Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
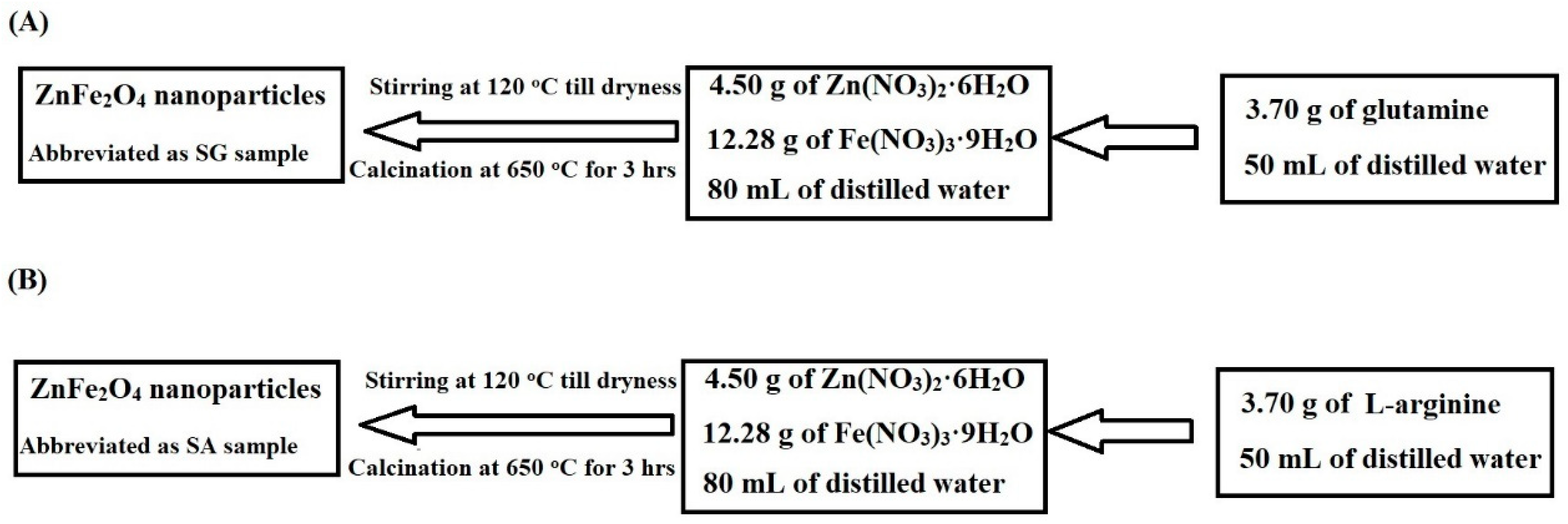
2.1. Synthesis and Characterization of ZnFe2O4 Nanoparticles
2.2. Removal of Rhodamine b Dye from Aqueous Media
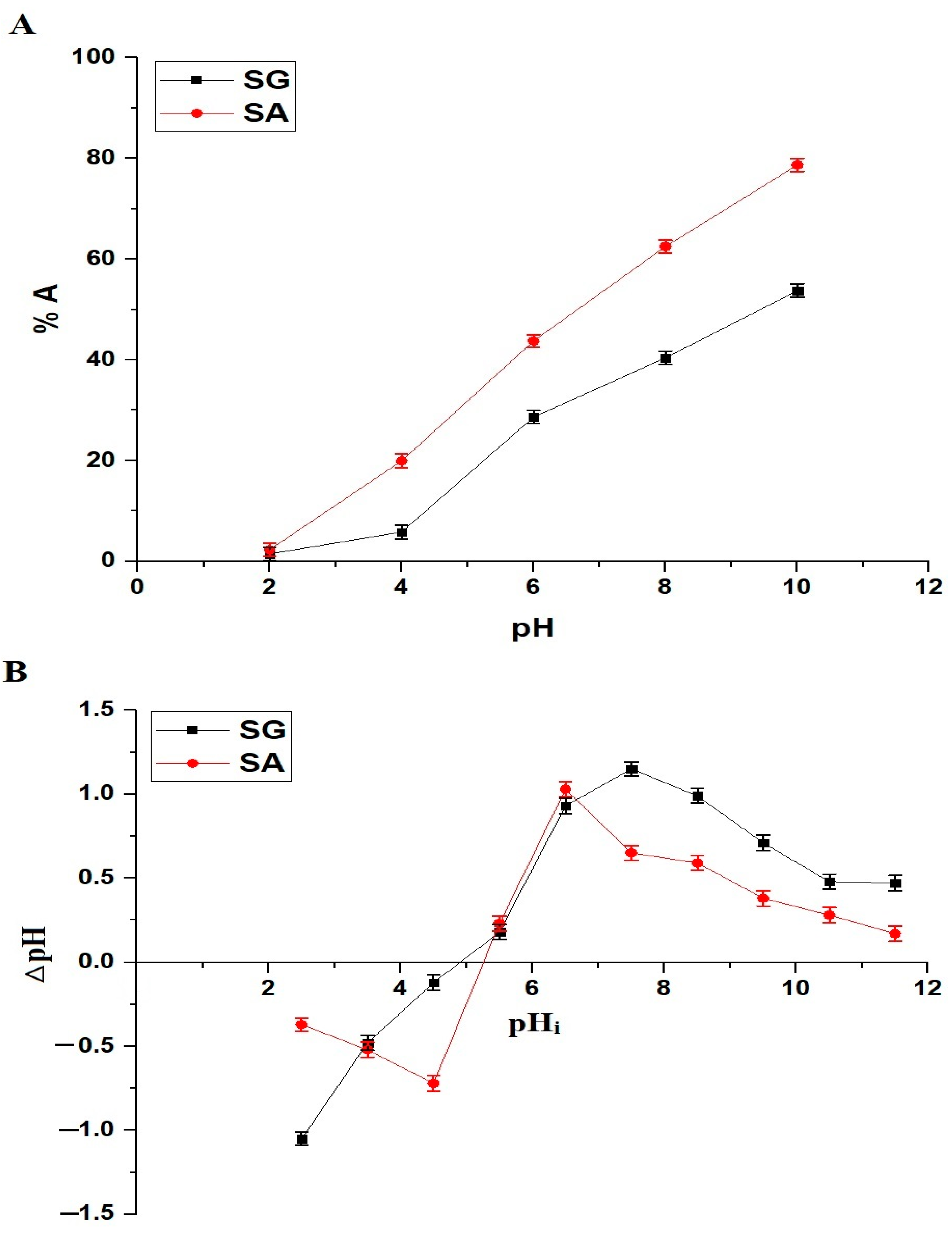
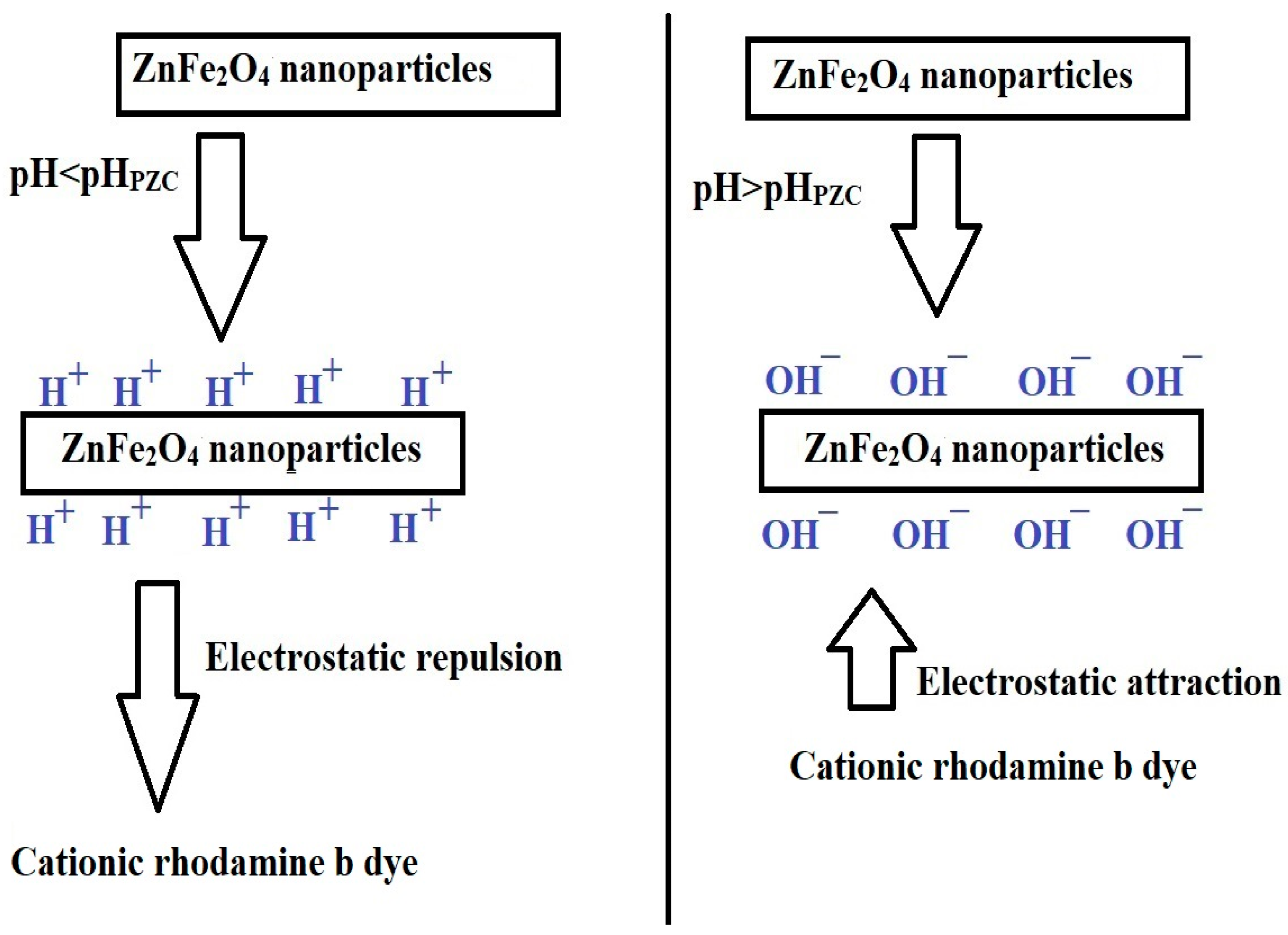
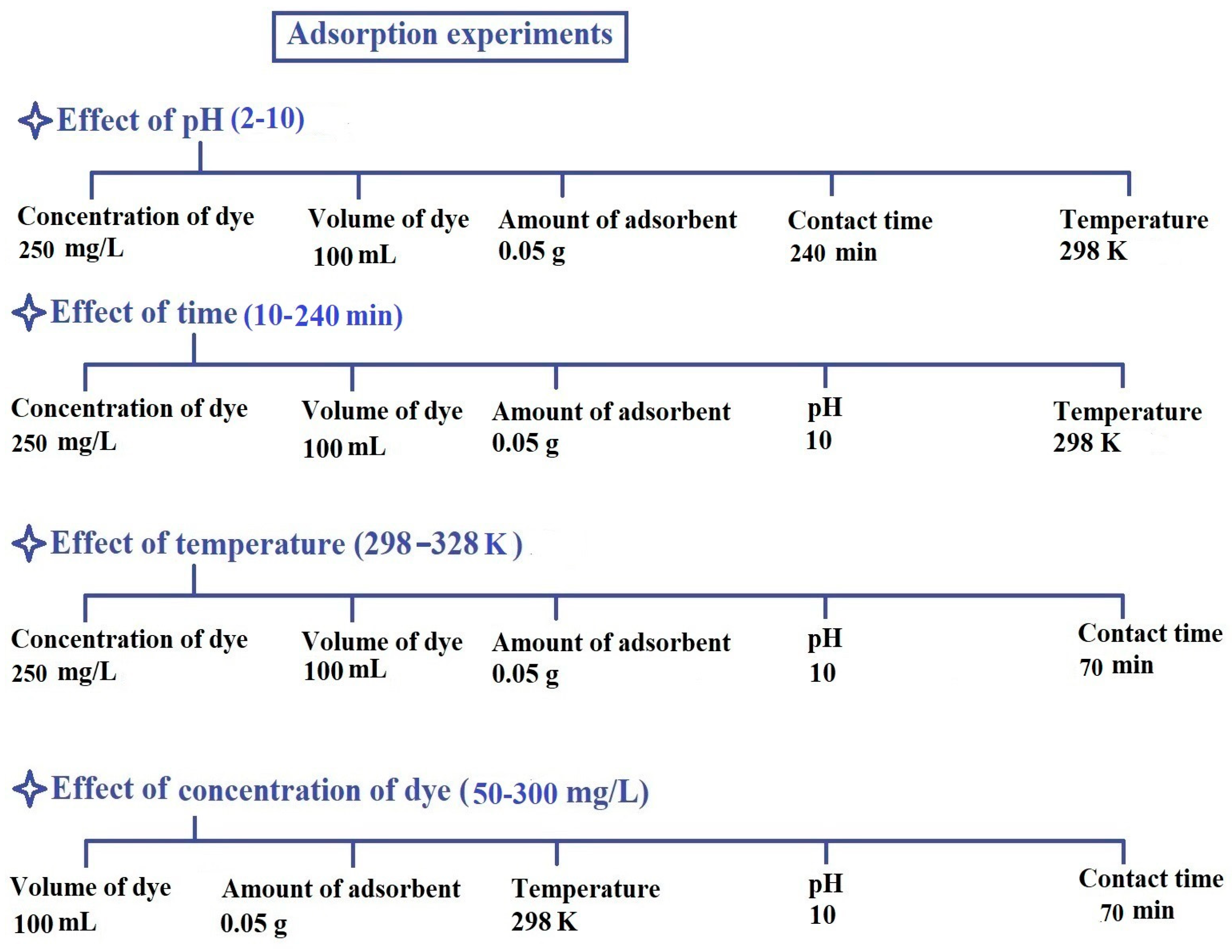
2.2.1. Influence of pH
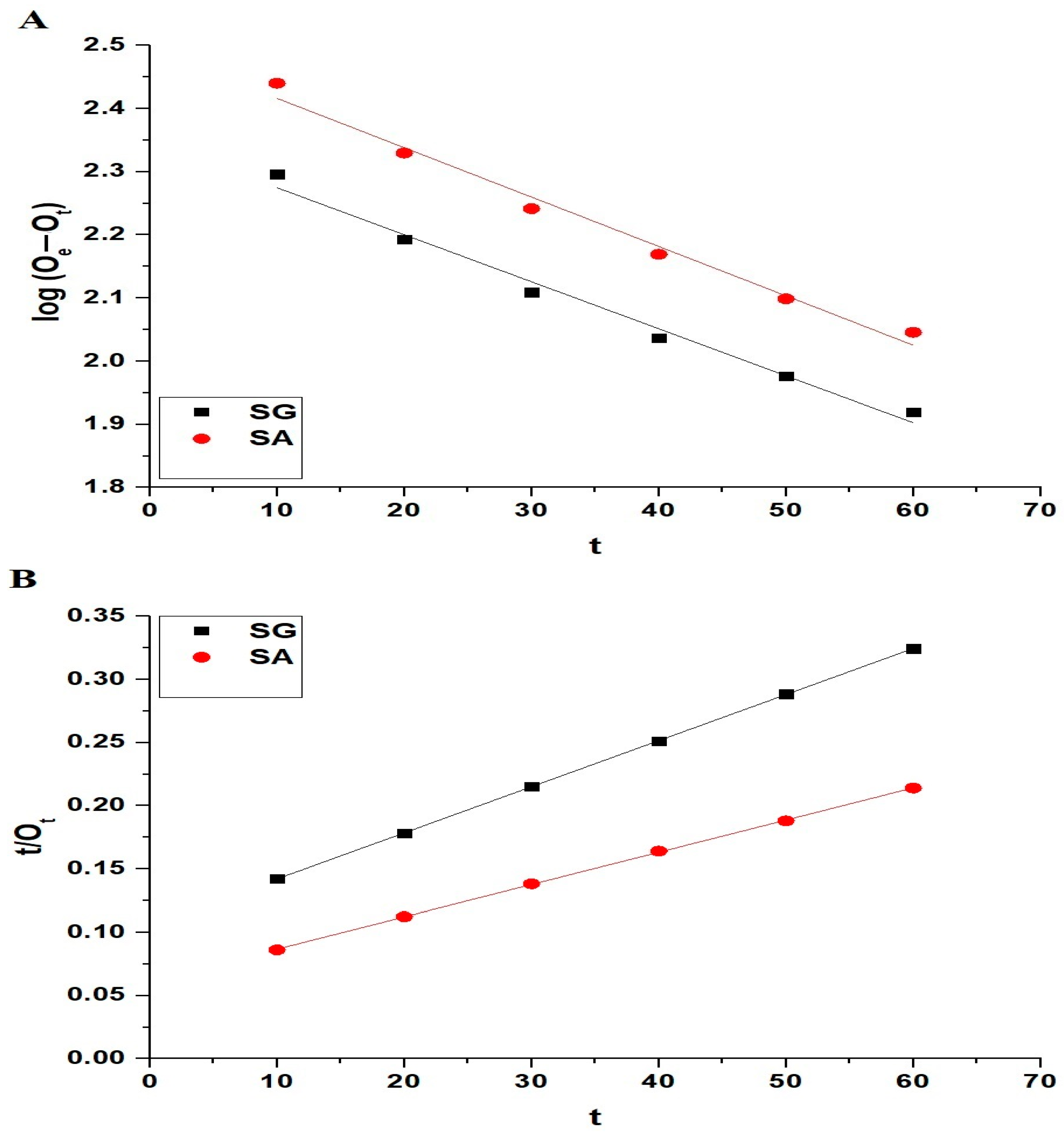
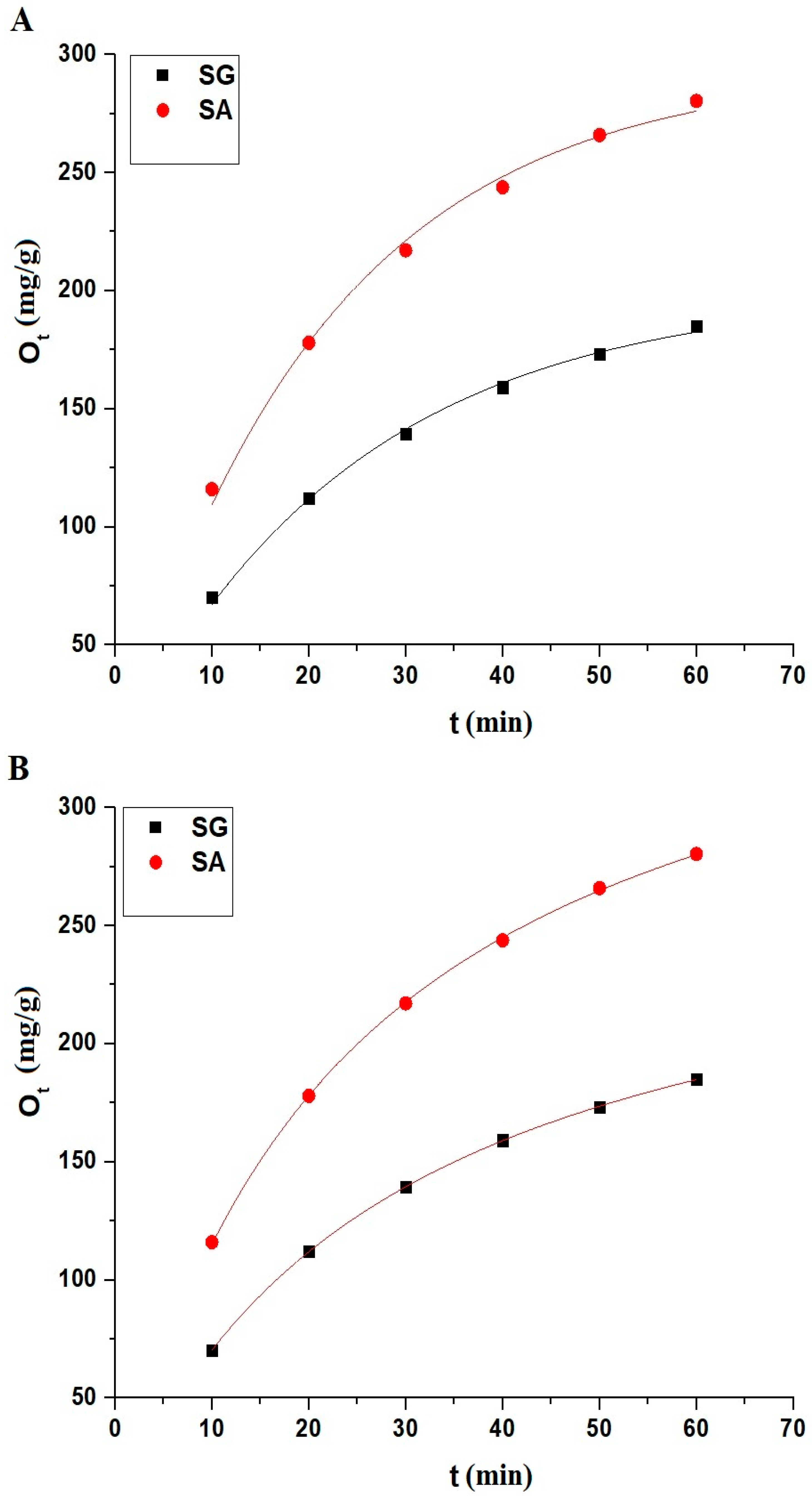
2.2.2. Influence of Contact Time
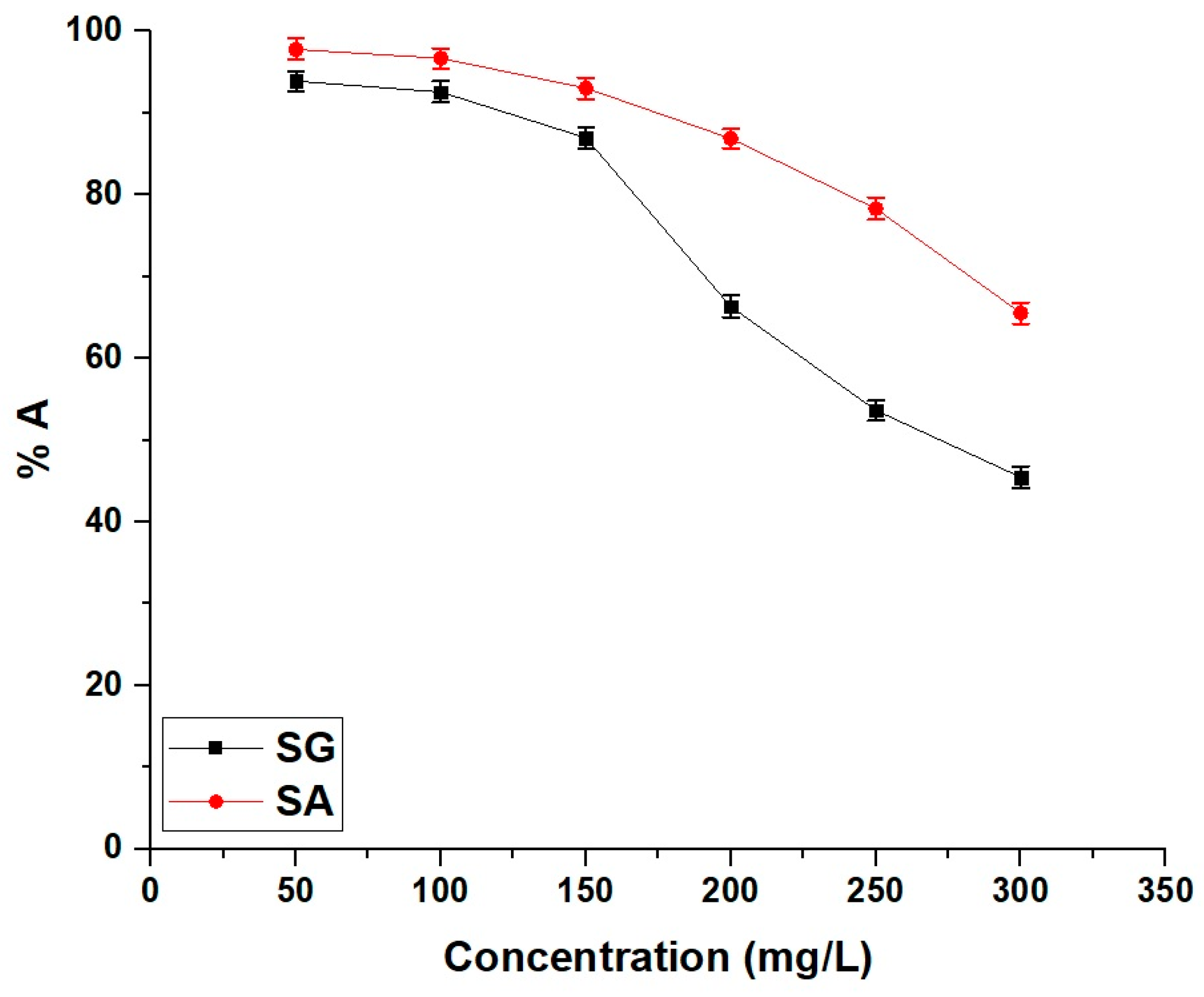
2.2.3. Influence of Temperature
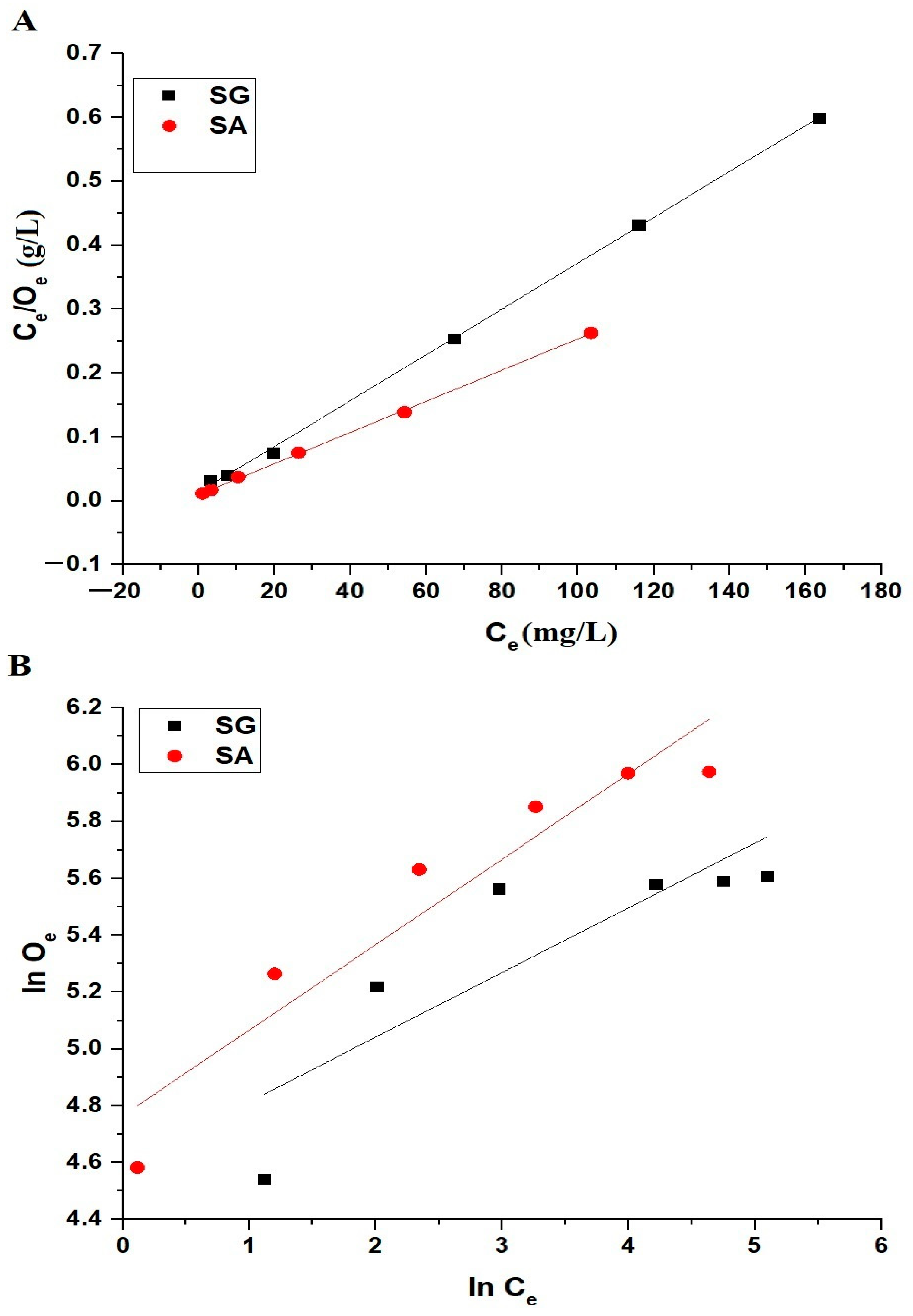
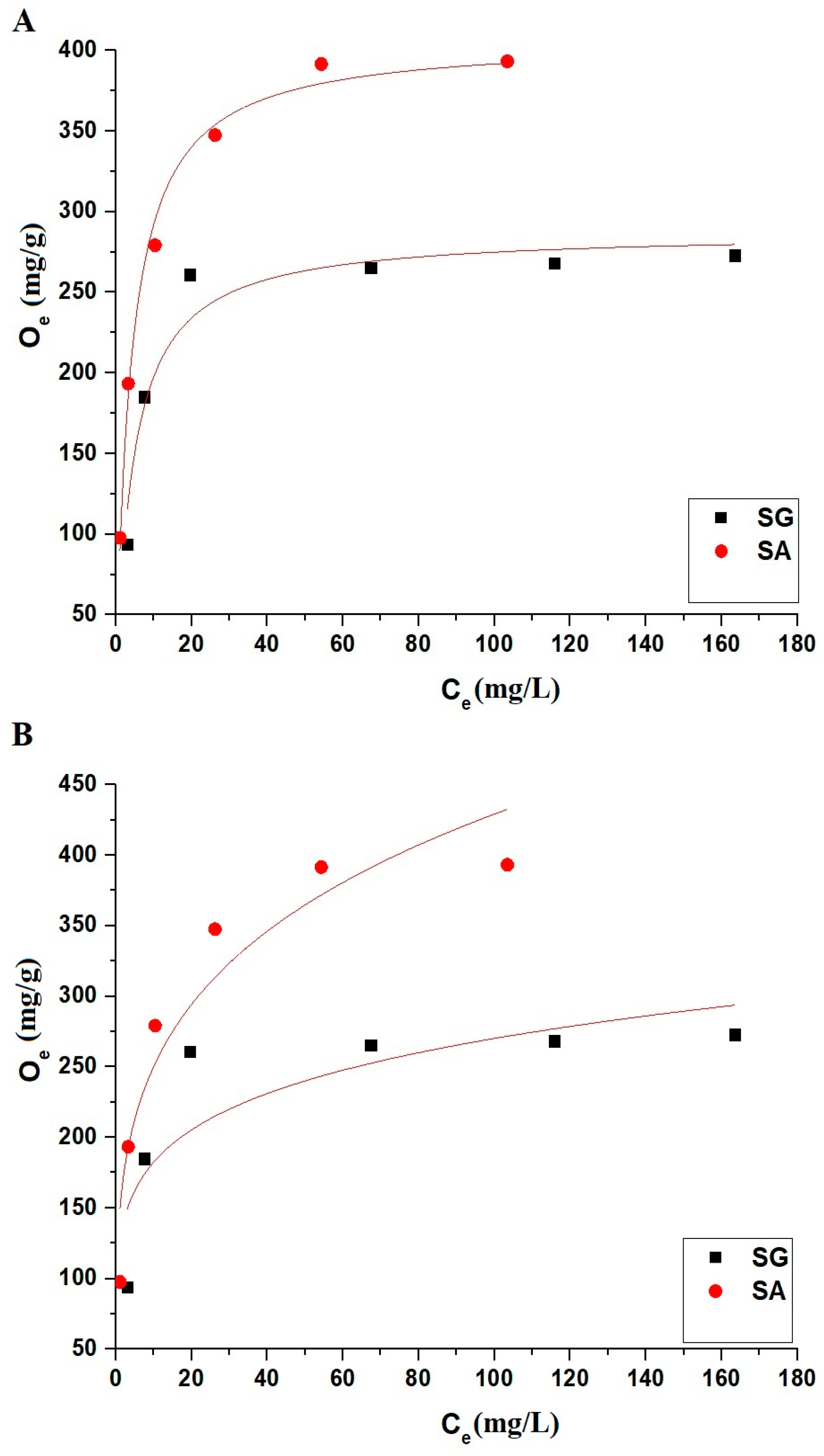
2.2.4. Influence of Concentration
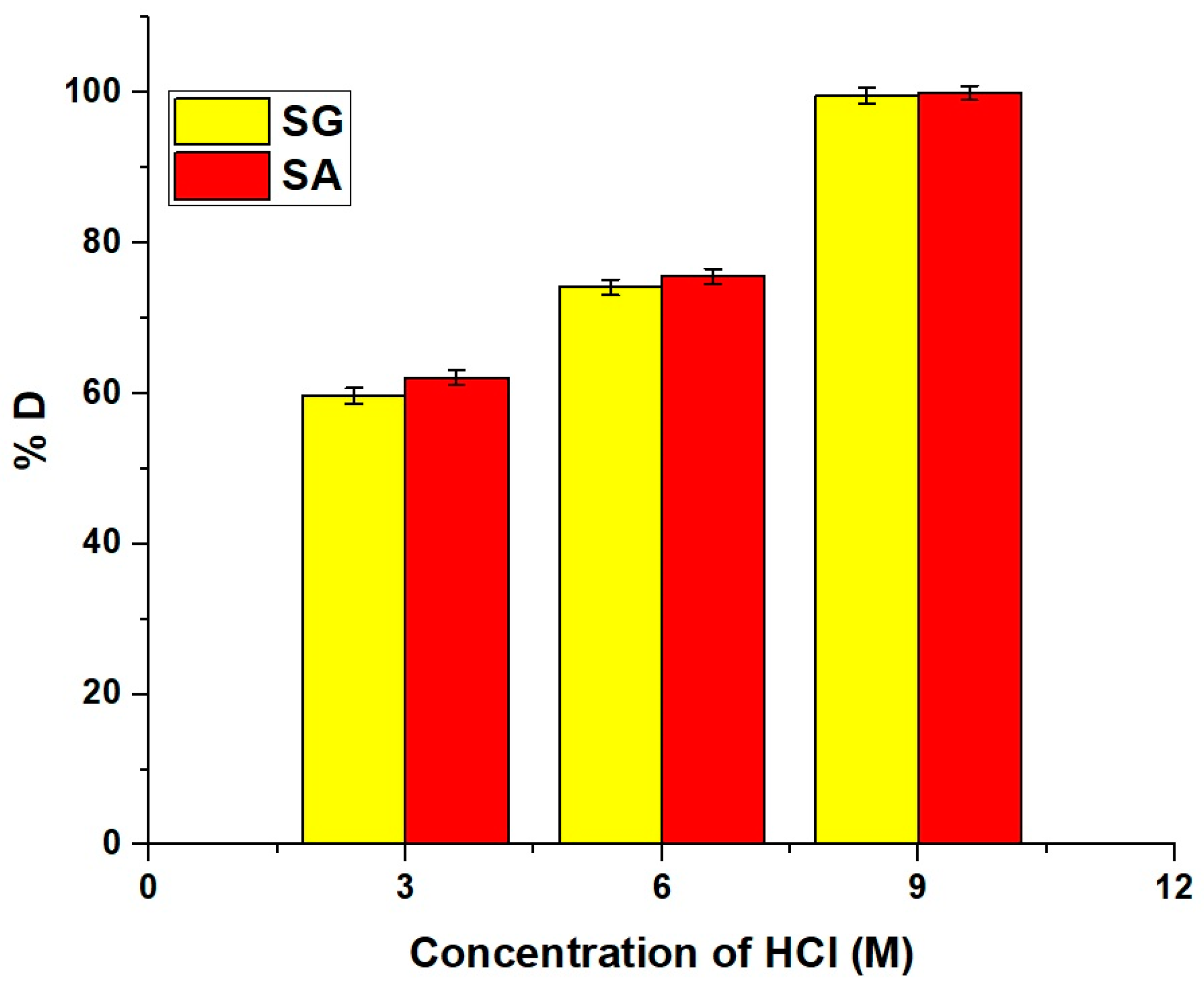
2.2.5. Effect of Regeneration and Reusability
2.2.6. Effect of Selectivity
2.2.7. Practical Application
3. Experimental Section
3.1. Materials
3.2. Synthesis of Zinc Ferrite (ZnFe2O4) Nanoparticles
3.3. Instrumentation
3.4. Adsorption of Rhodamine b Dye from Aqueous Media
3.5. Point of Zero Charge (pHPZC) of the SG and SA Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhou, L.; Rao, C.; Wang, G.L.; Jiang, F.; Singh, A.; Kumar, A.; Liu, J. Two 3D Supramolecular Isomeric Zn(II)-MOFs as Photocatalysts for Photodegradation of Methyl Violet Dye. Dye. Pigment. 2021, 190, 109285. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.; Kumar, A. Syntheses, Design Strategies, and Photocatalytic Charge Dynamics of Metal-Organic Frameworks (MOFs): A Catalyzed Photo-Degradation Approach towards Organic Dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Wang, J.; Rao, C.; Lu, L.; Zhang, S.; Muddassir, M.; Liu, J. Efficient Photocatalytic Degradation of Methyl Violet Using Two New 3D MOFs Directed by Different Carboxylate Spacers. CrystEngComm 2021, 23, 741–747. [Google Scholar] [CrossRef]
- El-habacha, M.; Miyah, Y.; Lagdali, S.; Mahmoudy, G.; Dabagh, A.; Chiban, M.; Sinan, F.; Iaich, S.; Zerbet, M. General Overview to Understand the Adsorption Mechanism of Textile Dyes and Heavy Metals on the Surface of Different Clay Materials. Arab. J. Chem. 2023, 16, 105248. [Google Scholar] [CrossRef]
