Abstract
In the periodic table of the elements, ruthenium occupies an excellent position, just below iron. And like iron, it possesses several oxidation states, with +2 and +3 being the most common. Accordingly, ruthenium chemistry is extremely rich and well developed, and ruthenium complexes show excellent catalytic aptitude, tremendous redox capacity, and intriguing biological activity. However, in the design of sensors, the use of ruthenium complexes can be better exploited, as they possess valuable electro- and photochemical properties. Therefore, there is an opportunity here, and ruthenium-based complexes might become, one day, key players in sensing technology. Starting a new research project with ruthenium-based sensors ourselves, writing this review was essential to see the current state of research in the field, to better identify opportunities and to have an overview of state-of-the-art examples.
1. Introduction
When writing a review on sensors, the first challenge is to find the main thread. The domain is so large because chemical sensors not only come in all kinds of shapes and structures, but they also use different mechanisms to respond to external stimuli (physical, biological, and chemical), and they are generally designed to target different analytes. Therefore, it is important to take a decision early on; otherwise, one can be rapidly overwhelmed by the literature. Herein, we have focused our attention on sensors incorporating ruthenium-based complexes. Ruthenium is an extremely popular transition metal, linked to Nobel Prize-winning discoveries [1]. It is a d-block metal with several accessible oxidation states (−2 to +8), it possesses a rich redox chemistry, it offers structural diversity, its chemistry is often compatible with water, and accordingly, it is an excellent platform for the preparation of sensors. However, despite such positive attributes, sensors developed on ligand design and ruthenium-based coordination chemistry are still limited.
In this review, we will present ruthenium-based sensors from an analyte point of view, trying to give the readers not only state-of-the-art examples of ruthenium-based sensors, but also where ruthenium complexes can be of special interest for sensing. The different analytes will be divided and sub-divided using the most commonly accepted families, such as anions, cations, gases, sugars, pollutants, biomolecules, etc. [2]. And each family will be introduced separately, giving an overview of the situation; providing, when available, information on sensitivity, affinity, selectivity, and the limit of detection; and trying to pinpoint the remaining challenges associated with specific analytes.
2. Ruthenium-Based Sensors
2.1. Cations
In terms of cations, alkali and alkaline earth metals together with transition metal ions remain the most common cationic analytes that have been studied with ruthenium-based sensors. Of particular interest are those dealing with Cu2+, a common pollutant in the environment (pesticides and fertilizers), but more importantly, an interesting biomarker in metallomics [3]. All ruthenium-based sensors for metal ions are either tris(bipyridyl) or bis(terpyridyl) ruthenium(II) complexes with at least one of the pyridyl-based ligand being functionalized with a coordinating unit for metal ions. Then, upon the coordination of copper ions to the coordinating appendage, the photophysical property of the ruthenium complex is modified, thus triggering a signal which can be observed by the naked eye or by various spectroscopic methods (UV-visible, fluorescence, and NMR).
Almost 25 years ago, a bis(terpyridyl) ruthenium(II) complex was synthesized and tested as a fluorescent sensor for transition metal ions [4]. The introduction of a 1,4,8,11-tetraazacyclotetradecane macrocycle on the terpyridyl ligand has afforded a functionalized complex with a cyclic tetradentate coordinating appendage (Figure 1). Interestingly, in the presence of Ni2+, Zn2+, Cd2+, Hg2+, and Pb2+ at various pH in water/acetonitrile mixtures, no changes in the fluorescence behavior of the complex were observed. However, upon the addition of Cu2+ ions at neutral pH, a substantial quenching of the luminescent intensity of the complex was observed. The result suggests that the coordination of Cu2+ in the macrocycle triggers an energy transfer process between the two parts of the bimetallic system (Cu2+ and Ru2+), thus modifying the electronic properties of the ruthenium-based fluorophore.
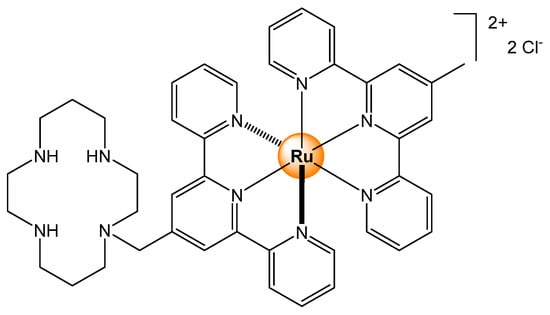
Figure 1.
Bis(terpyridyl) ruthenium complex functionalized with a tetraazacyclotetradecane macrocycle used for metal ions sensing [4].
Tris(bipyridyl) derivatives incorporating an analogous tetradentate ligand built from a flexible 3,7-diazabicyclo[3.3.1]nonane core functionalized with two co-planar pyridyl groups have been synthesized and tested as sensors (Figure 2) [5]. Like the terpyridyl derivatives, in the presence of Cu2+ ions, the luminescence intensity is significantly quenched, up to 40% with one equivalent of Cu2+, and even more at a higher stoichiometric ratio. Similarly, the quenching of the ruthenium-based fluorophore after the coordination of Cu2+ to the tetradentate ligand was interpreted in terms of an energy transfer mechanism.
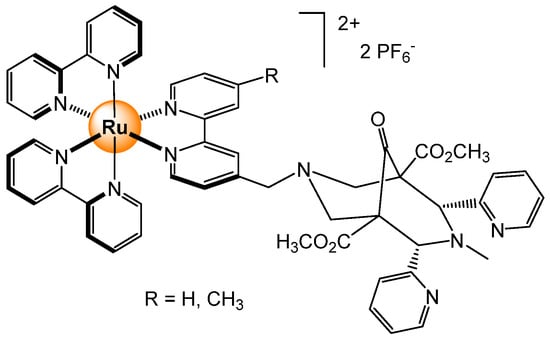
Figure 2.
Tris(bipyridyl) ruthenium complexes functionalized with a tetradentate ligand [5].
A system with two tris(bipyridyl) complexes, bridged by a bis-phenanthroline linker, has been synthesized and tested as a biological sensor for metal ions (Figure 3) [6]. The system is extremely sensitive to Cu2+ with a limit of detection of 3.33 × 10−8 M. However, in the presence of Na+, K+, Mg2+, Ca2+, Zn2+, Ag+, Fe2+, Fe3+, Ni2+, Mn2+, Co2+, Cd2+, Hg2+, and Cr3+, the luminescence intensity remains almost the same (>80%). The strong association constant (Ka ≈ 1.7 × 106 M−1) between Cu2+ and the dinuclear complex offers selectivity, and the water solubility of the system has provided an excellent probe for the detection of Cu2+ in zebrafish, taking, once again, advantage of the photophysical properties of ruthenium complexes.
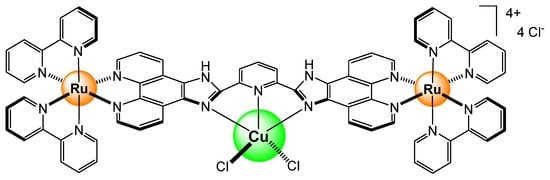
Figure 3.
Coordination of a Cu2+ ion in a bimetallic complex [6].
The affinity of functionalized tris(bipyridyl) ruthenium complexes for Cu2+ ions has been demonstrated on many other occasions [7,8,9]. In these systems, like those presented here, the strategy is very similar: the functionalization of one of the polypyridyl ligands on the ruthenium(II) center to be able to coordinate a cationic metal ion. Then, after coordination, the photophysical property of the polypyridyl ruthenium complex is affected, thus triggering a signal, which can be detected. However, adding selectivity and increasing affinity can only be performed upon ligand design, and too often, the ligand can coordinate different metal ions without discrimination.
Among other metal ions, Hg2+ is also an interesting target. Mercury is a volatile metal, which is toxic, and is often found in our environment due to mining activity or fungicidal and antiseptic applications. The most common oxidation state of mercury is +2, and therefore, developing sensors for Hg2+ is quite relevant. The nature of Hg2+, being a soft metal ion, requires different captors than Cu2+. For example, instead of using a functionalized polypyridyl ligand, thiocyanate derivatives have been prepared [10]. In these complexes (Figure 4), the soft metal ion reacts with the sulfur atom of the thiocyanate ligand to form mercury adducts, and upon the coordination of Hg2+, the photophysical property of the ruthenium complexes is modified. Indeed, with no metal, or in the presence of competing ions Cd2+, Pb2+, Fe2+, Cu2+, or Zn2+ (≈13 ppm), the aqueous solutions are green, while in the presence of Hg2+ (HgCl2) at the same concentration, the aqueous solution turns pink. In such a system, the limit of detection was determined for the best combination to be around 100 ppb (part per billion).
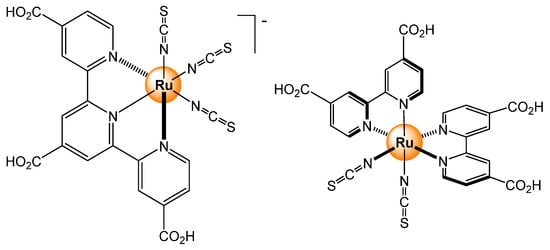
Figure 4.
Structures of two molecular probes for Hg2+ ions [10].
To obtain a reversible system, ruthenium complexes were also incorporated in a mesoporous nanocrystalline TiO2 support [10]. Like before, without Hg2+, the color of the material was initially green. However, when the TiO2 film loaded with the terpyridyl-tris(thiocyanato) ruthenium complex was dipped for an hour in an aqueous solution containing Hg2+ ions at 9 ppm concentration, the color changed from green to pink. Then, the ion-free ruthenium complex was regenerated almost instantly by dipping the film in a 10 mM aqueous solution of KI. This process was repeated several times, showing the reversibility of the system, which is crucial for commercial applications. Similar ruthenium complexes were loaded on other supports, such as nanophosphors [11], metal/organic frameworks (MOFs) [12], and mesoporous silica coating upconversion nanoparticles [13]. All the systems show great potential for the detection of the Hg2+ ion.
Other coordination strategies can be used to sense Hg2+, as well as other metal ions, exploiting, for example, ligands with both N∩N and N∩O chelating groups [14]. However, having chelates reduces selectivity, both being stronger ligands due to the chelating effect, unless one of the two units is coordinated to a metal prior to sensing. Indeed, when the N∩N chelating group is coordinated to a bis(bipyridyl) ruthenium unit (Figure 5), the remaining N∩O chelating site can selectively bind Cu2+ and Hg2+, which, like the previous examples, turns off the fluorescence property of the ruthenium complex through an intramolecular electron transfer photo-induced process, and accordingly, provides a visual signal for the detection of metal ions.
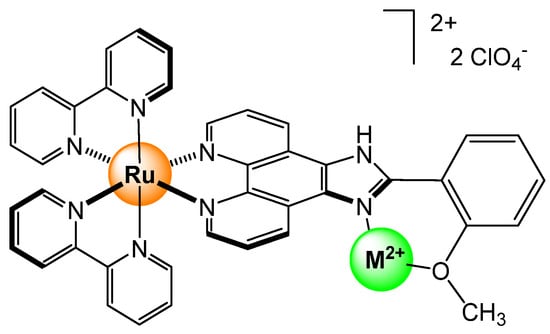
Figure 5.
Bis(bipyridyl) ruthenium complex with a pending N∩O chelating unit for sensing Hg2+ and Cu2+ metal ions [14].
Regarding alkali metals interacting with arene ruthenium-based complexes, the first example came from the group of Severin [15]. Trinuclear arene ruthenium metallacycles were prepared to mimic the cavity of crown ethers, thus allowing small ions to interact with the three perfectly positioned oxygen atoms of the bowl-shaped cavity (Scheme 1). In these systems, the selectivity for Li+ over Na+ was achieved by steric constraints from the arene ligands (p-cymene, toluene, triethylbenzene, hexamethylbenzene), which were located at the periphery of the cavity. The stability constant for these host–guest systems was quite high (>105 L·mol−1 in CD3OD for LiCl), but also very dependent on the solvent used. Interestingly, when the host cavity is occupied by a guest, the trinuclear complex is protected from oxidation. However, in the absence of LiCl or NaCl in the cavity of the host, a solution containing the trinuclear arene ruthenium metallacycle upon the addition of the oxidizing agent 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) will show a distinct color change, from yellow to orange–red. This color change is due to the oxidation of the ruthenium complex, which is extremely fast for the free receptor, and slow when occupied.
