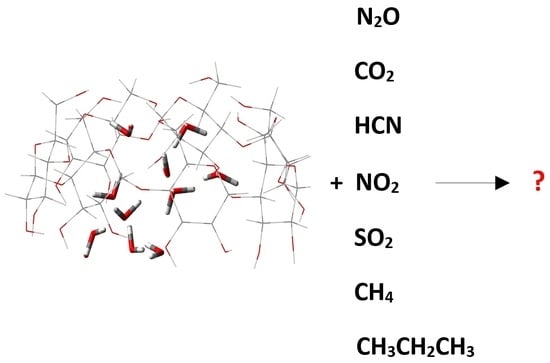
Inclusion Complexes between β-Cyclodextrin and Gaseous Substances—N2O, CO2, HCN, NO2, SO2, CH4 and CH3CH2CH3: Role of the Host’s Cavity Hydration
Abstract
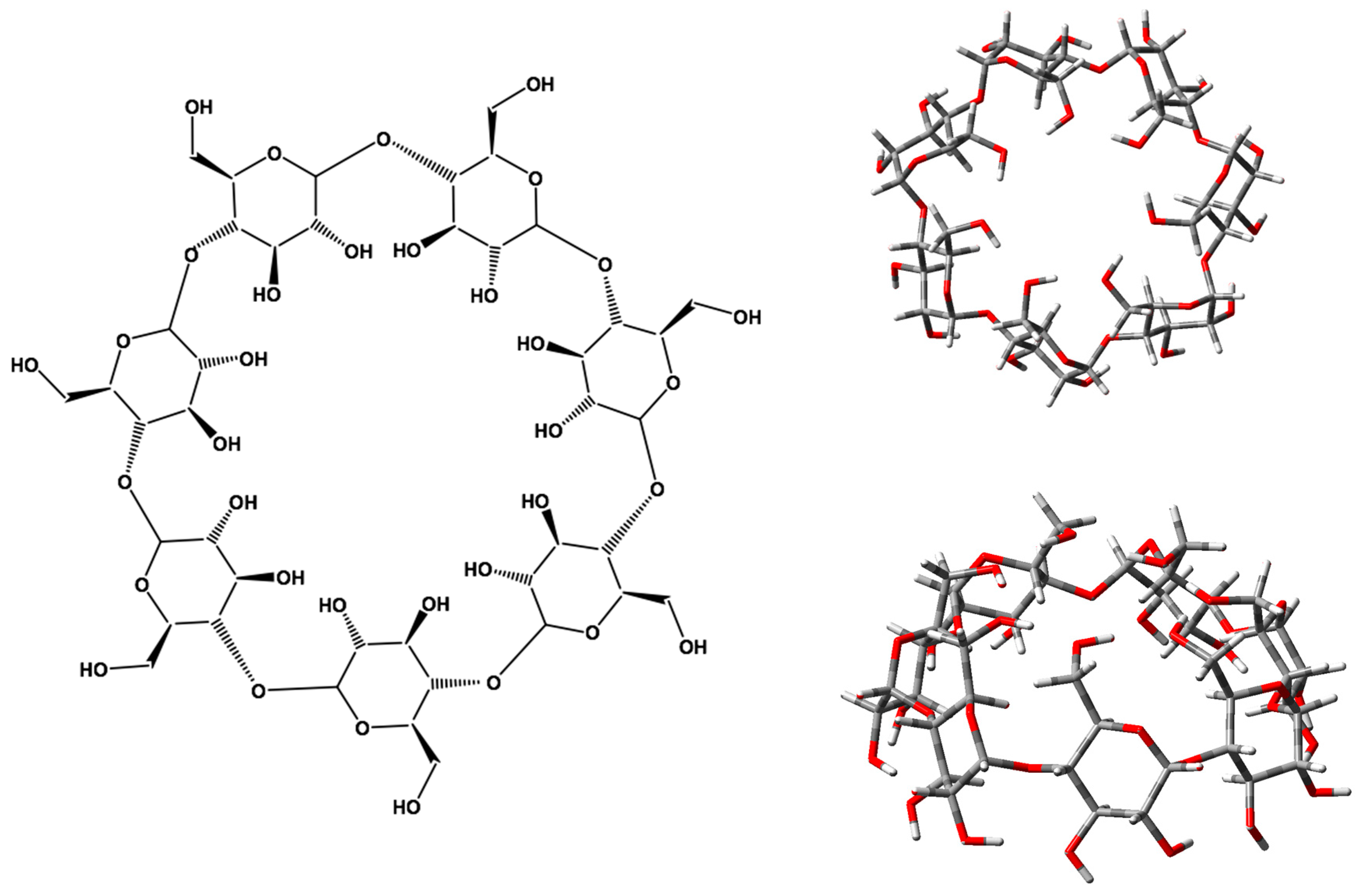
1. Introduction
2. Results and Discussion
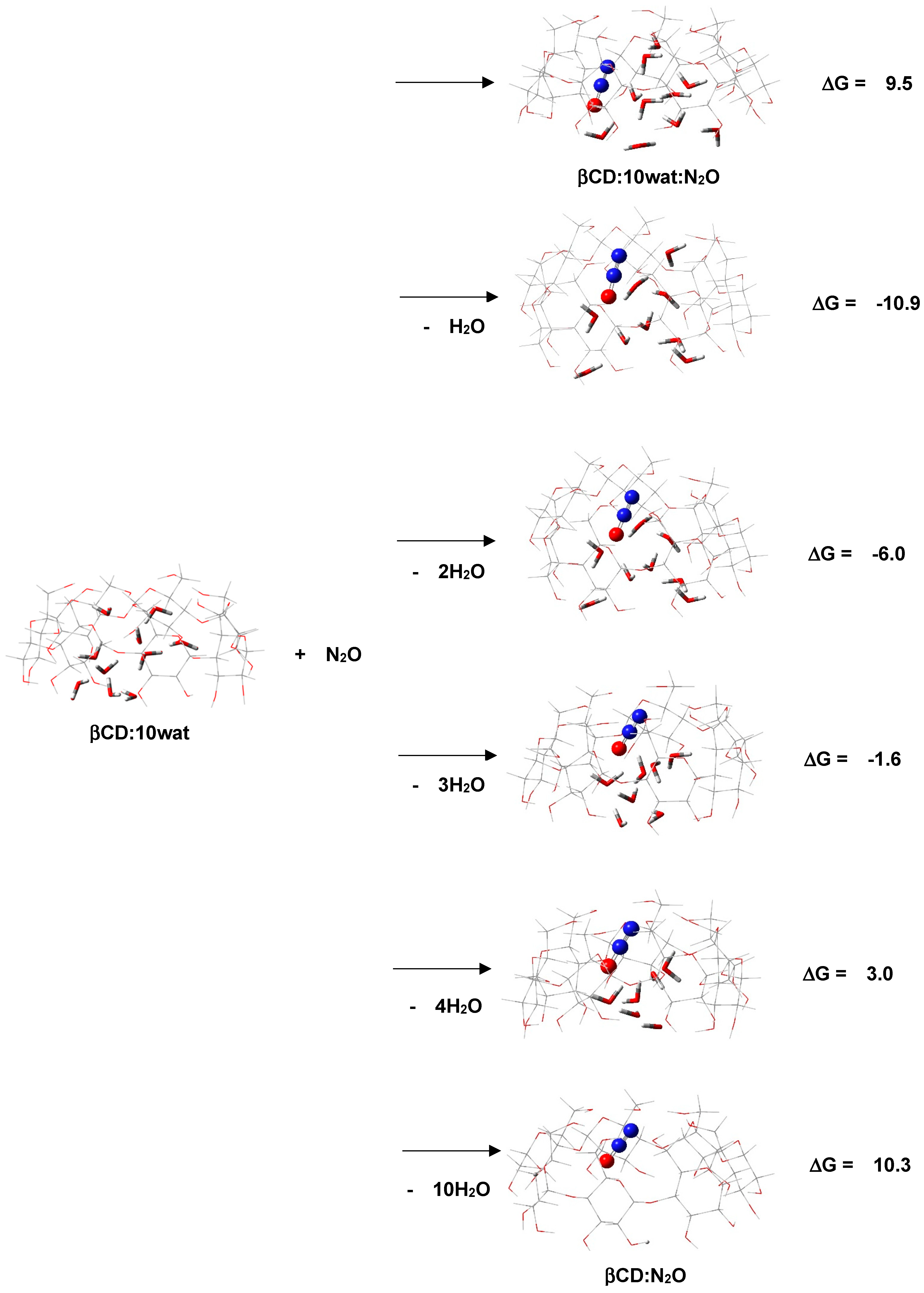
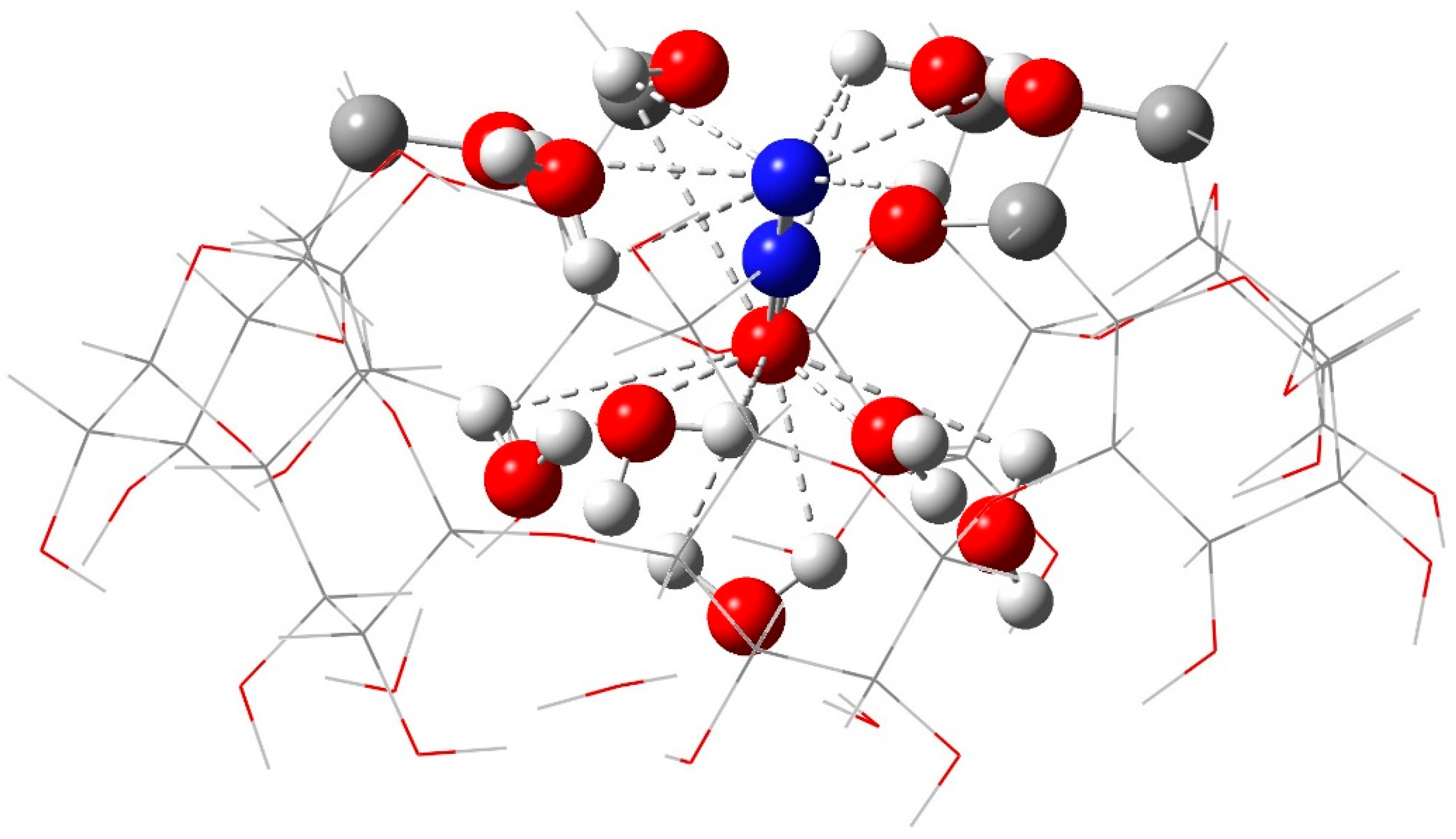
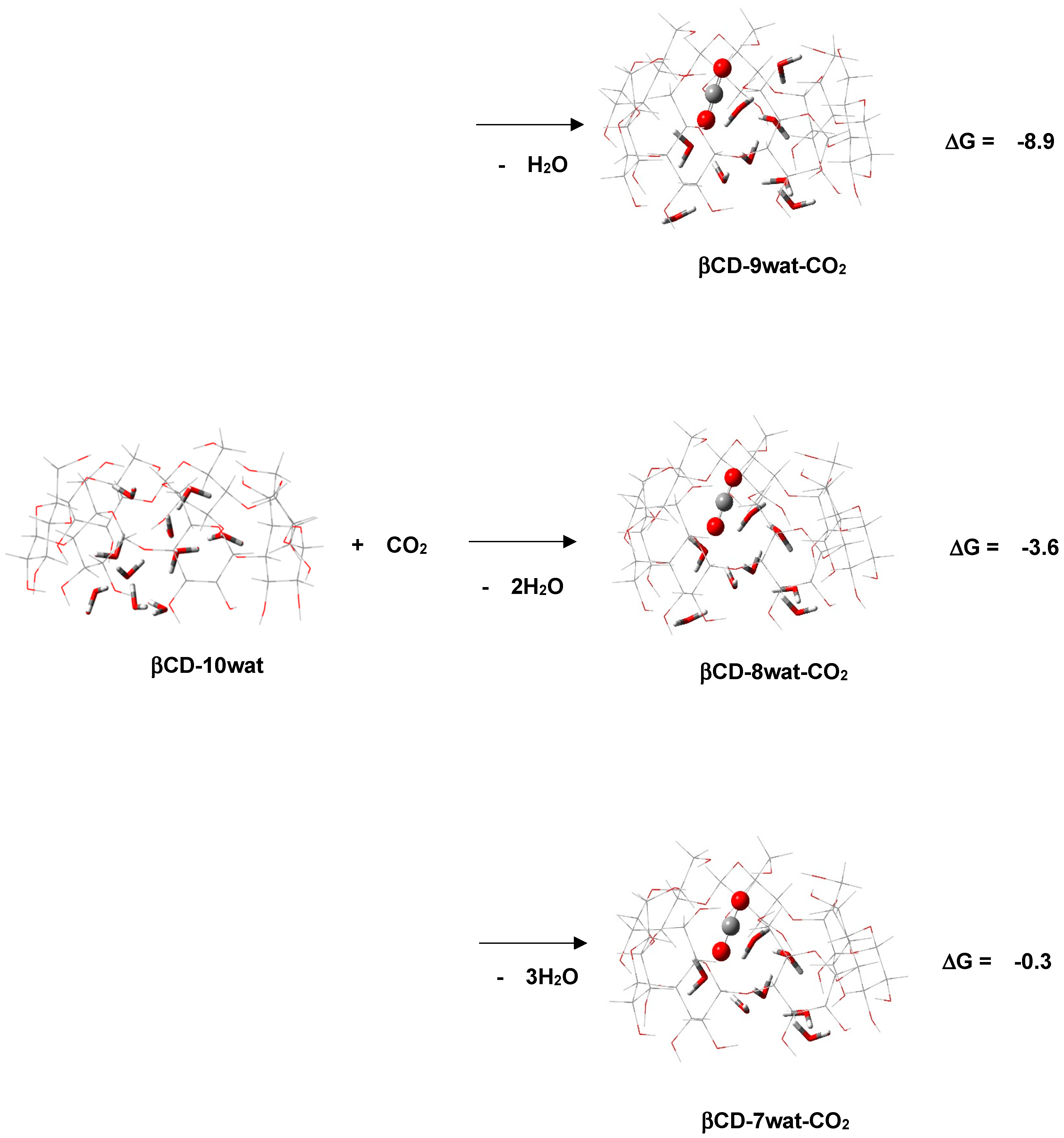
2.1. “Food/Beverage” Gases—N2O and CO2
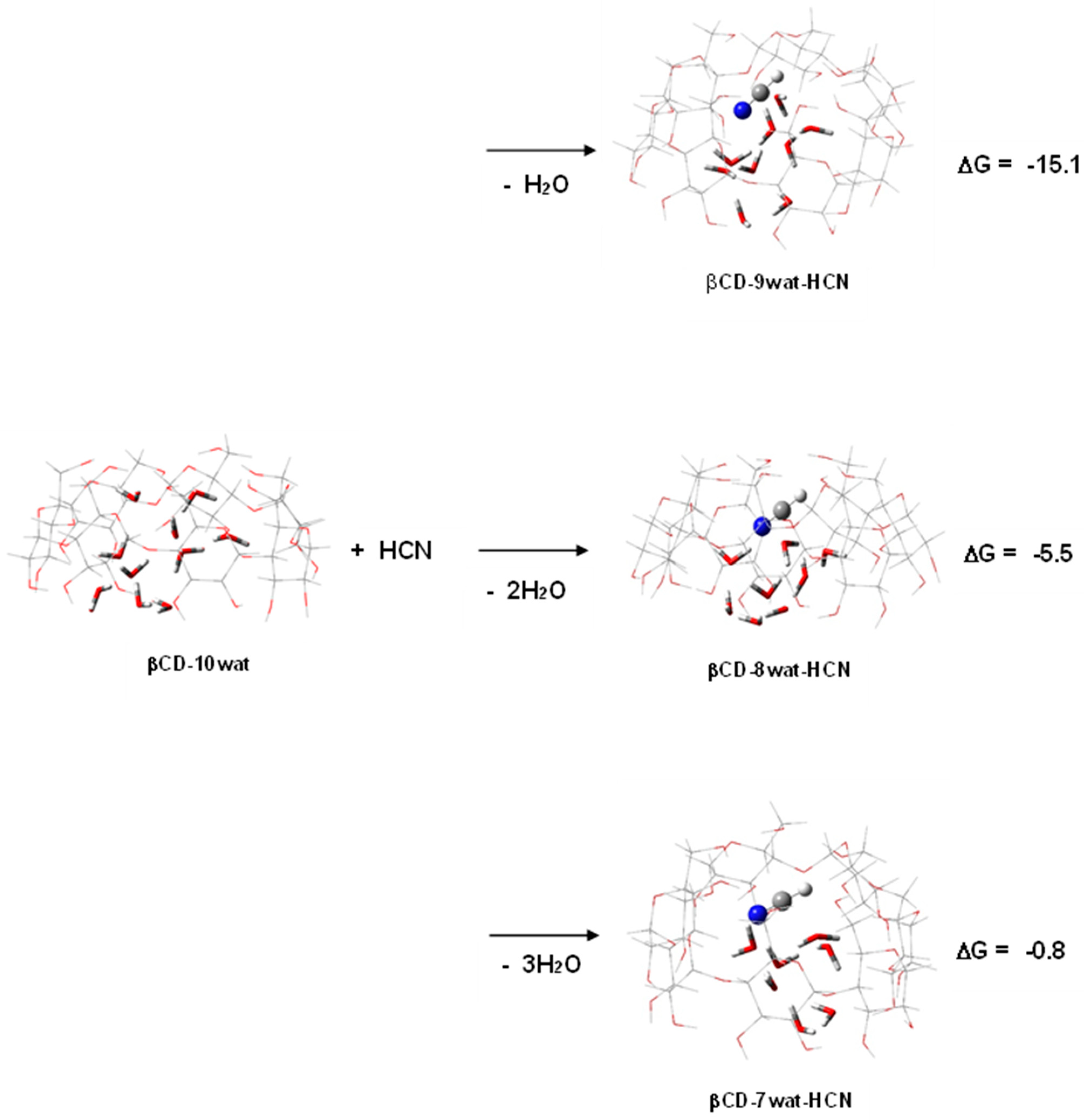
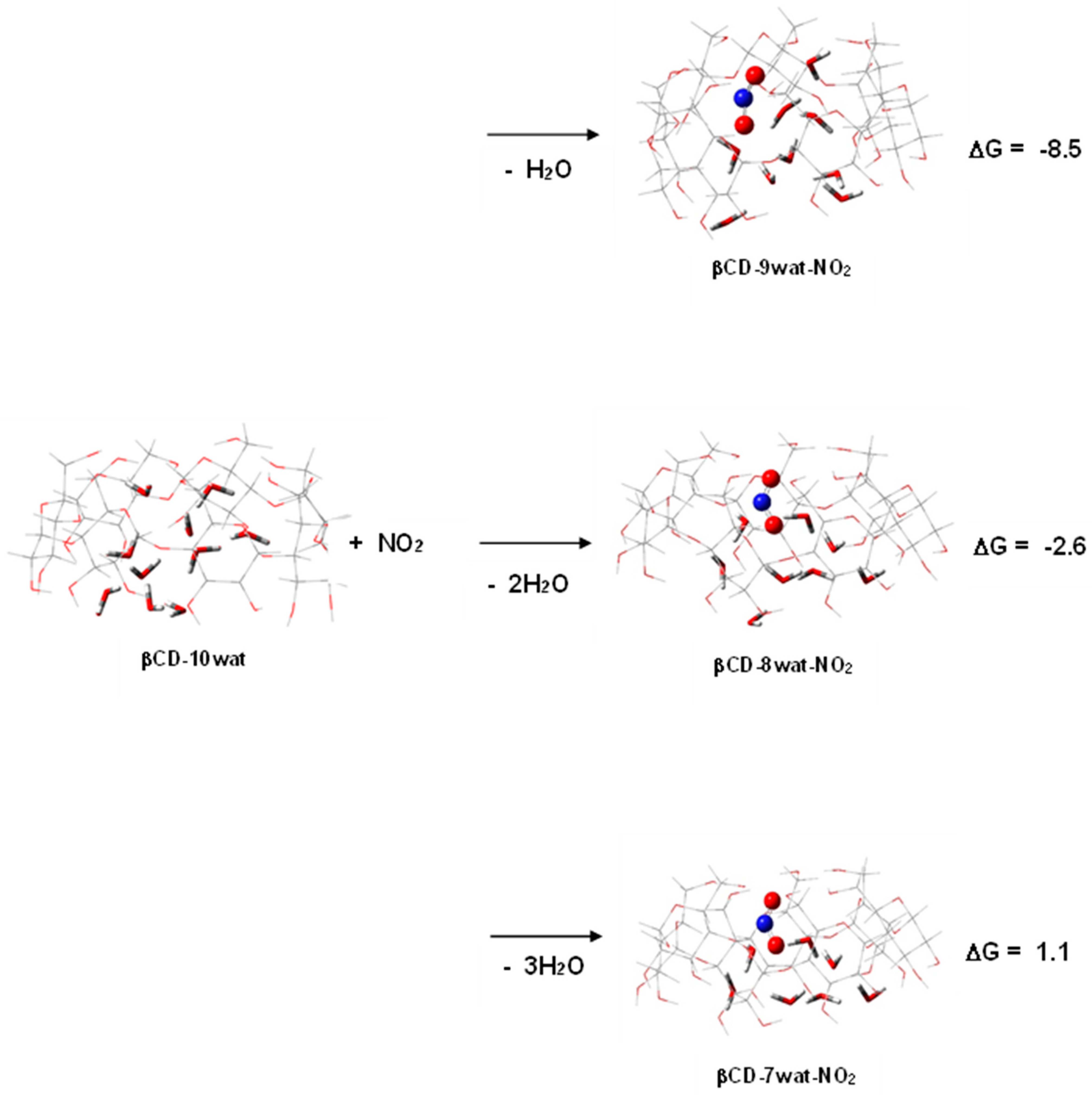
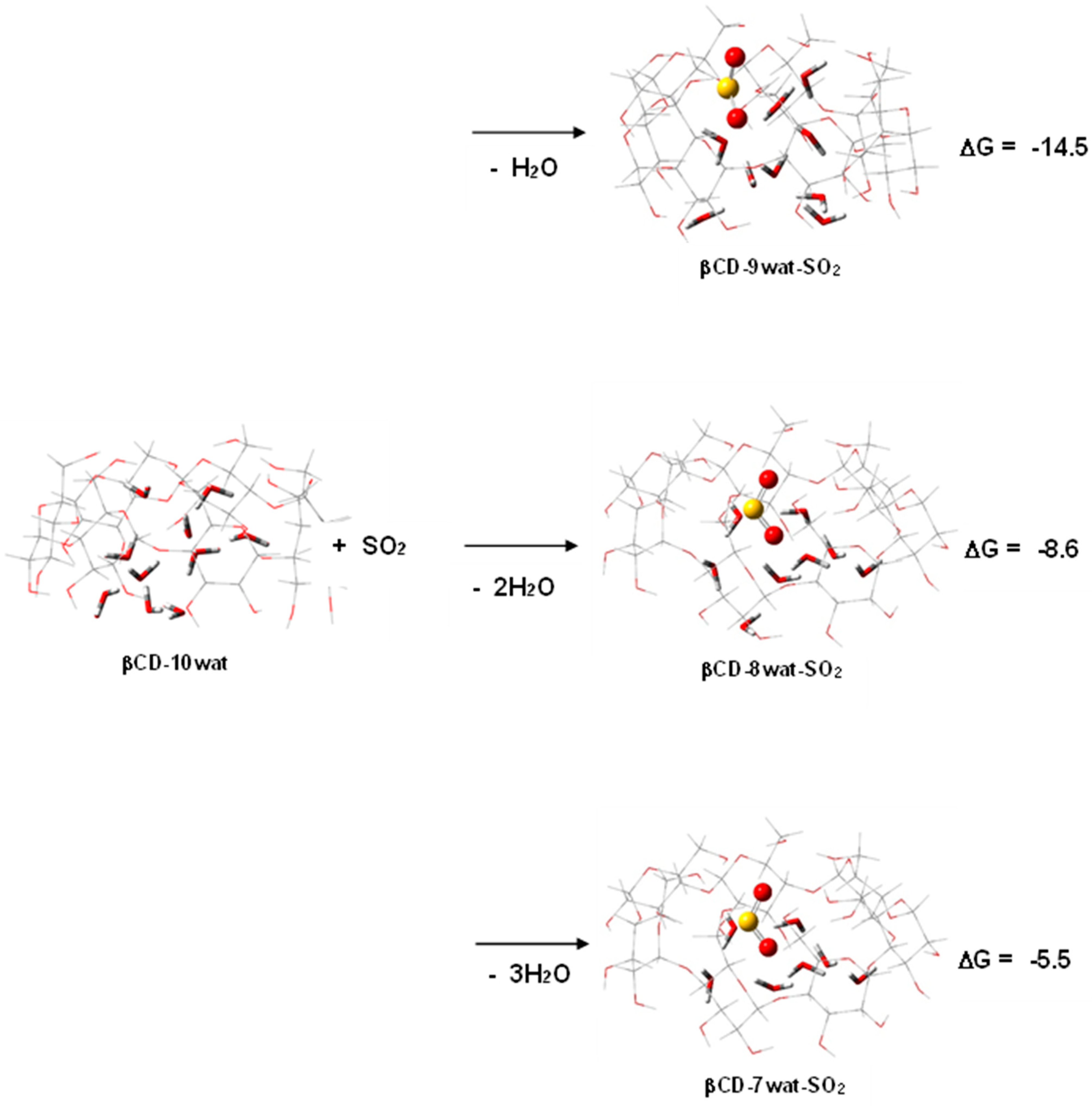
2.2. Poisonous Gases—HCN, NO2, and SO2
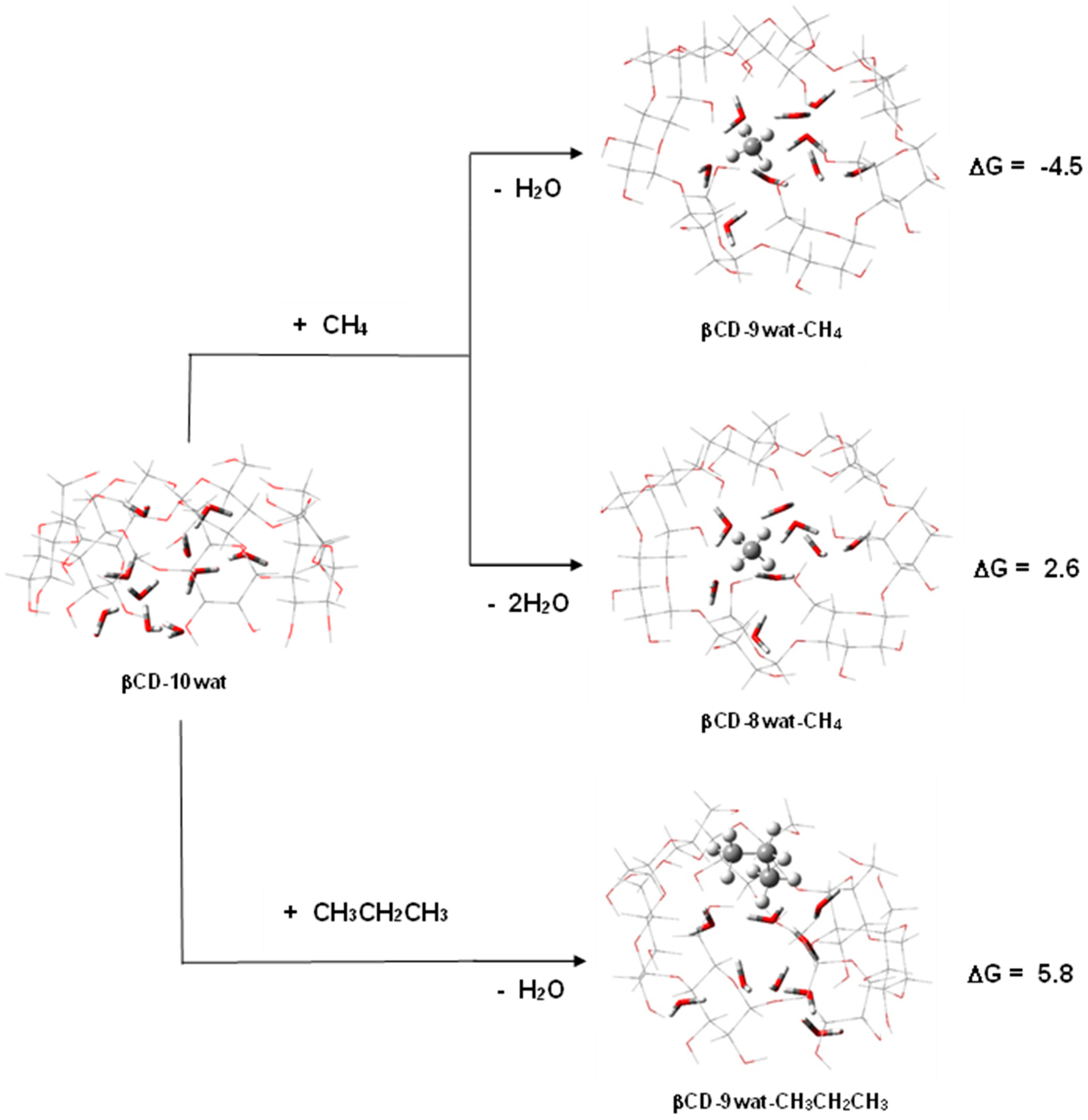
2.3. Saturated Hydrocarbons—Methane and Propane
3. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, R.K.; Garg, S. Current Status of Drug Delivery Technologies and Future Directions. Pharm. Technol. On-Line 2001, 25, 4. [Google Scholar]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host–Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.L.; Dias, D.C.; Justino, L.L.G.; Verissimo, L.M.P.; Valente, A.J.M.; Esteso, M.A.; Ribeiro, A.C.F.; Leaist, D.G.; Pina