- Sağlam, S.; Türk, F.N.; Arslanoğlu, H. Use and Applications of Metal-Organic Frameworks (MOF) in Dye Adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 109285. [Google Scholar] [CrossRef]
- Han, Z.; Sun, L.; Chu, Y.; Wang, J.; Wei, C.; Jiang, Q.; Han, C.; Yan, H.; Song, X. States of Graphene Oxide and Surface Functional Groups amid Adsorption of Dyes and Heavy Metal Ions. Chin. J. Chem. Eng. 2023, 63, 197–208. [Google Scholar] [CrossRef]
- Osagie, C.; Othmani, A.; Ghosh, S.; Malloum, A.; Kashitarash Esfahani, Z.; Ahmadi, S. Dyes Adsorption from Aqueous Media through the Nanotechnology: A Review. J. Mater. Res. Technol. 2021, 14, 2195–2218. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Fronczyk, J.; Świątkowski, A. Adsorptive Removal of Rhodamine B Dye from Aqueous Solutions Using Mineral Materials as Low-Cost Adsorbents. Water. Air. Soil Pollut. 2023, 234, 531. [Google Scholar] [CrossRef]
- He, H.; Chai, K.; Wu, T.; Qiu, Z.; Wang, S.; Hong, J. Adsorption of Rhodamine B from Simulated Waste Water onto Kaolin-Bentonite Composites. Materials 2022, 15, 4058. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Hermosillo-Nevárez, J.J.; Ramírez-Pereda, B.; Vaca, M.; Rangel-Peraza, J.G.; Bustos-Terrones, V.; Rojas-Valencia, M.N. Removal of BB9 Textile Dye by Biological, Physical, Chemical, and Electrochemical Treatments. J. Taiwan Inst. Chem. Eng. 2021, 121, 29–37. [Google Scholar] [CrossRef]
- Khasim, S.; Dastager, S.G.; Alahmdi, M.I.; Hamdalla, T.A.; Ulla, M.F.; Panneerselvam, C.; Makandar, M.B. Green Synthesis of Multifunctional Cu/MnO@Biochar 3D Structure as a High-Performance Anode Material in Li-Ion Batteries and Oxidative Removal of Cango-Red Dye. Case Stud. Chem. Environ. Eng. 2024, 9, 100561. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Omran, H.A.; Hassan, M.K.; Mohamed, A.F.; Backar, A.H.; Irfan, O.M.; Mohamed, O.A. Graphite-TiO2-Doped Coated Sand Granules for Efficient Continuous Removal of Methylene Blue Dye: Combining Adsorption and Photocatalytic Degradation. Results Eng. 2023, 20, 101646. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of Dye Structure on Color Removal Efficiency by Coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Abo-Ayad, Z.A.; Hussein, M.F.; Zayed, M.A.; Abdelraheem, O.H. GC–MS Identification of DR31 Textile Dye Degradation Products during Its Efficient Electrochemical Removal from Wastewater Media. J. Mol. Liq. 2024, 400, 124408. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Al-Senani, G.M.; Algethami, F.K.; Shah, R.K.; Saad, F.A.; ur Rehman, K.; Khezami, L.; Abdelrahman, E.A. Facile Synthesis of MgO/ZnO Nanocomposite for Efficient Removal of Alizarin Red S Dye from Aqueous Media. Inorg. Chem. Commun. 2024, 162, 112233. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Abdelrahman, E.A. Simple Production and Characterization of ZnO/MgO Nanocomposite as a Highly Effective Adsorbent for Eliminating Congo Red Dye from Water-Based Solutions. Inorg. Chem. Commun. 2024, 161, 112137. [Google Scholar] [CrossRef]
- Abdelrahman, E.A. Synthesis of Zeolite Nanostructures from Waste Aluminum Cans for Efficient Removal of Malachite Green Dye from Aqueous Media. J. Mol. Liq. 2018, 253, 72–82. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Saad, F.A.; Munshi, A.M.; Abdelrahman, E.A. Facile Synthesis and Characterization of Magnesium and Manganese Mixed Oxides for the Efficient Removal of Tartrazine Dye from Aqueous Media. RSC Adv. 2023, 13, 5656–5666. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Basha, M.T.; Alghanmi, R.M.; Al-Farraj, E.S.; Abdelrahman, E.A. Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd(II) and Cu(II) Ions from Aqueous Media. Separations 2023, 10, 88. [Google Scholar] [CrossRef]
