Catalytic Conversion of Jatropha curcas Oil to Biodiesel Using Mussel Shell-Derived Catalyst: Characterization, Stability, and Comparative Study
Abstract
1. Introduction
2. Results
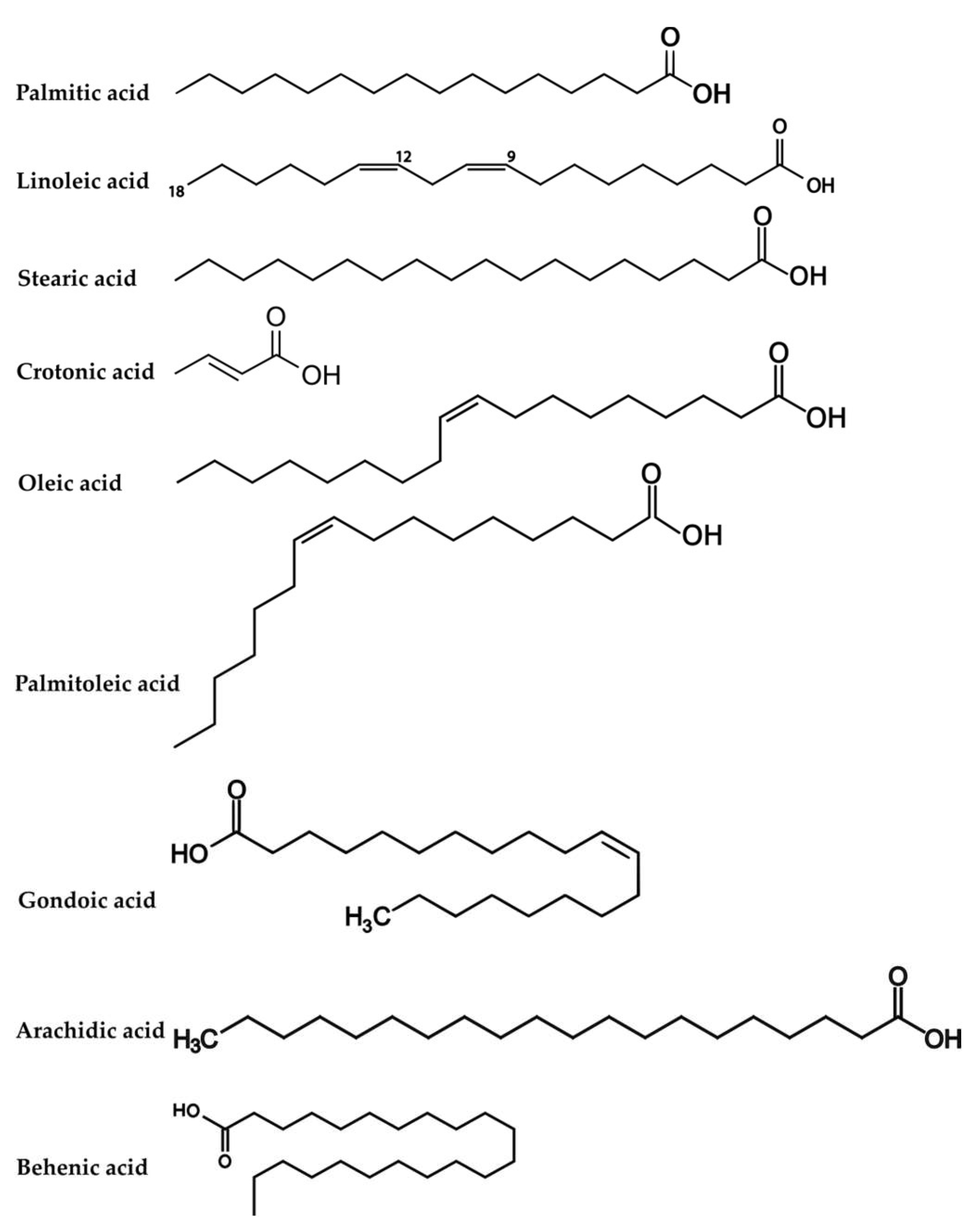
2.1. Determination of Fatty Acid Compositions by XRF
2.2. Composition of Methyl Ester in Biodiesel at Different Reaction Times by GC–MS
2.3. Composition of Methyl Ester in Biodiesel at Different Reaction Temperatures by GC–MS
2.4. Composition of Methyl Ester in Biodiesel at Different Methanol-to-Oil Ratios Using GC–MS
2.5. Composition of Methyl Ester at Different Calcined Catalyst Temperatures by GC–MS
2.6. Compositions of Methyl Ester at Different Calcined Catalyst Concentrations by GC–MS
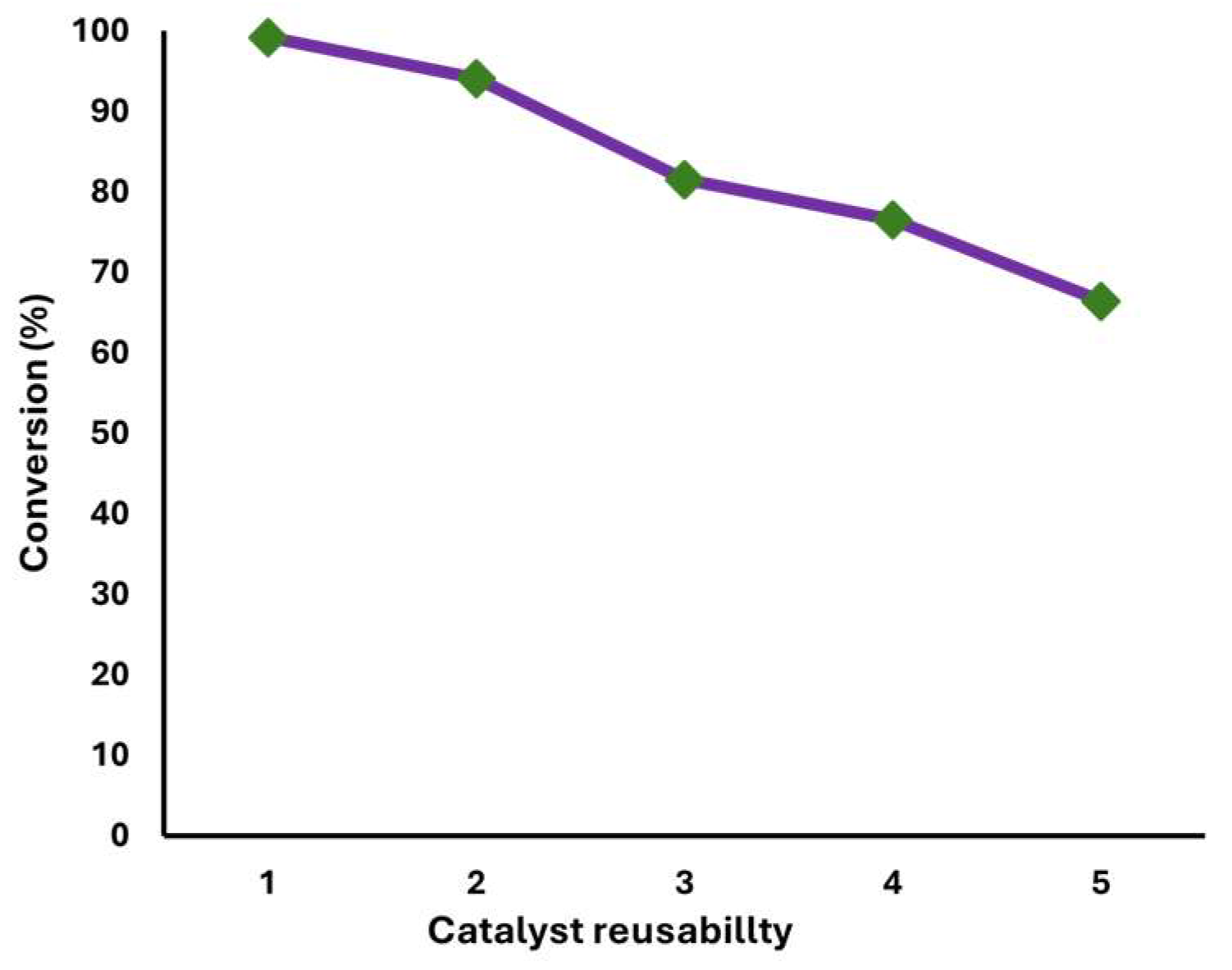
2.7. Catalyst Stability
2.8. Biodiesel Characterization
2.9. Comparison of Homogeneous and Heterogeneous Catalysts
3. Discussion
3.1. Determination of Fatty Acid Composition
3.2. Optimal Reaction Times for J. curcas Oil Biodiesel Manufacturing
3.3. Optimal Reaction Temperature for Biodiesel Production from J. curcas Oil
3.4. Ideal Methanol-to-Oil Ratio for J. curcas Oil Biodiesel Production
3.5. Optimal Calcined Catalyst Temperature for J. curcas Oil Biodiesel
3.6. Optimal Calcined Catalyst Concentration for Biodiesel Production from J. curcas Oil
3.7. Catalyst Stability
3.8. Biodiesel Characterization
3.9. Comparison of Homogeneous and Heterogeneous Catalysts
4. Materials and Methods
4.1. Mussel Shell-Derived CaO Catalyst Preparation and Oil Extraction
4.2. Evaluation of Reaction Parameters
4.3. Determination of Fatty Acid Compositions
4.4. Catalyst Stability
4.5. Comparison of Homogeneous and Heterogeneous Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raven, S.; Ekka, S.B.; Chattree, S.E.; Sadanand, S.S.; Rina, L.; Tiwari, A. Microbial technology for biofuel production. In Bioenergy Research: Evaluating Strategies for Commercialization and Sustainability; John Wiley & Sons Ltd: London, UK, 2021; pp. 19–45. [Google Scholar]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Olivetti, E.; Gülşen, E.; Malca, J.; Castanheira, E.; Freire, F.; Dias, L.; Kirchain, R. Impact of policy on greenhouse gas emissions and economics of biodiesel production. Environ. Sci. Technol. 2014, 48, 7642–7650. [Google Scholar] [CrossRef] [PubMed]
- Bhan, C.; Verma, L.; Singh, J. Alternative fuels for sustainable development. In Environmental Concerns and Sustainable Development: Volume 1: Air, Water and Energy Resources; Springer: Singapore, 2020; pp. 317–331. [Google Scholar]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Kwon, E.E.; Nadagouda, M.N.; Aminabhavi, T.M. Biomass utilization and production of biofuels from carbon neutral materials. Environ. Pollut. 2021, 276, 116731. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.; Pandey, A. Glycerol waste to value added products and its potential applications. Syst. Microbiol. Biomanufacturing 2021, 1, 378–396. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ejikeme, P.; Anyaogu, I.; Ejikeme, C.; Nwafor, N.; Egbuonu, C.; Ukogu, K.; Ibemesi, J. Catalysis in biodiesel production by transesterification processes-an insight. J. Chem. 2010, 7, 1120–1132. [Google Scholar] [CrossRef]
