Highly Biocompatible Hemoglobin-Stabilized Gold Nanoparticles for an Enhanced Catalytic Reduction of 4-Nitrophenol
Abstract
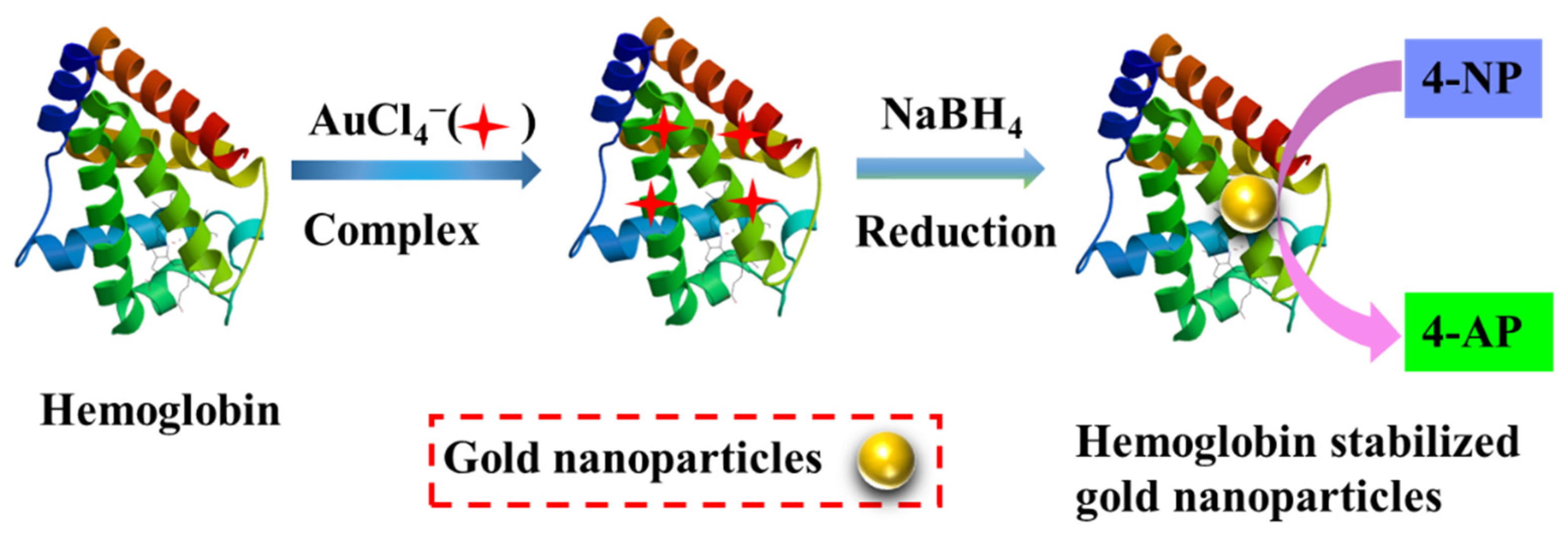
1. Introduction
2. Results and Discussion
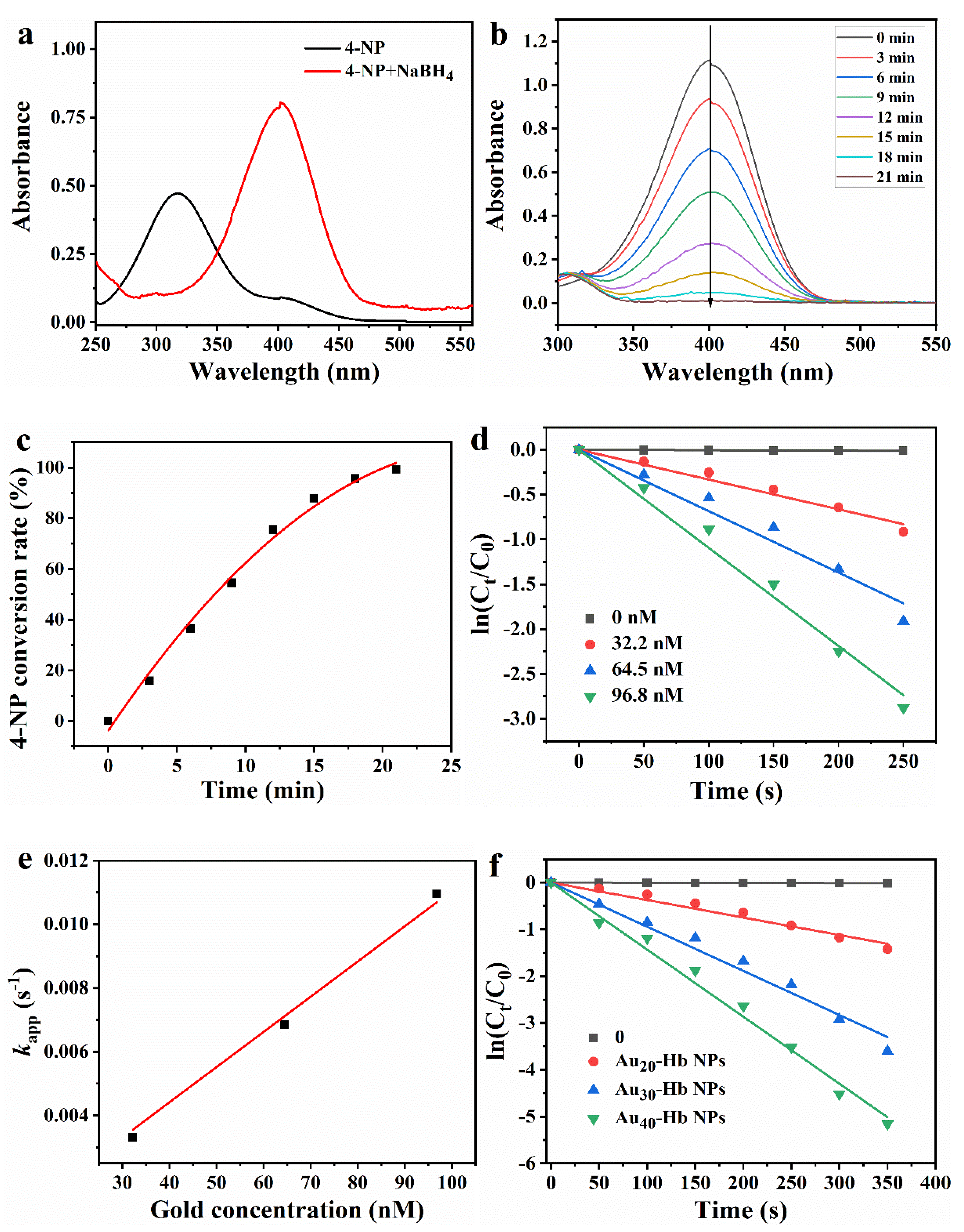
2.1. UV-Vis Spectra Analysis
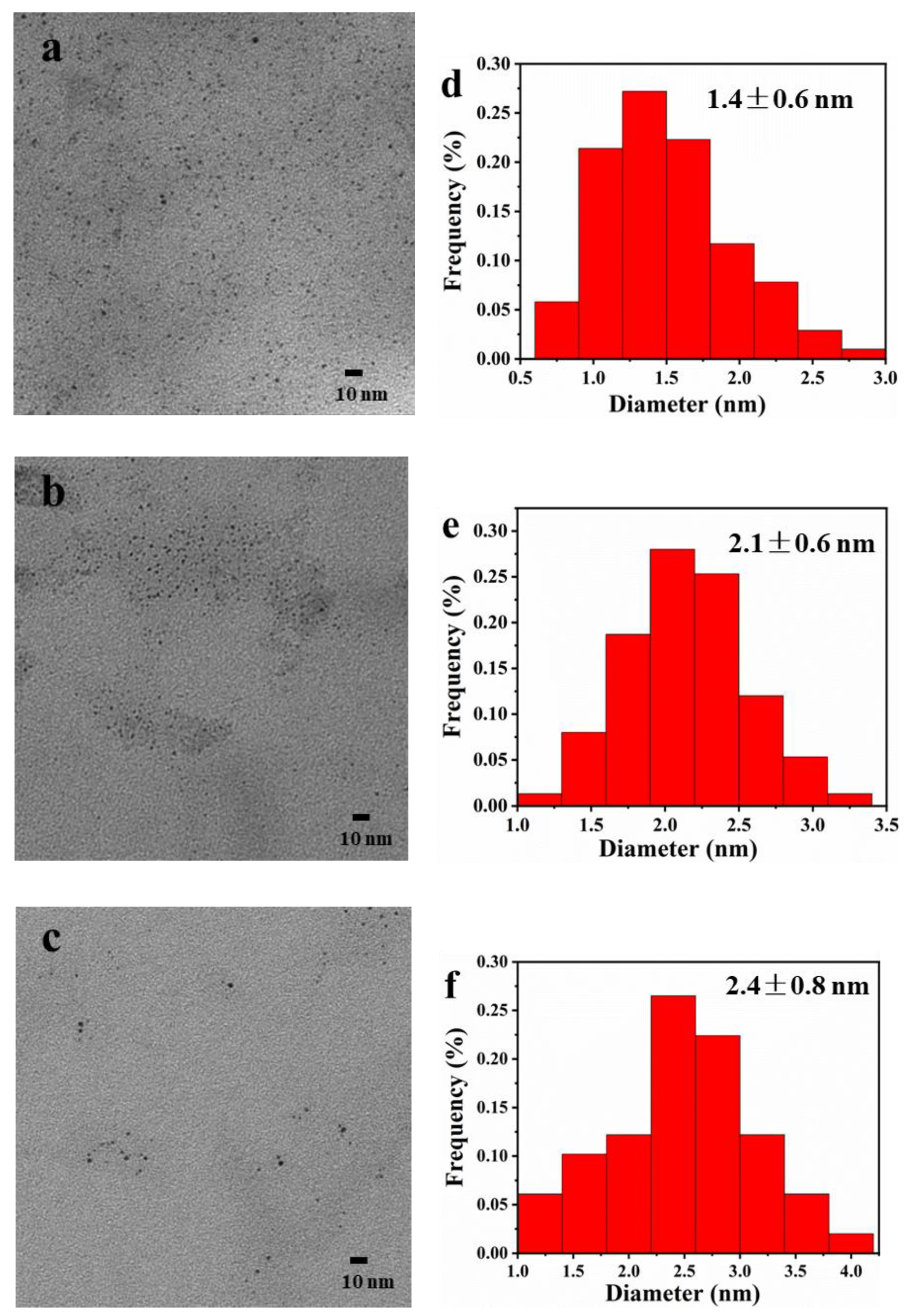
2.2. TEM Observation
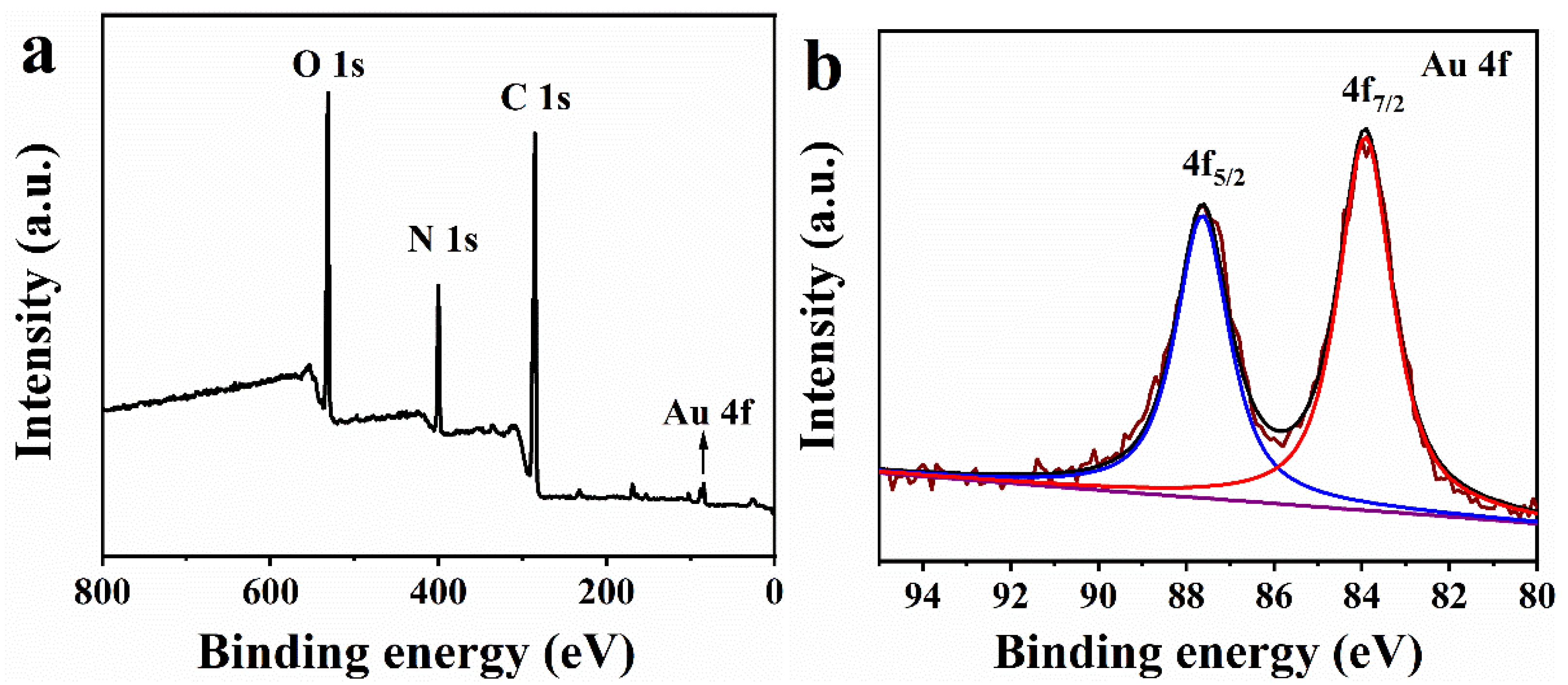
2.3. XPS Analysis
2.4. Stability in Solution
2.5. Catalytic Performance
| Catalyst | Au Size (nm) | kapp (×10−3 s−1) | knor (s−1 mmol−1) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|
| Au20-Hb | 1.4 | 3.32 | 3.32 × 104 | 6768 | This work |
| Au/graphene | 14.6 | 3.17 | 6.25 | 12 | [31] |
| Au NPs | 80 | 7.42 | 1.46 × 102 | 94 | [23] |
| GO@NH2-Au NPs | 14 | 35.6 | 5.85 × 102 | 595 | [32] |
| Au10-LP | 7.8 | 4.65 | 1.31 × 103 | 6053 | [33] |
| Au/Fe2O3@HAP | 10 | 7.12 | 1.27 × 103 | 241.3 | [34] |
| Au NPs/AOBC | 10.6 | 4.47 | 2.98 × 103 | 1198 | [35] |
| Cu-Au BNSs | 78 | 30.2 | 2.01 × 104 | 536.4 | [36] |
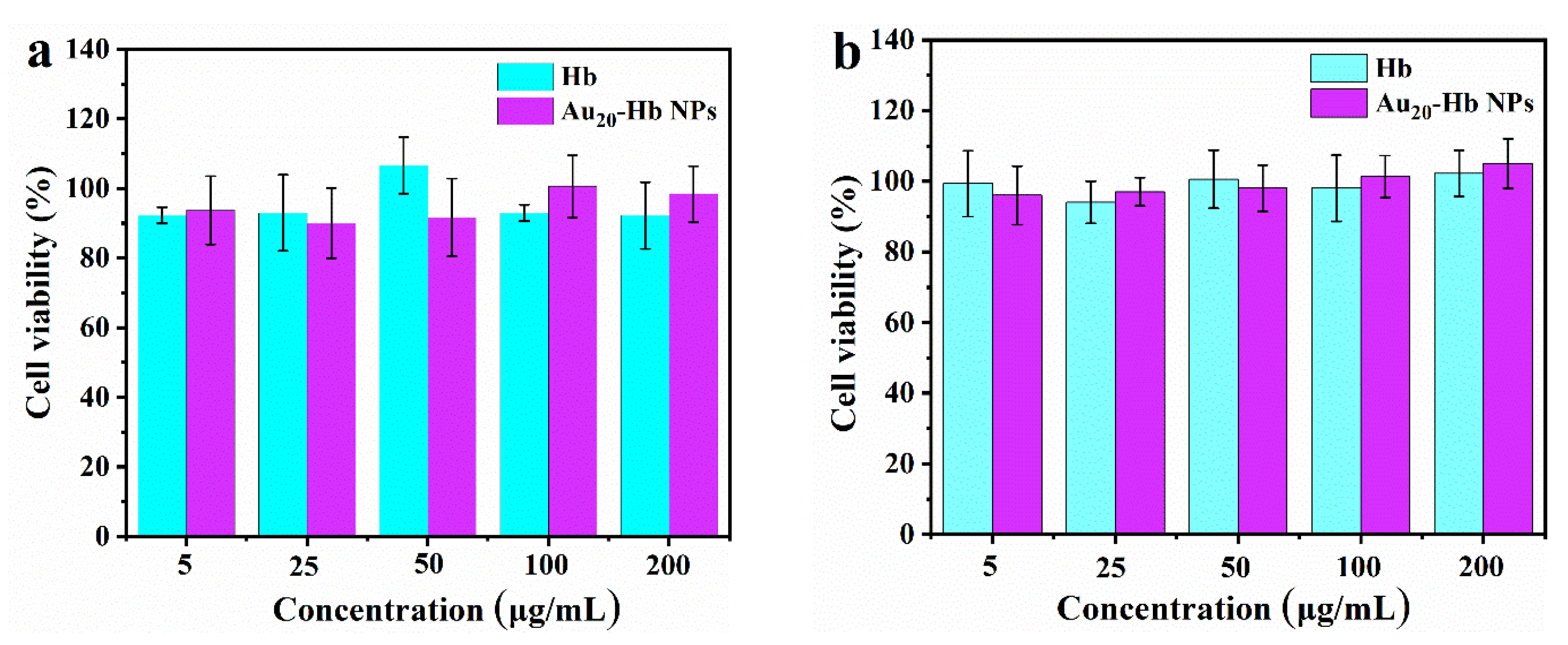
2.6. Biocompatibility
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Aun-Hb NPs
3.3. Characterization of Aun-Hb NPs
3.4. Catalytic Performance of Aun-Hb NPs
3.5. MTT Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mejía, Y.R.; Bogireddy, N.K.R. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Wu, J.; Jiang, G.; Qiao, Y.; Wang, Y.; Wu, D.; Zhong, Y.; Zhang, T.; Song, J.; Jin, Y.; et al. A hierarchically porous and hygroscopic carbon-based catalyst from natural wood for efficient catalytic reduction of industrial high-concentration 4-nitrophenol. Sep. Purif. Technol. 2022, 300, 121823. [Google Scholar] [CrossRef]
