for these elements. A small S signal was suggested for the EDS spectrum for SPE-AuMBs/-NH2 and not for SPE-AuMBs. The presence of sulfur only in SPE-AuMBs/-NH2 was corroborated through X-ray fluorescence (XRF) analysis, which is presented in Table 1.

Table 1.
Elemental percentage occurrence obtained by XRF for SPE-AuMBs and SPE-AuMBs/-NH2.
Some elements determined for the electrodes by XRF differ from those observed in the EDS spectra. EDS analyses were carried out, focusing only on the working electrode area. That was not possible using the XRF. Thus, regions of the reference electrode, counter-electrode, and SPE physical structure were also evaluated. Furthermore, XRF equipment is only able to detect elements heavier than Na. Thus, the C and O signals are not detected, but Al, Si, and Ti signals (present in the SPE physical structure) occurred in a large percentage.
Finally, EDS performed an elemental mapping for Au and S on the SPE-AuMBs/-NH2 electrode (Figure 6). The orange and light green regions represent Au and S, respectively. It is evident that the clusters in the image have a chemical composition consisting of gold. Furthermore, it was observed that the small occurrence of S is concentrated in regions where AuMBs are present. This strongly suggests that cysteamine immobilization occurs through chemical adsorption onto the electrode by establishing Au—S-type bonds on the AuMBs.
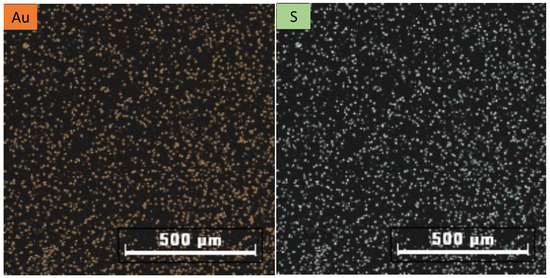
Figure 6.
Elemental mapping of Au (orange region) and S (light green) by EDS in an image obtained by SEM for the SPE-AuMBs/-NH2.
2.3. AB Detection
Ant was immobilized on NHS-activated SPE-AuMBs/-NH2 to become able to receive AB. Voltammograms were obtained by CV and DPV after each modification step and are shown in Figure 7.
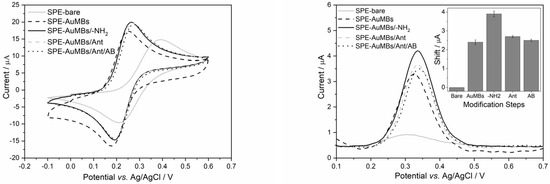
Figure 7.
Voltammograms were obtained for potassium ferrocyanide 2 mM in PBS (pH = 7.43) by CV (left) and DPV (right) using unmodified SPE and SPE after each modification step. CV was performed with Estep = 2 mV and v of 25 mV s−1. The parameters for DPV were Estep = 4 mV, Epulse = 2 mV, tpulse = 10 ms, and v = 10 mV s−1. The AB concentration tested was 0.25 µg mL−1.
As discussed previously, the cyclic voltammograms in Figure 7 (left) show that Iap increases until cysteamine adsorption. Then, the Iap of the electrode experiences two successive reductions due to the adsorptions of Ant and AB. Organic substances hinder electron transfer on the electrode surface. Thus, the presence of AB against the SARS-CoV-2 virus can be assessed by monitoring Iap using an Ant-activated SPE.
Voltammetric measurements by DPV were performed to monitor the modification steps through the potassium ferrocyanide anode signal. DPV is a technique that minimizes background current and is more advantageous for quantitative analysis [32]. The same behavior was observed, that is, Iap increases until cysteamine immobilization and shows successive reductions after Ant and AB immobilizations. The inset in Figure 7 shows a bar graph with the Iap shift that occurred after each modification step. The shift observed after the immobilization of AB on Ant was 0.2 µA.
