Two Perovskite Modifications of BiFe0.6Mn0.4O3 Prepared by High-Pressure and Post-Synthesis Annealing at Ambient Pressure
Abstract
1. Introduction
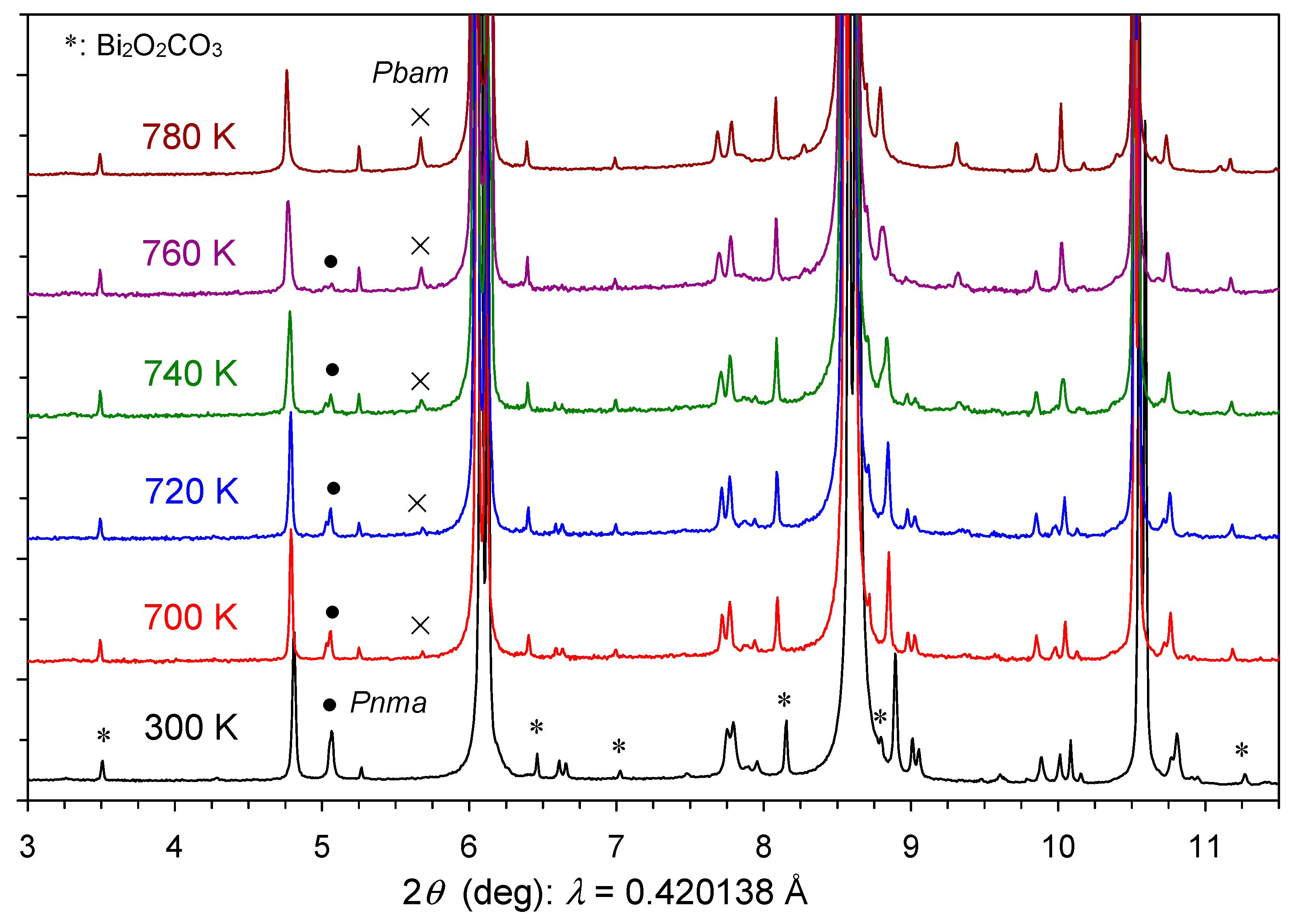
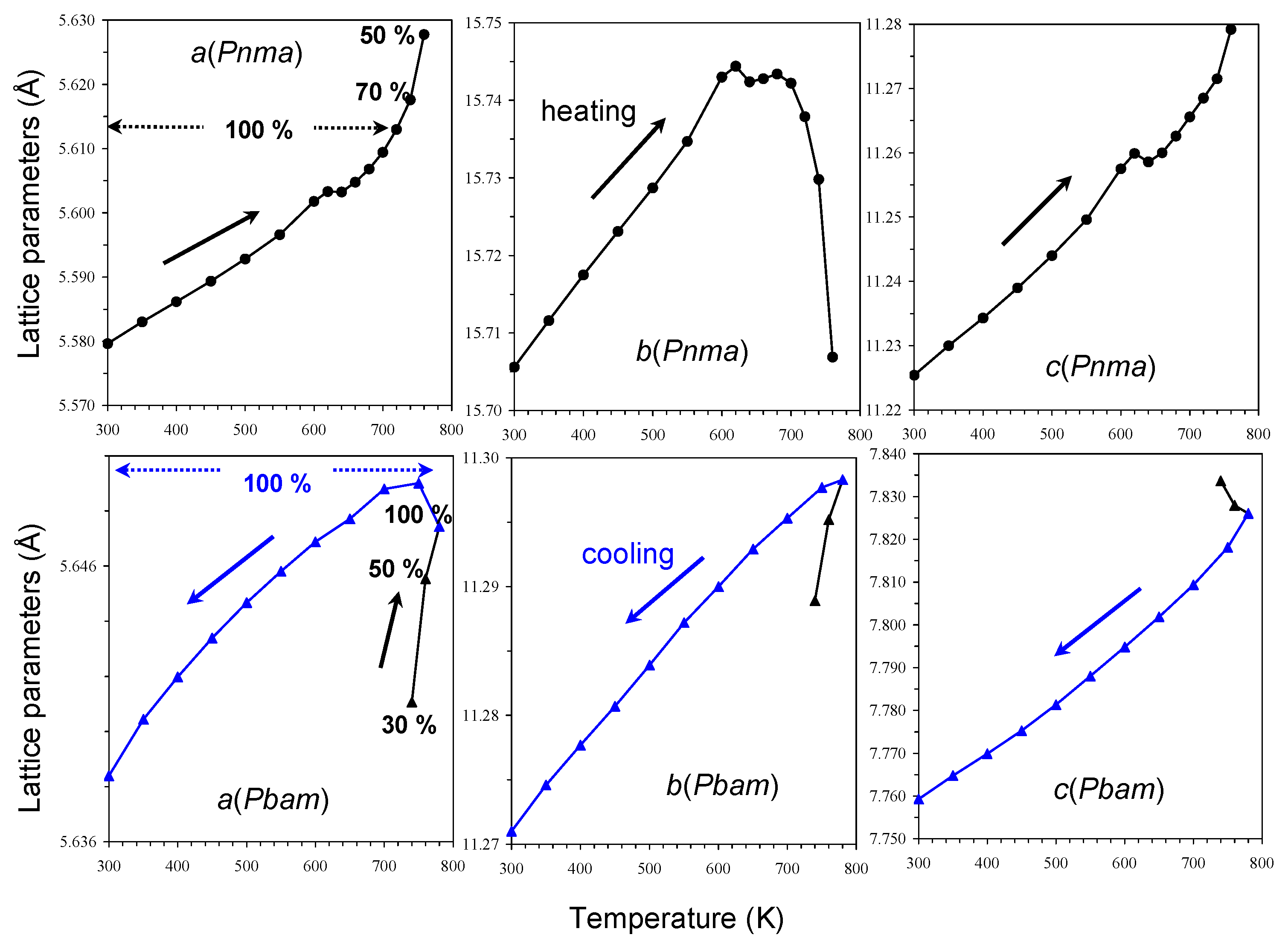
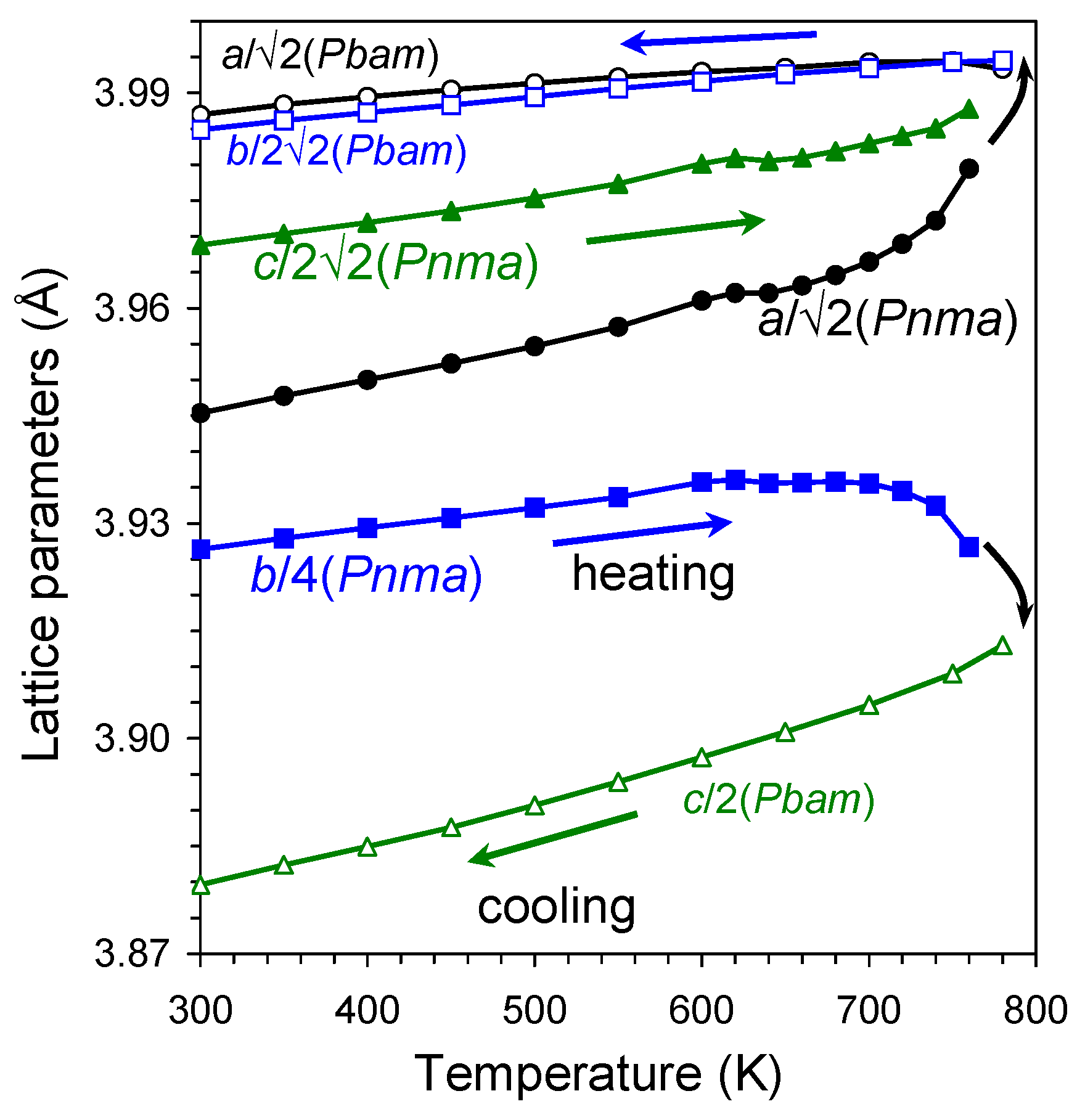
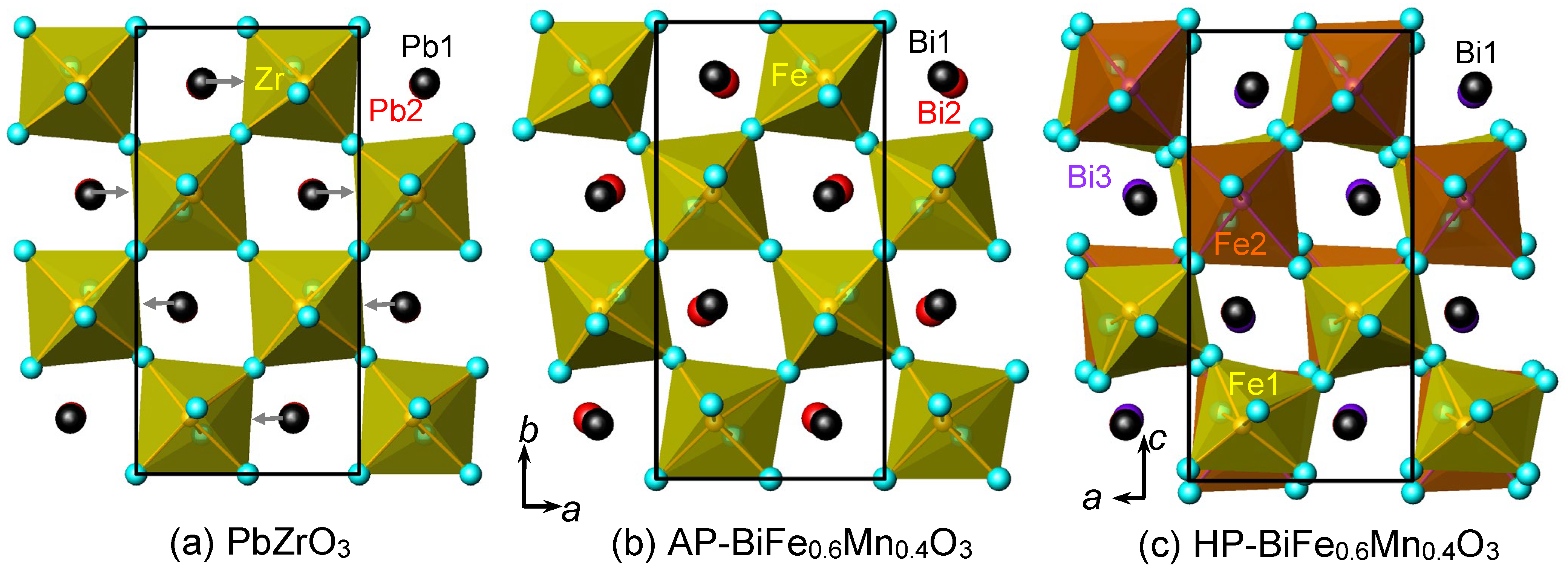
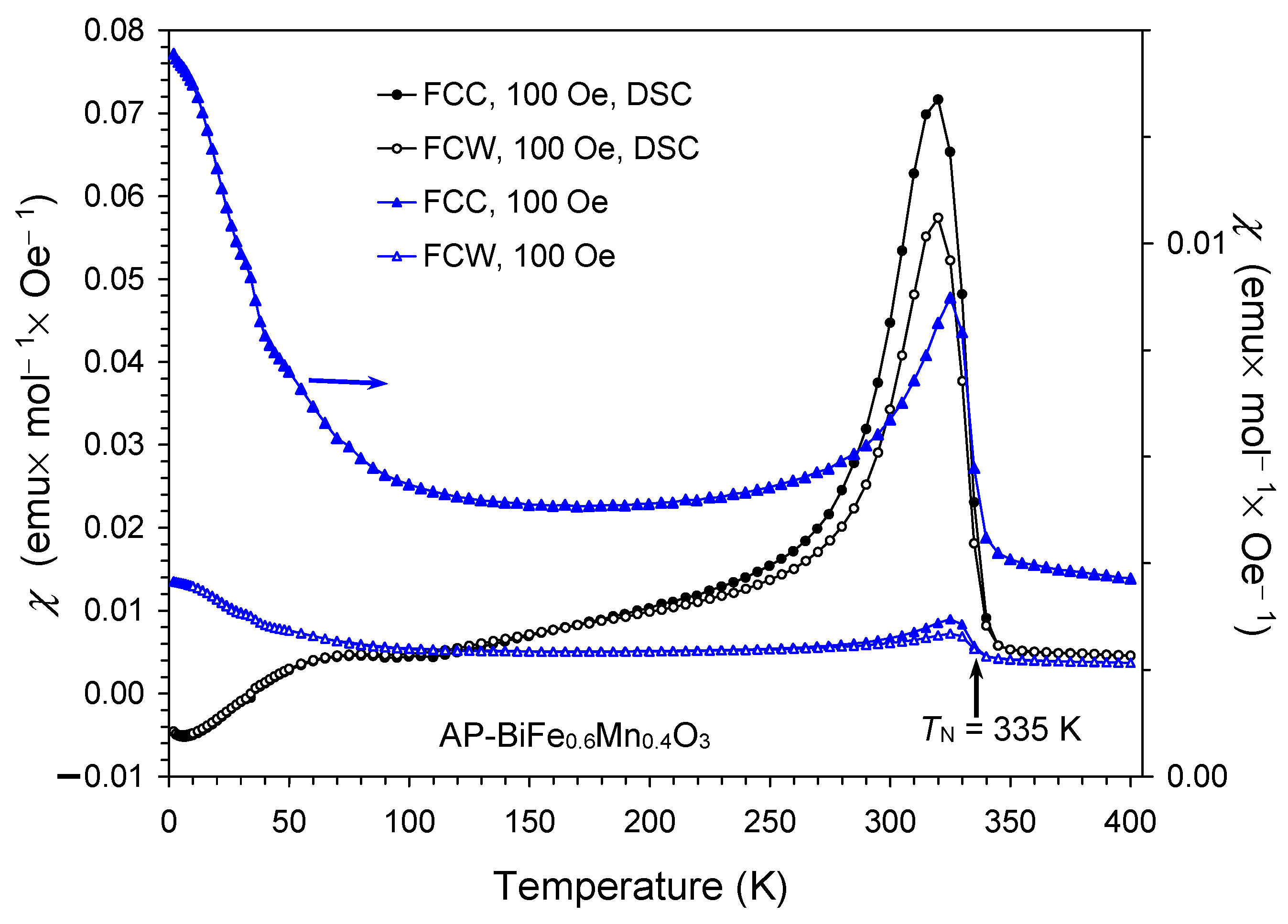
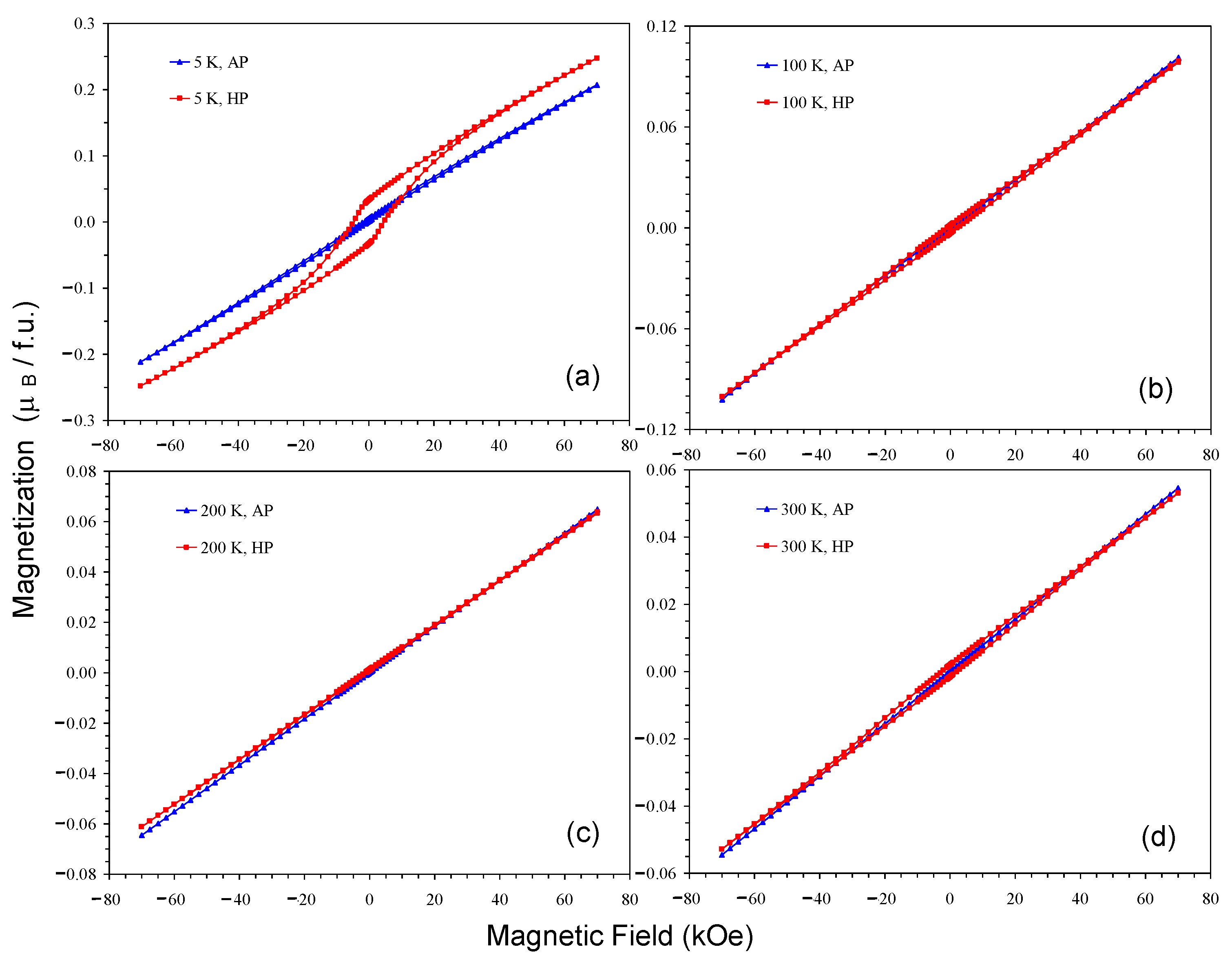
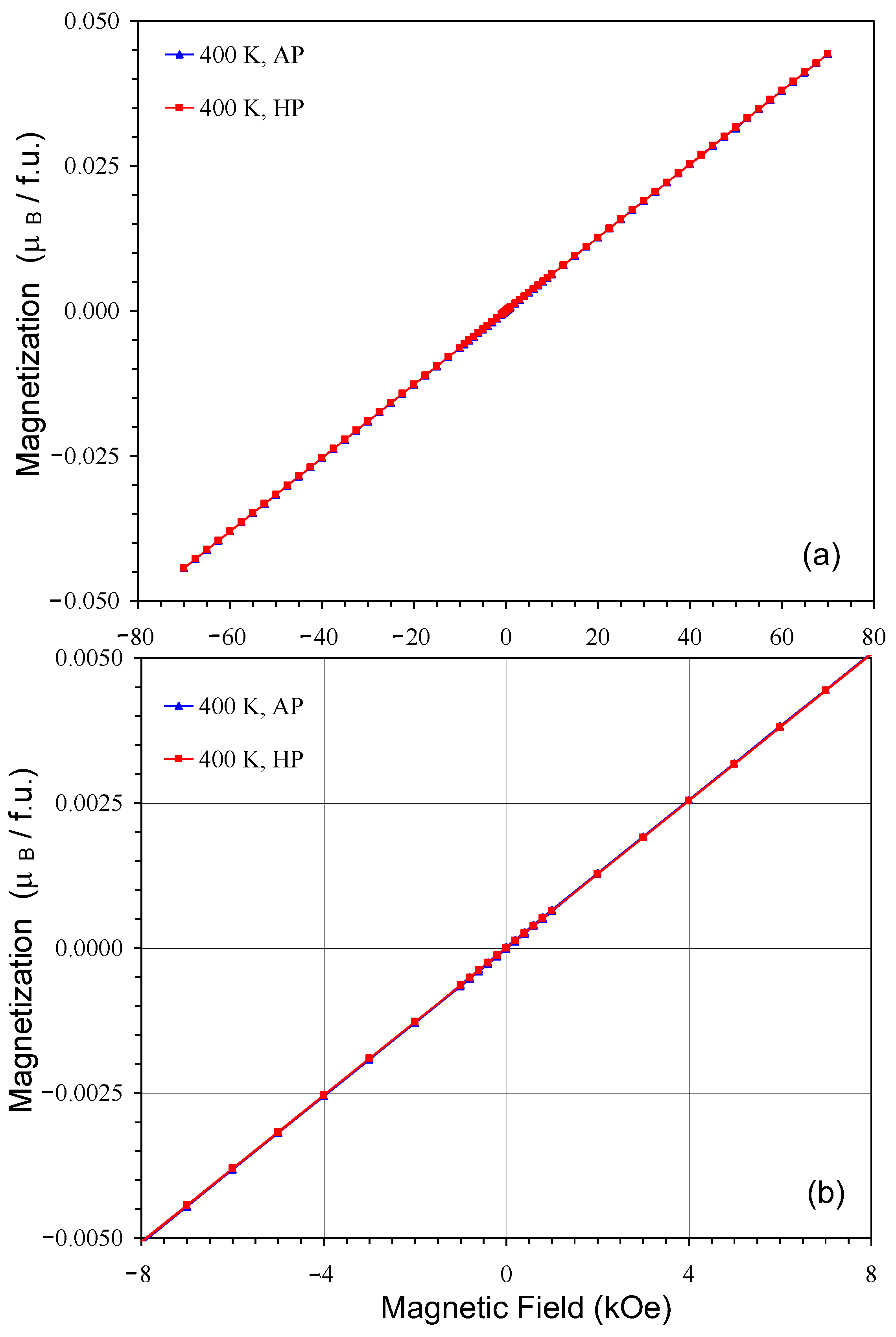
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokura, Y.; Seki, S.; Nagaosa, N. Multiferroics of spin origin. Rep. Prog. Phys. 2014, 77, 076501. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nature Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Khomskii, D.I. Multiferroics: Different ways to combine magnetism and ferroelectricity. J. Magn. Magn. Mater. 2006, 306, 1–8. [Google Scholar] [CrossRef]
- Khomskii, D. Classifying Multiferroics: Mechanisms and Effects. Physics 2009, 2, 20. [Google Scholar] [CrossRef]
- Schmid, H. Multi-ferroic Magnetoelectrics. Ferroelectrics 1994, 162, 317–338. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, E.; Cano, A. Non-collinear magnetism in multiferroic perovskites. J. Phys. Condens. Matter 2016, 28, 123001. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Dong, H.F.; Wen, M.R.; Wu, F.G. Pressure-Induced Phase Diagram and Electronic Structure Evolves during the Insulator-Metal Transition of Bulk BiFeO3. Inorg. Chem. 2023, 62, 16059–16067. [Google Scholar] [CrossRef]