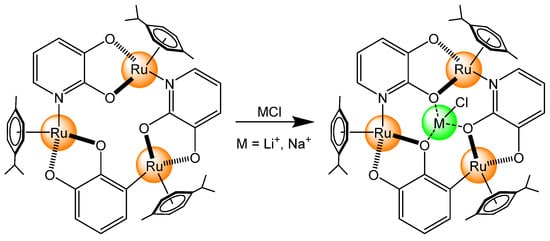
Scheme 1.
Sensing mechanism of alkali metal ions in a trinuclear arene ruthenium complex [15].
2.2. Anions
From halides to phosphates, nitrates, etc., and because of acid–base chemistry, there is a plethora of anions, and we have divided this section into four sub-groups: halides and small anions, phosphates, carboxylates, and others. In these sections, some ruthenium-based sensors have interacted with different types of anions and could have been presented in two or three sub-sections; however, we have discussed and located these complexes in the section where they have shown the best selectivity and/or affinity for a specific anion to avoid repetitions.
2.2.1. Halides and Small Anions
Halides and small anions play important roles in biology, and following industrialization, they can also be considered as environmental pollutants at high concentrations. Therefore, the development of rapid and accurate sensors for small anions is crucial, and several groups have worked on the matter for years, including inorganic and organometallic chemists. Accordingly, ruthenium-based sensors for small anions have been developed, taking advantage of the coordination and electronic properties of the ruthenium center.
The perturbation on the fluorescence behavior of polypyridyl ruthenium complexes upon the complexation of metal ions has also been exploited for small anions sensing [16]. Indeed, a series of hetero-dinuclear complexes in which Ni2+, Cu2+, or Zn2+ are coordinated to a tris(bipyridyl) ruthenium unit has been prepared (Figure 6) [16]. In the presence of coordinating anions (AcO−, H2PO4−, Cl−, Br−, or I−), the dicationic metal is displaced from the thioacetate pocket, thus modifying the fluorescence spectrum of the ruthenium complex. The increase in luminescence was especially strong when a source of Cl− was added to a solution containing the zinc derivative.
Figure 6.
Sensing of small anions by hetero-dinuclear complexes [16].
Arene ruthenium complexes were also used to sense small anions (AcO−, H2PO4−, HSO4−, CF3SO3−, NO3−, Cl−, and Br−) [17]. The presence of amino groups on the pyridine-based ligands can interact with small anions in a 1:1 or 1:2 ratio. Under a 1:2 ratio (anion–complex), the anion is surrounded by two arene ruthenium complexes, forming four weak N-H…anion interactions (Scheme 2). In such an environment, the ligand-to-metal charge transfer which takes place in the free complex is disrupted, thus modifying the fluorescence spectrum. Similarly, upon the addition of anions, some 1H NMR signals become diastereotopic, providing another spectroscopic method to study the host-guest systems, and if desired, to allow the determination of binding constants.
Scheme 2.
Sensing of small anions by arene ruthenium complexes (X− = AcO−, H2PO4−, HSO4−, CF3SO3−, NO3−, Cl−, Br−) upon formation of hydrogen-bonded dimeric systems [17].
Similar arene ruthenium complexes incorporating two bis-imidazolyl tetramethylbenzene ligands have also been prepared [18]. The parallel orientation of the two ligands provides a bowl-shaped cavity that can host anions. When trapped in that cavity, the anion forms several hydrogen-bonded interactions with the side arms of the complex, these interactions being observed by 1H NMR. Depending on the nature of the anion, up to 6 hydrogen bonds are formed, thus creating strong binding systems via a second coordination sphere approach [19].
Bis(terpyridyl) ruthenium complexes have been used as well for anion sensing [20]. In the presence of an excess of anions (AcO−, BF4−, ClO4−, PF6−, H2PO4−, CN−, SO42−, F−, Cl−, Br−, or I−), the imidazolyl N-H groups in the terpyridyl ligands are deprotonated (Figure 7), which triggers a photochromic change in the complex in a solution. In addition to this naked-eye observation, absorption, emission, 1H NMR, and cyclic voltammetry techniques can also be used to determine the presence of anions in a solution. The best results were observed with fluoride and cyanide, the binding constants being ≈2.0 × 105 M−1 in dimethylsulfoxide; however, most anions showed interactions with the proton of the imidazolyl-based ligands, thus offering the possibility to use such complexes as proton-driven molecular switches.
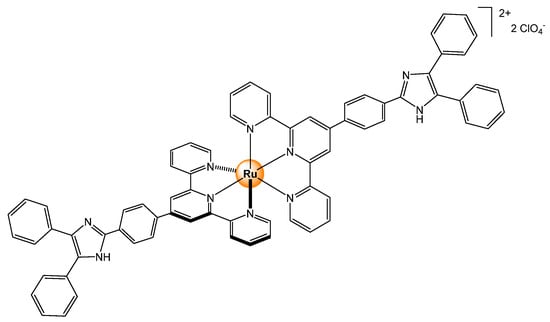
Figure 7.
Sensing of small anions from a bis(terpyridyl) ruthenium complex [20].
Similar interactions between anions and the N-H proton of 2,2′-dipyridylamine ligands have been observed on tris(bipyridyl) ruthenium complexes (Figure 8) [21]. Among several anions (AcO−, ClO4−, PF6−, NO3−, CN−, HSO4−, F−, Cl−, and Br−), only CN− and F− show strong interaction in acetonitrile (25 °C), with the binding constants of 104 dm3·mol−1 for the mono-substituted derivative (Figure 8I), 1010 dm6·mol−2 for the di-substituted (Figure 8II), and 1014 dm9 · mol−3 for the tris-2,2′-dipyridylamine ruthenium complex (Figure 8III). The naked-eye detection of anions occurs by deprotonation of the N-H groups, as demonstrated by a combination of 1H NMR, electrochemistry, and emission spectroscopy studies.
Figure 8.
Sensing of small anions from tris(bipyridyl) ruthenium complexes, incorporating one (I), two (II), or three (III) 2,2′-dipyridylamine ligands [21].
Cationic cyclometalated ruthenium complexes with a Lewis acid appendage at the periphery for anion sensing have been synthesized and characterized [22,23]. For the boryl derivative (Figure 9A), in the presence of F− and CN−, the corresponding zwitterionic fluoroborate and cyanoborate species are formed, with the binding constants (9/1 THF/DMF mixture) of 8.0 × 106 M−1 for fluoride and >107 M−1 for cyanide, respectively [22]. The antimony analog (Figure 9B) shows in acetonitrile with slightly weaker binding constants, being 6.8 × 105 M−1 for CN− and 1.4 × 106 M−1 for F− [23]. In both systems, the coordination of an anion on the Lewis acid moiety modifies the photophysical and electrochemical properties of the ruthenium polypyridyl complex, which is used to sense the presence of small anions in a solution.
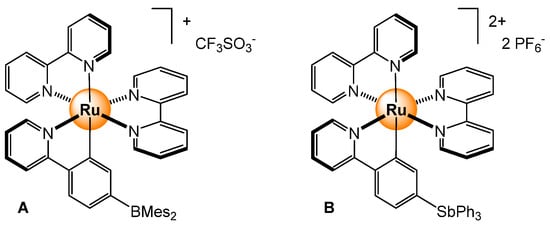
Figure 9.
Sensing of cyanide and fluoride from cyclometalated ruthenium complexes (Mes = mesityl, Ph = phenyl) [22,23].
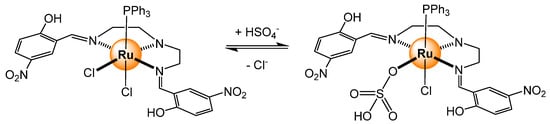
The anion exchanged capability of an inorganic [RuCl2(PPh3)L] (L = N,N-bis(2-hydroxy-5-nitrobenzaldehyde)-2,2′-diaminodiethylamine) complex has been used to sense small anions [24]. In the presence of bisulfate, a strong enhancement of the fluorescence (λex = 310 nm, λem = 347 nm) was observed, which was attributed to the replacement of one coordinated chloride with a bisulfate anion (Scheme 3). The binding constant was estimated at 2.3 × 105 M−1 (8/2 H2O/CH3CN mixture). The fluorescence remains high in the presence of an excess of other anions (AcO−, ClO4−, PF6−, NO3−, CN−, H2PO4−, S2O32−, F−, Cl−, Br−, or I−), showing a specificity for the bisulfate anion.
Scheme 3.
Anion exchange in a dichloro ruthenium complex [24].
2.2.2. Phosphates
Phosphates are fundamental molecules in biology and essential to living organisms. Phosphates are also critical in various cellular mechanisms, and participate in the formation of membranes, DNA, RNA, and proteins [25]. The selective binding and recognition of phosphate derivatives by proteins is a highly regulated event in living systems and of great interest for medicinal or analytical purposes. Therefore, sensing phosphates in aqueous media has been under scrutiny for decades, and several systems and strategies have been deployed to develop sensors. Accordingly, some ruthenium-based sensors for phosphates have been designed.
Among the first ruthenium-based sensors for phosphates, the calix[4]arene ruthenium bipyridyl complexes are worth mentioning [26]. In these systems, the phosphate anion interacts with amido groups, thus forming multiple hydrogen bonds. The strong affinity between the cationic complex (Figure 10) and phosphates is highlighted by the formation of single crystals, in which the ionic interactions are well defined. The stability constant between H2PO4− and the calix[4]arene ruthenium complex in DMSO is estimated at 2.8 × 104 M−1. The interaction is also reflected by a higher quantum yield in a solution of the ruthenium–phosphate adduct as compared to the free complex, providing a rapid detection tool.
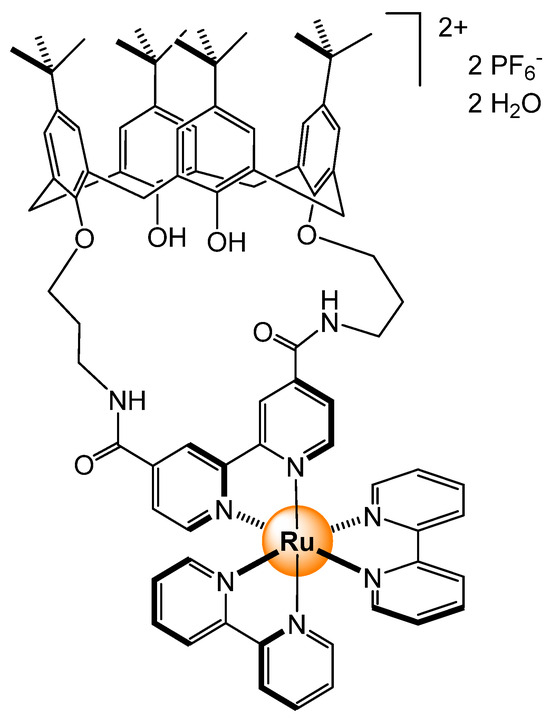
Figure 10.
Sensing of phosphates from a calix[4]arene-functionalized ruthenium complex [26].
A few years later, a series of analogous amido-pyridyl ligands coordinated to bis(bipyridyl) ruthenium unit was prepared [27]. The addition of imidazolium groups at the end of the amido-containing linkers (Figure 11) has increased the luminescence response for H2PO4− (by 226%). In terms of binding constants, the affinity for Cl−, as well as other anions (Br− and AcO−), were higher (7.6 × 104 M−1 for Cl− in a 9/1 CH3CN/H2O mixture), thus limiting selectivity, but interestingly, a blue shift of 11% was observed in the presence of Na2ATP (ATP = adenosine triphosphate). The ability of sensing ATP2− in a water/acetonitrile mixture opens new perspectives for biological applications.
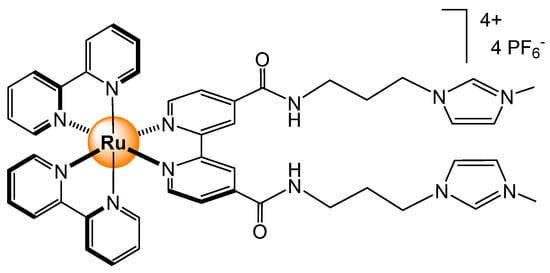
Figure 11.
Imidazolium-amido bipyridyl ligands attached to ruthenium bis(bipyridyl) center for phosphates’ sensing [27].