- Gorji, A.; Ghanei, R. A review on catalytic biodiesel production. J. Biodivers. Environ. Sci. 2014, 5, 48–59. [Google Scholar]
- Dagne, H.; Karthikeyan, R.; Feleke, S. Waste to energy: Response surface methodology for optimization of biodiesel production from leather fleshing waste. J. Energy 2019, 2019, 7329269. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.K.; Aziz, A.A.; Sulaiman, N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Sahu, G.; Datta, S.; Saha, S.; Chavan, P.D.; Yadav, D.; Chauhan, V. Efficiency of Catalysts During Biofuel Extraction. In Biofuel Extraction Techniques; Wiley-Scrivener Publishing LLC: Salem, MA, USA, 2023; pp. 441–493. [Google Scholar]
- Zhou, Y.B.; Zhan, Z.P. Conjugated microporous polymers for heterogeneous catalysis. Chem.–Asian J. 2018, 13, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Y.; Han, H. Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production. Biomass Bioenergy 2011, 35, 3627–3635. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental catalysis: Present and future. ChemCatChem. 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.; Khalid, M.; Abdullah, E.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 103219. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Boey, P.-L.; Maniam, G.P.; Abd Hamid, S. Biodiesel production via transesterification of palm olein using waste mud crab (Scylla serrata) shell as a heterogeneous catalyst. Bioresour. Technol. 2009, 100, 6362–6368. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Abd Hamid, S. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ma, X.; Wu, Z.; Cui, P.; Lu, W.; Liu, F.; Chu, H.; Wang, Y. A novel magnetic CaO-based catalyst synthesis and characterization: Enhancing the catalytic activity and stability of CaO for biodiesel production. Chem. Eng. J. 2020, 391, 123549. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, C.; Lv, P.; Yuan, H.; Yao, J. Catalytic applications of calcium rich waste materials for biodiesel: Current state and perspectives. Energy Convers. Manag. 2016, 127, 273–283. [Google Scholar] [CrossRef]
- Ooi, H.K.; Koh, X.N.; Ong, H.C.; Lee, H.V.; Mastuli, M.S.; Taufiq-Yap, Y.H.; Alharthi, F.A.; Alghamdi, A.A.; Asikin Mijan, N. Progress on modified calcium oxide derived waste-shell catalysts for biodiesel production. Catalysts 2021, 11, 194. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohadesi, M.; Moradi, G. Optimization of biodiesel production using waste mussel shell catalyst. Fuel 2013, 109, 534–541. [Google Scholar] [CrossRef]