A calibration curve (Figure 8) was obtained for Iap measurements from DPV voltammograms with respect to the application of different concentrations of AB.
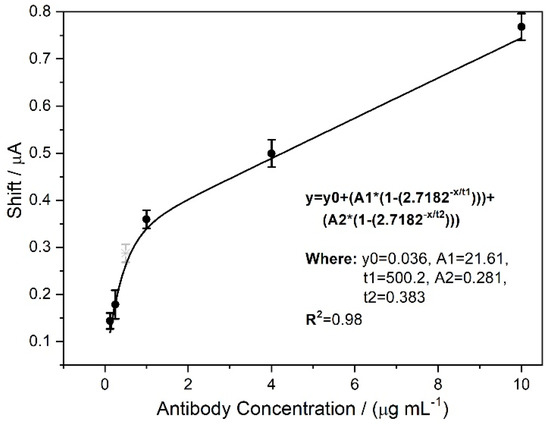
Figure 8.
Calibration curve for Iap variation as a function of AB concentration. The points were adjusted to exponential behavior.
The applied AB concentration range was from 0.250 to 10.0 μg mL−1, and the device responses were adjusted to exponential behavior. The equation and its parameters are shown in Figure 8. The electrode responses increased rapidly up to a concentration of 1.00 μg mL−1 and clearly lost sensitivity for higher concentrations. This is a typical behavior of loss of analyte adsorption capacity (surface saturation). For concentrations greater than 10.0 μg mL−1, the surface is completely saturated by AB molecules. This can be suggested because applying a concentration of 25.0 μg mL−1 caused a shift of 0.75 µA, which is approximately equal to the current variation observed for the concentration of 10.0 μg mL−1 of AB (0.77 ± 0.3 µA).
The analytical performance of the proposed device was compared with the recently reported SPE-based electrochemical biosensors. Table 2 presents the construction characteristics and the limit of detection (LOD) for the different sensor arrangements. The performance obtained here was comparable with another device with similar characteristics reported by Drobysch et al. [29] (first study in the list), considering a LOD obtained from the calibration curve’s linear range. However, the LOD was higher than those of devices that used modified SPEs and/or more sophisticated electrochemical techniques (such as EIS). Despite this, the performance shown here is fully compatible with antibodies against SARS-CoV-2 virus for analysis. A study reported by Nature showed that the concentration of IgM antibody against SARS-CoV-2 Spike (related to the active phase of the disease) in the serum is 1.5 μg mL−1 between 4 and 10 days after the onset of the disease and reaches maximum values around 8.0 μg mL−1 after 16–20 days since infection [30]. Thus, the proposed device covers all concentration ranges achieved by AB in infected patients. Furthermore, the biosensor has some distinct characteristics as it is a portable and energy-autonomous device that could easily perform rapid qualitative testing of AB (positive/negative type) from biological fluids in point-of-care and rapid analysis. The required time is around 20 min using an electrode previously prepared with Ant.

Table 2.
Characteristics of electrochemical biosensors for COVID-19 diagnosis based on screen-printed electrodes.
Additionally, it was shown that the biosensor could be used to infer the infection evolution stage by quantitative AB measurements. The accuracy test of the proposed calibration curve of Figure 8 was carried out by applying an AB solution with an intermediate concentration close to the inflection point in the curve (gray point in Figure 8). The variation in Iap after applying this solution was 0.287 µA. The fitting equation (insert in Figure 8) suggests that this response corresponds to a concentration of 0.574 μg mL−1 of AB. The actual AB concentration of the applied sample was 0.500 μg mL−1 (corresponds to half the AB concentration found in patients in the initial infection phase) and is close to that obtained by curve interpolation.
The estimated cost of electrode production for SARS-CoV-2 AB quantification is approximately 10.00 USD/unit. The disposable commercial SPE costs 6.00 USD/unit, and the reagents cost 4.00 USD/unit (including SARS-CoV-2 Ant, cysteamine, PBS, HAuCl4, NHS, and 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)). Costs could also be reduced with industrial-scale production. Comparatively, a serological or nasal test costs approximately USD 15.00. An additional advantage of the method proposed here is the possibility of acquiring a quantitative analysis of AB.