- Wu, Y.; Han, X.; Huang, H.J. Structural transformation pathways of multiferroic BiFeO3 under high pressures. J. Phys. Chem. C 2018, 122, 6852–6857. [Google Scholar] [CrossRef]
- Knee, C.S.; Tucker, M.G.; Manuel, P.; Cai, S.Z.; Bielecki, J.; Borjesson, L.; Eriksson, S.G. High pressure crystal and magnetic phase transitions in multiferroic Bi0.9La0.1FeO3. Chem. Mater. 2014, 26, 1180–1186. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Shvartsman, V.V.; Borisov, P.; Kleemann, W.; Kiselev, D.A.; Bdikin, I.K.; Vieira, J.M.; Kholkin, A.L. Effect of Gd substitution on the crystal structure and multiferroic properties of BiFeO3. Acta Mater. 2009, 57, 5137–5145. [Google Scholar] [CrossRef]
- Rusakov, D.A.; Abakumov, A.M.; Yamaura, K.; Belik, A.A.; Van Tendeloo, G.; Takayama-Muromachi, E. Structural evolution of the BiFeO3−LaFeO3 system. Chem. Mater. 2011, 23, 285–292. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Karpinsky, D.V.; Bushinsky, M.V.; Khomchenko, V.A.; Kakazei, G.N.; Araujo, J.P.; Tovar, M.; Sikolenko, V.; Efimov, V.; Kholkin, A.L. Isothermal structural transitions, magnetization and large piezoelectric response in Bi1−xLaxFeO3 perovskites. Phys. Rev. B 2011, 83, 054109. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Troyanchuk, I.O.; Tovar, M.; Sikolenko, V.; Efimov, V.; Efimova, E.; Shur, V.Y.; Kholkin, A.L. Temperature and composition-induced structural transitions in Bi1−xLa(Pr)xFeO3 ceramics. J. Am. Ceram. Soc. 2014, 97, 2631–2638. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Troyanchuk, I.O.; Tobbens, D.M.; Sikolenko, V.; Paixao, J.A. Composition- and temperature-driven structural transitions in Bi1−xCaxFeO3 multiferroics: A neutron diffraction study. J. Phys. Condens. Matter 2013, 25, 135902. [Google Scholar] [CrossRef]
- Belik, A.A.; Abakumov, A.M.; Tsirlin, A.A.; Hadermann, J.; Kim, J.; Van Tendeloo, G.; Takayama-Muromachi, E. Structure and magnetic properties of BiFe0.75Mn0.25O3 perovskite prepared at ambient and high pressure. Chem. Mater. 2011, 23, 4505–4514. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Pandey, D. Stability of the various crystallographic phases of the multiferroic (1−x)BiFeO3−xPbTiO3 system as a function of composition and temperature. J. Appl. Phys. 2010, 107, 124112. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, S.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Khomchenko, V.A.; Alikin, D.O.; Abramov, A.S.; et al. Peculiarities of the crystal structure evolution of BiFeO3–BaTiO3 ceramics across structural phase transitions. Nanomaterials 2020, 10, 801. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrud, M.A.; Grande, T. Structure and properties of multiferroic oxygen hyperstoichiometric BiFe1-xMnxO3+δ. Chem. Mater. 2009, 21, 5176–5186. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrud, M.A.; Grande, T. High-temperature semiconducting cubic phase of BiFe0.7Mn0.3O3+δ. Phys. Rev. B 2009, 79, 214113. [Google Scholar] [CrossRef]
- Belik, A.A. Polar and nonpolar phases of BiMO3: A review. J. Solid State Chem. 2012, 195, 32–40. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Salak, A.N.; Fertman, E.L.; Kotlyar, O.V.; Eardley, E.; Olekhnovich, N.M.; Pushkarev, A.V.; Radyush, Y.V.; Fedorchenko, A.V.; Desnenko, V.A.; et al. The phenomenon of conversion polymorphism in Bi-containing metastable perovskites. Chem. Commun. 2019, 55, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.A. Origin of magnetization reversal and exchange bias phenomena in solid solutions of BiFeO3–BiMnO3: Intrinsic or extrinsic? Inorg. Chem. 2013, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Sundaresan, A.; Rao, C.N.R.; Iyo, A.; Shirage, P.M.; Tanaka, Y.; Simon, C.; Pralong, V.; Lebedev, O.I.; Caignaert, V.; et