A ferrocene-imidazophenanthroline ligand was also designed for anion recognition [28]. The ligand and the corresponding ruthenium complex (Figure 12) interact with various anions. The redox-active ferrocenyl group is used for ADP (ADP = adenosine diphosphate) and ATP sensing through different signaling pathways, electrochemical, spectral, and fluorescence. The hetero-bimetallic complex showed high affinity for small anions like Cl− (K = 4.5 × 104 M−1 in CH3CN), having an important fluorescence enhancement, as well as a cathodic shift of the ferrocenyl oxidation wave. Moreover, the ferrocenyl-functionalized ligand can selectively sense HP2O73− anions over ADP and ATP due to a deprotonation process involving the imidazolyl moiety. The binding constant between the ferrocenyl complex and hydrogen-pyrophosphate was estimated to be 6.2 × 104 M−1 in CH3CN.
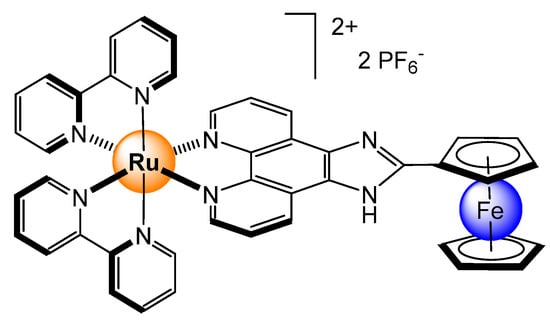
Figure 12.
A ferrocene-imidazophenanthroline ligand coordinated to a ruthenium bis(pyridyl) unit for sensing HP2O73−, ADP, and ATP [28].
Amino-benzenesulphonamido phenanthroline ligands have been coordinated to bis(bipyridyl) ruthenium unit to afford a series of functionalized complexes [29]. Like the previous sensors, the amino and amido groups are sensitive to the presence of various anions (AcO−, F−, and H2PO4−). In acetonitrile, the binding constants are in the range 3 × 103 to 5 × 104 M−1, which is comparable to those found for imidazolyl-phenanthrolin-(nitrophenyl)diazenylphenol [30] and aryl urea derivatized 2,2′-bipyridyl analogs [31]. In all cases, hydrogen bonds are observed between the anions and the functionalized ruthenium polypyridyl complex, thus disturbing the electronic properties of the ruthenium(II) center.
Coordination-driven self-assembly was used to develop luminescence hosts for the selective sensing of ATP in water [32]. The water-soluble hetero-bimetallic assemblies were built from two ruthenium bis-dipyridylphenazine units and two rhenium chloro triscarbonyl metal centers linked by two tetrapyridyl connectors (Figure 13). This particular host shows a specific emission response to ATP, while with the structurally analog GTP (guanosine triphosphate), no such emission behavior was observed. Computational studies suggest a different host-guest binding geometry, thus modulating the host-guest stacking interactions, and ultimately, modifying the luminescence response of the ruthenium(II) sensor.
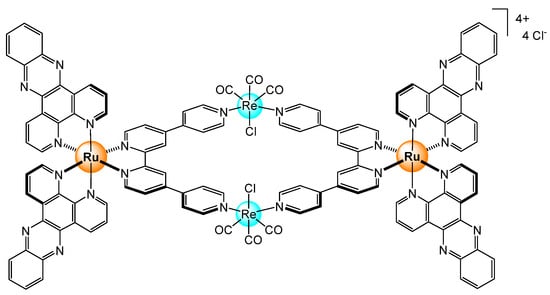
Figure 13.
A water-soluble bimetallic metalla-assembly to selectively sense ATP [32].
Another imidazolyl-polypyridyl ruthenium-based complex has been used to sense anions and cations [33,34]. Despite the dicationic nature of the ruthenium complex, the presence of a tris-pyridyl unit at the periphery allows coordination to cationic metal ions (Figure 14), thus offering to some extent the possibility of sensing both anions and cations. Indeed, Fe2+ and H2PO4− have shown a specific affinity for the complex, the metal ions bind to the terpyridyl unit, while H2PO4− forms hydrogen bonds with the imidazolyl group. Both host-guest systems show a naked-eye response in solution, offering a dual functional sensor. The terpyridyl group was also used to coordinate Re(CO)3Cl unit [34]. The hetero-bimetallic complex shows a higher affinity for H2PO4−, with a binding constant of 1.5 × 1010 M−2 in CH3CN. The enhanced binding affinity and increased emission were exploited for imaging cancer cells [34].
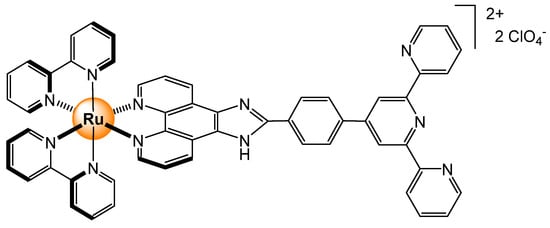
Figure 14.
Ruthenium-based sensor with dual imidazolyl and terpyridyl functions [33].
Other functional groups can be inserted at the periphery of polypyridyl ruthenium complexes. For example, ruthenium(II) complexes incorporating two phenanthrolines and a triazolo-pyridyl functionalized ligand have been designed for anion sensing [35,36]. The iodo derivative (Figure 15, left) interacts strongly with H2PO4− through halogen-bond interactions, with an association constant of 1.94 × 105 M−1 (CH3CN solution) [35]. Similarly, the 4-fluorophenyl urea functionalized complex (Figure 15, right) selectively sensed phosphates (H2PO4− and HP2O73−) in the presence of competitive anions (AcO−, ClO4−, PhCO2−, NO3−, HSO4−, CO32−, F−, Cl−, Br−, or I−) [36]. Interestingly, a titration of dihydrogen phosphate with the ruthenium complex showed a two-site binding system, while with hydrogen pyrophosphate a single 1:1 binding system was observed.
Figure 15.
Ruthenium-based sensor with dual functions [35,36].
Analogous complexes, with two bipyridyls and a triazolo-pyridyl functionalized ligand with an iodo group, have also been used to sense phosphates [37]. In these systems, a phthalimide group was anchored to the triazolo-pyridyl unit (Figure 16). Like its predecessors, the binding affinity for H2PO4− and HP2O73− was excellent, with a limit of detection being in the nanomolar range (CH3CN solution). The presence of an iodo group was demonstrated to be crucial for increasing the selectivity for phosphates via halogen–anion interactions.
Figure 16.
Ruthenium-based sensor with a phthalimide-anchored triazolo-pyridyl ligand [37].
A tris(bipyridyl) ruthenium(II) complex, in which a triazolo group is coupled with a benzothiazole, is part of the functionalization of a bi-pyridyl ligand (Figure 17), which was used for sensing phosphate anions [38]. The binding constants in acetonitrile for H2PO4− and H2P2O72− were estimated to be 7.7 × 104 mol−1 and 4.4 × 104 mol−1, respectively. The luminescence-enhancing phenomena in the presence of phosphates were exploited for cancer cell imaging. After 24 h of incubation, the ruthenium complex (50 μM) was internalized, showing strong red fluorescence in MCF-7 cancer cells. Interestingly, the fluorescence was quenched by adding four equivalents of Cu2+ ions to the cellular supernatant, thus suggesting that this fluorescence response can be used to evaluate levels of Cu2+ in cells.
Figure 17.
Ruthenium-based sensor with dual functions, detection of phosphate and evaluation of Cu2+ levels in cells [38].
In view of the importance of phosphates in biology and alarming phosphate concentrations in some lakes and the environment, the sensing of phosphates, and especially to selectively discriminate between the various types of phosphate anions, will remain an active field of research in the future.
2.2.3. Carboxylates
Carboxylates are intermediates or by-products in several biological and industrial processes [39]. The most common carboxylates are those of the conjugate bases of organic acids, such as acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, citric acid, lactic acid, formic acid, malonic acid, tartaric acid, and succinic acid. Therefore, these anions can interact with sensors by forming hydrogen bonds, and all the ruthenium-based sensors for carboxylates are taking advantage of that property. However, different strategies to introduce donor groups around a ruthenium-based sensor have been employed, showing in this section a great structural diversity.
One possibility is to use coordination-driven self-assembly to generate hosts with a cavity capable to form multiple hydrogen bonds, and this can be easily achieved with arene ruthenium complexes [40]. The donor groups can be attached to different building blocks, and even the arene ligand, thus offering a great diversity of structures and functions [41]. For sensing carboxylates, arene ruthenium metalla-rectangles incorporating bi-pyridyl functionalized linkers have been designed (Figure 18). In this particular case, mono-carboxylate anions could interact either inside or outside the cavity with limited affinity (Ka < 103 M−1) [42]. However, multi-carboxylate anions could not only interact with the multiple donor groups but also sit in the cavity of the cationic metalla-rectangle host, and consequently, the binding affinity in methanol was significantly amplified for these anions (oxalate Ka = 5 × 104 M−1, citrate Ka = 1.4 × 105 M−1, and tartrate Ka = 1.8 × 105 M−1). The strong affinity offers a degree of selectivity and suggests an inside-the-cavity interacting system, which is ideal for multi-carboxylate anions.
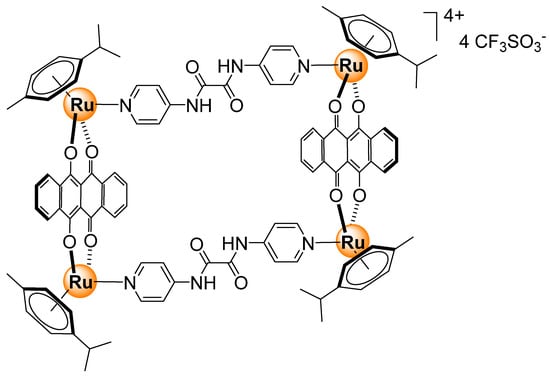
Figure 18.
Ruthenium-based metalla-rectangle for sensing multi-carboxylate anions [42].
Following the same strategy, other arene ruthenium metalla-rectangles were synthesized, and their ability to sense carboxylate anions was evaluated [43,44]. In all the cases, the donor groups were located on a bipyridyl linker. They consist of amido derivatives with different flexibility and geometry. In the case of 2,6-bis{N-(4-pyridyl)carbamoyl}pyridine, a bowl-shaped structure was obtained in which oxalate, tartrate, and citrate could sit and interact with NH-amido groups (Ka > 104 M−1 in methanol) [43]. On the other hand, with the unsymmetrical linker N-{4-(pyridin-4-ylethynyl)phenyl}isonicotinamide, a distorted rectangular geometry of the assembly was obtained, thus having isomeric mixtures [44]. Nevertheless, the metalla-rectangle showed a similar affinity for multi-carboxylate anions in solution thanks to the flexibility of the linkers. The binding constant for tartrate in methanol was estimated to be 5.5 × 104 M−1.
Other ruthenium-based complexes incorporating donor groups have been synthesized and studied for their binding affinity for carboxylates [45]. An amido-amino functionalized 3,3′-bipyridyl ligand was coordinated to a bis(bipyridyl) ruthenium complex in view to have multiple hydrogen bonds (Figure 19). The tetracationic complex shows great flexibility, and despite some distance between the binding site and the metal center, upon interactions with carboxylates, electrochemical and photoluminescence responses were observed in solution. Dihydrogen phosphate was detected by electrochemiluminescence, increasing the intensity of the signal and modifying the electronic behavior even in the presence of competing anions such as glutamate. However, with glutamate, the intensity of the photoluminescence increased, but no changes in the electrochemical process were observed, thus showing the different binding modes and consequently offering a degree of selectivity according to the technique used.
Figure 19.
Photoluminescent ruthenium complex with multiple hydrogen bond receptors [45].
Mononuclear arene ruthenium complexes were also used for sensing carboxylate anions [46]. Once again, the ruthenium complexes possess functional groups that allow the formation of hydrogen-bonded adducts between a functionalized ligand of the ruthenium complex and anions. Carbonate showed the strongest binding affinity (Ka = 3.97 × 104 M−1 in DMSO), followed by sulfate (Ka = 2.59 × 104 M−1). The geometry and double negative charge of these anions appear to be ideal for forming adducts (Figure 20), while all the other ions (SCN−, N3−, BPh4−, NO2−, S2O82−, Cl−, Br−, I−, Mg2+, K+, Ca2+, Sr2+, Hg2+, Cu2+, Fe3+, Mn2+, Zn2+, NH4+, Ni2+, Ba2+, Al3+, and Cd2+) have shown no specific interaction that could be detected by spectroscopic methods.