3. Materials and Methods
3.1. AuMBs Synthesis on SPE
Initially, water was dripped on a commercial carbon SPE (Metrohm, DPR-110, São Paulo, Brazil), and it was cleaned by applying a constant potential of 1.7 V vs. Ag/AgCl for 5 min. The AuMBs on the SPE were produced using the method reported in the literature [37]. For this, the SPE was rinsed with deionized water, and a 1.0 M HAuCl4/0.5 M H2SO4 aqueous solution was dripped onto the cleaned SPE. Then, a constant potential of −0,4 V vs. Ag/AgCl was applied for 20, 30, 50, and 300 s. CV measurements were performed for the SPE and SPE-AuMBs. The parameters used for all CV tests were a potential range of −0.1 to +0.6 V (begin potential 0.0 V), a 25 mV s−1 scan rate, and a 10 mV step potential. A 2.0 mM potassium ferrocyanide solution in phosphate buffer (PBS, pH = 7.4) was consistently used to evaluate the CV measurements’ electrochemical signal. The volume of liquids used to cover the SPEs fully was always 60 μL unless another volume was mentioned.
The electroactive area of the SPE and SPE-AuMBs was evaluated by acquiring cyclic voltammograms for potassium ferrocyanide 5.0 mM in KCl 0.5 M at different scan rates of 10, 25, 50, 75, 100, 125, and 150 mV s−1. The electrodes were characterized by scanning SEM-EDS (TESCAN, Libušina tř., Brno, Czech Republic). All electrochemical measurements used a bipotentiostat/galvanostat (Metrohm, μStat 400, São Paulo, Brazil).
3.2. SPE-AuMBs Amination
A 20 mM cysteamine solution was dropped on rinsed SPE-AuMBs. Then, a constant potential of 1.2 V vs. Ag/AgCl was applied on the electrode under cysteamine solution for 20 min. The aminated electrode was rinsed with water. Alternatively, an amination obtained by drop coating was also tested [31]. An aliquot of 10 μL 60 mM cysteamine solution was dropped only onto the SPE’s working electrode area. After incubation for 30 min, the aminated electrode was rinsed with water. The electrodes (SPE-AuMBs/-NH2) were characterized by SEM-EDS and XRF (Malvern Panalytical, Epsilon 1, São Paulo, Brazil).
3.3. Immobilization of the Spike Protein (Antigen) on the Electrode
Solutions of EDC (Sigma-Aldrich, Barueri, Brazil) and NHS (Sigma-Aldrich) were prepared using ultrapure water. The antigen and antibody solutions were prepared using PBS solution (pH = 7.4). Aliquots of 30 μL of 200 mM EDC and 20 μL of 200 mM NHS were mixed with 50 μL of 1 μg mL−1 SARS-CoV-2 spike antigen protein (Ant, Sigma-Aldrich) in an Eppendorf to activate the Ant carboxylic acid groups. After a 20 min reaction, the mixture was dropped (10 μL) onto the working electrode area of the SPE-AuMBs/-NH2 and incubated for 15 min to obtain SPE-AuMBs/Ant. The reaction between NHS-activated carboxylic acid (Ant) and the amine (cysteamine) groups results in amide formation. A 10 μL aliquot of 1 g mL−1 bovine serum albumin (BSA, Sigma-Aldrich) PBS solution was also dropped on the SPE-AuMBs/Ant for 20 min to block the non-specific sites. All incubations were conducted at 21 ± 3 °C.