- Chen, C.-S.; Chen, T.-C.; Chiu, K.-L.; Wu, H.-C.; Pao, C.-W.; Chen, C.-L.; Hsu, H.-C.; Kao, H.-M. Silver particles deposited onto magnetic carbon nanofibers as highly active catalysts for 4-nitrophenol reduction. Appl. Catal. B 2022, 315, 121596. [Google Scholar] [CrossRef]
- Hu, C.; Yang, C.; Wang, X.; Wang, X.; Zhen, S.; Zhan, L.; Huang, C.; Li, Y. Rapid and facile synthesis of Au nanoparticle-decorated porous MOFs for the efficient reduction of 4-nitrophenol. Sep. Purif. Technol. 2022, 300, 121801. [Google Scholar] [CrossRef]
- Ermis, S.; Kaya, K.; Topuz, F.; Yagci, Y. In-Situ and green photosynthesis of PVP-stabilized palladium nanoparticles as efficient catalysts for the reduction of 4-nitrophenol. Inorg. Chem. Commun. 2023, 152, 110626. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Li, G.Y.; Ye, Y.X.; Xia, D.S. Efficient catalytic reduction of 4-nitrophenol by magnetic Cu/Fe nanocomposite catalyst. Desalin. Water Treat. 2022, 272, 138–143. [Google Scholar]
- Xia, W.; Zhao, F.; Fang, P.; An, M.; Zhu, J.; Cheng, K.; Xia, M. Magnetic Fe3O4@C nanoparticles separated from cold rolling mill sludge for 4-nitrophenol reduction. Sep. Purif. Technol. 2023, 309, 123018. [Google Scholar] [CrossRef]
- He, H.; Tao, G.; Wang, Y.; Cai, R.; Guo, P.; Chen, L.; Zuo, H.; Zhao, P.; Xia, Q. In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater. Sci. Eng. C 2017, 80, 509–516. [Google Scholar] [CrossRef]