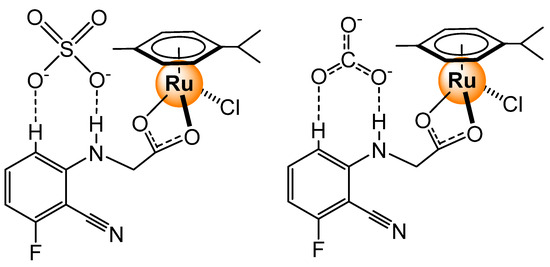
Figure 20.
Arene ruthenium complex interacting with CO32− (or SO42−) [46].
2.2.4. Others
Among the other small anions, peroxynitrite (ONOO−) is also a biologically relevant anion [47]. Peroxynitrite is a reactive biological oxidant which is involved in multiple biological processes where it acts as a peroxide, Lewis base, or free radical generator. However, because it is a short-lived and very reactive oxidant, sensing peroxynitrite remains a challenge. Nevertheless, a ruthenium-based polypyridyl complex was used to sense ONOO− in a methanol/water mixture [48]. In the presence of peroxynitrite, the functionalized bipyridyl ligand was oxidized, thus generating 1,4-benzoquinone (Scheme 4). The dealkylation reaction quenched the fluorescence signal of the complex, a phenomenon that was not observed with other ROS/RNS species (·OH, O2−, NO3−, NO2−, H2O2, 1O2, ·O2−, and NO). The probe was water soluble and showed a good response in the range of 10–50 μM at physiological pH.
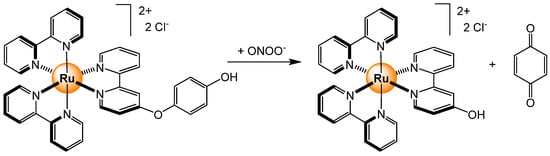
Scheme 4.
Oxidation reaction of a ruthenium-based sensor in the presence of peroxynitrite [48].
2.3. Carbohydrates
The development of boronic acid-based sensors for carbohydrates remains one of the most popular strategies to detect sugars [49]. Boronic acids can reversibly bind diols, a ubiquitous feature of sugars, and therefore, boronic acid functions are often introduced in the molecular sensors of carbohydrates. Consequently, it is not surprising that the first ruthenium-based sensor for glucose has taken advantage of that property [50,51]. The bis-bipyridyl 5,6-dyhydroxy-1,10-phenanthroline ruthenium complex forms at pH 8 a boronic acid adduct, which in the presence of glucose is broken up (Scheme 5). In this displacement assay, the luminescent intensity of the ruthenium boronic acid complex is extremely high, but it decreases gradually in the presence of glucose. An analogous ruthenium-based probe has been developed to sense glucose by the luminescence decay of a long-lifetime ruthenium tris(bipyridyl) complex [52]. The ruthenium center was coupled with Concanavalin A, a mannose residue binder, and the system was based on competitive displacement in the presence of glucose, once again, taking advantage of the disruption of the MLCT energy transfer process at the ruthenium center.
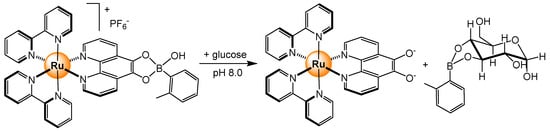
Scheme 5.
Boronic acid functionalized ruthenium complex to sense glucose by displacement assay [51].
Redox-active dinuclear ruthenium-based complexes (Figure 21) have been prepared using 1,2-dicarbonylhydrazido bridging ligands [53]. The dinuclear complex can be oxidized, thus switching on near-infrared (NIR) absorption. Under the right conditions, the response was particularly strong when the diruthenium complex reacted with hydrogen peroxide and glucose under physiological conditions (acetonitrile/Tris buffer).
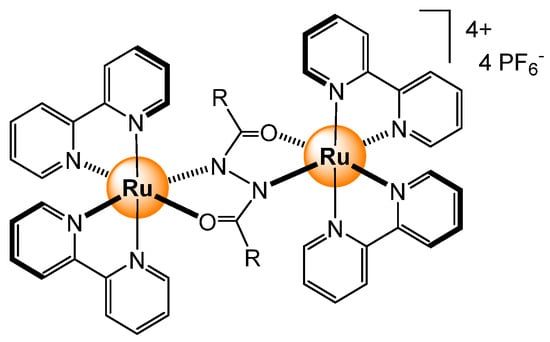
Figure 21.
Redox-active dinuclear ruthenium-based sensor for sugars [53].
2.4. Biomolecules
In biology, sensing is crucial for diagnostics, therapy, and more generally for understanding how living organisms function. Generating an interaction between a biomolecule and a metal-based complex is easy to obtain and quite common. However, showing specificity and selectivity remains extremely difficult to achieve in biological systems due to competition [54,55]. Indeed, to determine the degree of selectivity, a plethora of control experiments is required. Such control experiments involve not only competitive analytes, but also varying physical and physiological conditions (T°, pH, concentration, light, buffer, solvent, etc.) according to the sensor and the target. Unfortunately, it is unthinkable to run all the possible conditions. Nevertheless, the more you do, the more your system will show robustness, and therefore, usefulness.
2.4.1. Proteins
The aggregation of amyloid-β has been associated with Alzheimer’s disease, thus preventing or sensing such aggregations can help save lives. A dipyridophenazine ruthenium complex (Figure 22) has been used to detect the aggregation of amyloid-β [56]. In the presence of amyloid-β, a strong photoluminescence is observed in solution, which is associated with the large Stoke shift and long-lived photochemical process of the ruthenium complex when interacting with aggregates. Functionalized dipyridophenazine analogs were studied a few years later, confirming the sensing ability of ruthenium-based complexes for the aggregation detection of the amyloid-β peptides [57].
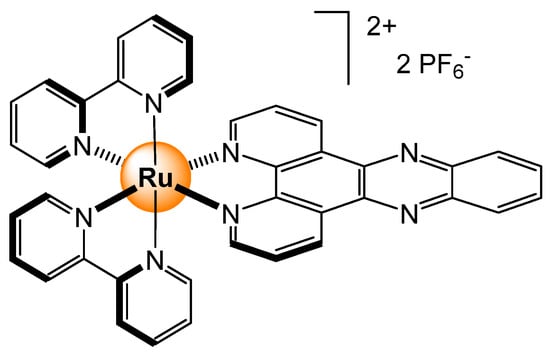
Figure 22.
Dipyridophenazine ruthenium complex used to detect aggregation of amyloid-β [56].
Nanorods grafted with tris(bipyridine) ruthenium complexes were used as electrochemiluminescence materials and incorporated into an immunosensor array in view to detect N-acetyl-β-D-glucosaminidase (NAG) [58], NAG being a biomarker for diabetic nephropathy. The luminescent signal shows linearity in concentrations from 1 ng mL−1 to 0.5 pg·mL−1, with the limit of detection being below 0.2 pg·mL−1. Similar devices were built with titanium dioxide nanoparticles decorated with tris(bipyridine) functionalized complexes. The tris(bipy)Ru-TiO2 nanoparticle/electrode system shows a linear response to protein kinase activity from 0.5 to 40 U·mL−1 [59]. Then, following a similar approach, the human heart-type fatty-acid-binding protein was also detected by electrochemiluminescence using cationic tris(bipy)ruthenium [Ru(bpy)3]2+ complexes incorporated in organic framework thin films [60]. The immunosensing tool has shown an extremely sensitive response with linearity from 150 ng·mL−1 to 150 fg·mL−1 and a limit of detection at 2.6 fg·mL−1. The high cationic ruthenium loading content in the films was crucial for the corresponding modified electrode to show an outstanding sensitivity.
Arene ruthenium metalla-assemblies, which are generally positively charged, have shown interactions with several proteins. In addition to electrostatic interactions, such assemblies have cavities that can also interact with proteins to potentially increase the binding affinity and the stability of the host-guest systems [19]. However, in such systems, other properties have to be exploited to study the protein–sensor interactions (NMR, mass, UV-vis, and fluorescence). For example, a tetracationic arene ruthenium metalla-rectangle (Figure 23), with bis-amido pyridyl linkers, has shown interaction with enhanced green fluorescence protein (EGFP) [61]. The binding constant (7.5 × 108 M−1) was estimated by UV-visible spectroscopy. Likewise, a hexacationic arene ruthenium metalla-prism, which interacted strongly with albumin, transferrin, cytochrome c, myoglobin, and ubiquitin by forming aggregates [62], has suggested that proteins are a possible target, which might explain the in vitro activity of arene ruthenium assemblies [63].
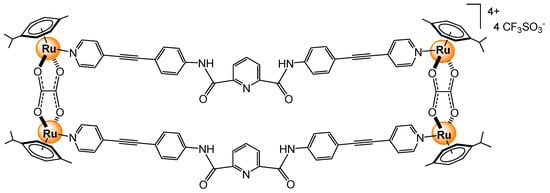
Figure 23.
Tetranuclear arene ruthenium metalla-rectangle interacting with EGFP protein [61].
2.4.2. DNA/RNA
Like proteins, DNA and RNA tend to interact strongly with positively charged compounds, thus making cationic ruthenium-based complexes ideal for sensing nucleic acids, DNA, and RNA. Accordingly, different mechanisms were exploited to generate a chemical response in the presence of such analytes, among them displacement assays. For example, a tagged-DNA sequence forms with polypyridyl ruthenium complexes as an adduct (tagged-DNA@Ru), which can be dismantled in the presence of a complementary RNA sequence (Scheme 6). When linked to the ruthenium complex, the fluorescence of the tagged DNA is quenched, but recovered upon dissociation [64]. The best detection limit was obtained for the 3-amino-1,2,4-triazino[5,6-f]-1,10-phenanthroline derivative, showing a value of 0.28 nM. A few years later, analogous ruthenium phenanthroline-functionalized complexes were tested, showing affinity for other microRNA sequences, and confirming the versatility of this strategy [65].
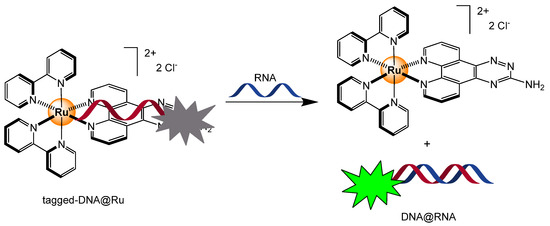
Scheme 6.
Mechanism involved in the displacement assay of a tagged-DNA ruthenium adduct in the presence of the complementary RNAs to form a DNA/RNA duplex [64].
The natural luminescence of tris(bipyridyl) ruthenium(II) complexes was also taken advantage of in electrochemiluminescence (ECL) nanohybrid materials [66]. Branched polyethylenimine-coated carbon dots covalently linked to ruthenium complexes have shown enhanced ECL in the presence of microRNA-133a, a target for acute myocardial infarction diagnosis. The detection limit was 60 fM in human serum samples, thus showing potential as biosensors. Gold nanoparticles were also coated with tris(bipyridyl) ruthenium complexes, thus adding redox-active centers to these hybrid materials to generate electrochemical indicators [67]. In the presence of a double-stranded DNA, the electrochemical behavior of the adduct is exploited to detect over the limit of 25 pmol the sequence associated with the stomach Helicobacter pylori bacterial infection. In the case of amino-linked ruthenium complexes on gold nanoparticles, the ruthenium complex (Figure 24) is largely displaced in the presence of cysteine, thus regaining its fluorescence (>30%) [68]. With other amino acids, the fluorescence intensity is at best at 5%, showing a clear selectivity for cysteine.
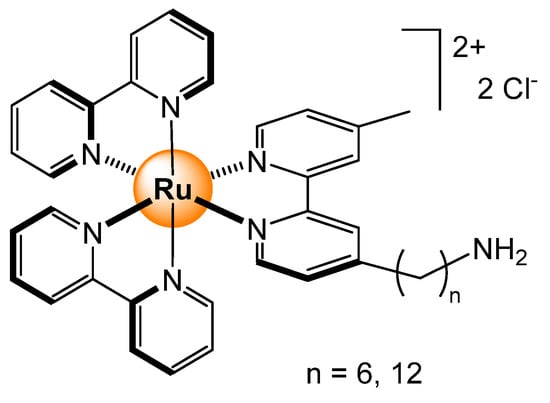
Figure 24.