3.4. Antibody Detection
After the biosensor preparation, 20 μL of the 0.250 μg mL−1 SARS-CoV-2 spike antibody protein (AB, Sigma-Aldrich) for the receptor-binding domain region, prepared in PBS, was incubated on the SPE-AuMBs/Ant surface for 15 min for an initial test. After AB immobilization, CV and DPV measurements in the potassium ferrocyanide probe were obtained after rinsing the surface with water to remove non-immobilized AB. Sequentially, a calibration curve regarding AB detection was also obtained using the same methodology. AB solutions were prepared at concentrations 0.125, 0.250, 1.00, 4.00, 10.0, and 25.0 μg mL−1 in commercial human serum (Sigma-Aldrich, ref. SAE0012). Each AB solution was detected on an individual SPE-AuMBs/Ant, and the tests were carried out in duplicate. The SPE-AuMBs/Ant electrodes were also used to assess AB in commercial human serum from a 0.500 μg mL−1 AB-spiked solution. The assays were carried out using the portable experimental setup illustrated in Figure 9 by DPV measurements.
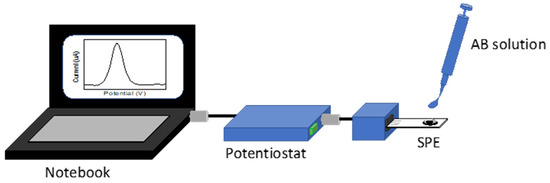
Figure 9.
Fully portable experimental setup for SARS-CoV-2 AB detection assays by DPV measurements.
4. Conclusions
Here, we proposed a simple and cheap electrochemical method using disposable SPEs, which had optimized responses by applying AuMBs. AuMBs production on SPEs was optimized, and the shortest electrochemical synthesis time tested (30 s) was the condition that showed the best signal for the electrode. The AuMBs-improved electrodes were aminated using two strategies: electrochemical and drop coatings. The first proved to be more effective for immobilizing cysteamine on gold, exploiting the strong chemical affinity of the metal with –SH groups. Aminated AuMBs–electrodes were tested to detect COVID-19 antibodies. It was then demonstrated that an antigen-activated SPE could qualitatively and quantitatively evaluate the presence of SARS-CoV-2 antibodies. The antibody analysis took around 15 min and was cheaper than traditional rapid tests. Thus, the method proved to be a good alternative for both qualitative analyses (quick “yes” or “no” test) and proved to be capable of estimating the evolution of the infection through quantitative analyses.
Author Contributions
Conceptualization, J.P.M. and A.F.M.; methodology, M.M.G.; formal analysis, M.M.G.; investigation, O.O.S.J. and P.H.M.B.; resources, J.P.M., E.G.B. and A.F.M.; data curation, E.G.B.; writing—original draft preparation, J.P.M. and M.M.G.; writing—review and editing, J.P.M. and P.H.M.B.; visualization, O.O.S.J.; supervision, J.P.M.; project administration, J.P.M.; funding acquisition, J.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development—CNPq (Brazil), grant number 305290/2020-7.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the CNPq—Brazil and the Laboratório Multiusuário de Apoio à Pesquisa da UTFPR Campus Apucarana (LAMAP)—UTFPR, Apucarana, Brazil. J.P.M. thanks the CNPq’s researcher grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, T.; Zhang, G.; Chai, H.; Qu, L.; Zhang, X. Flexible Biosensors Based on Colorimetry, Fluorescence, and Electrochemistry for Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 753692. [Google Scholar] [CrossRef] [PubMed]