- Nogueira, S.S.; de Araujo-Nobre, A.R.; Mafud, A.C.; Guimarães, M.A.; Alves, M.M.M.; Plácido, A.; Carvalho, F.A.A.; Arcanjo, D.D.R.; Mascarenhas, Y.; Costa, F.G.; et al. Silver nanoparticle stabilized by hydrolyzed collagen and natural polymers: Synthesis, characterization and antibacterial-antifungal evaluation. Int. J. Biol. Macromol. 2019, 135, 808–814. [Google Scholar] [CrossRef]
- San, B.H.; Moh, S.H.; Kim, K.K. The effect of protein shells on the antioxidant activity of protein-encapsulated platinum nanoparticles. J. Mater. Chem. 2012, 22, 1774–1780. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Zhang, T.; Ju, Z.; Ji, X.; Cui, Y.; Wang, L.; Xiao, H. Pd nanoparticles stabilized by bitter gourd polysaccharide with peroxidase properties for H2O2 detection. Int. J. Biol. Macromol. 2023, 233, 123513. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, M.; Cui, Y.; Ji, X.; Zhang, L.; Lan, X.; Wang, L.; Han, Z.; Xiao, H. Silver-palladium bimetallic nanoparticles stabilized by elm pod polysaccharide with peroxidase-like properties for glutathione detection and photothermal anti-tumor ability. Int. J. Biol. Macromol. 2024, 264, 130673. [Google Scholar] [CrossRef] [PubMed]
- Noël, S.; Bricout, H.; Addad, A.; Sonnendecker, C.; Zimmermann, W.; Monflier, E.; Léger, B. Catalytic reduction of 4-nitrophenol with gold nanoparticles stabilized by large-ring cyclodextrins. New J. Chem. 2020, 44, 21007–21011. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martinez-Antonio, A. Synthesis of gold nanoparticles by tetrachloroaurate reduction with cyclodextrins. Química Nova 2018, 41, 926–932. [Google Scholar] [CrossRef]