Amino-functionalized tris(pyridyl) ruthenium complexes fixed to gold nanoparticle surfaces [68].
2.4.3. Primary and Secondary Metabolites
Like most ruthenium-based sensors involving tris(bipyridyl) ruthenium derivatives, the electrochemical or electrochemiluminescence properties of these complexes have been used to trigger a signal in the presence of metabolites. Among electrochemical recognition mechanisms, a carbon nanotube decorated with ruthenium complexes was used as an electrode for sensing D-penicillamine, 6-thioguanine, and catecholamines by differential pulse voltammetry [69]. The limit of detection was estimated to be at 80 mM for penicillamine over a concentration range of 0.2–750 nM. In another system, an electrode consisting of a gold surface grafted with ruthenium complexes has been employed to sense dopamine [70]. The electro-catalytic process of oxidation in the presence of the analyte was studied in solution, with the lowest potential of detection being 0.2 V and the limit of detection being 3.3 μM.
When dealing with electrochemiluminescence, different hybrid materials incorporating various tris(bipyridyl) ruthenium complexes have been developed. In the case of a polymeric system, built from poly(methacrylic acid) crossed-linked to [Ru(bpy)3]2+ units, the detection range was 5 × 10−13 to 5 × 10−6 M (limit of detection = 1 × 10−13 M) for melamine [71]. Interestingly, the ruthenium units act as catalysts as well as sensing probes, thus showing a bifunctional behavior. For sensing estradiol (E2), an estrogen steroid hormone associated with breast cancer, functionalized perylene-DNA strands coupled with CuO nanoparticles modified with carbohydrazide and [Ru(dcpy)3]2+ complexes (dcpy = 4,4′-dicarboxylicacid-2,2′-bipyridyl) were prepared [72]. The dual-mode sensing system interacts with E2 in the 0.001–100 nM range. Following a similar electrochemiluminescence process, tenuazonic acid (pKa = 3.5), a food and feed mycotoxin, has shown a strong quenching of the fluorescence when a hydrogen-bonded adduct is formed (Scheme 7); with two equivalents of analyte, the quenching reaches 82% [73].
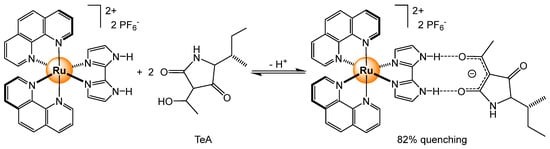
Scheme 7.
Electrochemiluminescence quenching of a ruthenium complex upon the addition of tenuazonic acid [73].
2.4.4. Biothiols
Reactions at the periphery of tris(bipyridyl) ruthenium complexes have been exploited for sensing and imaging biothiols [74,75]. For example, the replacement of the dinitrophenyl group in [Ru(bpy)2(DNS-bpy)]2+ (DNS-bpy = 4-(2,4-dinitrophenylthio)-2,2′-bipyridine) by glutathione (GSH) allows the emission of the ruthenium center to be recovered upon the annihilation of the intramolecular donor-acceptor photo-induced electron transfer (PET), thus providing visual evidence of the presence in vitro of thiol compounds (Scheme 8). A similar disruption of a PET process was used for the in vitro and in vivo visualization of biothiols [76]. The system shows discrimination between GSH and cysteine, forming different species in solution, a non-emissive compound with GSH or a short-lived green-emitting derivative with cysteine (or homocysteine). The same strategy was used with mononuclear and dinuclear complexes [77]. In these systems, the acceptor group is ortho-2,4-dinitrobenzenesulfonate, which produces the PET effect, and when displaced by biothiols, the strong luminescence of the complex is regained.
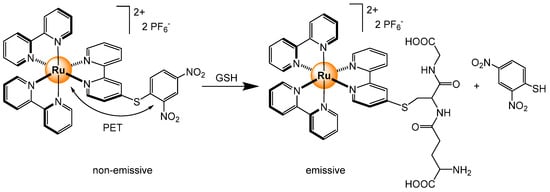
Scheme 8.
Nucleophilic substitution of dinitrophenyl with GSH to recover the natural MLCT emission of the tris(pyridyl) ruthenium unit [75].
2.5. Gases
The development of oxygen sensors is in great demand for both industrial and biological applications. Oxygen can be beneficial or detrimental to chemical and biological processes, so knowing rapidly and effectively the level of oxygen is key [78]. Among the first oxygen probes built from ruthenium are the [Ru(dpp)(dmch)2]2+ and [Ru(dpp)2(dmch)]2+ (dpp = 4,7-diphenyl-1,10-phenanthroline; dmch = 6,7-dihydro-5,8-dimethyl-dibenzo[i,j][1,10]-phenanthroline) complexes, both isolated as their dodecylsulfate salts [79]. The complexes were immobilized on different membranes, showing for the best system an oxygen-sensing capacity between 0 and 200 Torr of partial pressure, with visual luminescent response times being around 1 min for O2 in a solution and seconds in the gas phase. A few years later, analogous phenanthroline-based ruthenium complexes were linked to porous glass materials or Nafion membranes to provide non-aqueous solvent luminescent probes for oxygen [80,81]. The commercially available [Ru(bpy)3]2+ was also incorporated in polyelectrolyte microshells [82] and mesoporous silica spheres [83], in view to develop robust ruthenium-based oxygen indicators. Both showed luminescent response to oxygen in an aqueous solution, thus offering new perspectives in biological applications. Indeed, to monitor oxygen levels in cells, a coumarin-functionalized [84] and a Hoechst-tagged [85] ruthenium complexes have been synthesized (Figure 25). In both systems, the lipophilic appendage helped to internalize the fluorescent probe into cells, thus allowing an in situ visualization of oxygen content in living cells, in the mitochondrion for the coumarin derivative and in the nucleus for the Hoechst analog.
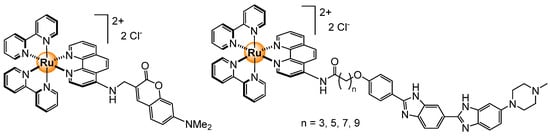
Figure 25.
Structure of a coumarin-functionalized ruthenium complex (left) [84] and a Hoechst-tagged analog (right) [85].
Not only tris(bipyridyl) ruthenium scaffolds can be used to prepare oxygen sensors. For example, the neutral dichloro-{2,6-bis[1-(4-dimethylaminophenylimino)ethyl]pyridine}ruthenium complex (Figure 26) has been used to sense O2 in perfluorochemical matrices [86]. The perfluorochemicals increase the solubility of O2 by three, thus intensifying the fluorescent response of the ruthenium complexes. Analogous dichloro polypyridyl complexes, in which the acetonitrile ligand was replaced by a bipyridyl linker (pyrazine, 4,4′-bipyridine), have generated dinuclear systems [87]. The bimetallic sensors show a 56% fluorescence intensity decrease in solution in the presence of O2. More biologically pertinent, a ruthenium carbonyl mesoporphyrin IX dye (Figure 26) was incorporated in myoglobin and the heme nitric oxide binding site within Thermoanaerobacter tengcongensis bacteria [88]. These protein-based sensors show intense red emission (λex = 550 nm) in the absence of O2, which can be strongly quenched in the presence of oxygen. The sensors even responded in E. coli, and the level of detection for O2 was biologically relevant, thus offering new avenues for biological applications in living organisms.
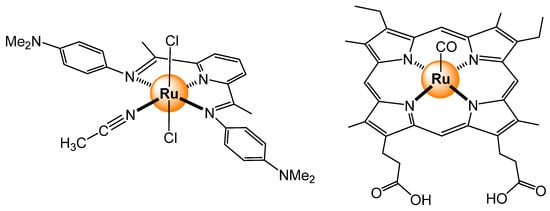
Figure 26.
Dichloro-{2,6-bis[1-(4-dimethylaminophenylimino)ethyl]pyridine}ruthenium complex (left) [85] and rutheniumcarbonyl mesoporphyrin IX (right) [88].
In view to generate other types of sensors for O2, polypyridyl ruthenium complexes have been incorporated into all kinds of hybrid materials, for example, in various forms of silicate-based materials, such as mesoporous silicates [89,90,91], core-shell nanospheres [92], and nanoparticles [93,94]. In addition, other matrices and supports were also used to develop ruthenium-based oxygen probes, like Langmuir-Blodgett film on glass [95], quantum dots in sol-gel matrix [96], zeolite [97], or zinc-coordination polymers [98]. These materials exploit the possibility of quenching the MLCT excited state of the ruthenium center in the presence of oxygen, thus triggering a visual effect within the hybrid materials, which can be integrated into devices.
Other gasses can also be detected by ruthenium-based sensors. In the case of N2, a nitrogen-bearing macrocycle was coordinated to a ruthenium diaqua complex to generate the dicationic complex [Ru(H2O)2(tmc)]2+ (tmc = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) [99]. The complex is pH-sensitive, showing a strong absorbance at 235 nm at pH > 10, which is associated with the formation of [Ru(OH)(N2)(tmc)]+ (Scheme 9). The limit of detection was determined to be at a N2 partial pressure of 0.01 MPa. Also pH-sensitive, a carbon dioxide sensor involving [Ru(pzth)3]2+ (pzth = 2-(2-pyrazinyl)thiazole) has been tested [100]. In the presence of CO2, a pH transduction via excited-state proton transfer to the ruthenium complex takes place, thus creating a Stokes shift that can be detected by an optic fiber.
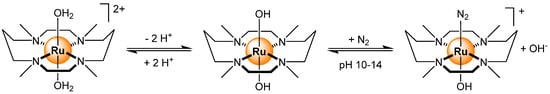
Scheme 9.
pH-dependent structural changes and equilibria during the sensing of N2 from [Ru(H2O)2(tmc)]2+ [99].
For carbon monoxide, a series of inorganic ruthenium complexes has been synthesized [101,102]. The ruthenium complexes possess two key elements, a vinyl-functionalized ligand linked to a fluorophore, and a labile 5-(3-thienyl)-2,1,3-benzothiadiazolo (tbtd) ligand. Upon the gradual addition of CO, the tbtd ligand is replaced by CO, which turns on the fluorescence of the newly formed complex. The naked-eye limit of detection is estimated to be at 0.005 ppm of CO in the air, while for the fluorescence, the limit of detection is 0.001 ppm. Control experiments show that acetonitrile and nitric oxide (NOx) can interfere at high concentrations, much higher than the general range of concentrations that require most CO detection applications.
Nitric oxide is another gas that can trigger an electronic response from tris(bipyridyl)ruthenium complexes [103]. In the presence of NO and oxygen, the [Ru(bipy)2(dapby)]2+ complex (dapby = 4-3,4-diaminophenoxy)-2,2′-bipyridine) forms a benzotriazole derivative (Scheme 10). The triazolo complex shows a much stronger luminescence at pH > 5, thus being compatible with biological applications. Indeed, in cells, the production of NO was monitored, thus showing the versatility of this probe.
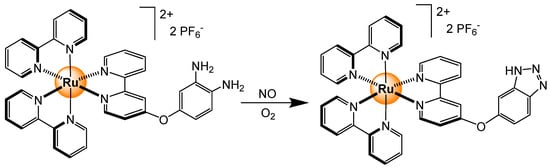
Scheme 10.
Reaction of NO with [Ru(bipy)2(dapby)]2+ to form a trizolo derivative [103].
2.6. Explosives-Pollutants
Among pollutants that have not been discussed previously in the review, we can mention organotin compounds with the general formula RnSnX(4−n) where R is an alkyl or aryl group and X a halide or hydroxide anion, both entities surrounding a tetrahedral Sn center. Accordingly, a chromophore-bridged dinuclear complex was synthesized, and its ability to generate aggregation-induced emission in the presence of organotin halides was studied [104]. The fluorescence at 645 nm (λex = 510 nm) is increased significantly upon the addition of PhSnCl3 in dichloromethane, a phenomenon that can also be followed by NMR spectroscopy.