Cabral, A.M.T.D.P.V.; Rodrigo, M.M. Interactions between glycyl-L-phenylalanine and β-cyclodextrin from diffusion, spectroscopic and computational studies. J. Mol. Liq. 2020, 315, 113704. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Paduano, L.; Costantino, L.; Ortona, O.; Vitagliano, V. Mutual Diffusion Coefficients in Systems with Inclusion Compounds: A-Cyclodextrin-Polyoxyethylene 5 Hexylether-Water and a-Cyclodextrin—Sodium 1 Hexylsulfonate-Water. J. Mol. Liq. 1998, 75, 169–180. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit[n]Uril Family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. Cucurbituril Chemistry: A Tale of Supramolecular Success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef]
- Kim, K. Cucurbiturils and Related Macrocycles. Molecules 2023, 28, 8130. [Google Scholar]
- El-Sheshtawy, H.S.; Chatterjee, S.; Assaf, K.I.; Shinde, M.N.; Nau, W.M.; Mohanty, J. Supramolecular Approach for Enhanced Antibacterial Activity and Extended Shelf-Life of Fluoroquinolone Drugs with Cucurbit[7]Uril. Sci. Rep. 2018, 8, 13925. [Google Scholar] [CrossRef]
- Homden, D.M.; Redshaw, C. The Use of Calixarenes in Metal-Based Catalysis. Chem. Rev. 2008, 108, 5086–5130. [Google Scholar] [CrossRef]
- Vicens, J.; Bohmer, V. Calixarenes: A Versatile Class of Macrocyclic Compounds; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Bukhzam, A.; Bader, N. Crown Ethers: Their Complexes and Analytical Applications. J. Appl. Chem. 2017, 3, 237–244. [Google Scholar]
- Kralj, M.; Tušek-Božić, L.; Frkanec, L. Biomedical Potentials of Crown Ethers: Prospective Antitumor Agents. ChemMedChem 2008, 3, 1478–1492. [Google Scholar] [CrossRef]
- Wupper, S.; Luersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.-S.; Lee, S.; Bin, H. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E. (Ed.) Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Trotta, F.; Cavalli, R.; Martina, K.; Biasizzo, M.; Vitillo, J.; Bordiga, S.; Vavia, P.; Ansari, K. Cyclodextrin nanosponges as effective gas carriers. J. Incl. Phenom. Mol. Recognit. Chem. 2011, 71, 189. [Google Scholar] [CrossRef]