al. Temperature-induced magnetization reversal in BiFe0.5Mn0.5O3 synthesized at high pressure. Phys. Rev. B 2010, 82, 100416. [Google Scholar] [CrossRef]
- Levin, I.; Tucker, M.G.; Wu, H.; Provenzano, V.; Dennis, C.L.; Karimi, S.; Comyn, T.; Stevenson, T.; Smith, R.I.; Reaney, I.M. Displacive phase transitions and magnetic structures in Nd-substituted BiFeO3. Chem. Mater. 2011, 23, 2166–2175. [Google Scholar] [CrossRef]
- Corker, D.L.; Glazer, A.M.; Dec, J.; Roleder, K.; Whatmore, R.W. A re-investigation of the crystal structure of the perovskite PbZrO3 by X-ray and neutron diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 135–142. [Google Scholar] [CrossRef]
- Teslic, S.; Egami, T. Atomic structure of PbZrO3 determined by pulsed neutron diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 750–765. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Troyanchuk, I.O.; Maria, T.M.R.; Karpinsky, D.V.; Das, S.; Amaral, V.S.; Paixao, J.A. Mn doping-induced structural and magnetic transformations in the antiferroelectric phase of the Bi1−xNdxFeO3 perovskites. J. Appl. Phys. 2012, 112, 064105. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Latushka, S.I.; Zhaludkevich, D.V.; Sikolenko, V.V.; Al-Ghamdi, H.; Almuqrin, A.H.; Sayyed, M.I.; Belik, A.A. Structural and magnetic phase transitions in BiFe1−xMnxO3 solid solution driven by temperature. Nanomaterials 2022, 12, 1565. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Latushka, S.I.; Zhaludkevich, D.V.; Sikolenko, V.V.; Svetogorov, R.; Sayyed, M.I.; Almousa, N.; Trukhanov, A.; Trukhanov, S.; et al. Temperature-driven transformation of the crystal and magnetic structures of BiFe0.7Mn0.3O3 ceramics. Nanomaterials 2022, 12, 2813. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.K.; Yusuf, S.M.; Shukla, R.; Tyagi, A.K. Exchange bias in BiFe0.8Mn0.2O3 nanoparticles with an antiferromagnetic core and a diluted antiferromagnetic shell. Phys. Rev. B 2011, 83, 184412. [Google Scholar] [CrossRef]
- Fertman, E.L.; Fedorchenko, A.V.; Desnenko, V.A.; Shvartsman, V.V.; Lupascu, D.C.; Salamon, S.; Wende, H.; Vaisburd, A.I.; Stanulis, A.; Ramanauskas, R.; et al. Exchange bias effect in bulk multiferroic BiFe0.5Sc0.5O3. AIP Adv. 2020, 10, 045102. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111. [Google Scholar] [CrossRef] [PubMed]
- Izumi, F.; Ikeda, T. A Rietveld-analysis program RIETAN-98 and its applications to zeolites. Mater. Sci. Forum 2000, 321–324, 198–205. [Google Scholar] [CrossRef]




| Atom | WP | x/a | y/b | z/c | Biso (Å2) |