Multiple arene ruthenium metallacycles have been built from dinuclear complexes and various ditopic ligands [41]. The size, shape, flexibility, and electronic properties of such assemblies can be controlled, thus providing a modulating approach for preparing supramolecular sensors. Indeed, such arene ruthenium metallacycles have been tested for sensing nitroaromatic molecules [105]. The bowl-shaped derivative, obtained by combining 1,3-bis(3-pyridylethynyl)benzene linkers and [{(p-cymene)Ru}2(μ-oxalato)]2+ dinuclear complexes (Figure 27), showed a binding affinity for 2,4,6-trinitrotoluene (TNT) in methanol at a room temperature of 3.3 × 103 M−1. Analogous tetranuclear arene ruthenium metallacycles have also interacted with nitroaromatic compounds, and therefore, could potentially act as sensors [106,107].
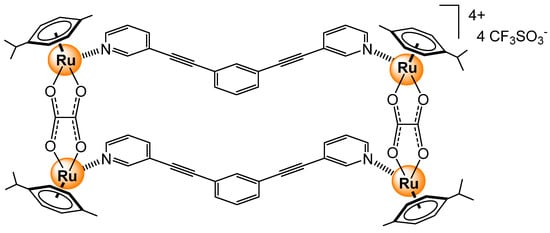
Figure 27.
Tetranuclear arene ruthenium metalla-rectangle interacting with TNT [105].
Encapsulated luminescent ruthenium complexes in molecular organic frameworks (MOF) have been developed to sense volatile nitroaromatic compounds (NAC) [108]. In the presence of NAC vapors, the energy transfer occurring between the MOF and the ruthenium complex upon light excitation is disrupted, thus providing a fluorescent signal. Similar photochemical processes have been used to sense humidity from air. Indeed, [Ru(phen)2(dppz)]2+ (phen = 1,10-phenanthroline, dppz = dipyrido[3,2-a:2′-3′-c]phenazine) has been immobilized on poly(tetrafluoroethylene), thus generating an optode for moisture, ranging from 4 to 100% relative humidity at 20 °C [109]. The recovery time is less than 2 min, and it can be performed repeatedly for years without the loss of sensitivity. Langmuir films coated on quartz and incorporating phenanthroline-derived ruthenium complexes have been used to measure the adsorption and desorption of humidity rates using the quartz crystal microbalance technique [110]. The precision range was determined to be between 11 and 97% of relative humidity at room temperature. Ruthenium loaded in mesoporous WO3 microflowers has been used to sense H2S in the gas phase [111]. Ruthenium chloride hydrate (RuCl3 · n H2O) was added during the preparation of the mesoporous materials, reaching up to 0.5 wt% of ruthenium. The ratio between the resistance in the air (Ra) and the resistance in the presence of a gas (Rg) for this hybrid material was quite high at 142 for a 10 ppm concentration of H2S.
2.7. Miscellaneous
Detecting traces of water in solvents is often crucial for conducting successful organic syntheses, and therefore, sensors are needed. In that regard, polyurethane-silica thin films doped with tris(bipyridyl) ruthenium complexes have been prepared [112]. The luminescence response to water ranges (v/v) from 0–6% in acetone (LOD = 0.13%) and 0–12% in THF (LOD = 0.49%). The doped membranes not only sense water molecules, but they can also extract water from the solvent, thus showing dual applications.
Monitoring hydrogen sulfide in vivo is essential for better understanding biological processes as H2S is naturally produced in mammals and can act as a mediator. Therefore, a ruthenium-based probe has been designed for sensing H2S in biological media [113]. The tris(bipyridyl) ruthenium complex was linked to a quenching group 2-((2,4-dinitrophenyl)thio)benzoate, which can be released in the presence of H2S (Scheme 11). The red luminescence (λex = 456 nm, λem = 612 nm) can be monitored in living organisms, such as zebrafish and mice, thus allowing the visualization of exogenous–endogenous H2S activity in vitro and in vivo.
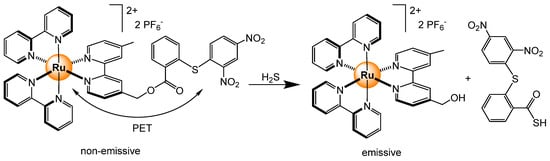
Scheme 11.
Cleavage of the dinitrophenyl quencher in the presence of H2S to recover the natural MLCT emission of the tris(pyridyl) ruthenium unit [113].
Luminescence thermometry has been achieved with the bis(1,10-phenanthroline)(4-chloro-1,10-phenanthroline)ruthenium complex embedded into poly(ethyl cyanoacrylate) [114]. The ruthenium dye incorporated in polymeric materials has been implemented in optical fiber tips for macroscopic applications, showing a 0.05 °C resolution with linear response ranging from 0 to 40 °C.
3. Conclusions
As illustrated in this review, ruthenium complexes and sensing have a long history, and several analytes have triggered different responses from ruthenium-based sensors. Nevertheless, even after 30 years or so of research involving ruthenium-based sensors, the subject has been covered in a single review. Moreover, it can also be seen in this review that among these examples, only a handful of them have shown proficiency in water, despite the relative stability of ruthenium complexes in aqueous solutions. Working in water is essential for detecting species in living cells and tissues [115]. Therefore, it is clear that more sensors developed around ruthenium chemistry should appear in the future, especially those compatible with water, a more challenging environment, but certainly more rewarding.
Funding
This research received no external funding.
Acknowledgments
Thibaud Rossel for fruitful discussions.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Higgins, S. Regarding ruthenium. Nat. Chem. 2010, 2, 1100. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Trevino, K.M.; Wagner, C.R.; Tamura, E.K.; Garcia, J.; Louie, A.Y. Small molecule sensors for the colorimetric detection of Copper(II): A review of the literature from 2010–2022. Dye. Pigment. 2023, 214, 110881. [Google Scholar] [CrossRef]
- Padilla-Tosta, M.E.; Lloris, J.M.; Martínez-Máñez, R.; Benito, A.; Soto, J.; Pardo, T.; Miranda, M.A.; Marcos, M.D. Bis(terpyridyl)-Ruthenium(II) Units Attached to Polyazacycloalkanes as Sensing Fluorescent Receptors for Transition Metal Ions. Eur. J. Inorg. Chem. 2000, 741–748. [Google Scholar] [CrossRef]
- Busche, C.; Comba, P.; Mayboroda, A.; Wadepohl, H. Novel RuII Complexes with Bispidine-Based Bridging Ligands: Luminescence Sensing and Photocatalytic Properties. Eur. J. Inorg. Chem. 2010, 1295–1302. [Google Scholar] [CrossRef]
- Zhang, P.; Pei, L.; Chen, Y.; Xu, W.; Lin, Q.; Wang, J.; Wu, J.; Shen, Y.; Ji, L.; Chao, H. A Dinuclear Ruthenium(II) Complex as a One- and Two-Photon Luminescent Probe for Biological Cu2+ Detection. Chem. Eur. J. 2013, 19, 15494–15503. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Kang, S.-Y.; Zhao, Y.; Zhang, N.; Yi, X.; Wang, K.-Z. pH and copper ion luminescence on/off sensing by a dipyrazinylpyridine-appended ruthenium complex. Sens. Actuators B Chem. 2015, 221, 614–624. [Google Scholar] [CrossRef]
- Shao, J.-Y.; Yao, C.-J.; Cui, B.-B.; Gong, Z.-L.; Zhong, Y.-W. Electropolymerized films of redox-active ruthenium complexes for multistate near-infrared electrochromism, ion sensing, and information storage. Chin. Chem. Lett. 2016, 27, 1105–1114. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Beletskaya, I.P.; Meyer, M.; Bessmertnykh-Lemeune, A. Ruthenium(II) Complexes with (3-Polyamino)phenanthrolines: Synthesis and Application in Sensing of Cu(II) Ions. Chemosensors 2022, 10, 79. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Marti-Gastaldo, C.; Palomares, E.; Durrant, J.R.; Vilar, R.; Gratzel, M.; Nazeeruddin, M.K. Reversible Colorimetric Probes for Mercury Sensing. J. Am. Chem. Soc. 2005, 127, 12351–12356. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, J.; Sun, L.; Li, F. High-Efficiency Upconversion Luminescent Sensing and Bioimaging of Hg(II) by Chromophoric Ruthenium Complex-Assembled Nanophosphors. ACS Nano 2011, 5, 8040–8048. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Zr-Based MOFs integrated with a chromophoric ruthenium complex for specific and reversible Hg2+ sensing. Dalton Trans. 2018, 47, 5570–5574. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sun, L.; Ma, B.; Jin, D.; Dong, L.; Shi, L.; Li, N.; Chen, H.; Huang, W. Simultaneous realization of Hg2+ sensing, magnetic resonance imaging and upconversion luminescence in vitro and in vivo bioimaging based on hollow mesoporous silica coating UCNPS and ruthenium complex. Nanoscale 2015, 7, 13877–13887. [Google Scholar] [CrossRef] [PubMed]
- Alreja, P.; Kaur, N. Modulation in Photophysical Properties of Fluorescent Imidazole Possessing 1,10-Phenanthroline on Introduction of Ru(bipy)22+ towards Cation Sensing. ChemistrySelect 2017, 2, 8638–8642. [Google Scholar] [CrossRef]
- Piotrowski, H.; Hilt, G.; Schulz, A.; Mayer, P.; Polborn, K.; Severin, K. Self-Assembled Organometallic [12]Metallacrown-3 Complexes. Chem. Eur. J. 2001, 7, 3196–3208. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.D.; Beer, P.D. Heterodinuclear ruthenium(II) bipyridyl-transition metal dithiocarbamate macrocycles for anion recognition and sensing. Tetrahedron 2004, 60, 11227–11238. [Google Scholar] [CrossRef]
- Dickson, S.J.; Paterson, M.J.; Willans, C.E.; Anderson, K.M.; Steed, J.W. Anion binding and luminescent sensing using cationic ruthenium(II) aminopyridine complexes. Chem. Eur. J. 2008, 14, 7296–7305. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; Xu, Y.; Su, Z.; Qian, Y.; Li, S.; Yu, T.; Sadler, P.J.; Liu, H.-K. A novel strategy to construct Janus metallamacrocycles with both a Ru-arene face and an imidazolium face. Dalton Trans. 2017, 46, 16205–16215. [Google Scholar] [CrossRef]
- Therrien, B. The role of the second coordination sphere in the biological activity of arene ruthenium metalla-assemblies. Front. Chem. 2018, 6, 602. [Google Scholar] [CrossRef]
- Bhaumik, C.; Maity, D.; Das, S.; Baitalik, S. Anion sensing studies of luminescent bis-tridentate ruthenium(II) and osmium(II) complexes based on terpyridyl-imidazole ligand through different channels. Polyhedron 2013, 52, 890–899. [Google Scholar] [CrossRef]
- Patil, S.K.; Ghosh, R.; Kennedy, P.; Mobin, S.M.; Das, D. Potential anion sensing properties by a redox and substitution series of [Ru(bpy)3-n(Hdpa)n]2+, n = 1–3; Hdpa = 2,2′-dipyridylamine: Selective recognition and stoichiometric binding with cyanide and fluoride ions. RSC Adv. 2016, 6, 62310–62319. [Google Scholar] [CrossRef]
- Wade, C.R.; Gabbaï, F.P. Cyanide anion binding by a triarylborane at the outer rim of a cyclometalated ruthenium(II) cationic complex. Inorg. Chem. 2010, 49, 714–720. [Google Scholar] [CrossRef]
- Christianson, A.M.; Gabbaï, F.P. Fluoride and cyanide anion sensing by an Sb(V)-substituted cyclometalated Ru polypyridyl complex. J. Organomet. Chem. 2017, 847, 154–161. [Google Scholar] [CrossRef]
- Bazany-Rodríguez, I.J.; García-Rojas, L.M.; Hernández, J.G.; Thangarasu, P. Sequential recognition of bisulfate and acetate by a ruthenium(II) complex: Experimental and theoretical studies. ChemPhotoChem 2023, 8, e202300145. [Google Scholar] [CrossRef]
- Pal, S.; Ghosh, T.K.; Ghosh, R.; Mondal, S.; Ghosh, P. Recent advances in recognition, sensing and extraction of phosphates: 2015 onwards. Coord. Chem. Rev. 2020, 405, 213128. [Google Scholar] [CrossRef]
