Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Seed Extract-Mediated Biofabricated ZnO Nanoparticles
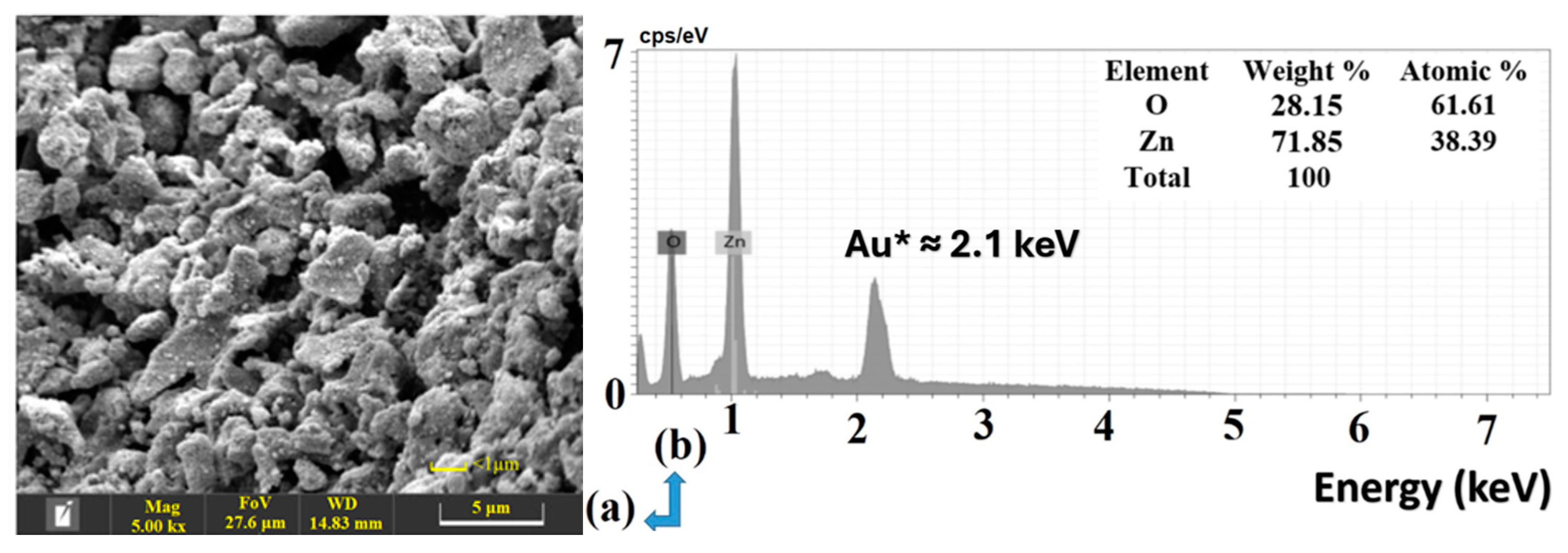
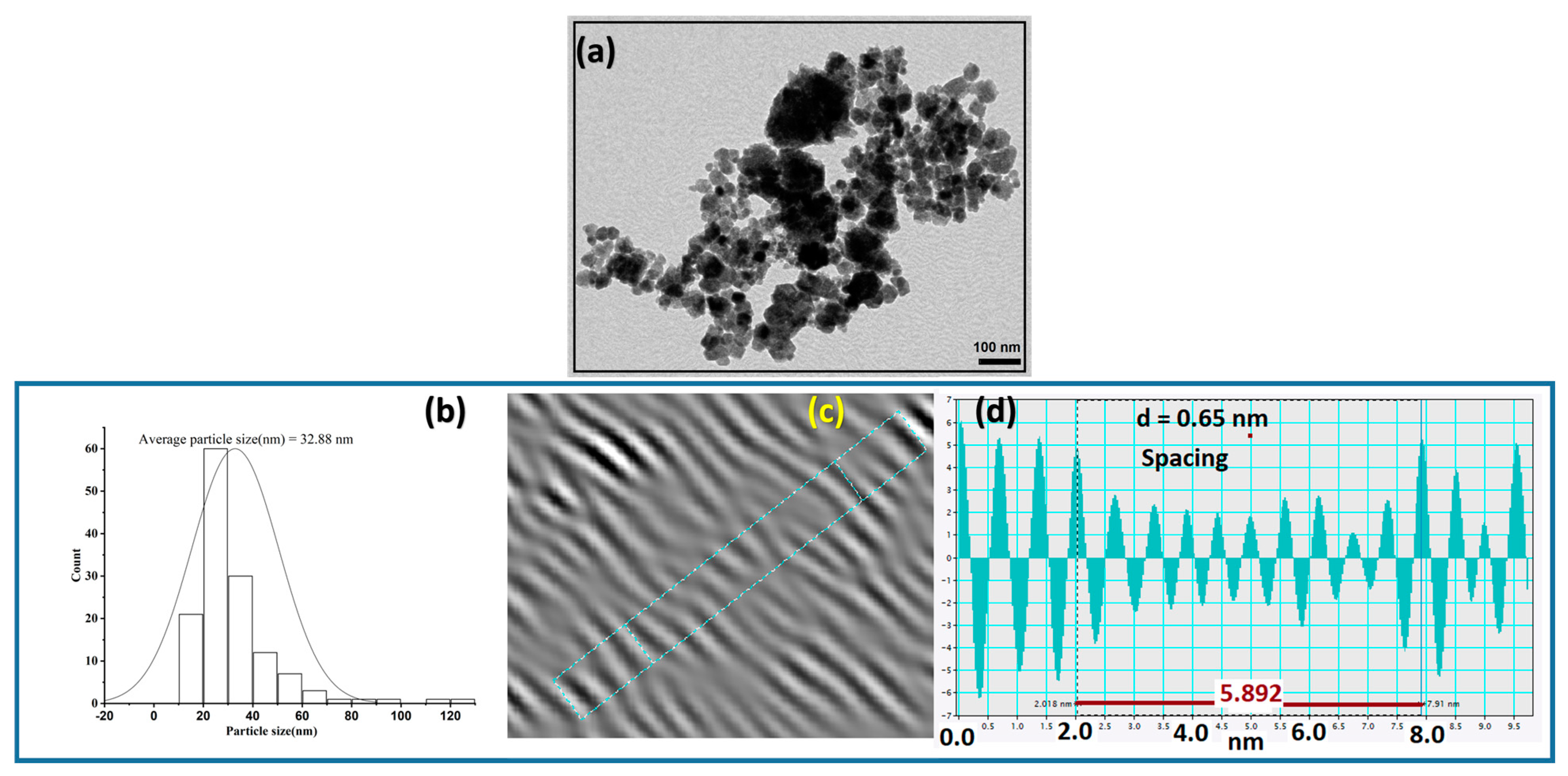
2.1.1. SEM, EDX, and TEM
2.1.2. FT-IR, XRD, and UV Analysis
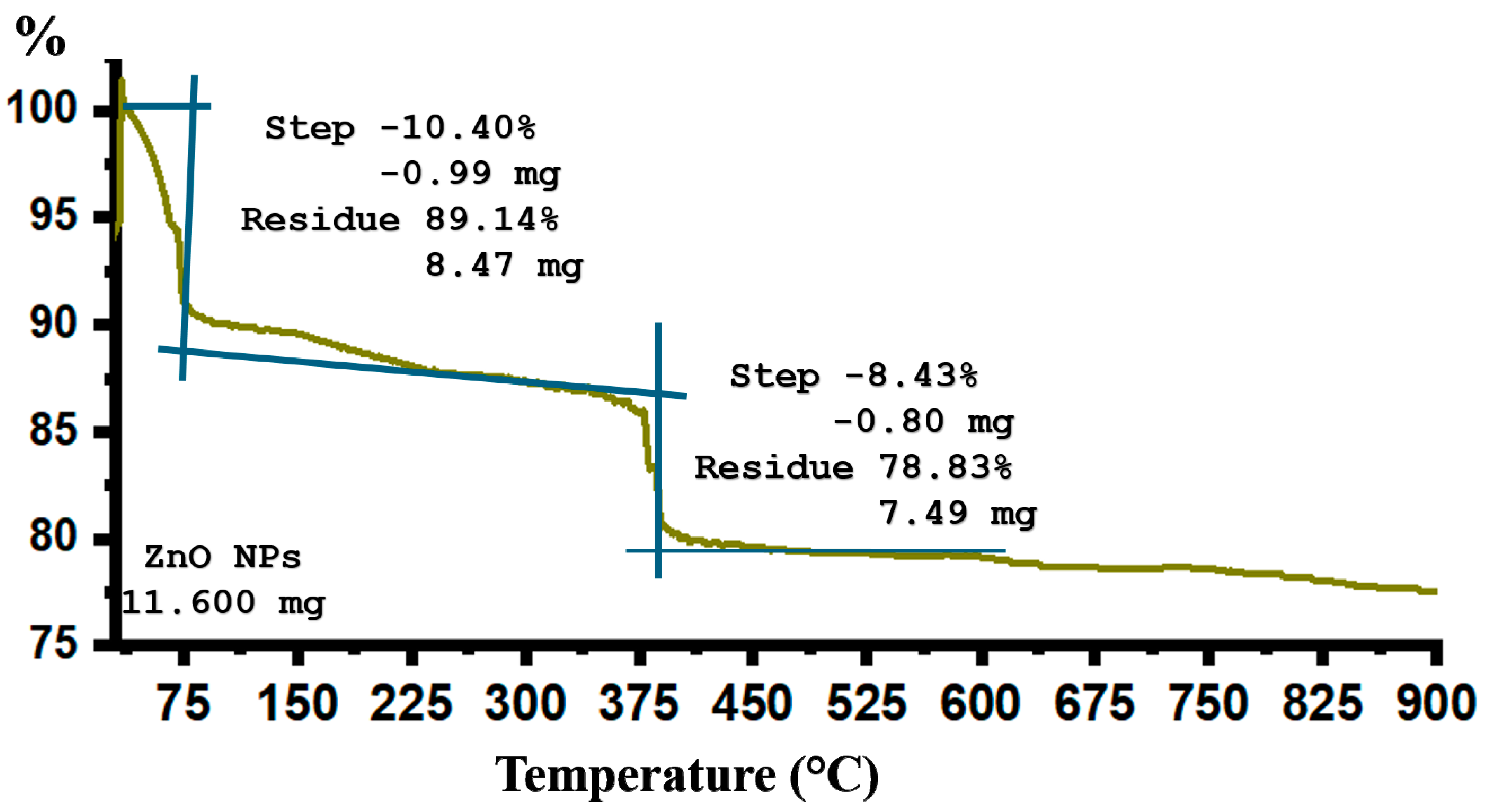
2.2. Thermogravimetric Analysis (TGA)
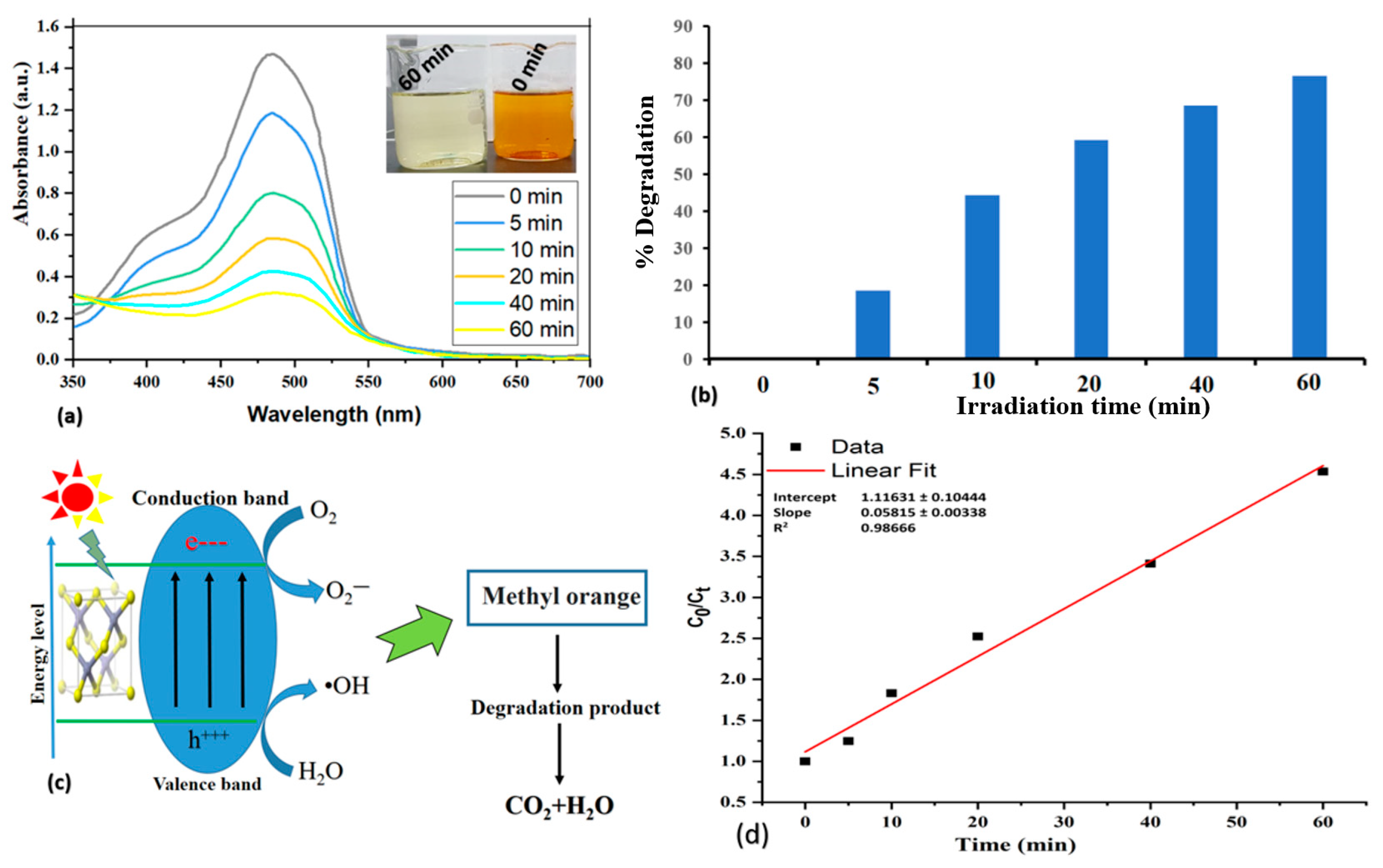
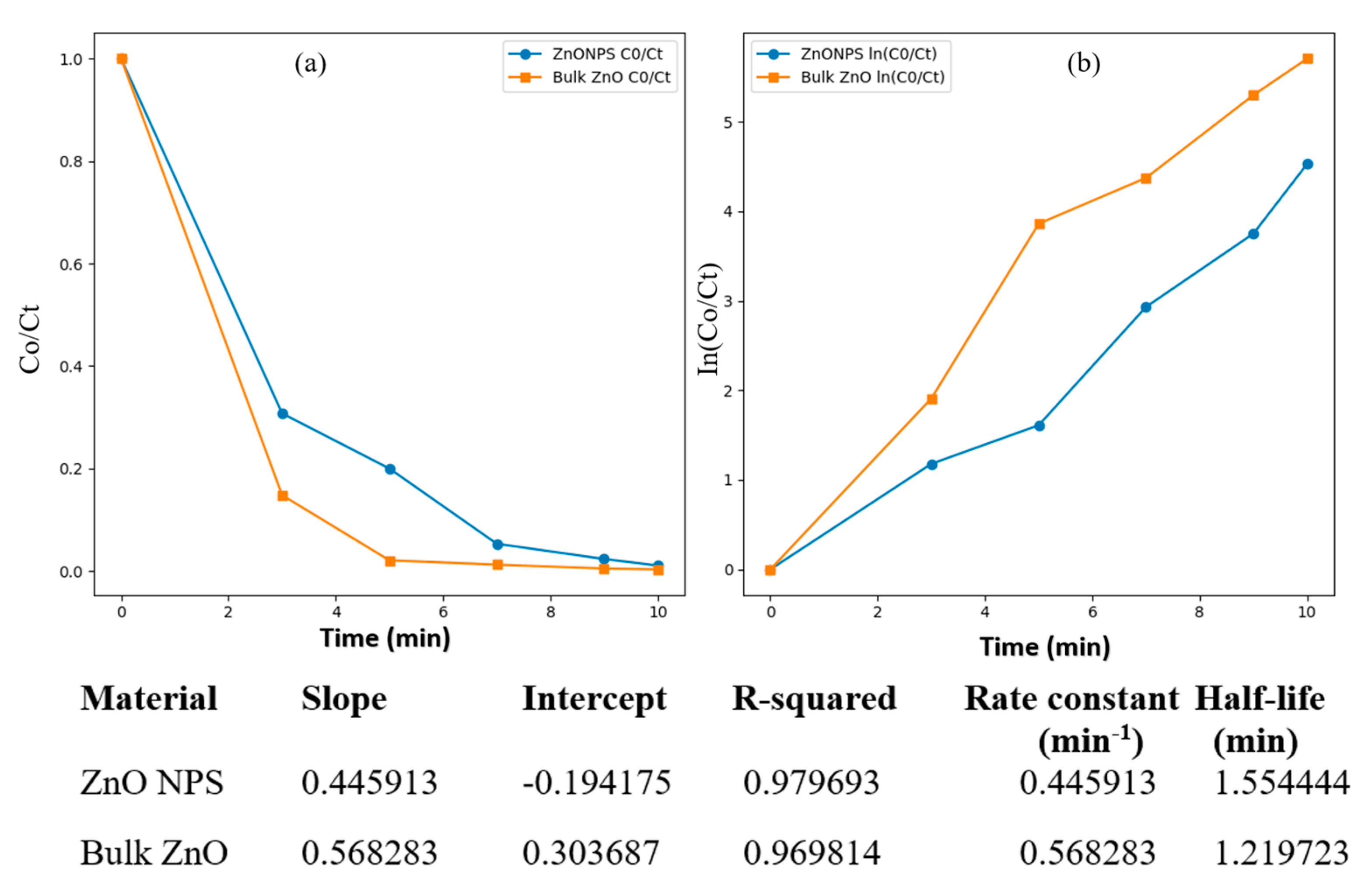
2.3. Analysis of Photocatalytic Activity
2.4. Stability and Reusability of ZnONPs
2.5. Analysis of Corrosion Studies
2.6. Evaluation of H2S Adsorption Capacity
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Plant Extract and Utilization in Biosynthesis of Zinc Oxide Nanoparticles
3.3. Characterization of Biofabricated ZnO NPs
3.4. Photocatalytic Experiment
3.5. Corrosion Studies
3.5.1. Samples Compositions and Preparation
3.5.2. Preparation for Corrosive Environments
3.5.3. Electrochemical Polarization Measurement
3.6. Adsorption Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J.J.E.T. Innovation. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and environmental applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Green nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 145–198. [Google Scholar]
- Khan, S.H. Green nanotechnology for the environment and sustainable development. In Green Materials for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 13–46. [Google Scholar]
- Sukul, P.K.; Kar, C. Green Conversion Methods to Prepare Nanoparticle. In Bioinspired and Green Synthesis of Nanostructures: A Sustainable Approach; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 115–139. [Google Scholar]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y.J.P. Zinc oxide nanoparticles: Synthesis, characterization, modification, and applications in food and agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Soltani, S.; Gacem, A.; Choudhary, N.; Yadav, V.K.; Alsaeedi, H.; Modi, S.; Patel, A.; Khan, S.H.; Cabral-Pinto, M.M.; Yadav, K.K. Scallion peel mediated synthesis of zinc oxide nanoparticles and their applications as nano fertilizer and photocatalyst for removal of organic pollutants from wastewater. Water 2023, 15, 1672. [Google Scholar] [CrossRef]
- Adraoui, I.; Mamouni, R.; Saffaj, N.; Achemchem, F. Eco-friendly synthesis of zinc oxide nanoparticles using saffron extract and their photocatalytic and antibacterial activities. J. Mater. Res. 2023, 38, 2874–2884. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Shnawa, B.H.; Jalil, P.J.; Al-Ezzi, A.; Mhamedsharif, R.M.; Mohammed, D.A.; Biro, D.M.; Ahmed, M.H. Evaluation of antimicrobial and antioxidant activity of zinc oxide nanoparticles biosynthesized with Ziziphus spina-christi leaf extracts. J. Environ. Sci. Health Part C 2024, 42, 93–108. [Google Scholar] [CrossRef]
- Sutharappa Kaliyamoorthy, T.; Subramaniyan, V.; Renganathan, S.; Elavarasan, V.; Ravi, J.; Prabhakaran Kala, P.; Subramaniyan, P.; Vijayakumar, S. Sustainable environmental-based ZnO nanoparticles derived from Pisonia grandis for future biological and environmental applications. Sustainability 2022, 14, 17009. [Google Scholar] [CrossRef]