- Stiufiuc, G.; Toma, V.; Moldovan, A.I.; Stiufiuc, R.; Lucaciu, C.M. One pot microwave assisted synthesis of cyclodextrins capped spherical gold nanoparticles. Dig. J. Nanomater. Biostruct. 2017, 12, 1089–1095. [Google Scholar]
- Faggiano, S.; Ronda, L.; Bruno, S.; Abbruzzetti, S.; Viappiani, C.; Bettati, S.; Mozzarelli, A. From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol. Asp. Med. 2022, 84, 101050. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; Alba, J.; D’abramo, M.; Vitagliano, L. Quaternary structure transitions of human hemoglobin: An atomic-level view of the functional intermediate states. J. Chem. Inf. Model. 2021, 61, 3988–3999. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, P.; Huang, D.; Zeng, G.; Lai, C.; Qin, L.; Li, B.; He, J.; Yi, H.; Cheng, M.; et al. Au nanoparticles decorated on activated coke via a facile preparation for efficient catalytic reduction of nitrophenols and azo dyes. Appl. Surf. Sci. 2019, 473, 578–588. [Google Scholar] [CrossRef]
- Hidayat, H.; Purwiandono, G.; Tohari, T.; Nugroho, B.H.; Jauhari, M.H.; Widyaputra, S.B.; Fatimah, I. Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract. Green Process. Synth. 2022, 11, 1072–1082. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J. Colloid Interface Sci. 2013, 389, 260–267. [Google Scholar] [CrossRef]