- Szemes, F.; Hesek, D.; Chen, Z.; Dent, S.W.; Drew, M.G.B.; Goulden, A.J.; Graydon, A.R.; Grieve, A.; Mortimer, R.J.; Wear, T.; et al. Synthesis and characterization of novel acyclic, macrocyclic, and calix[4]arene ruthenium(II) bipyridyl receptor molecules that recognize and sense anions. Inorg. Chem. 1996, 35, 5868–5879. [Google Scholar] [CrossRef]
- Vickers, M.S.; Martindale, K.S.; Beer, P.D. Imidazolium functionalized acyclic ruthenium(II) bipyridyl receptors for anion recognition and luminescent sensing. J. Mater. Chem. 2005, 15, 2784–2790. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tárraga, A.; Molina, P. Cation coordination induced modulation of the anion sensing properties of a ferrocene-imidazophenanthroline dyad: Multichannel recognition from phosphate-related to chloride anions. J. Org. Chem. 2008, 73, 4034–4044. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Gunupuru, R.; Maity, D.; Patra, S.; Suresh, E.; Paul, P. Synthesis and anion-sensing property of a family of Ru(II)-based receptors containing functionalized polypyridine as binding site. Inorg. Chem. Commun. 2010, 13, 1522–1526. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Razaeian, K. A catalyst-free approach to a novel imidazo [4,5-f][1,10] phenanthroline ligand and its corresponding ruthenium(II) complex: Insights into their applications in colorimetric anion sensing. New J. Chem. 2014, 38, 5536–5543. [Google Scholar] [CrossRef]
- Kitchen, J.A.; Boyle, E.M.; Gunnlaugsson, T. Synthesis, structural characterization and luminescent anion sensing studies of a Ru(II)polypyridyl complex featuring an aryl urea derivatized 2,2′-bpy auxiliary ligand. Inorg. Chim. Acta 2012, 381, 236–242. [Google Scholar] [CrossRef]
- Ahmad, H.; Hazel, B.W.; Meijer, A.J.H.M.; Thomas, J.A.; Wilkinson, K.A. A self-assembled luminescent host that selectively sense ATP in water. Chem. Eur. J. 2013, 19, 5081–5087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-B.; Duan, Z.-M.; Ma, Y.-Y.; Wang, K.-Z. Highly sensitive and selective difunctional ruthenium(II) complex-based chemosensor for dihydrogen phosphate anion and ferrous cation. Inorg. Chem. 2013, 52, 2306–2316. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Wu, Y.-Q.; Wang, K.-Z.; Li, F. pH luminescence switching, dihydrogen phosphate sensing, and cellular uptake of a heterobimetallic ruthenium(II)-rhenium(I) complex. Dalton Trans. 2014, 43, 3273–3284. [Google Scholar] [CrossRef]
- Chowdhury, B.; Sinha, S.; Ghosh, P. Selective sensing of phosphates by a new bis-heteroleptic RuII complex through halogen bonding: A superior sensor over its hydrogen-bonding analogue. Chem. Eur. J. 2016, 22, 18051–18059. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Chakraborty, S.; Chowdhury, B.; Ghosh, P. Bis-heteroleptic ruthenium(II) complex of pendent urea functionalized pyridyl triazole and phenanthroline for recognition, sensing, and extraction of oxyanions. Inorg. Chem. 2017, 56, 5371–5382. [Google Scholar] [CrossRef]
- Ramachandran, M.; Syed, A.; Marraiki, N.; Anandan, S. The aqueous dependent sensing of hydrazine and phosphate anions using a bis-heteroleptic Ru(II) complex with a phthalimide-anchored pyridine-triazole ligand. Analyst 2021, 146, 1430–1443. [Google Scholar] [CrossRef]
- Ramachandran, M.; Anandan, S. Triazole appending ruthenium(II) polypyridine complex for selective sensing of phosphate anions through C-H---anion interaction and copper(II) ions via cancer cells. New J. Chem. 2020, 44, 6186–6196. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.J. A review of carboxylate production and recovery from organic wastes. Bioresour. Technol. Rep. 2021, 16, 100826. [Google Scholar] [CrossRef]
- Linares, F.; Galindo, M.A.; Galli, S.; Romero, M.A.; Navarro, J.A.R.; Barea, E. Tetranuclear coordination assemblies based on half-sandwich ruthenium(II) complexes: Noncovalent binding to DNA and cytotoxicity. Inorg. Chem. 2009, 48, 7413–7420. [Google Scholar] [CrossRef]
- Therrien, B. Functionalised η6-arene ruthenium complexes. Coord. Chem. Rev. 2009, 253, 493–519. [Google Scholar] [CrossRef]
- Vajpayee, V.; Song, Y.H.; Lee, M.H.; Kim, H.; Wang, M.; Stang, P.J.; Chi, K.-W. Self-assembled arene-ruthenium-based rectangles for the selective sensing of multi-carboxylate anions. Chem. Eur. J. 2011, 17, 7837–7844. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Vajpayee, V.; Kim, H.; Lee, M.H.; Jung, H.; Wang, M.; Stang, P.J.; Chi, K.-W. Self-assembled metalla-bowls for selective sensing of multi-carboxylate anions. Dalton Trans. 2012, 41, 1195–1201. [Google Scholar] [CrossRef]
- Mishra, A.; Lee, S.; Kim, H.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Selective detection of multicarboxylate anions based on “Turn on” electron transfer by self-assembled molecular rectangles. Chem. Asian J. 2012, 7, 2592–2599. [Google Scholar] [CrossRef]
- Berni, E.; Gosse, I.; Badocco, D.; Pastore, P.; Sojic, N.; Pinet, S. Differential photoluminescent and electrochemiluminescent detection of anions with a modified ruthenium(II)-bipyridyl complex. Chem. Eur. J. 2009, 15, 5145–5152. [Google Scholar] [CrossRef]
- Sonkar, C.; Sarkar, S.; Malviya, N.; Kuznetsov, M.L.; Mukhopadhyay, S. Recognition and mechanistic investigation of anion sensing by ruthenium(II) arene complexes and bio-imaging application. Dalton Trans. 2022, 51, 13071–13084. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, J.; Liu, W.; Wang, P.; Fan, Z. Ruthenium(II) complex-based fluorescent sensor for peroxynitrite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 94, 340–345. [Google Scholar] [CrossRef]
- Williams, G.T.; Kedge, J.L.; Fossey, J.S. Molecular boronic acid-based saccharide sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, Z.; Lakowicz, J.R. Lifetime-based sensing of glucose using luminescent ruthenium(II) metal complex. In Proceedings of the SPIE Conference on Advances in Fluorescence Sensing Technology IV, San Jose, CA, USA, 24–27 January 1999; Volume 3602, pp. 326–334. [Google Scholar]
- Murtaza, Z.; Tolosa, L.; Harms, P.; Lakowicz, J.R. On the possibility of glucose sensing using boronic acid and a luminescent ruthenium metal-ligand complex. J. Fluoresc. 2002, 12, 187–192. [Google Scholar] [CrossRef]
- Xun, S.; LeClair, G.; Zhang, J.; Chen, X.; Gao, J.P.; Wang, Z.Y. Tuning the electrical and optical properties of dinuclear ruthenium complexes for near infrared optical sensing. Org. Lett. 2006, 8, 1697–1700. [Google Scholar] [CrossRef]
- Tolosa, L.; Szmacinski, H.; Rao, G.; Lakowicz, J.R. Lifetime-based sensing of glucose using energy transfer with a long lifetime donor. Anal. Biochem. 1997, 250, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R. Recent optical sensing technologies for the detection of various biomolecules: Review. Opt. Laser Technol. 2021, 134, 106620. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J.E. Supramolecular coordination complexes as optical biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.P.; Torres, V.; Jain, D.; Martí, A.A. Sensing amyloid-β aggregation using luminescent dipyridophenazine ruthenium(II) complexes. J. Am. Chem. Soc. 2011, 133, 1121–1123. [Google Scholar] [CrossRef]
- Babu, E.; Mareeswaran, P.M.; Sathish, V.; Singaravadivel, S.; Rajagopal, S. Sensing and inhibition of amyloid-β based on the simple luminescent aptamer-ruthenium complex system. Talanta 2015, 134, 343–353. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Zhuo, Y.; Chai, Y.; Yuan, R. Self-enhanced electrochemiluminescence nanorods of tris(bipyridine) ruthenium(II) derivative and its sensing application for detection of N-acetyl-β-D-glucosaminidase. Anal. Chem. 2016, 88, 2258–2266. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Liu, C.; Ding, F.; Li, Y.; Tang, H.; Ma, M. Phosphate group-derivated bipyridine-ruthenium complex and titanium dioxide nanoparticles for electrochemical sensing of protein kinase activity. ACS Sens. 2021, 6, 4451–4460. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, X.; Wang, M.; Dong, Y.; Liu, J.; Zhu, Z.; Li, M.; Yang, D.; Shao, Y. Fabrication of tris(bipyridine)ruthenium(II)-functionalized metal-organic framework thin films by electrochemically assisted self-assembly technique for electrochemiluminescent immunoassay. Anal. Chem. 2018, 90, 11622–11628. [Google Scholar] [CrossRef]
- Mishra, A.; Ravikumar, S.; Song, Y.H.; Prabhu, N.S.; Kim, H.; Hong, S.H.; Cheon, S.; Noh, J.; Chi, K.-W. A new arene-Ru based supramolecular coordination complex for efficient binding and selective sensing of green fluorescent protein. Dalton Trans. 2014, 43, 6032–6040. [Google Scholar] [CrossRef]
- Paul, L.E.H.; Therrien, B.; Furrer, J. Interactions of arene ruthenium metallaprisms with human proteins. Org. Biomol. Chem. 2015, 13, 946–953. [Google Scholar] [CrossRef]
- Therrien, B. Unmasking arene ruthenium building blocks. Chem. Rec. 2021, 21, 460–468. [Google Scholar] [CrossRef]
- Sun, B.; Liang, Z.; Xie, B.-P.; Li, R.-T.; Li, L.-Z.; Jiang, Z.-H.; Bai, L.-P.; Chen, J.-X. Fluorescence sensing platform based on ruthenium(II) complexes as high 3S (sensitivity, specificity, speed) and “on-off-on” sensors for the miR-185 detection. Talanta 2018, 179, 658–667. [Google Scholar] [CrossRef]
- Li, R.-T.; Liang, Z.; Li, M.-C.; Tan, Y.; Xie, B.-P.; Duan, W.-J.; Ning, C.-T.; Chen, J.-X.; Sun, B. Speedy, specific, synchronous sensing platforms with ruthenium complexes for multiplexed microRNA detection. Inorg. Chem. 2018, 58, 15126–15137. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, G.; Yan, M.; Zhu, Q.; Zhu, L.; Huang, J.; Yang, X. Highly luminescent and self-enhanced electrochemiluminescence of tris(bipyridine) ruthenium(II) nanohybrid and its sensing application for label-free detection of microRNA. Anal. Chem. 2019, 91, 13237–13243. [Google Scholar] [CrossRef]
- García, T.; Casero, E.; Revenga-Parra, M.; Martín-Benito, J.; Pariente, F.; Vázquez, L.; Lorenzo, E. Architectures based on the use of gold nanoparticles and ruthenium complexes as a new route to improve genosensor sensitivity. Biosens. Bioelectron. 2008, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Zhan, C.-Q.; Zheng, Y.-M.; Chen, G.-N.; Chen, X. Simple and selective sensing of cysteine using gold nanoparticles modified by ruthenium(II) complexes. J. Nanosci. Nanotechnol. 2011, 11, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Sheikh-Mohseni, M.A.; Salavati-Niasari, M. A ruthenium complex/carbon nanotube based electrode as the first electrochemical sensor for simultaneous sensing D-penicillamine, 6-thioguanine and catecholamines. Electroanalysis 2016, 28, 1370–1376. [Google Scholar] [CrossRef]