- Tabrez, S.; Khan, A.U.; Hoque, M.; Suhail, M.; Khan, M.I.; Zughaibi, T.A. Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer. Nanotechnol. Rev. 2022, 11, 2714–2725. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Rehman, A.; Mehmood, A.; Ali, A.; Zahra, S.M.; Roobab, U.; Singh, A.; Ibrahim, S.A.; Siddiqui, S.A. Biocompatible nanomaterials in food science, technology, and nutrient drug delivery: Recent developments and applications. Front. Nutr. 2022, 8, 778155. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Apu, M.A.I.; Ashrafudoulla, M.; Rahman, M.M.; Parvez, M.A.K.; Balusamy, S.R.; Akter, S.; Rahman, M.S. Bioactive ZnO Nanoparticles: Biosynthesis, Characterization and Potential Antimicrobial Applications. Pharmaceutics 2023, 15, 2634. [Google Scholar] [CrossRef] [PubMed]
- Despotović, V.; Finčur, N.; Bognar, S.; Šojić Merkulov, D.; Putnik, P.; Abramović, B.; Panić, S. Characterization and Photocatalytic Performance of Newly Synthesized ZnO Nanoparticles for Environmental Organic Pollutants Removal from Water System. Separations 2023, 10, 258. [Google Scholar] [CrossRef]
- Chemingui, H.; Missaoui, T.; Mzali, J.C.; Yildiz, T.; Konyar, M.; Smiri, M.; Saidi, N.; Hafiane, A.; Yatmaz, H. Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): Antibacterial and photocatalytic activities. Mater. Res. Express 2019, 6, 1050b4. [Google Scholar] [CrossRef]
- Chemingui, H.; Lafi, R.; Missaoui, T.; Montasser, I.; Hafiane, A.; Kamoun, M. Eco-friendly approach for the synthesis of zinc oxide nanoparticles (ZnO NPs) using Verbena officinalis L: Enhancement of photocatalytic activity under UV light and application of response surface methodology. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–19. [Google Scholar]
- Chemingui, H.; Moulahi, A.; Missaoui, T.; Al-Marri, A.H.; Hafiane, A. A novel green preparation of zinc oxide nanoparticles with Hibiscus sabdariffa L.: Photocatalytic performance, evaluation of antioxidant and antibacterial activity. Environ. Technol. 2024, 45, 926–944. [Google Scholar] [CrossRef]
- Wal, A.; Singh, M.R.; Gupta, A.; Rathore, S.; Rout, R.R.; Wal, P. Pumpkin Seeds (Cucurbita spp.) as a Nutraceutical Used in Various Lifestyle Disorders. Nat. Prod. J. 2024, 14, 118–137. [Google Scholar] [CrossRef]
- Ethiraj, S.; Balasundaram, J. Phytochemical and biological activity of Cucurbita seed extract. J. Adv. Biotechnol. 2016, 6, 813–821. [Google Scholar] [CrossRef]
- Muchirah, P.N.; Waihenya, R.; Muya, S.; Abubakar, L.; Ozwara, H.; Makokha, A. Characterization and anti-oxidant activity of Cucurbita maxima Duchesne pulp and seed extracts. J. Phytopharm. 2018, 7, 134–140. [Google Scholar] [CrossRef]
- Zhao, X.; Baharinikoo, L.; Farahani, M.D.; Mahdizadeh, B.; Farizhandi, A.A. Experimental modelling studies on the removal of dyes and heavy metal ions using ZnFe2O4 nanoparticles. Sci. Rep. 2022, 12, 5987. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green synthesis of nanoparticles and their applications in water and wastewater treatment. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 349–379. [Google Scholar]
- Alam, M. Analyses of biosynthesized silver nanoparticles produced from strawberry fruit pomace extracts in terms of biocompatibility, cytotoxicity, antioxidant ability, photodegradation, and in-silico studies. J. King Saud Univ.-Sci. 2022, 34, 102327. [Google Scholar] [CrossRef]