- Khan, S.G.; Hassan, M.; Anwar, M.; Khan, U.M.; Zhao, C. Mussel shell based CaO nano-catalyst doped with praseodymium to enhance biodiesel production from castor oil. Fuel 2022, 330, 125480. [Google Scholar] [CrossRef]
- Buasri, A.; Lukkanasiri, M.; Nernrimnong, R.; Tonseeya, S.; Rochanakit, K.; Wongvitvichot, W.; Masa-ard, U.; Loryuenyong, V. Rapid transesterification of Jatropha curcas oil to biodiesel using novel catalyst with a microwave heating system. Korean J. Chem. Eng. 2016, 33, 3388–3400. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Lee, H.V.; Lau, P.L. Transesterification of jatropha curcas oil to biodiesel by using short necked clam (Orbicularia orbiculata) shell derived catalyst. Energy Explor. Exploit. 2012, 30, 853–866. [Google Scholar] [CrossRef]
- Alsabi, H.A.; Shafi, M.E.; Almasoudi, S.H.; Mufti, F.A.; Alowaidi, S.A.; Sharawi, S.E.; Alaswad, A.A. From Waste to Catalyst: Transforming Mussel Shells into a Green Solution for Biodiesel Production from Jatropha curcas Oil. Catalysts 2024, 14, 59. [Google Scholar] [CrossRef]
- Lee, H.; Yunus, R.; Juan, J.C.; Taufiq-Yap, Y.H. Process optimization design for jatropha-based biodiesel production using response surface methodology. Fuel Process. Technol. 2011, 92, 2420–2428. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef]
- Suwannasingha, N.; Kantavong, A.; Tunkijjanukij, S.; Aenglong, C.; Liu, H.-B.; Klaypradit, W. Effect of calcination temperature on structure and characteristics of calcium oxide powder derived from marine shell waste. J. Saudi Chem. Soc. 2022, 26, 101441. [Google Scholar] [CrossRef]
- Semwal, S. Process Optimization for Biodiesel Preparation from Vegetable Oils Using Heterogeneous Catalysts; College of Engineering, University of Petroleum & Energy Studies: Haryana, India, 2011. [Google Scholar]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.-H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Sun, H.; Sun, K.; Wang, F.; Liu, Y.; Ding, L.; Xu, W.; Sun, Y.; Jiang, J. Catalytic self-activation of Ca-doped coconut shell for in-situ synthesis of hierarchical porous carbon supported CaO transesterification catalyst. Fuel 2021, 285, 119192. [Google Scholar] [CrossRef]
- Micic, R.D.; Kiralj, M.S.B.; Panic, S.N.; Tomic, M.D.; Jovic, B.D.; Boskovic, G.C. Activation temperature imposed textural and surface synergism of CaO catalyst for sunflower oil transesterification. Fuel 2015, 159, 638–645. [Google Scholar] [CrossRef]
- Esipovich, A.; Danov, S.; Belousov, A.; Rogozhin, A. Improving methods of CaO transesterification activity. J. Mol. Catal. A Chem. 2014, 395, 225–233. [Google Scholar] [CrossRef]