- Al, N.S.; Fawaz, K.; Reem, A.S.; Eida, K.S.; Farraj, S.A.; El, G.S.; Abdelrahman, E.A. A Facile Sol-Gel Synthesis and Characterization of MgCO3/MnCO3 and MgMn2O4/Mn2O3 Novel Nanostructures With Remarkably High Adsorption Activity Toward Eriochrome Black T Dye. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2046–2057. [Google Scholar] [CrossRef]
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient Removal of Rhodamine B Dye Using Biochar as an Adsorbent: Study the Performance, Kinetics, Thermodynamics, Adsorption Isotherms and Its Reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef]
- Pedebos, M.E.S.; Druzian, D.M.; Oviedo, L.R.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; Espinosa, D.C.R.; da Silva, W.L. Removal of Rhodamine B Dye by Adsorption onto an Eco-Friendly Zeolite and Machine Learning Modeling. J. Photochem. Photobiol. A Chem. 2024, 449, 115404. [Google Scholar] [CrossRef]
- El Hassani, A.A.; Tanji, K.; El Mrabet, I.; Fahoul, Y.; El Gaidoumi, A.; Benjelloun, A.T.; Sfaira, M.; Zaitan, H.; Kherbeche, A. A Combined Molecular Dynamics Simulation, DFT Calculations, and Experimental Study of the Adsorption of Rhodamine B Dye on Kaolinite and Hydroxyapatite in Aqueous Solutions. Surfaces Interfaces 2023, 36, 102647. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Haouti, R.; El Guerdaoui, A.; Haounati, R.; Amaterz, E.; Addi, A.A.; Akbal, F.; Taha, M.L. Experimental and Molecular Dynamics Simulation Study on the Adsorption of Rhodamine B Dye on Magnetic Montmorillonite Composite γ-Fe2O3@Mt. J. Mol. Liq. 2020, 309, 113142. [Google Scholar] [CrossRef]
- Al-Rashed, S.M.; Al-Gaid, A.A. Kinetic and Thermodynamic Studies on the Adsorption Behavior of Rhodamine B Dye on Duolite C-20 Resin. J. Saudi Chem. Soc. 2012, 16, 209–215. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Liu, Y. Synthesis of a Hierarchical SnS2 Nanostructure for Efficient Adsorption of Rhodamine B Dye. J. Colloid Interface Sci. 2017, 507, 225–233. [Google Scholar] [CrossRef]