- Huda Abd Kadir, N.; Ali Khan, A.; Kumaresan, T.; Khan, A.U.; Alam, M. The impact of pumpkin seed-derived silver nanoparticles on corrosion and cytotoxicity: A molecular docking study of the simulated AgNPs. Green Chem. Lett. Rev. 2024, 17, 2319246. [Google Scholar] [CrossRef]
- Jose, L.M.; Raj, R.A.; Sajan, D.; Aravind, A. Adsorption and photocatalytic activity of biosynthesised ZnO nanoparticles using Aloe Vera leaf extract. Nano Express 2021, 2, 010039. [Google Scholar] [CrossRef]
- Kadir, N.H.A.; Murugan, N.; Khan, A.A.; Sandrasegaran, A.; Khan, A.U.; Alam, M. Evaluation of the cytotoxicity, antioxidant activity, and molecular docking of biogenic zinc oxide nanoparticles derived from pumpkin seeds. Microsc. Res. Tech. 2024, 87, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies. Nanotechnol. Rev. 2021, 10, 1079–1091. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Hashem, A.H.; Elhussieny, N.I.; Saied, E. Green biosynthesis of zinc oxide nanoparticles using Pluchea indica leaf extract: Antimicrobial and photocatalytic activities. Molecules 2023, 28, 4679. [Google Scholar] [CrossRef] [PubMed]
- Al Rahbi, A.S.; Al Mawali, A.H.; Al Rawahi, S.S.; Al Dighishi, R.K.; Al Abri, F.A.; Ahmed, A.; Rahman, S. Green synthesis of zinc oxide nanoparticles from Salvadora persica leaf extract: Characterization and studying methyl orange removal by adsorption. Water Pract. Technol. 2024, 19, 1219–1231. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: Synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J. Photochem. Photobiol. B Biol. 2016, 162, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grandiflorum for antimicrobial activity and adsorption of methylene blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Akhir, R.M.; Norashikin, M.H.; Mahat, M.M.; Bonnia, N.N. Biosynthesis of Zinc Oxide Nanoparticles for Corrosion Protection Application. Int. J. Eng. Technol 2018, 4, 488–492. [Google Scholar]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Hanini, K.; Tamfu, A.N.; Boudiba, L.; Soltani, H.; Laouer, H.; Akkal, S. Evaluation of the Potential of Daucus crinitus Extracts and Their Synthesized ZnO Nanoparticles in Inhibiting the Corrosion of Carbon Steel. J. Chem. 2024, 2024, 7703829. [Google Scholar] [CrossRef]
- Al-Senani, G.M. Synthesis of ZnO-NPs using a Convolvulus arvensis leaf extract and proving its efficiency as an inhibitor of carbon steel corrosion. Materials 2020, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, Z.; Vunduk, J.; Bartolic, D.; Miskovic, G.; Ognjanovic, M.; Tadic, N.B.; Nikolic, M.V. An Eco-friendly Approach to ZnO NP Synthesis Using Citrus reticulata Blanco Peel/Extract: Characterization and Antibacterial and Photocatalytic Activity. ACS Appl. Bio Mater. 2024, 7, 3014–3032. [Google Scholar] [CrossRef] [PubMed]
- Gamedze, N.P.; Mthiyane, D.M.; Mavengahama, S.; Singh, M.; Onwudiwe, D.C. Biosynthesis of ZnO nanoparticles using the aqueous extract of Mucuna pruriens (utilis): Structural characterization, and the anticancer and antioxidant activities. Chem. Afr. 2024, 7, 219–228. [Google Scholar] [CrossRef]
- Vishnupriya, B.; Nandhini, G.E.; Anbarasi, G. Biosynthesis of zinc oxide nanoparticles using Hylocereus undatus fruit peel extract against clinical pathogens. Mater. Today Proc. 2022, 48, 164–168. [Google Scholar] [CrossRef]