- Toledo, K.C.F.; Bonacin, J.A. Preferential coordination of ruthenium complex as an electroactive self-assembled monolayer on gold substrate and its application in sensing of dopamine. Inorg. Chem. Commun. 2019, 99, 52–59. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Xu, Y.; Wei, S.; Huang, W.; Liu, R.; Liu, J. A versatile signal-enhanced ECL sensing platform based on molecular imprinting technique via PET-RAF cross-linking polymerization using bifunctional ruthenium complex as both catalyst and sensing probes. Biosens. Bioelectron. 2019, 124–125, 15–24. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Q.; Yang, L.; Ma, H.; Wu, D.; Liu, L.; Ren, X.; Ju, H.; Wei, Q. Dual-mode sensing platform guided by intramolecular electrochemiluminescence of a ruthenium complex and cationic N,N-bis(2-(trimethylammonium iodide)propylene) perylene-3,4,9,10-tetracarboxydiimide for estradiol assay. Anal. Chem. 2021, 93, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Alburquerque, J.; García-Iriepa, C.; Marazzi, M.; Descalzo, A.B.; Orellana, G. Interaction of a 1,3-dicarbonyl toxin with Ru(II)-biimidazole complexes for luminescence sensing: A spectroscopic and photochemical experimental study rationalized by time-dependent density functional theory calculations. Inorg. Chem. 2022, 61, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Gao, Q.; An, X.; Song, B.; Yuan, J. A functional ruthenium(II) complex for imaging biothiols in living bodies. Dalton Trans. 2015, 44, 8278–8283. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Zhang, W.; Wang, Y.-L.; Liu, Q.; Song, B.; Yuan, J.; Zhang, R. “Two birds with one stone” ruthenium(II) complex probe for biothiols discrimination and detection in vitro and in vivo. Adv. Sci. 2020, 7, 2000458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, Z.; Yin, Y.; Wang, G.; Jin, D.; Yuan, J.; Piper, J.A. Developing red-emissive ruthenium(II) complex-based luminescent probes for cellular imaging. Bioconjug. Chem. 2012, 23, 725–733. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Han, Y.-F.; Ge, Y.-Q.; Cui, J.-C.; Zuo, J.; Nie, K. Rapid and selective detection of biothiols by novel ruthenium(II) complex-based phosphorescence probes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 328–334. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mat. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Klimant, I.; Belser, P.; Wolfbeis, O.S. Novel metal-organic ruthenium(II) diimin complexes for use as longwave excitable luminescent oxygen probes. Talanta 1994, 41, 985–991. [Google Scholar] [CrossRef]
- Xavier, M.P.; Garcia-Fresnadillo, D.; Moreno-Bondi, M.C.; Orellana, G. Oxygen sensing in nonaqueous media using porous glass with covalently bound luminescent Ru(II) complexes. Anal. Chem. 1998, 70, 5184–5189. [Google Scholar] [CrossRef]
- Garcia-Fresnadillo, D.; Marazuela, M.D.; Moreno-Bondi, M.C.; Orellana, G. Luminescent Nafion membranes dyed with ruthenium(II) complexes as sensing materials for dissolved oxygen. Langmuir 1999, 15, 6451–6459. [Google Scholar] [CrossRef]
- McShane, M.J.; Brown, J.Q.; Guice, K.B.; Lvov, Y.M. Polyelectrolyte microshells as carriers for fluorescent sensors: Loading and sensing properties of a ruthenium-based oxygen indicator. J. Nanosci. Nanotechnol. 2002, 2, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, J.; Wang, Y.; Pang, W. Incorporation of luminescent tris(pyridine)ruthenium(II) complex in mesoporous silica spheres and their spectroscopic and oxygen-sensing properties. Mat. Lett. 2002, 53, 400–405. [Google Scholar] [CrossRef]
- Hara, D.; Komatsu, H.; Son, A.; Nishimoto, S.-I.; Tanabe, K. Water-soluble phosphorescent ruthenium complex with a fluorescent coumarin unit for ratiometric sensing of oxygen levels in living cells. Bioconjug. Chem. 2015, 26, 645–649. [Google Scholar] [CrossRef]
- Hara, D.; Umehara, Y.; Son, A.; Asahi, W.; Misu, S.; Kurihara, R.; Kondo, T.; Tanabe, K. Tracking the oxygen status in the cell nucleus with a Hoechst-tagged phosphorescent ruthenium complex. ChemBioChem 2018, 19, 956–962. [Google Scholar] [CrossRef]
- Ertekin, K.; Kocak, S.; Ozer, M.S.; Aycan, S.; Cetinkaya, B. Enhanced optical oxygen sensing using a newly synthesized ruthenium complex together with oxygen carriers. Talanta 2003, 61, 573–579. [Google Scholar] [CrossRef]
- Oter, O.; Ertekin, K.; Dayan, O.; Cetinkaya, B. Photocharacterization of novel ruthenium dyes and their utilities as oxygen sensing materials in presence of perfluorochemicals. J. Fluoresc. 2008, 18, 269–276. [Google Scholar] [CrossRef]
- Winter, M.B.; McLaurin, E.J.; Reece, S.Y.; Olea, C., Jr.; Nocera, D.G.; Marletta, M.A. Ru-porphyrin protein scaffolds for sensing O2. J. Am. Chem. Soc. 2010, 132, 5582–5583. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Li, B.; Yue, S.; Li, W. Optical oxygen sensing materials based on trinuclear starburst ruthenium(II) complexes assembled in mesoporous silica. J. Lumin. 2008, 128, 341–347. [Google Scholar] [CrossRef]
- Wu, X.; Song, L.; Li, B.; Liu, Y. Synthesis, characterization, and oxygen sensing properties of Ru(II) complex covalently grafted to mesoporous MCM-41. J. Lumin. 2010, 130, 374–379. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Song, H. Multifunctional mesoporous nanocomposites with magnetic, optical, and sensing features: Synthesis, characterization, and their oxygen-sensing performance. Langmuir 2013, 29, 1273–1279. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, L.; Liu, L.; Wang, Y. Photoluminescent and oxygen sensing properties of core-shell nanospheres based on a covalently grafted ruthenium(II) complex. Appl. Organometal. Chem. 2011, 25, 21–26. [Google Scholar] [CrossRef]
- Liu, L.; Li, B.; Qin, R.; Zhao, H.; Ren, X.; Su, Z. Synthesis and characterization of new bifunctional nanocomposites possessing upconversion and oxygen-sensing properties. Nanotechnology 2010, 21, 285701. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, Y.; Gao, Y.; Zhang, Y.; Li, X.; Zhang, J.; Wang, Y.; Chen, B. Mesoporous silica coating NaYF4:Yb,Er@NaYF4 upconversion nanoparticles loaded with ruthenium(II) complex nanoparticles: Fluorometric sensing and cellular imaging of temperature by upconversion and oxygen by downconversion. Microchim. Acta 2018, 185, 454. [Google Scholar] [CrossRef]
- Chu, B.W.-K.; Yam, V.W.-W. Sensitive single-layered oxygen-sensing systems: Polypyridyl ruthenium(II) complexes covalently attached or deposited as Langmuir-Blodgett monolayer on glass surfaces. Langmuir 2006, 22, 7437–7443. [Google Scholar] [CrossRef]
- Jorge, P.A.S.; Maule, C.; Silva, A.J.; Benrashid, R.; Santos, J.L.; Farahi, F. Dual sensing of oxagen and temperature using quantum dots and a ruthenium complex. Anal. Chim. Acta 2008, 606, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruda-Eberenz, T.A.; Nagy, A.; Waldman, W.J.; Dutta, P.K. Entrapment of ionic tris(2,2′-bipyridyl) ruthenium(II) in hydrophobic siliceous zeolite: O2 sensing in biological environments. Langmuir 2008, 24, 9140–9147. [Google Scholar] [CrossRef]
- Qi, X.-L.; Liu, S.-Y.; Lin, R.-B.; Liao, P.-Q.; Ye, J.-W.; Lai, Z.; Guan, Y.; Cheng, X.-N.; Zhang, J.-P.; Chen, X.-M. Phosphorescence doping in a flexible ultramicroporous framework for high and tunable oxygen sensing efficiency. Chem. Commun. 2013, 49, 6864–6866. [Google Scholar] [CrossRef]
- Kizaki, T.; Matsumoto, T.; Ogo, S. Dissolved N2 sensing by pH-dependent Ru Complexes. Dalton Trans. 2010, 39, 1339–1344. [Google Scholar] [CrossRef]
- Orellana, G.; Moreno-Bondi, M.C.; Segovia, E.; Marazuela, M.D. Fiber-optic sensing of carbon dioxide based on excited-state proton transfer to a luminescent ruthenium(II) complex. Anal. Chem. 1992, 64, 2210–2215. [Google Scholar] [CrossRef]
- Toscani, A.; Marín-Hernández, C.; Moragues, M.E.; Sancenón, F.; Dingwall, P.; Brown, N.J.; Martínez-Máñez, R.; White, A.J.P.; Wilton-Ely, J.D.E.T. Ruthenium(II) and osmium(II) vinyl complexes as highly sensitive and selective chromogenic and fluorogenic probes for the sensing of carbon monoxide in air. Chem. Eur. J. 2015, 21, 14529–14538. [Google Scholar] [CrossRef]
- Toscani, A.; Marín-Hernández, C.; Robson, J.A.; Chua, E.; Dingwall, P.; White, A.J.P.; Sancenón, F.; de la Torre, C.; Martínez-Máñez, R.; Wilton-Ely, J.D.E.T. Highly sensitive and selective molecular probes for chromo-fluorogenic sensing of carbon monoxide in air, aqueous solution and cells. Chem. Eur. J. 2019, 25, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, Z.; Wang, G.; Zhang, W.; Yuan, J. Development of a ruthenium(II) complex based luminescent probe for imaging nitric oxide production in living cells. Chem. Eur. J. 2010, 16, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Han, F.; Zhang, Q.; Xie, T.; Lu, L.; Li, S.; Xia, H. Off/On fluorescent chemosensors for organotin halides based on binuclear ruthenium complexes. Angew. Chem. Int. Ed. 2013, 52, 5599–5603. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; Bar, A.K.; Joshi, S.A.; Patil, Y.P.; Mukherjee, P.S. Constructions of 2D-metallamacrocycles using half-sandwich RuII2 precursors: Synthesis, molecular structures, and self-selection for a single linkage isomer. Organometallics 2011, 30, 1951–1960. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Jadhav, H.; Mukherjee, P.S. Self-assembly of chloro-bridged arene-ruthenium based rectangle: Synthesis, structural characterization and sensing study. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2014, 84, 197–203. [Google Scholar] [CrossRef]
- Vajpayee, V.; Bivaud, S.; Goeb, S.; Croué, V.; Allain, M.; Popp, B.V.; Garci, A.; Therrien, B.; Sallé, M. Electron-rich arene-ruthenium metalla-architectures incorporating tetrapyridyl-tetrathiafulvene donor moieties. Organometallics 2014, 33, 1651–1658. [Google Scholar] [CrossRef]
- Zhao, H.; Ni, J.; Zhang, J.-J.; Liu, S.-Q.; Sun, Y.-J.; Zhou, H.; Li, Y.-Q.; Duan, C.-Y. A trichromatic MOF composite for multidimensional ratiometric luminescent sensing. Chem. Sci. 2018, 9, 2918–2926. [Google Scholar]
- Bedoya, M.; Díez, M.T.; Moreno-Bondi, M.C.; Orellana, G. Humidity sensing with a luminescent Ru(II) complex and phase-sensitive detection. Sens. Actuators B 2006, 113, 573–581. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Okur, S. Humidity sensing properties of novel ruthenium polypyridyl complex. Sens. Actuators B 2010, 151, 223–228. [Google Scholar] [CrossRef]
- Mehta, S.S.; Nadargi, D.Y.; Tamboli, M.S.; Chaudhary, L.S.; Patil, P.S.; Mulla, I.S.; Suryavanshi, S.S. Ru-loaded mesoporous WO3 microflowers for dual applications: Enhanced H2S sensing and sunlight-driven photocatalysis. Dalton Trans. 2018, 47, 16840–16845. [Google Scholar] [CrossRef]
- Peng, H.-S.; Li, X.-H.; You, F.-T.; Teng, F.; Huang, S.-H. Sensing water in organic solvent using a polyurethane-silica hybrid membrane doped with a luminescent ruthenium complex. Microchim. Acta 2013, 180, 807–812. [Google Scholar] [CrossRef]
- Du, Z.; Song, B.; Zhang, W.; Duan, C.; Wang, Y.-L.; Liu, C.; Zhang, R.; Yuan, J. Quantitative monitoring and visualization of hydrogen sulfide in vivo using a lumiscent probe based on a ruthenium(II) complex. Angew. Chem. Int. Ed. 2018, 57, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, N.; Ielasi, G.; Bedoya, M.; Orellana, G. Optimization of temperature sensing with polymer-embedded luminescent Ru(II) complexes. Polymers 2018, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.C.-L.; Yam, V.W.-W. Luminescent cation sensors: From host-guest chemistry, supramolecular chemistry to reaction-based mechanisms. Chem. Soc. Rev. 2015, 44, 4192–4202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).