- Bingwa, N.; Meijboom, R. Evaluation of catalytic activity of Ag and Au dendrimer-encapsulated nanoparticles in the reduction of 4-nitrophenol. J. Mol. Catal. A Chem. 2015, 396, 1–7. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Y.; Wang, S. ABC triblock copolymer-stabilized gold nanoparticles for catalytic reduction of 4-nitrophenol. J. Catal. 2015, 329, 425–430. [Google Scholar] [CrossRef]
- Guo, M.; He, J.; Li, Y.; Ma, S.; Sun, X. One-step synthesis of hollow porous gold nanoparticles with tunable particle size for the reduction of 4-nitrophenol. J. Hazard. Mater. 2016, 310, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Ma, E.; Zeng, F.; Wu, T.; Chen, K.; Fan, P.; Wen, X.; Li, L.; Qu, Q. Control-synthesized ultrafine Au nanoparticles by Aspergillus niger extracellular metabolites from SIM cards as high-effective 4-nitrophenol degradation catalyst. J. Environ. Chem. Eng. 2022, 10, 108676. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F. Bimetallic (AuAg, AuPd and AgPd) nanoparticles supported on cellulose-based hydrogel for reusable catalysis. Carbohydr. Polym. 2023, 310, 120726. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Dikshit, P.K.; Kim, B.S. Green in situ immobilization of gold and silver nanoparticles on bacterial nanocellulose film using Punica granatum peels extract and their application as reusable catalysts. Int. J. Biol. Macromol. 