Unraveling the Crystal Structure of Sodium Tetrabenzylborate: Synthesis through the Sodium Borohydride Reduction of Benzaldehyde in the Solid State
Abstract
1. Introduction
2. Results
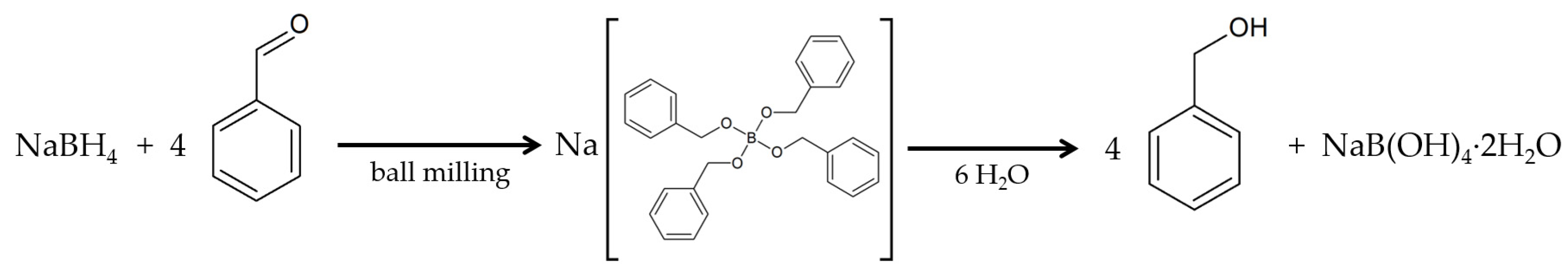
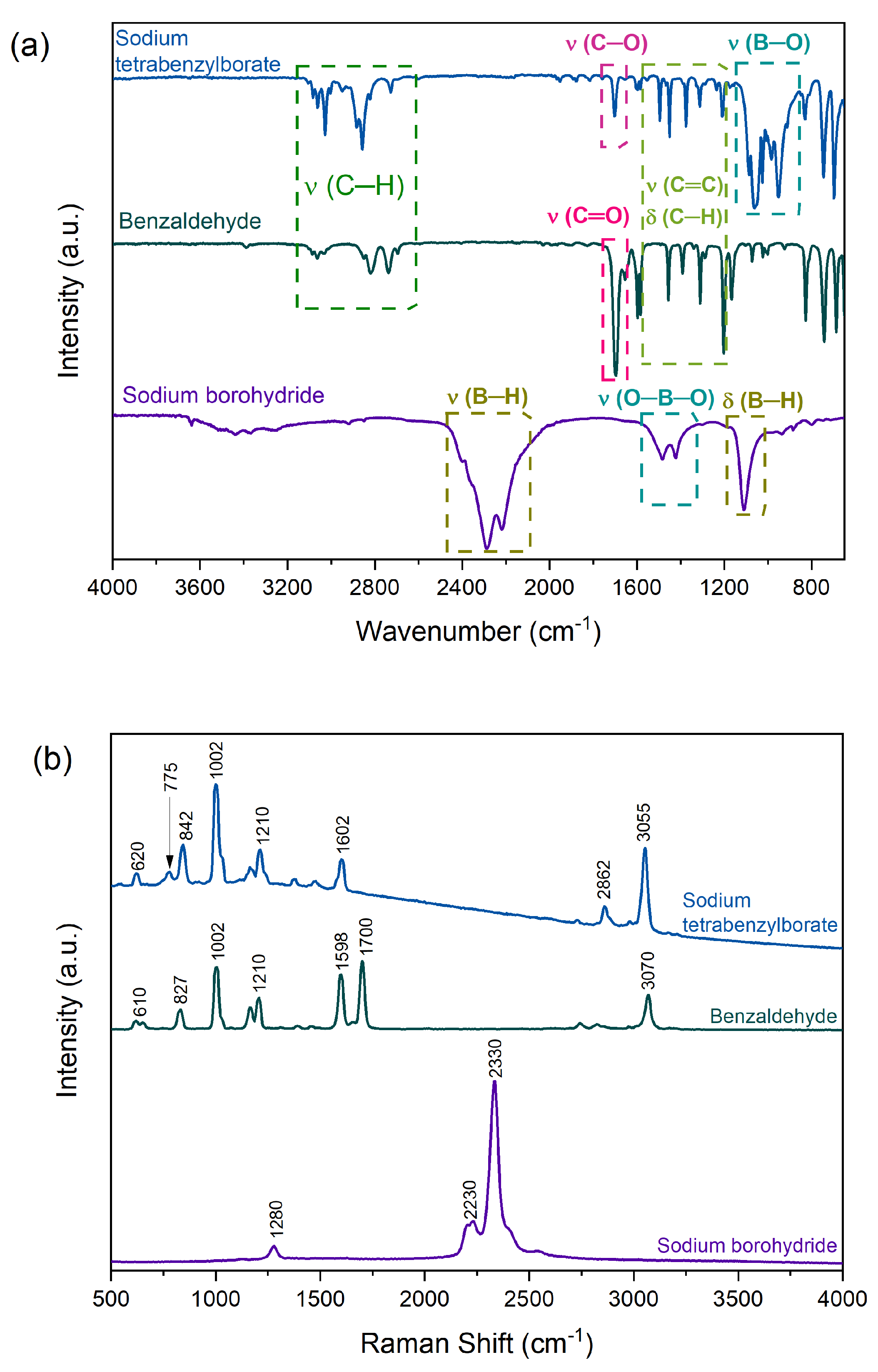
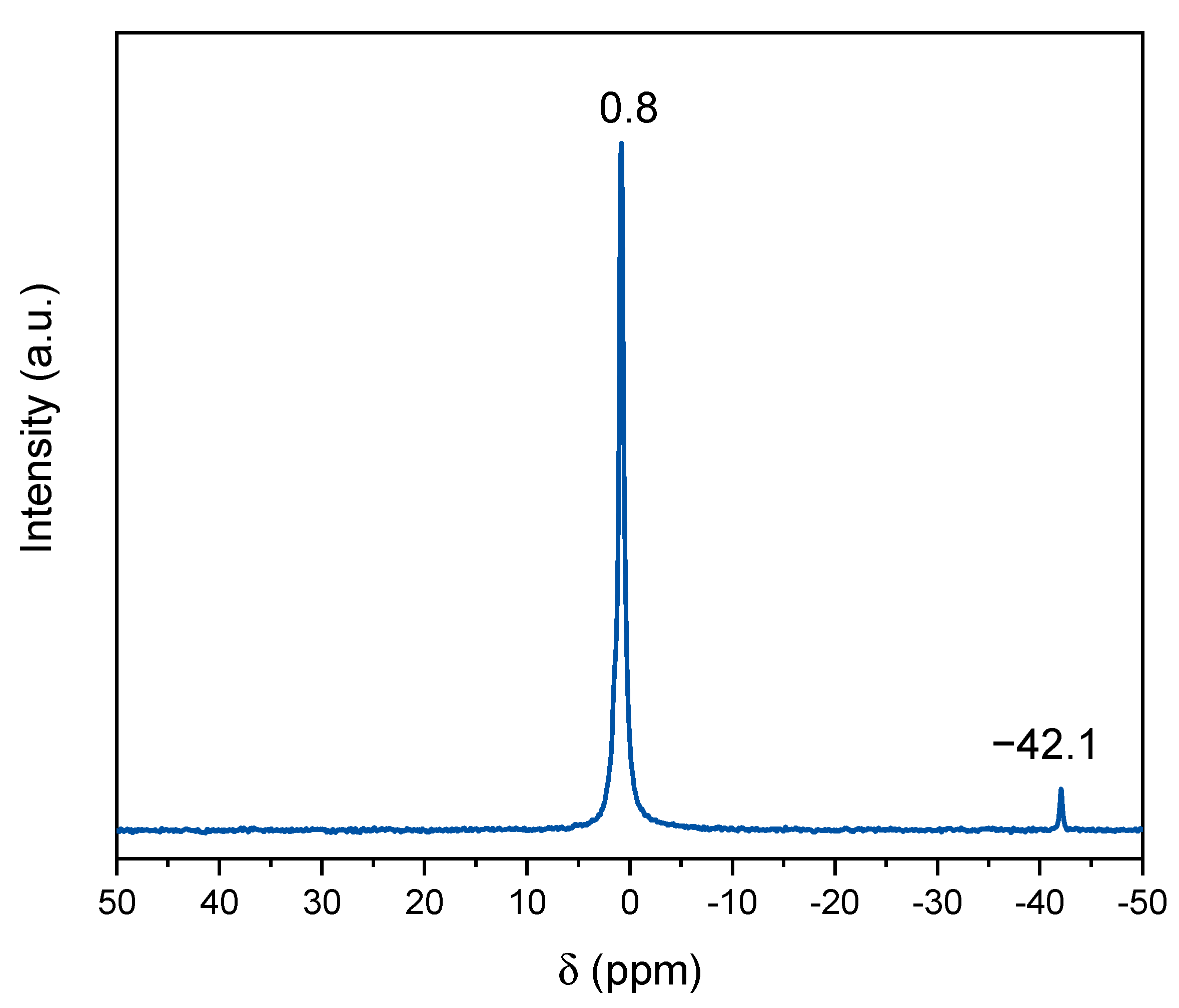
2.1. Sodium Tetrabenzylborate
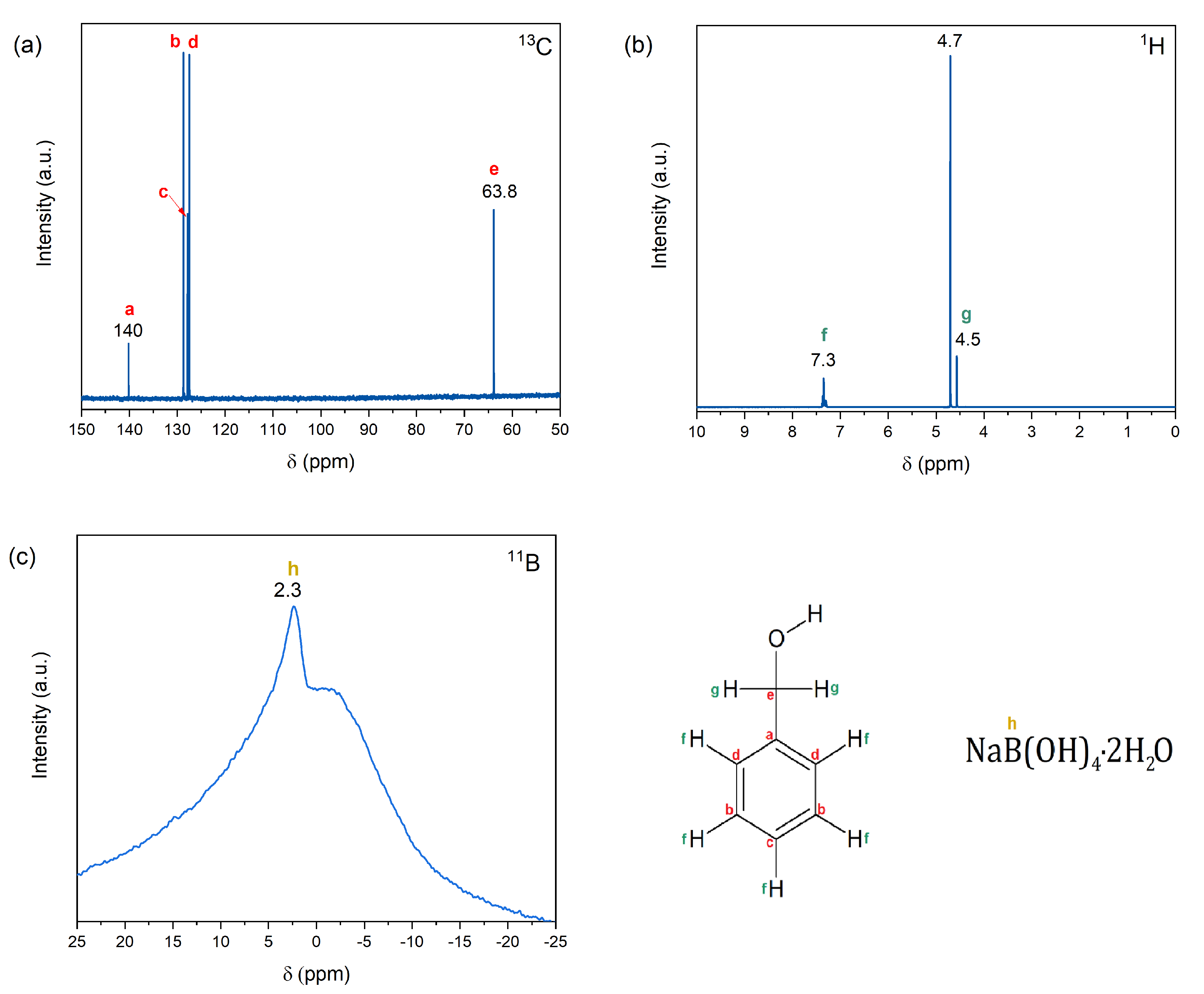
2.2. Molecular Structure
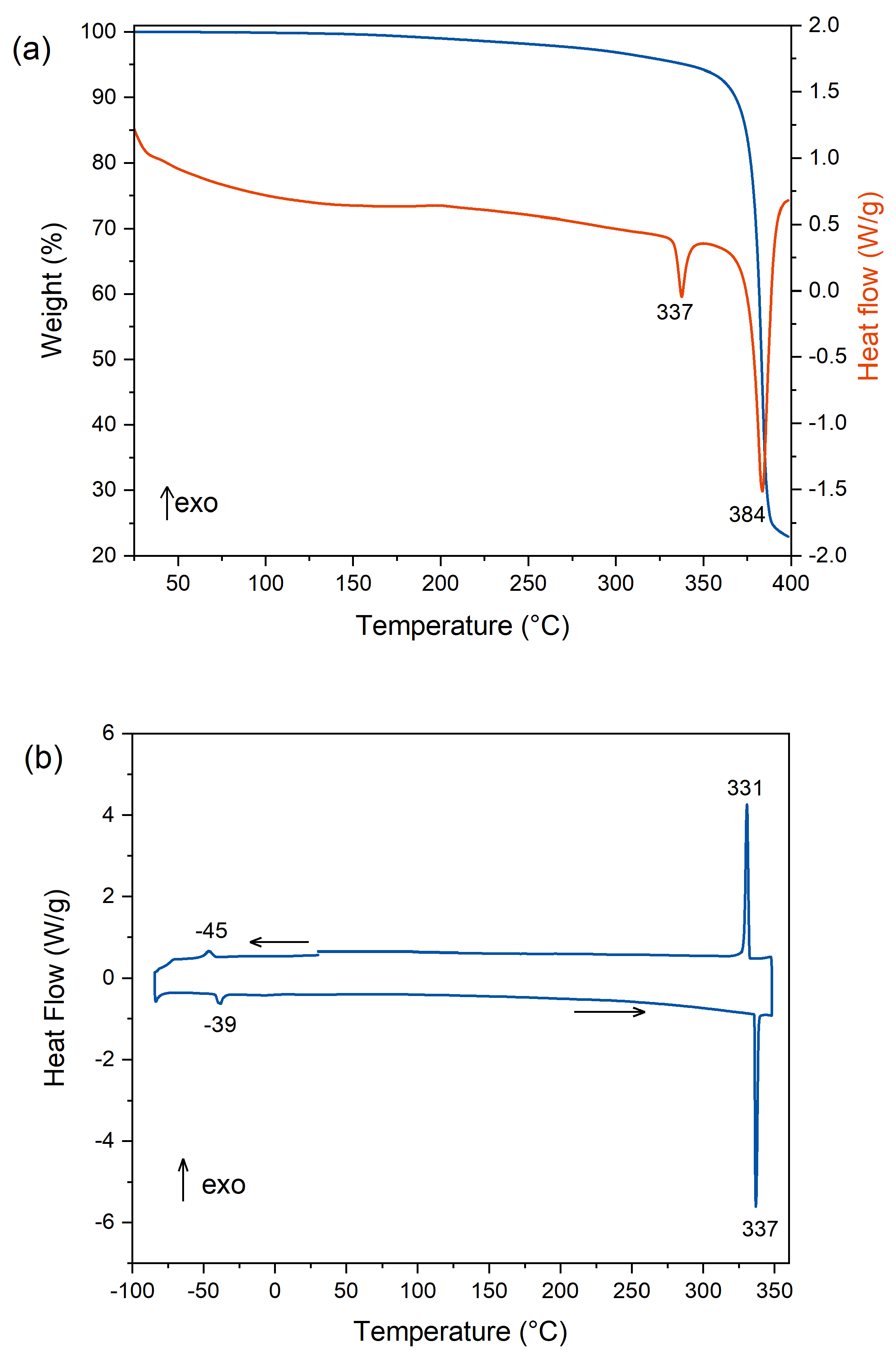
2.3. Thermal Analysis
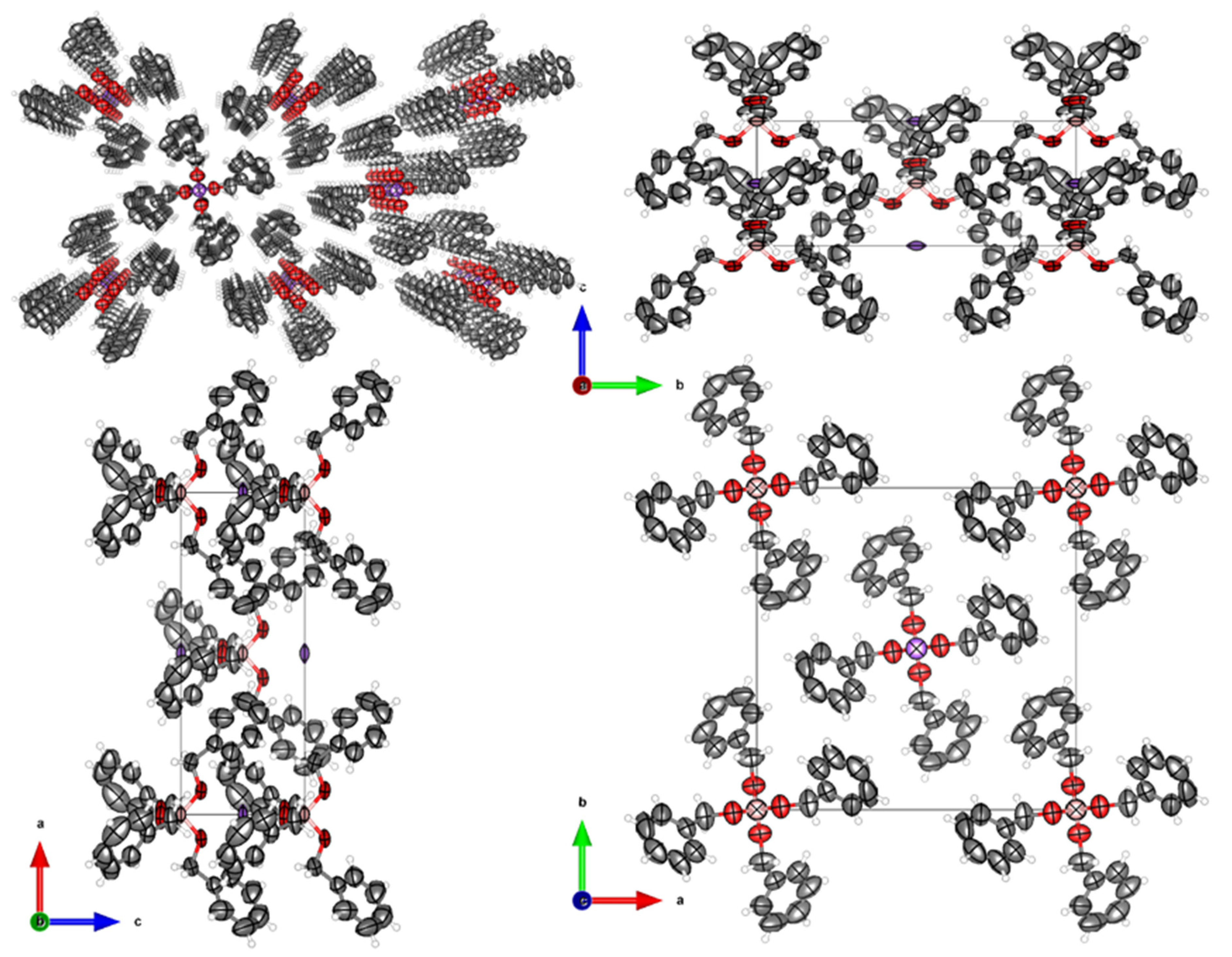
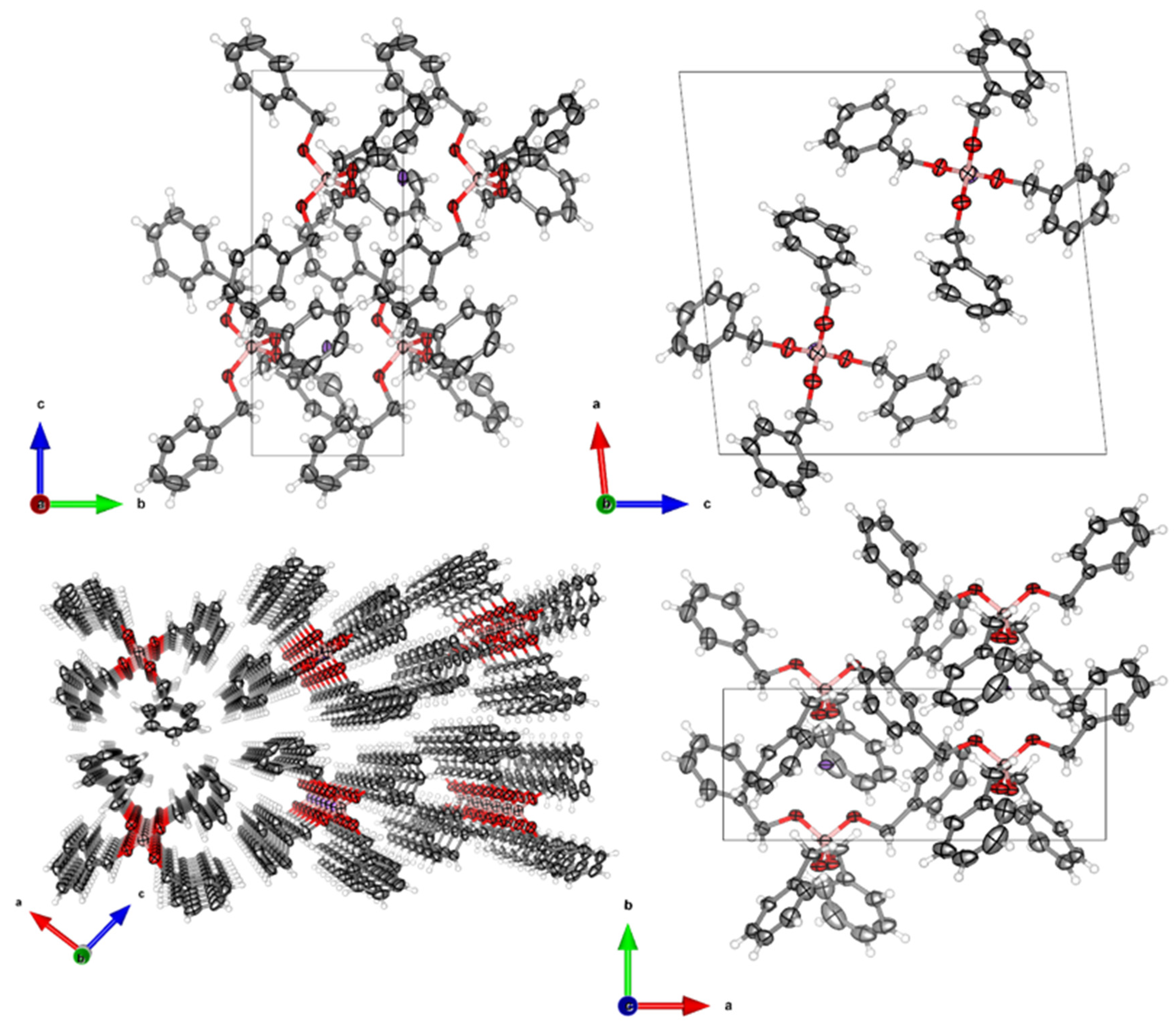
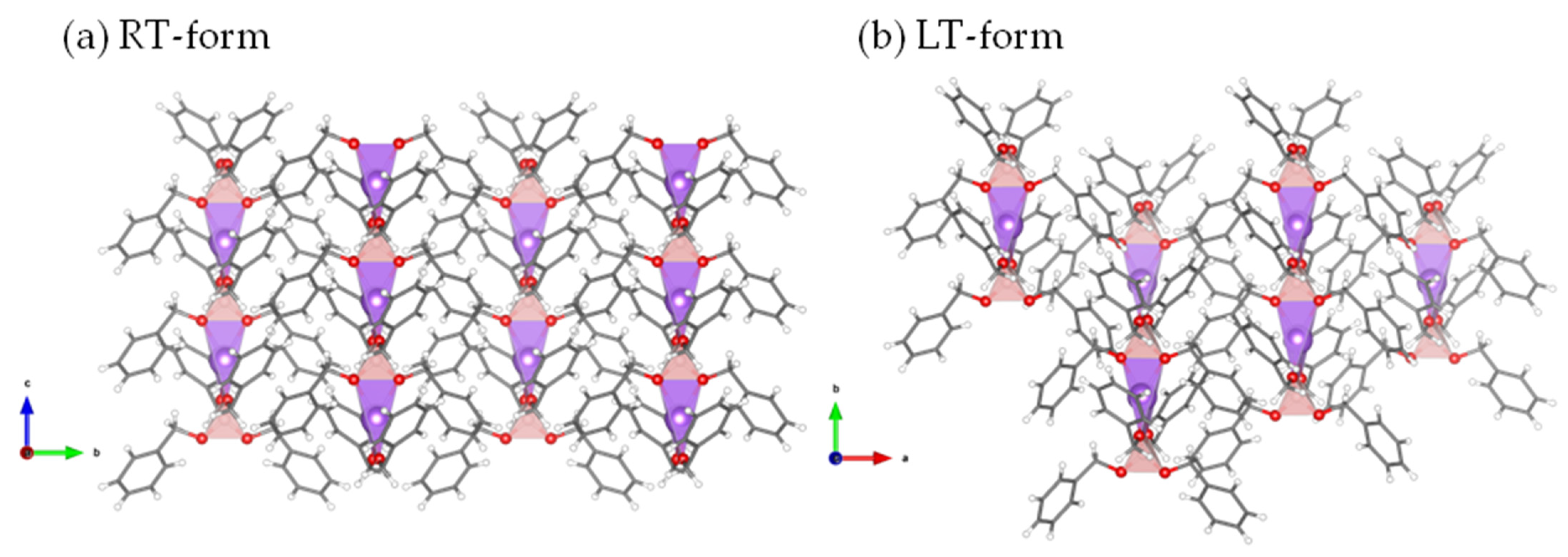
2.4. Crystal Structure Determination
2.5. Hydrolysis of the Tetraalkoxyborate into Benzyl Alcohol
3. Materials and Methods
3.1. Reagents
3.2. Synthesis
3.3. Growth of Single Crystals
3.4. Characterizations
3.5. Hydrolysis Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaikin, S.W.; Brown, W.G. Reduction of Aldehydes, Ketones and Acid Chlorides by Sodium Borohydride. J. Am. Chem. Soc. 1949, 71, 122–125. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and Its Use as a Reducing Agent and in the Generation of Hydrogen1. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Brown, H.C.; Wheeler, O.H.; Ichikawa, K. Chemical Effects of Steric Strains—XIII. Tetrahedron 1957, 1, 214–220. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.C.; Stewart, J.A.; Pérez-Ramírez, J. Status and Prospects in Higher Alcohols Synthesis from Syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, K.M.; Paramparambath, S.; Satheesh, A.; Selvam, S.; Kandasamy, E. Reduction of Aldehydes and Ketones by NaBH4 in Presence of 1-Alkyl-1,2,4-Triazolium Salts. Mater. Today Proc. 2020, 33, 2381–2384. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Yahyaei, S. A Mild and Convenient Method for the Reduction of Carbonyl Compounds with NaBH4 in the Presence of Catalytic Amounts of MoCl5. Bull. Korean Chem. Soc. 2003, 24, 1664–1670. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Shrinidhi, A. Selective Aldehyde Reduction in Ketoaldehydes with NaBH4-Na2CO3-H2O at Room Temperatures. Synth. Commun. 2014, 44, 2051–2056. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Chemoselective Reduction of Aldehydes in the Presence of Ketones with NaBH 4 in Polyethylene Glycol Dimethyl Ethers. Synth. Commun. 2005, 35, 867–872. [Google Scholar] [CrossRef]
- Naimi-Jamal, M.R.; Mokhtari, J.; Dekamin, M.G.; Kaupp, G. Sodium Tetraalkoxyborates: Intermediates for the Quantitative Reduction of Aldehydes and Ketones to Alcohols through Ball Milling with NaBH4. Eur. J. Org. Chem. 2009, 2009, 3567–3572. [Google Scholar] [CrossRef]
- Renaudin, G.; Gomes, S.; Hagemann, H.; Keller, L.; Yvon, K. Structural and Spectroscopic Studies on the Alkali Borohydrides MBH4 (M = Na, K, Rb, Cs). J. Alloys Compd. 2004, 375, 98–106. [Google Scholar] [CrossRef]
- D’Anna, V.; Spyratou, A.; Sharma, M.; Hagemann, H. FT-IR Spectra of Inorganic Borohydrides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Botasini, S.; Méndez, E. On the Purity Assessment of Solid Sodium Borohydride. J. Power Sources 2012, 197, 218–223. [Google Scholar] [CrossRef]