- Kumar, R.; Das, N. Seed oil of Jatropha curcas L. germplasm: Analysis of oil quality and fatty acid composition. Ind. Crops Prod. 2018, 124, 663–668. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- Likozar, B.; Pohar, A.; Levec, J. Transesterification of oil to biodiesel in a continuous tubular reactor with static mixers: Modelling reaction kinetics, mass transfer, scale-up and optimization considering fatty acid composition. Fuel Process. Technol. 2016, 142, 326–336. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Wu, T. The Impact of Long Chain Unsaturated Fatty Acids on LDL Oxidative Susceptibility and Oxidized LDL-Induced Cell Death; The University of North Carolina at Greensboro: Greensboro, NC, USA, 2001. [Google Scholar]
- Sari, E. Green Diesel Production Via Catalytic Hydrogenation/Decarboxylation of Triglycerides and Fatty Acids of Vegetable Oil and Brown Grease; Wayne State University: Detroit, MI, USA, 2013. [Google Scholar]
- Haile, M. Biofuel Energy: Spent Coffee Grounds Biodiesel, Bioethanol and Solid Fuel; Anchor Academic Publishing: Hamburg Germany, 2015. [Google Scholar]
- Alsaiari, M.; Bokhari, A.; Chuah, L.F.; Mubashir, M.; Harraz, F.A.; Almohana, A.I.; Show, P.L.; Awasthi, M.K.; Rizk, M.A. Synthesis of methyl esters from Hippophae rhamnoides via pilot scale hydrodynamic cavitation intensification reactor. Renew. Energy 2023, 205, 238–247. [Google Scholar] [CrossRef]
- Gopinath, A.; Sairam, K.; Velraj, R.; Kumaresan, G. Effects of the properties and the structural configurations of fatty acid methyl esters on the properties of biodiesel fuel: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2015, 229, 357–390. [Google Scholar] [CrossRef]
- Dunn, R. Effects of high-melting methyl esters on crystallization properties of fatty acid methyl ester mixtures. Trans. ASABE 2012, 55, 637–646. [Google Scholar] [CrossRef]
- DeLong, J.P.; Gibert, J.P.; Luhring, T.M.; Bachman, G.; Reed, B.; Neyer, A.; Montooth, K. The combined effects of reactant kinetics and enzyme stability explain the temperature dependence of metabolic rates. Ecol. Evol. 2017, 7, 3940–3950. [Google Scholar] [CrossRef]
- Izquierdo, N.; Aguirrezábal, L. Genetic variability in the response of fatty acid composition to minimum night temperature during grain filling in sunflower. Field Crops Res. 2008, 106, 116–125. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Biodiesel production from waste cooking oil via supercritical methanol: Optimisation and reactor simulation. Renew. Energy 2018, 124, 144–154. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sawangkeaw, R.; Ngamprasertsith, S. Response surface methodology for the optimization of biofuel production at a low molar ratio of supercritical methanol to used palm olein oil. Asia-Pac. J. Chem. Eng. 2016, 11, 539–548. [Google Scholar] [CrossRef]
- Mujtaba, M.; Cho, H.M.; Masjuki, H.; Kalam, M.; Ong, H.; Gul, M.; Harith, M.; Yusoff, M. Critical review on sesame seed oil and its methyl ester on cold flow and oxidation stability. Energy Rep. 2020, 6, 40–54. [Google Scholar] [CrossRef]
- Encinar, J.; Pardal, A.; Martínez, G. Transesterification of rapeseed oil in subcritical methanol conditions. Fuel Process. Technol. 2012, 94, 40–46. [Google Scholar] [CrossRef]