2022, 205, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Wang, L.; Qiang, X.; Song, Y.; Wang, X.; Gu, W.; Niu, J.; Sun, Y.; Srinuanpan, S.; Wang, G. Green synthesis of gold nanoparticles by phycoerythrin extracted from Solieria tenuis as an efficient catalyst for 4-nitrophenol reduction and degradation of dyes in wastewater. Mater. Today Sustain. 2023, 23, 100435. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.-Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Ju, Y.; Li, X.; Feng, J.; Ma, Y.; Hu, J.; Chen, X. One pot in situ growth of gold nanoparticles on amine-modified graphene oxide and their high catalytic properties. Appl. Surf. Sci. 2014, 316, 132–140. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Cui, Y.; Cui, T.; Chen, S.; Ma, G.; Hou, W.; Wang, L. Green synthesis of gold nanoparticles using longan polysaccharide and their reduction of 4-nitrophenol and biological applications. Nano 2020, 15, 2050002. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Shi, N.; Zhang, X. Highly efficient reduction of 4-nitrophenolate to 4-aminophenolate by Au/-Fe2O3@HAP magnetic composites. RSC Adv. 2019, 9, 10272–10281. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kang, H.; Gong, Y.; Guo, J.; Zhang, H.; Liu, R. Bacterial cellulose supported gold nanoparticles with excellent catalytic properties. ACS Appl. Mater. Interfaces 2015, 7, 21717–21726. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Fu, S.; Song, W.; Zhang, X.; Liu, B.; Zhang, F.; Chai, F. Controllable synthesis of sea urchin-like Cu-Au bimetallic nanospheres and their utility as efficient catalyst for hydrogenation of 4-nitrophenol. J. Solid State Chem. 2023, 322, 123968. [Google Scholar] [CrossRef]