- Burkholder, T.R.; Andrews, L. Reactions of Boron Atoms with Molecular Oxygen. Infrared Spectra of BO, BO2, B2O2, B2O3, and BO−2 in Solid Argon. J. Chem. Phys. 1991, 95, 8697–8709. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, Q. FT-IR Spectroscopy and DFT Calculation Study on the Solvent Effects of Benzaldehyde in Organic Solvents. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Sinha, N.; Kumar, B. Growth and Properties of Sodium Tetraborate Decahydrate Single Crystals. Mater. Res. Bull. 2013, 48, 1632–1636. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Marques, M.P.M.; Batista de Carvalho, L.a.E.; Castro, J.L.; Otero, J.C. Anomalous Surface-Enhanced Raman Scattering of Aromatic Aldehydes and Carboxylic Acids. J. Raman Spectrosc. 2017, 48, 413–417. [Google Scholar] [CrossRef]
- Applegarth, L.M.S.G.A.; Pye, C.C.; Cox, J.S.; Tremaine, P.R. Raman Spectroscopic and Ab Initio Investigation of Aqueous Boric Acid, Borate, and Polyborate Speciation from 25 to 80 °C. Ind. Eng. Chem. Res. 2017, 56, 13983–13996. [Google Scholar] [CrossRef]