- De Boer, K.; Bahri, P.A. Supercritical methanol for fatty acid methyl ester production: A review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar] [CrossRef]
- Takase, M. A Critical Review of Croton as a Multipurpose Nonedible Tree Plant for Biodiesel Production towards Feedstock Diversification for Sustainable Energy. Adv. Agric. 2022, 2022, 5895160. [Google Scholar] [CrossRef]
- Satya, S.; Kolakoti, A.; Rao, R. Optimization of palm methyl ester and its effect on fatty acid compositions and cetane number. Math. Models Eng. 2019, 5, 25–34. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Krzemińska, I.; Szymańska, M.; Ciempiel, W.; Piasecka, A. Auxin supplementation under nitrogen limitation enhanced oleic acid and MUFA content in Eustigmatos calaminaris biomass with potential for biodiesel production. Sci. Rep. 2023, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Negm, N.A.; Abou Kana, M.T.; Youssif, M.A.; Mohamed, M.Y. 13 Biofuels from Vegetable Oils as Alternative Fuels. Surfactants Tribol. Vol. 5 2017, 5, 289. [Google Scholar]
- Likozar, B.; Levec, J. Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride transesterification to biodiesel: Experimental and modeling based on fatty acid composition. Fuel Process. Technol. 2014, 122, 30–41. [Google Scholar] [CrossRef]
- Csernica, S.N.; Hsu, J.T. The phase behavior effect on the kinetics of transesterification reactions for biodiesel production. Ind. Eng. Chem. Res. 2012, 51, 6340–6349. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crops Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Madhu, D.; Chavan, S.B.; Singh, V.; Singh, B.; Sharma, Y.C. An economically viable synthesis of biodiesel from a crude Millettia pinnata oil of Jharkhand, India as feedstock and crab shell derived catalyst. Bioresour. Technol. 2016, 214, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kharas, K.C.; Croley, B.J.; Datye, A.K. The sintering of supported Pd automotive catalysts. ChemCatChem 2011, 3, 1004–1014. [Google Scholar] [CrossRef]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ Transmission Electron Microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhao, H.; Tong, D.S.; Wu, L.M.; Yu, W.H. Recent advances in catalytic conversion of glycerol. Catal. Rev. 2013, 55, 369–453. [Google Scholar] [CrossRef]
- Houache, M.S.; Hughes, K.; Baranova, E.A. Study on catalyst selection for electrochemical valorization of glycerol. Sustain. Energy Fuels 2019, 3, 1892–1915. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J.D. Future challenges in heterogeneous catalysis: Understanding catalysts under dynamic reaction conditions. ChemCatChem 2017, 9, 17–29. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-s. Purification to remove leached CaO catalyst from biodiesel with the help of cation-exchange resin. Fuel 2013, 105, 318–324. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Chen, G.; Shan, R.; Ma, W.; Liu, C. The utilization of hydroxyapatite-supported CaO-CeO2 catalyst for biodiesel production. Energy Convers. Manag. 2016, 130, 156–164. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.; Lee, H.; Hussein, M.; Yunus, R. Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenergy 2011, 35, 827–834. [Google Scholar] [CrossRef]
- Kouzu, M.; Tsunomori, M.; Yamanaka, S.; Hidaka, J. Solid base catalysis of calcium oxide for a reaction to convert vegetable oil into biodiesel. Adv. Powder Technol. 2010, 21, 488–494. [Google Scholar] [CrossRef]
- de Sousa, F.P.; dos Reis, G.P.; Cardoso, C.C.; Mussel, W.N.; Pasa, V.M. Performance of CaO from different sources as a catalyst precursor in soybean oil transesterification: Kinetics and leaching evaluation. J. Environ. Chem. Eng. 2016, 4, 1970–1977. [Google Scholar] [CrossRef]
- Yusuff, A.S. Parametric optimization of solvent extraction of Jatropha curcas seed oil using design of experiment and its quality characterization. South Afr. J. Chem. Eng. 2021, 35, 60–68. [Google Scholar] [CrossRef]
- Sitorus, H.B.H.; Setiabudy, R.; Bismo, S.; Beroual, A. Jatropha curcas methyl ester oil obtaining as vegetable insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2021–2028. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Liaquat, A.M.; Shahabuddin, M.; Varman, M. Prospects of biodiesel from Jatropha in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 5007–5020. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Fattah, I.M.R.; Maitra, S.; Mahlia, T.M.I. A ranking scheme for biodiesel underpinned by critical physicochemical properties. Energy Convers. Manag. 2021, 229, 113742. [Google Scholar] [CrossRef]
- Sudsakorn, K.; Saiwuttikul, S.; Palitsakun, S.; Seubsai, A.; Limtrakul, J. Biodiesel production from Jatropha Curcas oil using strontium-doped CaO/MgO catalyst. J. Environ. Chem. Eng. 2017, 5, 2845–2852. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Mumtaz, M.W.; Mukhtar, H.; Najeeb, J.; Irfan, A.; Akram, S.; Touqeer, T.; Nabi, G. Lipase-PDA-TiO2 NPs: An emphatic nano-biocatalyst for optimized biodiesel production from Jatropha curcas oil. Renew. Energy 2021, 169, 1026–1037. [Google Scholar] [CrossRef]
- Adepoju, T.F. Optimization processes of biodiesel production from pig and neem (Azadirachta indica a.Juss) seeds blend oil using alternative catalysts from waste biomass. Ind. Crops Prod. 2020, 149, 112334. [Google Scholar] [CrossRef]
- Ayetor, G.K.; Sunnu, A.; Parbey, J. Performance evaluation of biodiesel-biodiesel blends in a dedicated CIDI engine. Int. J. Renew. Energy Res. 2015, 5, 168–176. [Google Scholar]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Biodiesel production from palm oil using hydrated lime-derived CaO as a low-cost basic heterogeneous catalyst. Energy Convers. Manag. 2016, 108, 459–467. [Google Scholar] [CrossRef]