- Madhurima, V.P.; Kumari, K.; Jain, P.K. Synthesis and Study of Carbon Nanomaterials through Arc Discharge Technique for Efficient Adsorption of Organic Dyes. Diam. Relat. Mater. 2024, 141, 110538. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Enhanced Removal of Indigo Carmine Dye from Textile Effluent Using Green Cost-Efficient Nanomaterial: Adsorption, Kinetics, Thermodynamics and Mechanisms. Sustain. Chem. Pharm. 2022, 29, 100753. [Google Scholar] [CrossRef]
- Kumari, S.; Khan, A.A.; Chowdhury, A.; Bhakta, A.K.; Mekhalif, Z.; Hussain, S. Efficient and Highly Selective Adsorption of Cationic Dyes and Removal of Ciprofloxacin Antibiotic by Surface Modified Nickel Sulfide Nanomaterials: Kinetics, Isotherm and Adsorption Mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124264. [Google Scholar] [CrossRef]
- Singla, P.; Goel, N.; kumar, V.; Singhal, S. Boron Nitride Nanomaterials with Different Morphologies: Synthesis, Characterization and Efficient Application in Dye Adsorption. Ceram. Int. 2015, 41, 10565–10577. [Google Scholar] [CrossRef]
- Hegazey, R.M.; Abdelrahman, E.A.; Kotp, Y.H.; Hameed, A.M.; Subaihi, A. Facile Fabrication of Hematite Nanoparticles from Egyptian Insecticide Cans for Efficient Photocatalytic Degradation of Rhodamine B Dye. J. Mater. Res. Technol. 2020, 9, 1652–1661. [Google Scholar] [CrossRef]
- Abkenar, S.D.; Hassannezhad, M.; Hosseini, M.; Ganjali, M.R. Efficient Removal of Malachite Green from Aqueous Solution by Adsorption on Carbon Nanotubes Modified with ZnFe2O4 Nanoparticles. J. Serbian Chem. Soc. 2019, 84, 701–712. [Google Scholar] [CrossRef]
- Nguyen, U.T.P.; Bui, D.X.M.; Nguyen, T.T.T.; Nguyen, N.H.; Nguyen, D.T.C.; Tran, T.V. Green Synthesis of ZnFe2O4 Nanoparticles Using Chrysanthemum Spp. Flower Extract for the Adsorption and Photocatalytic Degradation of Malachite Green Dye. J. Chem. Technol. Biotechnol. 2022, 98, 2639–2654. [Google Scholar] [CrossRef]