- de Eguilaz, M.R.; Cumba, L.R.; Forster, R.J. Electrochemical Detection of Viruses and Antibodies: A Mini Review. Electrochem. Commun. 2020, 116, 106762. [Google Scholar] [CrossRef] [PubMed]
- Brazaca, L.C.; dos Santos, P.L.; de Oliveira, P.R.; Rocha, D.P.; Stefano, J.S.; Kalinke, C.; Abarza Muñoz, R.A.; Bonacin, J.A.; Janegitz, B.C.; Carrilho, E. Biosensing Strategies for the Electrochemical Detection of Viruses and Viral Diseases—A Review. Anal. Chim. Acta 2021, 1159, 338384. [Google Scholar] [CrossRef]
- Wan, X.; Du, H.; Tuo, D.; Qi, X.; Wang, T.; Wu, J.; Li, G. UiO-66/Carboxylated Multiwalled Carbon Nanotube Composites for Highly Efficient and Stable Voltammetric Sensors for Gatifloxacin. ACS Appl. Nano Mater. 2023, 6, 19403–19413. [Google Scholar] [CrossRef]
- Wang, T.; Xia, Y.; Wan, X.; Zhang, Y.; Chen, N.; Jin, Y.; Li, G. A Facile and Efficient Voltammetric Sensor for Marbofloxacin Detection Based on Zirconium-Based Metal-Organic Framework UiO-66/Nitrogen-Doped Graphene Nanocomposite. Microchem. J. 2024, 201, 110673. [Google Scholar] [CrossRef]
- Safitri, E.; Heng, L.Y.; Ahmad, M.; Tan, L.L.; Nazaruddin, N.; Suhud, K.; Chiang, C.P.; Iqhrammullah, M. Electrochemical DNA Biosensor Based on Mercaptopropionic Acid-Capped ZnS Quantum Dots for Determination of the Gender of Arowana Fish. Biosensors 2022, 12, 650. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Wang, L.; Zhang, S.; Yan, L.; Zhang, X.; Sun, W. Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode. Molecules 2023, 28, 6151. [Google Scholar] [CrossRef]
- Shao, B.; Chen, W.; Yan, L.; Huang, Y.; Wang, B.; Zou, Q.; Xuan, Y.; Sun, W.; Niu, Y. Preparation and Electrocatalytic Study of Myoglobin Biosensor Based on Platinum-Gold-Three Dimensional Graphene Modified Electrode. Int. J. Electrochem. Sci. 2021, 16, 211039. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Nanda, P.; Sanati, A.; Pritzker, M.; Chen, P. Direct Electrochemistry of Hemoglobin/Peptide-Carbon Nanotube Modified Electrode for Hydrogen Peroxide Biosensing. Mater. Lett. 2023, 335, 133799. [Google Scholar] [CrossRef]
- Yan, L.; Shi, F.; Zhang, J.; Niu, Y.; Huang, L.; Huang, Y.; Sun, W. Electrochemical DNA Biosensor Based on Platinum-Gold Bimetal Decorated Graphene Modified Electrode for the Detection of Vibrio Parahaemolyticus Specific Tlh Gene Sequence. Curr. Anal. Chem. 2022, 18, 781–789. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.; Deng, Y.; Cheng, H.; Li, X.; Yan, L.; Li, G.; Sun, W. Reduced Graphene Oxide/Titanium Carbide MXene Nanocomposite-Modified Electrode for Electrochemical Hemoglobin Biosensor. J. Chin. Chem. Soc. 2021, 68, 2326–2336. [Google Scholar] [CrossRef]
- Wen, Z.; Niu, X.; Li, X.; Zhao, W.; Li, X.; Ma, D.; Deng, Y.; Sun, X.; Sun, W. Application of Nanosized LiFePO4 Modified Electrode to Electrochemical Sensor and Biosensor. Curr. Anal. Chem. 2018, 14, 452–457. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, R.; Chen, J.; Feng, Z.; Lin, S. A Highly Sensitivity Electrochemistry Biosensor for E. coli DNA Detection Based on Hollow and Porous DCuO@rGO Composite. Inorg. Chem. Commun. 2024, 166, 112621. [Google Scholar] [CrossRef]