- Rajbanshi, B.; Dutta, A.; Mahato, B.; Roy, D.; Maiti, D.K.; Bhattacharyya, S.; Roy, M.N. Study to explore host guest inclusion complexes of vitamin B1 with CD molecules for enhancing stability and innovative application in biological system. J. Mol. Liq. 2020, 298, 111952. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Sarafska, T.; Angelova, S.; Spassov, T.; Dudev, T. Inclusion complexes of ibuprofen and β-cyclodextrin: Supramolecular structure and stability. J. Mol. Struct. 2020, 1205, 127575. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside b-cyclodextrin cavity: Amount, stability and mechanism of binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. α-Cyclodextrin: How Effectively Can Its Hydrophobic Cavity Be Hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Angelova, S.E.; Nikolova, V.K.; Dudev, T.M. Determinants of the host-guest interactions between α-, β- and γ-cyclodextrins and group IA, IIA and IIIA metal cations: A DFT/PCM study. Phys. Chem. Chem. Phys. 2017, 19, 15129–15136. [Google Scholar] [CrossRef] [PubMed]
- Angelova, S.; Nikolova, V.; Molla, N.; Dudev, T. Factors Governing the Host−Guest Interactions between IIA/IIB Group Metal Cations and α-Cyclodextrin: A DFT/CDM Study. Inorg. Chem. 2017, 56, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185. [Google Scholar] [PubMed]
- Kohler, P.; Peterseann, R.-D.; Botcher, S. Stabilization of tea tree oil. SÖFW J. 1999, 125, 10. [Google Scholar]
- Kock, J. Storage of stable, powdered detergent and cleaning agent containing perfume. DE 1981, 30, 220–269. [Google Scholar]
- Bacon, D.R.; Trinh, T. Detergent Compositions Containing Enduring Perfume. U.S. Patent 5 500 154, 26 February 1996. [Google Scholar]
- Kubota, M.; Komaki, R. Long-lasting perfume compositions containing (−)muscone. Jpn. Kokai Tokkyo Koho JP 1995, 7, 196. [Google Scholar]