- Abdulhamid, Z.M.; Dabbawala, A.A.; Delclos, T.; Straubinger, R.; Rueping, M.; Polychronopoulou, K.; Anjum, D.H. Synthesis, Characterization, and Preliminary Insights of ZnFe2O4 Nanoparticles into Potential Applications, with a Focus on Gas Sensing. Sci. Rep. 2023, 13, 19705. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Abdelrahman, E.A. Efficient Photocatalytic Degradation of Malachite Green Dye Using Facilely Synthesized Hematite Nanoparticles from Egyptian Insecticide Cans. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 226, 117612. [Google Scholar] [CrossRef]
- Saafan, S.A.; El-Nimr, M.K.; Hussein, M.M.; Omar, M.K. FTIR, DC, and AC Electrical Measurements of Mg Zn Nano-Ferrites and Their Composites with Polybenzoxazine. Appl. Phys. A Mater. Sci. Process. 2021, 127, 800. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Al-Senani, G.M.; Algethami, F.K.; Shah, R.K.; Saad, F.A.; Munshi, A.M.; Rehman, K.; Khezami, L.; Abdelrahman, E.A. Calcium Ferrite Nanoparticles: A Simple Synthesis Approach for the Effective Disposal of Congo Red Dye from Aqueous Environments. Inorganics 2024, 12, 69. [Google Scholar] [CrossRef]
- Alhalili, Z.; Abdelrahman, E.A. Facile Synthesis and Characterization of Manganese Ferrite Nanoparticles for the Successful Removal of Safranine T Dye from Aqueous Solutions. Inorganics 2024, 15, 30. [Google Scholar] [CrossRef]
- Koohi, P.; Rahbar-kelishami, A.; Shayesteh, H. Efficient Removal of Congo Red Dye Using Fe3O4/NiO Nanocomposite: Synthesis and Characterization. Environ. Technol. Innov. 2021, 23, 101559. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Algethami, F.K.; Alsalem, H.S.; Binkadem, M.S. Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye From. Inorganics 2023, 11, 339. [Google Scholar] [CrossRef]
- Shafeeq, K.; El, S.M.; Mostafa, R.; Reem, M.H.K.; Fawaz, K.S.; Mohamed, A.S. Functionalization of Calcium Silicate/Sodium Calcium Silicate Nanostructures with Chitosan and Chitosan/Glutaraldehyde as Novel Nanocomposites for the Efficient Adsorption of Cd(II) and Cu(II) Ions from Aqueous Solutions. Silicon 2023, 16, 1713–1730. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Algethami, F.K.; Saad, F.A.; Abdelrahman, E.A. Remarkable High Adsorption of Methylene Blue Dye from Aqueous Solutions Using Facilely Synthesized MgFe2O4 Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2035–2045. [Google Scholar] [CrossRef]