- Alderman, O.L.G.; Iuga, D.; Howes, A.P.; Pike, K.J.; Holland, D.; Dupree, R. Spectral Assignments and NMR Parameter–Structure Relationships in Borates Using High-Resolution 11B NMR and Density Functional Theory. Phys. Chem. Chem. Phys. 2013, 15, 8208. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Vosegaard, T.; Jakobsen, H.J.; Skibsted, J. 11B Chemical Shift Anisotropies in Borates from 11B MAS, MQMAS, and Single-Crystal NMR Spectroscopy. J. Phys. Chem. A 2004, 108, 586–594. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment—Olex2 Dissected. Acta Crystallogr. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, G.L.; Pethő, B.; Králl, P.; Novák, Z. Palladium-Catalyzed Methoxylation of Aromatic Chlorides with Borate Salts. Adv. Synth. Catal. 2014, 356, 125–129. [Google Scholar] [CrossRef]
- Berger, A.; Ibrahim, A.; Hales, T.A.; D’Angelo, A.M.; Buckley, C.E.; Paskevicius, M. Alkali Metal Alkoxyborate Ester Salts; a Contemporary Look at Old Compounds. Dalton Trans. 2024, 53, 3638–3653. [Google Scholar] [CrossRef] [PubMed]
- Favre-Nicolin, V.; Černý, R. FOX, ‘free Objects for Crystallography’: A Modular Approach to Ab. Initio Structure Determination from Powder Diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Für Krist. Cryst. Mater. 2014, 229. [Google Scholar] [CrossRef]

| Empirical formula | C28H28BNaO4 | |
| Formula weight (g mol−1) | 188.945 | |
| Temperature (K/°C) | 298 (25) | 173 (−100) |
| Wavelength (Å) | 0.71073 | 1.54178 |
| Crystal system | tetragonal | monoclinic |
| Space group | (No. 82) | P21 (No. 4) |
| Unit cell dimensions: | ||
| a (Å) | 14.9406 (8) | 14.0787 (4) |
| b (Å) | 5.7733 (2) | |
| c (Å) | 5.7838 (4) | 14.8056 (4) |
| β (°) | 96.0210 (10) | |
| Cell volume (Å3) | 1291.07 (13) | 1250.32 (6) |
| Z | 2 | 2 |
| Calculated density (g cm−3) | 1.189 | 1.228 |
| Absorption coefficient (μ·mm−1) | 0.092 | 0.789 |
| F (000) | 488.3 | 488.0 |