- Pereva, S.; Himitliiska, T.; Spassov, T.; Stoyanov, S.D.; Arnaudov, L.N.; Dudev, T. Cyclodextrin-Based Solid-Gas Clathrates. J. Agric. Food Chem. 2015, 63, 6603–6613. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, R.; Wang, X.; Kong, L.; Xu, J.; Xiao, H.; Bedane, A.H. Porous Structure of β-Cyclodextrin for CO2 Capture: Structural Remodeling by Thermal Activation. Molecules 2022, 27, 7375. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Manteghian, M.; Mirzaei, M. Effect of β-cyclodextrin on dissolution of methane in water. Chem. Eng. Res. Des. 2011, 89, 421–427. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Mousavi Safavi, S.M. Solubility of Methane in Water at the Presence of β-Cyclodextrin, SDS, and Multi Wall Carbon Nanotubes at Low Temperatures. Pet. Sci. Technol. 2013, 31, 1085–1091. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, B.; Hu, C. Adsorption Properties of β-Cyclodextrin for Adsorbing Aromatic Hydrocarbons from the Gas Phase and Water. J. Macromol. Sci. Part B Phys. 2008, 47, 211–216. [Google Scholar] [CrossRef]
- Joseph, V.; Warhaftig, O.; Klein, S.; Levine, M. Paper-based manganese and β-cyclodextrin sensors for colorimetric sulfur dioxide detection. Anal. Chem. Acta 2022, 1200, 339629. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Kuo, K.-Y. β-Cyclodextrin Inclusion Complex Characterization and Application in the Remediation of Total Petroleum Hydrocarbons. Water 2022, 14, 1955. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2014, 114, 538–556. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Heitz, S.; Hese, A. Stark experiments on NO2: Determination of electric dipole moments and observation of perturbations by obscure background levels. J. Chem. Phys. 1993, 98, 6810–6819. [Google Scholar] [CrossRef]
- Lide, D.R., Jr. Microwave Spectrum, Structure, and Dipole Moment of Propane. J. Chem. Phys. 1960, 33, 1514–1518. [Google Scholar] [CrossRef]
- Sarafska, T.; Ivanova, S.; Dudev, T.; Petrov, V.; Spassov, T. Beta-cyclodextrin—Citric acid complexation by ball milling and annealing. J. Mol. Struct. 2024, 1295, 136701. [Google Scholar] [CrossRef]