- Mendow, G.; Veizaga, N.; Querini, C. Ethyl ester production by homogeneous alkaline transesterification: Influence of the catalyst. Bioresour. Technol. 2011, 102, 6385–6391. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. Parametric study of the alkali catalyzed transesterification of waste frying oil for Biodiesel production. Energy Convers. Manag. 2014, 79, 246–254. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy 2019, 136, 837–845. [Google Scholar] [CrossRef]
- Jamil, F.; Kumar, P.S.M.; Al-Haj, L.; Myint, M.T.Z.; Ala’a, H. Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Convers. Manag. X 2021, 10, 100047. [Google Scholar] [CrossRef]
- Das, V.; Tripathi, A.M.; Borah, M.J.; Dunford, N.T.; Deka, D. Cobalt-doped CaO catalyst synthesized and applied for algal biodiesel production. Renew. Energy 2020, 161, 1110–1119. [Google Scholar] [CrossRef]
- Todorović, Z.B.; Troter, D.Z.; Đokić-Stojanović, D.R.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S.; Veselinović, L.M.; Veljković, V.B. Optimization of CaO-catalyzed sunflower oil methanolysis with crude biodiesel as a cosolvent. Fuel 2019, 237, 903–910. [Google Scholar] [CrossRef]
- Lee, S.L.; Wong, Y.C.; Tan, Y.P.; Yew, S.Y. Transesterification of palm oil to biodiesel by using waste obtuse horn shell-derived CaO catalyst. Energy Convers. Manag. 2015, 93, 282–288. [Google Scholar] [CrossRef]
- Mohamad, M.; Ngadi, N.; Lani, N.S. Production of Biodiesel from Cooking Oil Using CaO Catalyst. Adv. Mater. Res. 2015, 1113, 518–522. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013, 460923. [Google Scholar] [CrossRef]
- Mohammed, N.I.; Kabbashi, N.A.; Alam, M.Z.; Mirghani, M.E.S. Esterification of Jatropha curcas hydrolysate using powdered niobic acid catalyst. J. Taiwan Inst. Chem. Eng. 2016, 63, 243–249. [Google Scholar] [CrossRef]
| Calcination Temperature * | CaO% | Fe2O3% | SrO% |
|---|---|---|---|
| 800 °C | 98.67 ± 0.08 | 0.06 ± 0.02 | 1.27 ± 0.05 |
| 900 °C | 98.80 ± 0.07 | 0.19 ± 0.04 | 1.01 ± 0.02 |
| 1000 °C | 98.82 ± 0.04 | 0.02 ± 0.01 | 1.17 ± 0.04 |
| 1100 °C | 98.85 ± 0.06 | 0.01 ± 0.01 | 1.14 ± 0.02 |
| Components Methyl Ester * | Concentration (%) | |||
|---|---|---|---|---|
| 3 h | 4 h | 5 h | 6 h | |
| Palmitoleic ME | – | – | – | 0.53 ± 0.02 |
| Palmitic Acid ME | 6.77 ± 0.14 | 7.44 ± 0.03 | 7.51 ± 0.05 | 10.72 ± 1.82 |
| Linoleic Acid ME | 14.69 ± 0.34 | 16.28 ± 0.23 | 15.95 ± 0.72 | 21.55 ± 2.95 |
| Oleic Acid ME | 15.53 ± 0.52 | 18.47 ± 0.61 | 16.81 ± 0.62 | 23.03 ± 3.25 |
| Stearic acid ME | 3.89 ± 0.02 | 4.33 ± 0.02 | 4.50 ± 0.15 | 6.16 ± 0.90 |
| Components Methyl Ester * | Concentration (%) | |||
|---|---|---|---|---|
| 90 °C | 100 °C | 110 °C | 120 °C | |
| Palmitoleic ME | – | – | 0.53 ± 0.03 | – |
| Palmitic Acid ME | 10.67 ± 0.90 | 9.42 ± 0.80 | 10.72 ± 1.30 | 7.31 ± 0.50 |