- Pusomjit, P.; Teengam, P.; Thepsuparungsikul, N.; Sanongkiet, S.; Chailapakul, O. Impedimetric Determination of Cortisol Using Screen-Printed Electrode with Aptamer-Modified Magnetic Beads. Microchim. Acta 2021, 188, 41. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Jiang, H.; Lee, E.C. Physical Surface Modification of Carbon-Nanotube/ Polydimethylsiloxane Composite Electrodes for High-Sensitivity Dna Detection. Nanomaterials 2021, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, J.; Jiang, T.; Li, B.; Chen, J. A Portable Electrochemical Impedance Spectroscopy Lab-on-Chip System for Biosensing Applications. In Proceedings of the IEEE International Symposium on Circuits and Systems, Austin, TX, USA, 27 May–1 June 2022; pp. 1347–1351. [Google Scholar] [CrossRef]
- Roberto de Oliveira, P.; Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Wuamprakhon, P.; Hurst, N.J.; Dempsey-Hibbert, N.C.; Sawangphruk, M.; Janegitz, B.C.; Banks, C.E. Low-Cost, Facile Droplet Modification of Screen-Printed Arrays for Internally Validated Electrochemical Detection of Serum Procalcitonin. Biosens. Bioelectron. 2023, 228, 115220. [Google Scholar] [CrossRef]
- Elewi, A.S.; Al-Shammaree, S.A.W.; AL Sammarraie, A.K.M.A. Hydrogen Peroxide Biosensor Based on Hemoglobin-Modified Gold Nanoparticles–Screen Printed Carbon Electrode. Sens. Biosensing Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Koudelková, Z.; Sedláčková, E.; Richtera, L.; Švec, P.; Adam, V. Gold Nanoparticles Modified Screen Printed Carbon Electrode as a Tool for Detection of TP53. In Proceedings of the NANOCON 11th International Conference on Nanomaterials—Research & Application, Brno, Czech Republic, 16–18 October 2020; pp. 402–405. [Google Scholar] [CrossRef]
- Mulyani, R.; Yumna, N.; Maksum, I.P.; Subroto, T.; Hartati, Y.W. Optimization of Aptamer-Based Electrochemical Biosensor for ATP Detection Using Screen-Printed Carbon Electrode/Gold Nanoparticles (SPCE/AuNP). Indones. J. Chem. 2022, 22, 1256–1268. [Google Scholar] [CrossRef]
- Hodson, R. Preparing the World for the next Pandemic. Nature 2022, 610, S33. [Google Scholar] [CrossRef]
- Nascimento, E.D.; Fonseca, W.T.; de Oliveira, T.R.; de Correia, C.R.S.T.B.; Faça, V.M.; de Morais, B.P.; Silvestrini, V.C.; Pott-Junior, H.; Teixeira, F.R.; Faria, R.C. COVID-19 Diagnosis by SARS-CoV-2 Spike Protein Detection in Saliva Using an Ultrasensitive Magneto-Assay Based on Disposable Electrochemical Sensor. Sens. Actuators B Chem. 2022, 353, 131128. [Google Scholar] [CrossRef]
- Triastuti, A.; Zakiyyah, S.N.; Gaffar, S.; Anshori, I.; Surawijaya, A.; Hidayat, D.; Wiraswati, H.L.; Yusuf, M.; Hartati, Y.W. CeO2@NH2 Functionalized Electrodes for the Rapid Detection of SARS-CoV-2 Spike Receptor Binding Domain. RSC Adv. 2023, 13, 5874–5884. [Google Scholar] [CrossRef] [PubMed]
- Brazaca, L.C.; Imamura, A.H.; Gomes, N.O.; Almeida, M.B.; Scheidt, D.T.; Raymundo-Pereira, P.A.; Oliveira, O.N.; Janegitz, B.C.; Machado, S.A.S.; Carrilho, E. Electrochemical Immunosensors Using Electrodeposited Gold Nanostructures for Detecting the S Proteins from SARS-CoV and SARS-CoV-2. Anal. Bioanal. Chem. 2022, 414, 5507–5517. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, E.; Erdemir, E.; Demirbilek, N.; Liv, L. Colorimetric and Electrochemical Detection of SARS-CoV-2 Spike Antigen with a Gold Nanoparticle-Based Biosensor. Anal. Chim. Acta 2021, 1182, 338939. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon Nanotube Field-Effect Transistor (CNT-FET)-Based Biosensor for Rapid Detection of SARS-CoV-2 (COVID-19) Surface Spike Protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Yang, X.; Yin, Z.Z.; Zheng, G.; Zhou, M.; Zhang, H.; Li, J.; Cai, W.; Kong, Y. Molecularly Imprinted Miniature Electrochemical Biosensor for SARS-CoV-2 Spike Protein Based on Au Nanoparticles and Reduced Graphene Oxide Modified Acupuncture Needle. Bioelectrochemistry 2023, 151, 108375. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Molecularly Imprinted Polymer Based Electrochemical Sensor for Quantitative Detection of SARS-CoV-2 Spike Protein. Sens. Actuators B Chem. 2022, 353, 131160. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Viter, R.; Chen, C.F.; Ramanavicius, A.; Ramanaviciene, A. Determination of RSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures. Biosensors 2022, 12, 593. [Google Scholar] [CrossRef]
- Zeng, W.; Ma, H.; Ding, C.; Yang, Y.; Sun, Y.; Huang, X.; He, W.; Xiang, Y.; Gao, Y.; Jin, T. Characterization of SARS-CoV-2-Specific Antibodies in COVID-19 Patients Reveals Highly Potent Neutralizing IgA. Signal Transduct. Target. Ther. 2021, 6, 35. [Google Scholar] [CrossRef]
- Liv, L.; Kayabay, H. An Electrochemical Biosensing Platform for the SARS-CoV-2 Spike Antibody Detection Based on the Functionalised SARS-CoV-2 Spike Antigen Modified Electrode. ChemistrySelect 2022, 7, e202200256. [Google Scholar] [CrossRef]
- Klein, R.S.; Taniguchi, M.M.; dos Santos, P.D.; Bonafe, E.G.; Martins, A.F.; Monteiro, J.P. Trans-Resveratrol Electrochemical Detection Using Portable Device Based on Unmodified Screen-Printed Electrode. J. Pharm. Biomed. Anal. 2022, 207, 114399. [Google Scholar] [CrossRef]
- Jaewjaroenwattana, J.; Phoolcharoen, W.; Pasomsub, E.; Teengam, P.; Chailapakul, O. Electrochemical Paper-Based Antigen Sensing Platform Using Plant-Derived Monoclonal Antibody for Detecting SARS-CoV-2. Talanta 2023, 251, 123783. [Google Scholar] [CrossRef] [PubMed]
- Primpray, V.; Kamsong, W.; Pakapongpan, S.; Phochakum, K.; Kaewchaem, A.; Sappat, A.; Wisitsoraat, A.; Lomas, T.; Tuantranont, A.; Karuwan, C. An Alternative Ready-to-Use Electrochemical Immunosensor for Point-of-Care COVID-19 Diagnosis Using Graphene Screen-Printed Electrodes Coupled with a 3D-Printed Portable Potentiostat. Talanta Open 2022, 6, 100155. [Google Scholar] [CrossRef] [PubMed]
- Gevaerd, A.; Carneiro, E.A.; Gogola, J.L.; Nicollete, D.R.P.; Santiago, E.B.; Riedi, H.P.; Timm, A.; Predebon, J.V.; Hartmann, L.F.; Ribeiro, V.H.A.; et al. Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor. Sensors 2024, 24, 3772. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.A.; Khan, S.A.; Rehman, A. Screen-Printed Graphene/Carbon Electrodes on Paper Substrates as Impedance Sensors for Detection of Coronavirus in Nasopharyngeal Fluid Samples. Diagnostics 2021, 11, 1030. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Crescitelli, A.; Pilloton, R.; Esposito, E. Impedimetric Label-Free Immunosensor on Disposable Modified Screen-Printed Electrodes for Ochratoxin A. Biosensors 2016, 6, 33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).