- Hayat, K.; Din, I.U.; Alam, K.; Khan, F.U.; Khan, M.; Mohamed, H.I. Green synthesis of zinc oxide nanoparticles using plant extracts of Fumaria officinalis and Peganum harmala and their antioxidant and antibacterial activities. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–15. [Google Scholar]
- Dappula, S.S.; Kandrakonda, Y.R.; Shaik, J.B.; Mothukuru, S.L.; Lebaka, V.R.; Mannarapu, M.; Amooru, G.D. Biosynthesis of zinc oxide nanoparticles using aqueous extract of Andrographis alata: Characterization, optimization and assessment of their antibacterial, antioxidant, antidiabetic and anti-Alzheimer’s properties. J. Mol. Struct. 2023, 1273, 134264. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, E.; Adimasu, Y.; Dessie, Y. Biosynthesis and optimization of ZnO nanoparticles using Ocimum lamifolium leaf extract for electrochemical sensor and antibacterial activity. ACS Omega 2023, 8, 27344–27354. [Google Scholar] [CrossRef]
- Mishra, D.; Chitara, M.K.; Negi, S.; Pal Singh, J.; Kumar, R.; Chaturvedi, P. Biosynthesis of zinc oxide nanoparticles via leaf extracts of Catharanthus roseus (L.) G. Don and their application in improving seed germination potential and seedling vigor of Eleusine coracana (L.) Gaertn. Adv. Agric. 2023, 2023, 7412714. [Google Scholar] [CrossRef]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar]
- Ramamoorthy, S.; Surendhiran, S.; Senthil Kumar, D.; Murugesan, G.; Kalaiselvi, M.; Kavisree, S.; Muthulingam, S.; Murugesan, S. Evaluation of photocatalytic and corrosion properties of green synthesized zinc oxide nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 9722–9731. [Google Scholar] [CrossRef]
- Shaghaghi, Z.; Mollaei, S.; Amani-Ghadim, A.R.; Abedini, Z. Green synthesis of ZnO nanoparticles using the aqueous extract of Platanus orientalis: Structural characterization and photocatalytic activity. Mater. Chem. Phys. 2023, 305, 127900. [Google Scholar] [CrossRef]
- Boora, A.; Duhan, S.; Rohilla, B.; Malik, P.; Sehrawat, S.; Goyat, M.; Mishra, Y.K.; Kumar, V. A three-dimensional ZnO/TUD-1 nanocomposite-based multifunctional sensor for humidity detection and wastewater remediation. Mater. Adv. 2024, 5, 4467–4479. [Google Scholar] [CrossRef]
- Plana-Ruiz, S.; Krysiak, Y.; Portillo, J.; Alig, E.; Estradé, S.; Peiró, F.; Kolb, U. Fast-ADT: A fast and automated electron diffraction tomography setup for structure determination and refinement. Ultramicroscopy 2020, 211, 112951. [Google Scholar] [CrossRef] [PubMed]
- Roslova, M.; Smeets, S.; Wang, B.; Thersleff, T.; Xu, H.; Zou, X. InsteaDMatic: Towards cross-platform automated continuous rotation electron diffraction. J. Appl. Crystallogr. 2020, 53, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Dwivedi, P.; Malik, A.; Hussain, H.Z.F.; Jatrana, I.; Imtiyaz, K.; Rizvi, M.A.; Mushtaque, M.; Khan, A.U.; Alam, M.; Rafatullah, M. Eco-Friendly CuO/Fe3O4 Nanocomposite synthesis, characterization, and cytotoxicity study. Heliyon 2024, 10, e27787. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Rajashekara, S.; Shrivastava, A.; Sumhitha, S.; Kumari, S. Biomedical Applications of Biogenic Zinc Oxide Nanoparticles Manufactured from Leaf Extracts of Calotropis gigantea (L.) Dryand. BioNanoScience 2020, 10, 654–671. [Google Scholar] [CrossRef]