- Al-wasidi, A.S.; Katouah, H.A.; Saad, F.A.; Abdelrahman, E.A. Functionalization of Silica Nanoparticles by 5-Chloro-8-Quinolinol as a New Nanocomposite for the Efficient Removal and Preconcentration of Al3+ Ions from Water Samples. ACS Omega 2023, 8, 15276–15287. [Google Scholar] [CrossRef]
- Algethami, F.K.; Al, A.S.; Eida, W.; Al Farraj, S.; Katouah, H.A.; Abdelrahman, E.A. Discover Nano Facile Synthesis and Characterization of Fe3O4/Analcime Nanocomposite for the Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Discov. Nano 2023, 18, 70. [Google Scholar] [CrossRef]
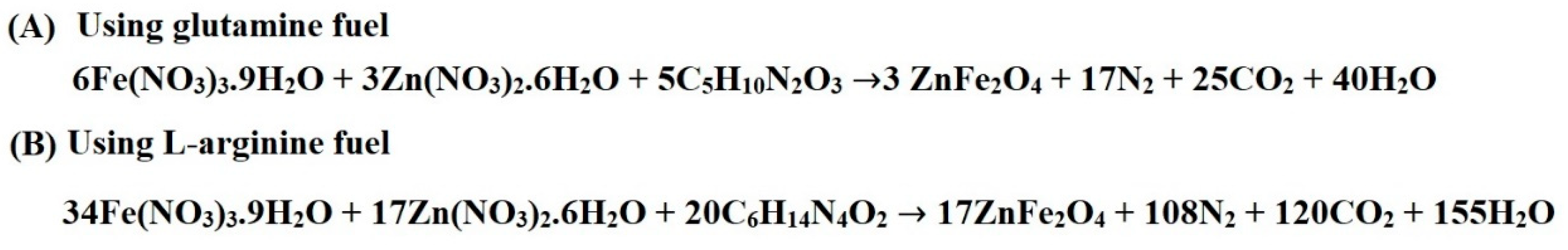
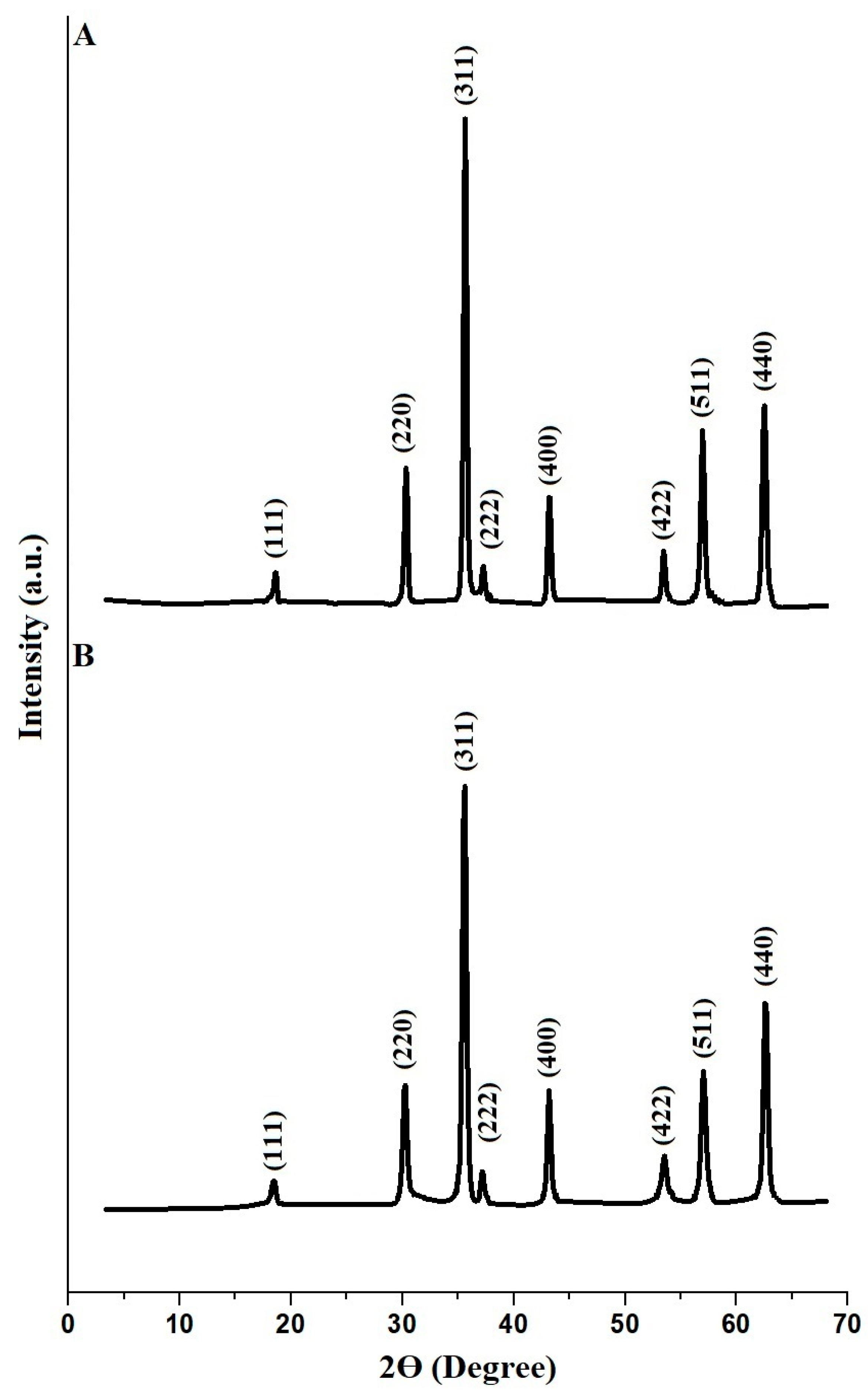
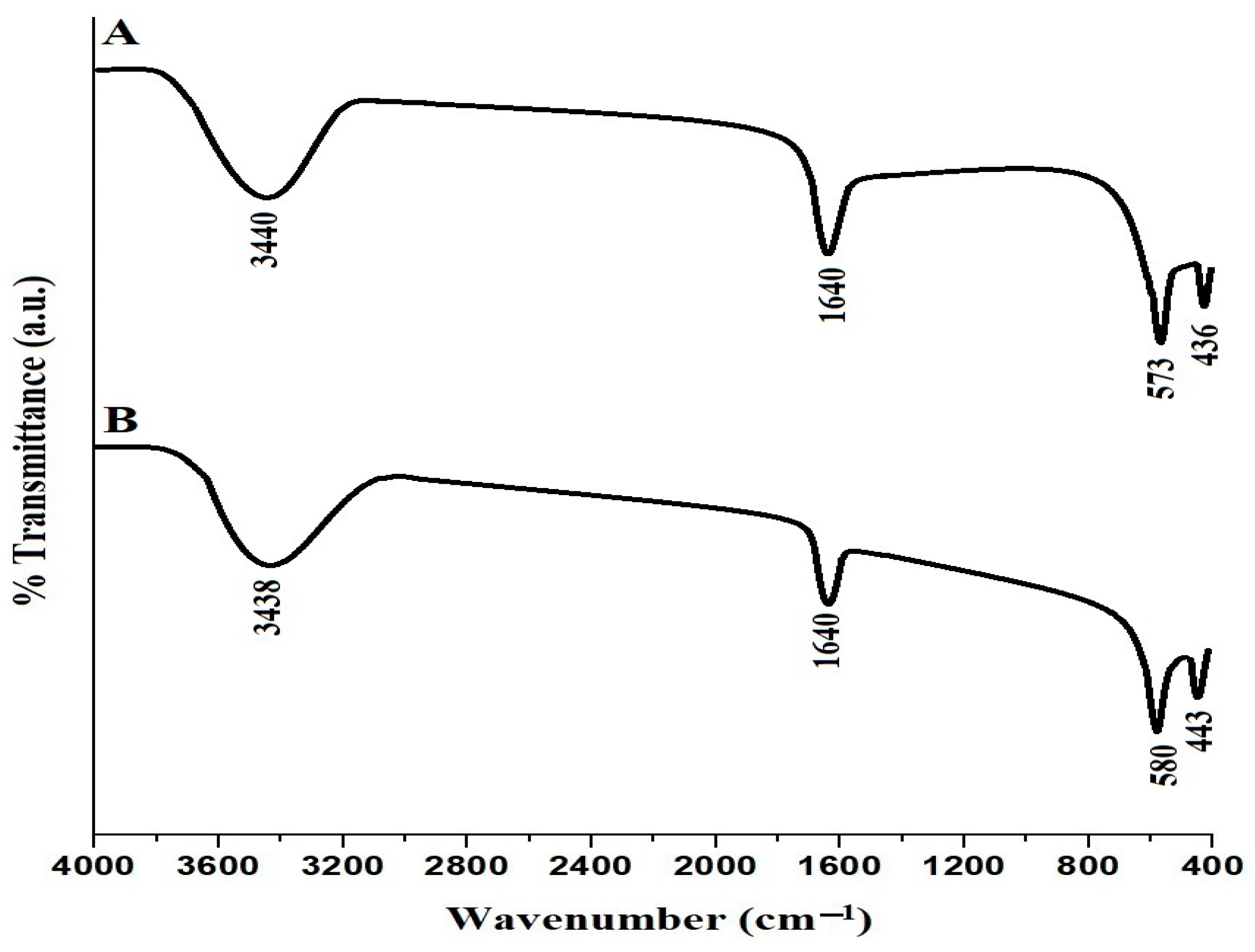
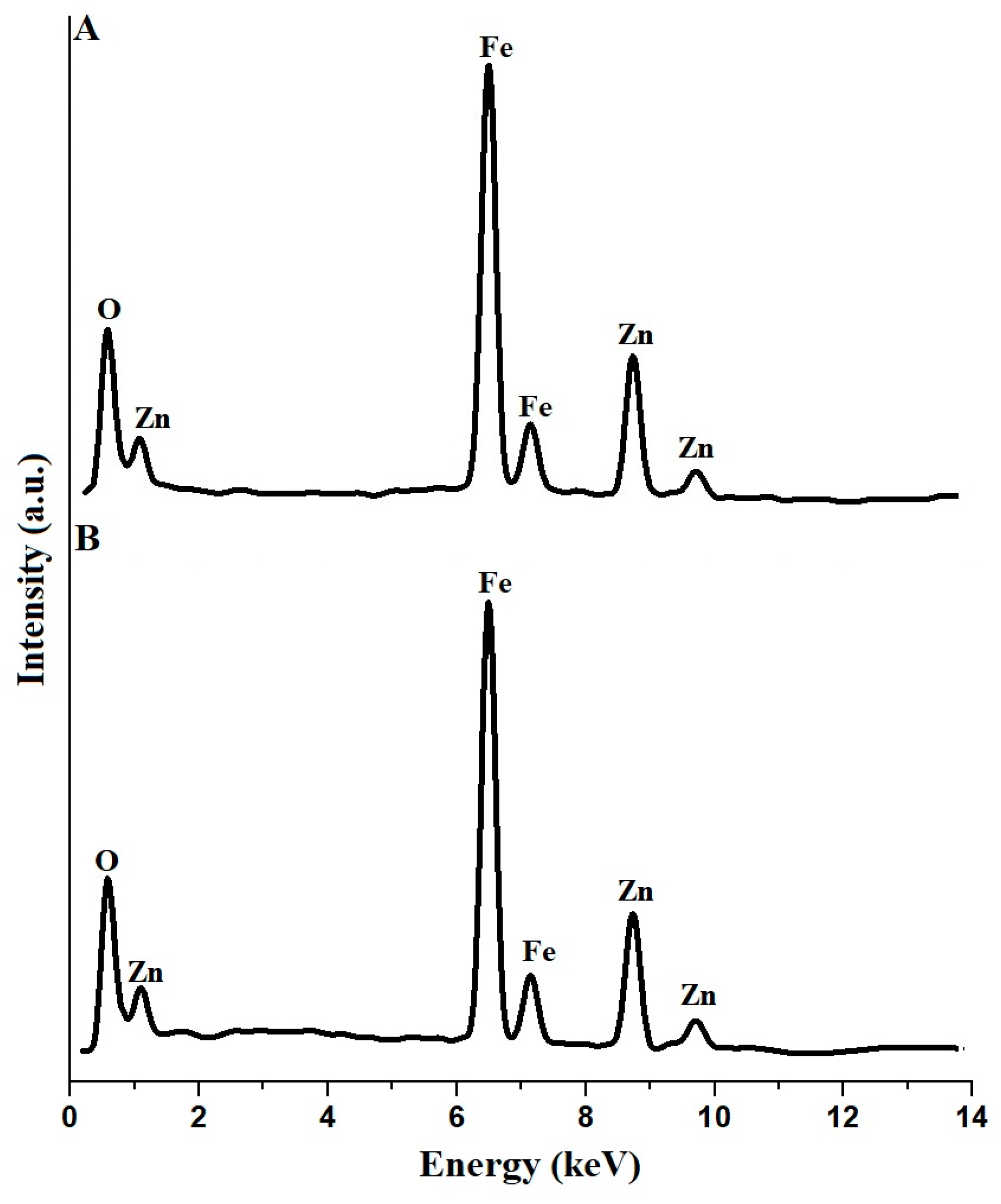

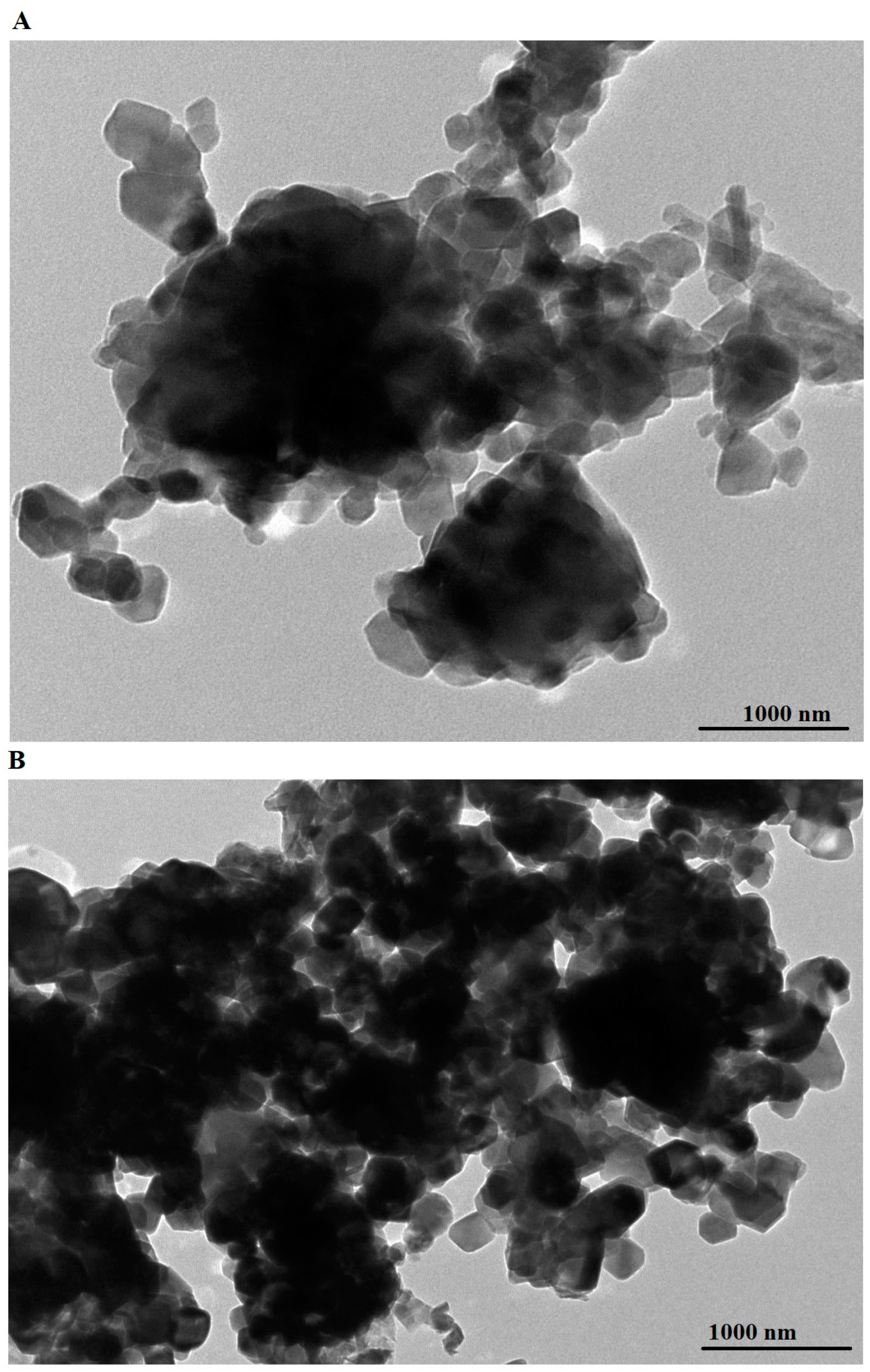
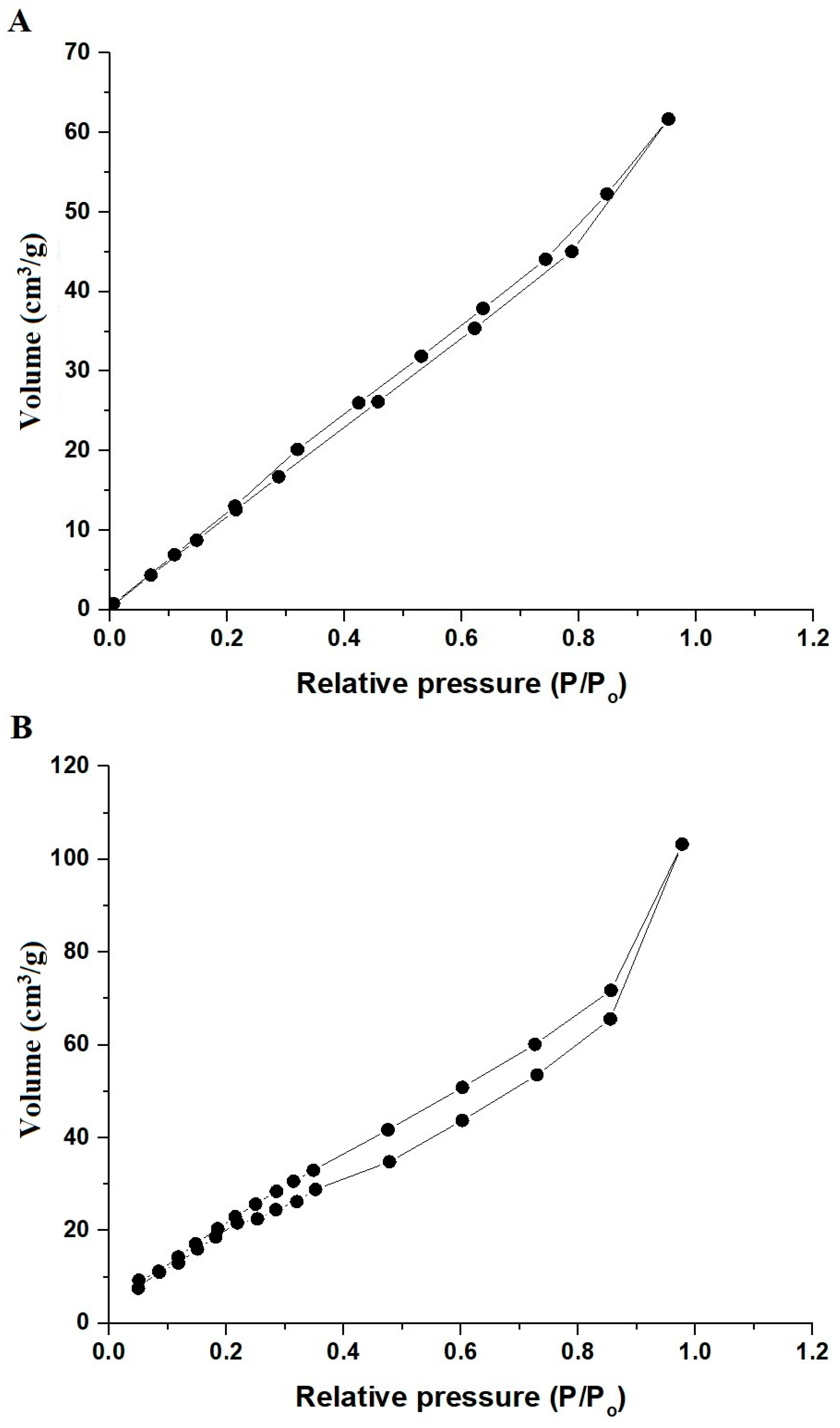




| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| SG | 60 | 0.0947 | 3.36 |
| SA | 85 | 0.1538 | 3.77 |
| Samples | OExp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L1 (1/min) | Oe (mg/g) | R2 | RSS | L2 (g/mg.min) | Oe (mg/g) | R2 | RSS | ||
| SG | 268.06 | 0.01713 | 223.38 | 0.9836 | 0.00129 | 0.00013 | 273.97 | 0.9999 | 3.12 × 10−7 |
| SA | 391.44 | 0.01801 | 312.08 | 0.9818 | 0.00158 | 0.00011 | 392.16 | 0.9998 | 1.70 × 10−6 |
| Samples | OExp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L1 (1/min) | Oe (mg/g) | R2 | χ2 | L2 (g/mg.min) | Oe (mg/g) | R2 | χ2 | ||
| SG | 268.06 | 0.04110 | 199.51 | 0.9969 | 5.60 | 1.26 × 10−4 | 274.00 | 0.9999 | 0.0279 |
| SA | 391.44 | 0.04661 | 294.02 | 0.9934 | 25.02 | 1.06 × 10−4 | 392.48 | 0.9998 | 0.8874 |
| Samples | ΔS° (kJ/molK) | ΔH° (kJ/mol) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| SG | 0.03499 | −12.54 | −22.97 | −23.32 | −23.67 | −24.02 |