|---|---|---|---|---|---|
| Bi1 | 8d | 0.2169(5) | 0.00935(9) | 0.62476(16) | 1.18(5) |
| Bi2 | 4c | 0.2135(7) | 0.25 | 0.6251(2) | 1.37(7) |
| Bi3 | 4c | 0.7729(7) | 0.25 | 0.3635(2) | 1.55(7) |
| FeMn1 | 8d | 0.2397(12) | 0.1227(6) | 0.8779(7) | 1.06(11) |
| FeMn2 | 8d | 0.2615(11) | 0.6249(5) | 0.8770(6) | 0.54(10) |
| O1 | 8d | 0.809(4) | −0.0026(12) | 0.6556(18) | 0.24(16) |
| O2 | 4c | 0.641(6) | 0.25 | 0.653(3) | =B(O1) |
| O3 | 4c | 0.177(7) | 0.25 | 0.425(3) | =B(O1) |
| O4 | 8d | 0.099(4) | 0.1267(17) | 0.7217(19) | =B(O1) |
| O5 | 8d | −0.003(4) | 0.3942(15) | −0.0260(21) | =B(O1) |
| O6 | 8d | 0.041(4) | 0.6458(14) | 0.7424(23) | =B(O1) |
| O7 | 8d | 0.019(5) | 0.3989(13) | 0.516(3) | =B(O1) |
| Atom | WP | x/a | y/b | z/c | Biso (Å2) |
|---|---|---|---|---|---|
| Bi1 | 4g | 0.2431(3) | 0.3807(3) | 0 | 2.15(6) |
| Bi2 | 4h | 0.2061(3) | 0.3676(2) | 0.5 | 0.32(3) |
| FeMn | 8i | 0.2552(6) | 0.1239(14) | 0.2487(13) | 0.80(5) |
| O1 | 4g | 0.241(4) | 0.162(3) | 0 | 1.8(9) |
| O2 | 4h | 0.338(5) | 0.093(3) | 0.5 | 1.2(7) |
| O3 | 8i | 0.078(4) | 0.2649(16) | 0.275(3) | 1.7(5) |
| O4 | 4f | 0 | 0.5 | 0.188(3) | 1.3(7) |
| O5 | 4e | 0 | 0 | 0.202(4) | 2.4(8) |
| Atom | WP | x/a | y/b | z/c | Biso (Å2) |
|---|---|---|---|---|---|
| Bi1 | 4g | 0.2383(4) | 0.3786(5) | 0 | 3.31(13) |