- Gaussian 09, Version D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudev, T.; Spassov, T. Inclusion Complexes between β-Cyclodextrin and Gaseous Substances—N2O, CO2, HCN, NO2, SO2, CH4 and CH3CH2CH3: Role of the Host’s Cavity Hydration. Inorganics 2024, 12, 110. https://doi.org/10.3390/inorganics12040110
Dudev T, Spassov T. Inclusion Complexes between β-Cyclodextrin and Gaseous Substances—N2O, CO2, HCN, NO2, SO2, CH4 and CH3CH2CH3: Role of the Host’s Cavity Hydration. Inorganics. 2024; 12(4):110. https://doi.org/10.3390/inorganics12040110
Chicago/Turabian StyleDudev, Todor, and Tony Spassov. 2024. "Inclusion Complexes between β-Cyclodextrin and Gaseous Substances—N2O, CO2, HCN, NO2, SO2, CH4 and CH3CH2CH3: Role of the Host’s Cavity Hydration" Inorganics 12, no. 4: 110. https://doi.org/10.3390/inorganics12040110
APA StyleDudev, T., & Spassov, T. (2024). Inclusion Complexes between β-Cyclodextrin and Gaseous Substances—N2O, CO2, HCN, NO2, SO2, CH4 and CH3CH2CH3: Role of the Host’s Cavity Hydration. Inorganics, 12(4), 110. https://doi.org/10.3390/inorganics12040110