| SA | 0.03318 | −14.82 | −24.71 | −25.04 | −25.37 | −25.70 |
| Samples | Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Omax (mg/g) | L3 (L/mg) | R2 | RSS | Omax (mg/g) | L4 (mg/g)(L/mg)1/n | 1/Z | R2 | RSS | |
| SG | 279.33 | 0.2615 | 0.9993 | 1.51 × 10−4 | 344.95 | 98.18 | 0.2276 | 0.6682 | 0.23738 |
| SA | 409.84 | 0.2503 | 0.9994 | 2.26 × 10−5 | 617.61 | 117.46 | 0.3006 | 0.8832 | 0.13744 |
| Samples | Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Omax (mg/g) | L3 (L/mg) | R2 | χ2 | Omax (mg/g) | L4 (mg/g)(L/mg)1/n | 1/Z | R2 | χ2 | |
| SG | 287.42 | 0.2192 | 0.9317 | 353.11 | 315.76 | 123.19 | 0.1705 | 0.6600 | 1757.88 |
| SA | 407.01 | 0.2536 | 0.9900 | 140.35 | 532.07 | 145.61 | 0.2347 | 0.8849 | 1621.50 |
| Adsorbent | Omax (mg/g) | Ref. |
|---|---|---|
| Biochar | 169.5 | [21] |
| Zeolite | 259.17 | [22] |
| Kaolinite | 19.19 | [23] |
| Magnetic montmorillonite composite | 209.20 | [24] |
| Duolite C-20 resin | 28.571 | [25] |
| SnS2 | 200.00 | [26] |
| SG | 279.33 | This study |
| SA | 409.84 | This study |
| Dye | Adsorption Capacity (mg/g) | |
|---|---|---|
| SG | SA | |
| Rhodamine b | 110.67 | 170.68 |
| Crystal violet | 90.56 | 138.24 |
| Methylene blue | 78.10 | 100.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wasidi, A.S.; AlReshaidan, S. Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles. Inorganics 2024, 12, 191. https://doi.org/10.3390/inorganics12070191
Al-Wasidi AS, AlReshaidan S. Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles. Inorganics. 2024; 12(7):191. https://doi.org/10.3390/inorganics12070191
Chicago/Turabian StyleAl-Wasidi, Asma S., and Salwa AlReshaidan. 2024. "Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles" Inorganics 12, no. 7: 191. https://doi.org/10.3390/inorganics12070191
APA StyleAl-Wasidi, A. S., & AlReshaidan, S. (2024). Enhanced Removal of Rhodamine b Dye from Aqueous Media via Adsorption on Facilely Synthesized Zinc Ferrite Nanoparticles. Inorganics, 12(7), 191. https://doi.org/10.3390/inorganics12070191