| Bi2 | 4h | 0.2141(5) | 0.3700(4) | 0.5 | 1.91(9) |
| FeMn | 8i | 0.2524(9) | 0.1246(25) | 0.2499(17) | 1.56(6) |
| O1 | 4g | 0.257(5) | 0.170(5) | 0 | 2.3 (3) |
| O2 | 4h | 0.304(6) | 0.091(5) | 0.5 | =B(O1) |
| O3 | 8i | 0.075(4) | 0.2678(19) | 0.271(4) | =B(O1) |
| O4 | 4f | 0 | 0.5 | 0.185(4) | =B(O1) |
| O5 | 4e | 0 | 0 | 0.206(4) | =B(O1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belik, A.A. Two Perovskite Modifications of BiFe0.6Mn0.4O3 Prepared by High-Pressure and Post-Synthesis Annealing at Ambient Pressure. Inorganics 2024, 12, 226. https://doi.org/10.3390/inorganics12080226
Belik AA. Two Perovskite Modifications of BiFe0.6Mn0.4O3 Prepared by High-Pressure and Post-Synthesis Annealing at Ambient Pressure. Inorganics. 2024; 12(8):226. https://doi.org/10.3390/inorganics12080226
Chicago/Turabian StyleBelik, Alexei A. 2024. "Two Perovskite Modifications of BiFe0.6Mn0.4O3 Prepared by High-Pressure and Post-Synthesis Annealing at Ambient Pressure" Inorganics 12, no. 8: 226. https://doi.org/10.3390/inorganics12080226
APA StyleBelik, A. A. (2024). Two Perovskite Modifications of BiFe0.6Mn0.4O3 Prepared by High-Pressure and Post-Synthesis Annealing at Ambient Pressure. Inorganics, 12(8), 226. https://doi.org/10.3390/inorganics12080226