- Othman, A.; Ali, M.A.; Ibrahim, E.; Osman, M. Influence of Cu doping on structural, morphological, photoluminescence, and electrical properties of ZnO nanostructures synthesized by ice-bath assisted sonochemical method. J. Alloys Compd. 2016, 683, 399–411. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef] [PubMed]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Moharram, A.; Mansour, S.; Hussein, M.; Rashad, M. Direct precipitation and characterization of ZnO nanoparticles. J. Nanomater. 2014, 2014, 20. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band gap narrowing and widening of ZnO nanostructures and doped materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Arumugam, J.; Ramu, P.; Pugazhenthiran, N.; Jothilakshmi, R.; Prabu, K. Sunlight assisted degradation of methylene blue dye by zinc oxide nanoparticles green synthesized using Vitex negundo plant leaf extract. Results Chem. 2024, 7, 101315. [Google Scholar] [CrossRef]
- Samavati, A.; Awang, A.; Samavati, Z.; Ismail, A.F.; Othman, M.; Velashjerdi, M.; Rostami, A. Influence of ZnO nanostructure configuration on tailoring the optical bandgap: Theory and experiment. Mater. Sci. Eng. B 2021, 263, 114811. [Google Scholar] [CrossRef]
- Pal, D.; Chattopadhyay, S. Surface and interface effects: Properties of nanostructured ZnO. In Nanostructured Zinc Oxide; Elsevier: Amsterdam, The Netherlands, 2021; pp. 253–287. [Google Scholar]
- Gaur, J.; Kumar, S.; Pal, M.; Kaur, H.; Batoo, K.M.; Momoh, J.O. Current trends: Zinc oxide nanoparticles preparation via chemical and green method for the photocatalytic degradation of various organic dyes. Hybrid Adv. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green synthesis of silver nanoparticles using Diospyros malabarica fruit extract and assessments of their antimicrobial, anticancer and catalytic reduction of 4-nitrophenol (4-NP). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef]
- Arroyo-Ortega, G.; Paz, J.; Armendariz, I.O.; Camacho-Montes, H.; León, C.; Reyes-Blas, H.; Rodríguez-González, C. Photocatalytical degradation of methyl orange (MO) using ZnO nanoparticles from alkaline wasted batteries. The effect of the MO, catalyst, and organic loads. Dig. J. Nanomater. Biostruct. (DJNB) 2022, 17, 1241–1248. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Souza, D.A.R.; Gusatti, M.; Sanches, C.; Moser, M.; Kuhnen, N.; Riella, H. Initial studies of photocatalytic discolouration of methyl orange by using ZnO nanostructures. Chem. Eng. Trans. 2013, 32, 2275–2280. [Google Scholar]
- Vasei, H.V.; Masoudpanah, S.; Adeli, M.; Aboutalebi, M.; Habibollahzadeh, M. Mesoporous honeycomb-like ZnO as ultraviolet photocatalyst synthesized via solution combustion method. Mater. Res. Bull. 2019, 117, 72–77. [Google Scholar] [CrossRef]
- Rahimi, R.; Yaghoubi-Berijani, M.; Zargari, S.; Rabbani, M.; Shariatinia, S. SnTCPP-modified ZnO nanorods prepared via a simple co-precipitation method: Application as a new photocatalyst for photodegradation and photoreduction processes. Res. Chem. Intermed. 2016, 42, 4697–4714. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and rapid photocatalytic degradation of methyl orange dye using Al/ZnO nanoparticles. Nanomaterials 2021, 11, 1059. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and characterization of Co–ZnO and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Ajil, A.H.; Ahmed, N.M.; Yam, F.; Zango, Z.U.; Wadi, I.A.; Binzowaimil, A.M.; Aldaghri, O.; Ibnaouf, K.H.; Cabrera, H. Enhancing methyl orange degradation with laser-generated ZnO and Ce-doped ZnO nanoparticles. Appl. Sci. 2023, 13, 11857. [Google Scholar] [CrossRef]