| Crystal size (mm) | 0.3 × 0.015 × 0.01 | 0.3 × 0.015 × 0.01 |
| 2θ range for data collection (°) | 3.86 to 50.7 | 6.002 to 114.898 |
| Limiting indices | −18 ≤ h ≤ 18, −18 ≤ k ≤ −17, −6 ≤ l ≤ 6 | −18 ≤ h ≤ 18, −6 ≤ k ≤ 6, −18 ≤ l ≤ 18 |
| Reflexion collected | 9324 | 23,291 |
| Completeness | 2θ = 50.5°: 100% | 2θ = 144.9°: 99% |
| Independent reflections | 1186 [Rint = 0.0545, Rsigma = 0.0458] | 4783 [Rint = 0.0423, Rsigma = 0.0332] |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 1186/0/77 | 4783/1/308 |
| Goodness-of-fit on F2 | 1.038 | 1.072 |
| Final R indices [I > 2σ(I)] | R1 = 0.0516, wR2 = 0.1673 | R1 = 0.0330, wR2 = 0.0822 |
| R indices (all data) | R1 = 0.0692, wR2 = 0.200 | R1 = 0.0363, wR2 = 0.0851 |
| LDPH (e Å−3) [a] | 0.26/−0.43 | 0.24/−0.14 |
| Flack parameter | 0 (220) | 0.05 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilla-Martinez, C.A.; Granier, D.; Yot, P.G.; Demirci, U.B. Unraveling the Crystal Structure of Sodium Tetrabenzylborate: Synthesis through the Sodium Borohydride Reduction of Benzaldehyde in the Solid State. Inorganics 2024, 12, 179. https://doi.org/10.3390/inorganics12070179
Castilla-Martinez CA, Granier D, Yot PG, Demirci UB. Unraveling the Crystal Structure of Sodium Tetrabenzylborate: Synthesis through the Sodium Borohydride Reduction of Benzaldehyde in the Solid State. Inorganics. 2024; 12(7):179. https://doi.org/10.3390/inorganics12070179
Chicago/Turabian StyleCastilla-Martinez, Carlos A., Dominique Granier, Pascal G. Yot, and Umit B. Demirci. 2024. "Unraveling the Crystal Structure of Sodium Tetrabenzylborate: Synthesis through the Sodium Borohydride Reduction of Benzaldehyde in the Solid State" Inorganics 12, no. 7: 179. https://doi.org/10.3390/inorganics12070179
APA StyleCastilla-Martinez, C. A., Granier, D., Yot, P. G., & Demirci, U. B. (2024). Unraveling the Crystal Structure of Sodium Tetrabenzylborate: Synthesis through the Sodium Borohydride Reduction of Benzaldehyde in the Solid State. Inorganics, 12(7), 179. https://doi.org/10.3390/inorganics12070179