- Zi, W.; Karmakar, B.; El-kott, A.F.; Al-Saeed, F.A.; Negm, S.; Salem, E.T. Green synthesized silver nanoparticles incorporated graphene oxide: Investigation of its catalytic activity, antioxidant and potential activity against colorectal cancer cells. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1693–1703. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Li, S.; Yu, N.; Yu, X.; Ji, X.; Wang, L. Highly Biocompatible Hemoglobin-Stabilized Gold Nanoparticles for an Enhanced Catalytic Reduction of 4-Nitrophenol. Inorganics 2024, 12, 136. https://doi.org/10.3390/inorganics12050136
Cui Y, Li S, Yu N, Yu X, Ji X, Wang L. Highly Biocompatible Hemoglobin-Stabilized Gold Nanoparticles for an Enhanced Catalytic Reduction of 4-Nitrophenol. Inorganics. 2024; 12(5):136. https://doi.org/10.3390/inorganics12050136
Chicago/Turabian StyleCui, Yanshuai, Shukai Li, Ning Yu, Xiaodong Yu, Xianbing Ji, and Longgang Wang. 2024. "Highly Biocompatible Hemoglobin-Stabilized Gold Nanoparticles for an Enhanced Catalytic Reduction of 4-Nitrophenol" Inorganics 12, no. 5: 136. https://doi.org/10.3390/inorganics12050136
APA StyleCui, Y., Li, S., Yu, N., Yu, X., Ji, X., & Wang, L. (2024). Highly Biocompatible Hemoglobin-Stabilized Gold Nanoparticles for an Enhanced Catalytic Reduction of 4-Nitrophenol. Inorganics, 12(5), 136. https://doi.org/10.3390/inorganics12050136