- Sultana, K.A.; Hernandez Ortega, J.; Islam, M.T.; Dorado, Z.N.; Alvarado-Tenorio, B.; Galindo-Esquivel, I.R.; Noveron, J.C. Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation. Sustain. Chem. 2023, 4, 321–338. [Google Scholar] [CrossRef]
- Algarni, T.S.; Abduh, N.A.; Aouissi, A.; Al Kahtani, A. Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract. Green Process. Synth. 2022, 11, 895–906. [Google Scholar] [CrossRef]
- Ali, J.; Irshad, R.; Li, B.; Tahir, K.; Ahmad, A.; Shakeel, M.; Khan, N.U.; Khan, Z.U.H. Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J. Photochem. Photobiol. B Biol. 2018, 183, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Salam, M.A. Green synthesis of zinc oxide nanoparticles by Ziziphus jujuba leaves extract: Environmental application, kinetic and thermodynamic studies. J. Phys. Chem. Solids 2021, 158, 110237. [Google Scholar] [CrossRef]
- Baruah, R.; Goswami, M.; Das, A.M.; Nath, D.; Talukdar, K. Multifunctional ZnO bionanocomposites in the treatment of polluted water and controlling of multi-drug resistant bacteria. J. Mol. Struct. 2023, 1283, 135251. [Google Scholar] [CrossRef]
- El Faroudi, L.; Saadi, L.; Barakat, A.; Mansori, M.; Abdelouahdi, K.; Solhy, A. Facile and Sustainable Synthesis of ZnO Nanoparticles: Effect of Gelling Agents on ZnO Shapes and Their Photocatalytic Performance. ACS Omega 2023, 8, 24952–24963. [Google Scholar] [CrossRef] [PubMed]
- Moja, M.M.; Mapossa, A.B.; Chirwa, E.M.N.; Tichapondwa, S. Photocatalytic degradation of 2, 4-dichlorophenol using nanomaterials silver halide catalysts. Environ. Sci. Pollut. Res. 2024, 31, 11857–11872. [Google Scholar] [CrossRef] [PubMed]
- Sarah, M.; Sihem, A.; Nabila, B.; Asma, Y.; Rafik, C.; Kamilia, M.; Karima, A. Corrosion inhibiting effects of biosynthesized ZnO nanoparticles by the extract of Plectranthus amboinicus leaves. Inorg. Chem. Commun. 2024, 112836, in press. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. ZnO nanoparticles: Synthesis and adsorption study. Nat. Sci. 2009, 1, 129. [Google Scholar] [CrossRef]
- Zewde, D.; Geremew, B. Biosynthesis of ZnO nanoparticles using Hagenia abyssinica leaf extracts; their photocatalytic and antibacterial activities. Environ. Pollut. Bioavailab. 2022, 34, 224–235. [Google Scholar] [CrossRef]




| S.No. | Plant Section | Characterization | Applications * | Ref | ||
|---|---|---|---|---|---|---|
| Biological Assay | PDA/E/AS | CIA/Ad | ||||
| 1 | Verbena offcinalis leaf extract | XRD, FTIR, SEM, TEM, DLS, UV, 19.44 nm, Eg = 3.3 eV, spherical | Congo Red/97% | [19] | ||
| 2 | Laurus nobilis plant extract | Photoluminescence, XRD, FTIR, SEM, TEM, UV, 20–30 nm, Eg = 3.30 eV. BET/BJH | Antibacterial (E. coli) | Remazol Brilliant Red F3B/99%/60 min | [18] | |
| 3 | Hibiscus sabdariffa L. flower extract | FTIR, XRD, 15 nm, SEM/EDX, DLS, BET, TEM, ZP, wurtzite, Eg = 3.21 eV | Antibacterial (E. coli, S. aureus) DPPH (IC50 value of 38 μg/mL) | MO under UV irradiation/99%/60 min | [20] | |
| 4 | Pluchea indica leaf extract | FTIR, UV, XRD, TEM, SAED, SEM/EDX, DLS, 21.9 nm | Antimicrobial (P. aeruginosa; E. faecalis, B. subtilis; S. aureus; C. albicans; C. neoformans | MB/95%/150 min | [31] | |
| 5 | Salvadora persica leaf extract | SEM-EDX, FTIR, XRD, UV-Vis, 32–68 nm, Langmuir isotherm model, hexagonal/rod-shaped | MO/68%/100 min | [32] | ||
| 6 | Jacaranda mimosifolia flowers extract | Microwave-assisted synthesis, XRD, HRTEM, 2–4 nm GC–MS, Eg = 4.03 eV | Antibacterial, E. coli, E. faecium | Adsorption by molecular modeling | [33] | |
| 7 | Becium grandiflorum | UV–Vis, FTIR, XRD and SEM–EDS | Antimicrobial, S. epidermidis, S. aureus, K. pneumonia, P. aeruginosa, E. coli | MB under UV irradiation/69%/200 min | [34] | |
| 8 | Pandanus amaryllifolius leaves extract | XRD, wurtzite, FESEM, EDX, 4.15 nm, 27.19 nm, 35.69 nm | Corrosion inhibition, 1.0 M HCl, 79.43% | [35] | ||
| 9 | Daucus crinitus extracts (DCE) | UV-vis, SEM-EDS | Corrosion inhibitors for carbon steel (CS) in HCl, inhibitory efficiencies; 80.20, 91.20% | [36] | ||
| 10 | Convolvulus arvensis leaf extract | FTIR, UV-vis | Inhibitor of carbon steel corrosion, 1 M HCl solution, 91% | [37] | ||
| 11 | Pinecone extract (PCE) | UV-Vis, FTIR, XRD, HRTEM, and SAED, 40 to 60 nm, sphere-like morphology | Antimicrobial, E. coli, B. subtilis, H. insolens, M. indicus, biocompatibility, cytotoxicity | Photocatalytic activity, MB, 60%. 30 min | [30] | |
| 12 | Fruit extracts of Myristica fragrans | XRD (41.23 nm), FTIR, SEM (43.3 to 83.1 nm), UV (Eg = 2.57 eV), semispherical shape, TEM (35.5 nm), DLS, Zeta (66 nm and −22.1 mV), TGA | Protein kinase inhibition assay. Antidiabetic, antioxidant, antilarvicidal K. pneumoniae, E. coli, P. aeruginosa, S. aureus | Photocatalytic, MB, 88% | [38] | |
| 13 | Citrus reticulata Blanco | FTIR, UV–vis, hexagonal wurtzite, Eg = 2.84–3.14 eV, 7 and 26 nm via XRD, ZP = −20 mV, * PL | Antimicrobial; E. coli, S. enteritidis, S. aureus, antioxidant; CUPRAC assay ABTS | Photocatalytic, acid green dye, 94%/90 min | [39] | |
| 14 | Pumpkin seed extract | FTIR, XRD, FESEM/, TEM, 48–50 nm | Anticancer breast cancer | [14] | ||
| 15 | Pumpkin seed extract | FTIR, UV, XRD, SEM–EDX, TEM/SAED, 50 to 100 nm, TGA | Anticancer HCT 116, DPPH (IC50 of 142.857 μg/mL) | [29] | ||
| 16 | Aqueous extract of Mucuna pruriens | FTIR, UV, XRD, SEM/EDX, Spherical, TEM/SAED, 30.50 nm, Eg = 3.75 eV | Anticancer HeLa, HEK 293, antioxidant, DPPH (IC50 = 4.10 µg mL−1) | [40] | ||
| 17 | Hylocereus undatus fruit peel extract | FTIR, UV, XRD, SEM/EDX, spherical shape, 10–100 nm; | Antimicrobial activity; E. coli; K. Pneumoniae; P. aeruginosa; B. subtilis; C. albicans | [41] | ||
| 18 | Fumaria officinalis and Peganum harmala | FTIR, UV, XRD, 25.10 nm, irregular rods, spherical | Antioxidant ABTS, (41.67 & 39.79%) antibacterial (S. aureus; C. michiganensis | [42] | ||
| 19 | Andrographis alata | UV–Vis, FT-IR, XRD, SEM, EDAX, HR-TEM, DLS, 35–53 nm | Antibacterial, antioxidant (DPPH, ABTS), antidiabetic, anti-Alzheimer | [43] | ||
| 20 | Sea lavender | UV–Vis, FT-IR, XRD, ~ 41 nm, hexagonal/cubic crystalline. SEM, EDAX, TEM, GC–MS, TGA, 41 nm | Anti-skin cancer IC50 = 409.7 µg/mL/cytotoxicity/antimicrobial activity E. coli; C. albicans/DPPH IC50 = 95.80 μg/mL | [44] | ||
| 21 | Ocimum lamifolium leaf extract | UV–vis, TGA/DTA, FTIR, XRD, SEM-EDX, TEM, HRTEM, SAED, 6.5–22.8 nm, Eg~3.2 eV | Antimicrobial, E. coli, S. aureus, P. aeruginosa, S. pyogen | Electrocatalytic activity | [45] | |
| 22 | Leaf extracts of Catharanthus roseus (L.) G. Don | UV-Vis FTIR, FE-SEM, EDX, and TEM, 44.5 nm, nonspherical, ZP (−18.8 mV) | Seed germination | [46] | ||
| 23 | Orange fruit peel extract | XRD, FTIR, TGA, TEM, 10–20 nm | Antibacterial, E. coli, S. aureus | [47] | ||
| 24 | Neem plant extracts | SEM/EDX, 23–40 nm, spherical-shaped, DLS (27.81 nm), Eg= 3.24 eV | Photocatalytic MO, 95%, 120 min; Rhodamine-B, 90%, 120 min | CIS Nyquist, Tafel, uncoated and ZnO NPs coated Zn metal plates (3.5% NaCl) | [48] | |
| 25 | Platanus orientalis | FT-IR, PXRD 23.48 nm, UV-Vis, PL, FESEM-EDX, TEM-SAED, spherical, BET | Photocatalytic acid red 14/85%/45 min | [49] | ||
| 26 | Cucurbita pepo L. seed extract | SEM, EDX, TEM 32.88 nm, FT-IR, PXRD 13.72, UV-Vis, TGA, Eg of 3.29 eV, d-spacing of 0.65 nm | MO dye, 75–80%/60 min | CSI for mild steel (MS) in 1.0 HCl, inhibitory efficiencies 83.66%; Nyquist plots; H2S AD capacity with ZnO NPs and bulk ZnO. | Present work | |
| Synthesis Method | Dye Used/Conc./Nature of Radiation | ZnO NPs Dosage | Exposure Time (min.) | % Removal | Ref. |
|---|---|---|---|---|---|
| Sol–gel | MO/40 mg·L−1/UV | 200 mg/L | 120 | 65 | [71] |
| Sol–gel | MO/100 ppm/UV | 1000 ppm | 120 | 42 | [72] |
| Solochemical | MO/0.02 g·L−1/UV | 0.1 g·L−1 | 12 h | 78–80 | [73] |
| Solution combustion | MO/15 mg·L−1/UV | 0.1 g | 180 | 52 | [74] |
| Hydrothermal synthesis | MO/10 mg·L−1/UV | 0.6 g L−1 | 240 | 40 | [75] |
| Sol–gel | MO/50 mg·L−1/UV | 30 mg | 240 | 80 | [76] |
| Chemical Precipitation | MO/100 mg·L−1/UV | 0.05 g | 120 | 50 | [77] |
| Laser-Generated | MO/20 ppm/Sunlight | 0.05 g | 120 | 89 | [78] |
| Green | MO/10 mg·L−1/Sunlight | 50 mg | 60 | 75–80 | Present |
| Inhibitor | Conc. (ppm) Inhibitor | Ecorr (mV) | icorr (μA/cm2) | βa (mV/dec) | βc (mV/dec) | θ | CR mmpy | ηPDP (%) or % I.Ep |
|---|---|---|---|---|---|---|---|---|
| blank | 0 | −440.52 | 211.588 | 110.2 | 202.4 | - | 2.47636 | - |
| ZnO NPs | 10 | −416.20 | 59.412 | 79.6 | 159.9 | 0.72 | 0.69534 | 71.92 |
| 20 | −415.92 | 56.561 | 78.9 | 143.6 | 0.73 | 0.66197 | 73.26 | |
| 40 | −422.84 | 40.056 | 76.1 | 144.2 | 0.81 | 0.46880 | 81.06 | |
| 100 | −446.03 | 34.560 | 82.7 | 110.1 | 0.84 | 0.40448 | 83.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmi, S.N.H.; Alam, M. Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles. Inorganics 2024, 12, 199. https://doi.org/10.3390/inorganics12070199
Azmi SNH, Alam M. Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles. Inorganics. 2024; 12(7):199. https://doi.org/10.3390/inorganics12070199
Chicago/Turabian StyleAzmi, Syed Najmul Hejaz, and Mahboob Alam. 2024. "Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles" Inorganics 12, no. 7: 199. https://doi.org/10.3390/inorganics12070199
APA StyleAzmi, S. N. H., & Alam, M. (2024). Exploring the Anti-Corrosion, Photocatalytic, and Adsorptive Functionalities of Biogenically Synthesized Zinc Oxide Nanoparticles. Inorganics, 12(7), 199. https://doi.org/10.3390/inorganics12070199