| Linoleic Acid ME | 12.39 ± 1.10 | 12.15 ± 1.30 | 21.55 ± 2.50 | 9.04 ± 0.90 |
| Oleic Acid ME | 25.93 ± 1.50 | 23.99 ± 2.15 | 23.03 ± 2.70 | 13.52 ± 1.30 |
| Stearic acid ME | 5.12 ± 0.5 | 4.88 ± 0.75 | 6.16 ± 0.80 | 4.034 ± 0.50 |
| Components Methyl Ester * | Concentration (%) | |||
|---|---|---|---|---|
| 12:1 | 15:1 | 18:1 | 21:1 | |
| Palmitoleic ME | – | 1.00 ± 0.02 | 0.97 ± 0.01 | – |
| Palmitic Acid ME | 10.67 ± 0.90 | 12.90 ± 1.30 | 16.12 ± 2.20 | 8.65 ± 0.80 |
| Linoleic Acid ME | 12.39 ± 1.50 | 33.25 ± 3.10 | 32.36 ± 3.30 | 19.22 ± 2.30 |
| Oleic Acid ME | 25.93 ± 2.30 | 34.80 ± 3.50 | 37.69 ± 3.50 | 27.32 ± 3.70 |
| Stearic acid ME | 5.12 ± 0.70 | 8.50 ± 0.95 | 8.281 ± 1.20 | 4.29 ± 0.30 |
| Crotonic acid ME | – | – | 0.11 ± 0.01 | – |
| Components Methyl Ester * | Concentration (%) | |||
|---|---|---|---|---|
| 800 °C | 900 °C | 1000 °C | 1100 °C | |
| Palmitoleic ME | 0.94 ± 0.02 | 0.97 ± 0.03 | – | – |
| Palmitic Acid ME | 18.65 ± 2.40 | 16.12 ± 1.50 | 16.14 ± 1.60 | 13.67 ± 1.50 |
| Linoleic Acid ME | 32.06 ± 3.30 | 32.36 ± 2.30 | 32.39 ± 3.50 | 28.33 ± 2.50 |
| Oleic Acid ME | 35.76 ± 3.50 | 37.69 ± 3.30 | 36.97 ± 3.20 | 36.66 ± 3.90 |
| Stearic acid ME | 8.06 ± 0.90 | 8.281 ± 0.90 | 8.28 ± 0.90 | 7.21 ± 1.10 |
| Crotonic acid ME | – | 0.11 ± 0.01 | – | – |
| Components Methyl Ester * | Concentration (%) | |||
|---|---|---|---|---|
| 3 wt% | 6 wt% | 9 wt% | 12 wt% | |
| Palmitoleic ME | – | 0.97 ± 0.02 | – | – |
| Palmitic Acid ME | 8.96 ± 0.60 | 16.12 ± 1.60 | 8.94 ± 0.60 | 9.56 ± 0.80 |
| Linoleic Acid ME | 19.49 ± 1.30 | 32.36 ± 3.30 | 19.84 ± 2.30 | 21.91 ± 2.30 |
| Oleic Acid ME | 25.35 ± 2.20 | 37.69 ± 3.20 | 23.02 ± 2.50 | 24.09 ± 2.90 |
| Stearic acid ME | 4.80 ± 0.70 | 8.281 ± 0.80 | 4.69 ± 0.70 | 4.79 ± 0.50 |
| Crotonic acid ME | – | 0.11 ± 0.01 | – | – |
| Contents | Unit | ASTM D-6751 | EN 14214 | Prepared Biodiesel |
|---|---|---|---|---|
| Viscosity | mm2/s | 1.9–6 | 3.5–5 | 4.89 |
| Flash point | °C | >120 | >120 | 110 |
| Cloud point | °C | –3 to 15 | –––– | 0 |
| Pour point | pp | –5 to 10 | –––– | –3 |
| Cetane number | 47–65 | 51 to 120 | 50.5 | |
| Density at 15 °C | g /cm3 | 0.82–0.9 | 0.86–0.9 | 0.856 |
| Calorific value | MJ/kg | ––– | 32.9 | 38.140 |
| Distillation profile Initial boiling point | Volume | Temp. °C | ||
| 10 mL | 98 | |||
| 20 mL | 106 | |||
| 30 mL | 154 | |||
| 40 mL | 172 | |||
| 50 mL | 191 | |||
| 60 mL | 228 | |||
| 70 mL | 268 | |||
| 80 mL | 296 | |||
| 90 mL | 309 | |||
| Residue % | 7 | |||
| Loss % | 1 |
| Components Methyl Ester * | Concentration of JCME (%) | |
|---|---|---|
| (CaO) Mussel Shell | NaOH | |
| Palmitic acid ME | 16.12 | – |
| Linoleic acid ME | 32.36 | – |
| Stearic acid ME | 8.28 | – |
| Crotonic acid ME | 0.11 | – |
| Oleic acid ME | 37.69 | 86.32 |
| Palmitoleic ME | 0.97 | 9.31 |
| Gondoic acid ME | – | 0.75 |
| Arachidic acid ME | – | 1.75 |
| Behenic acid ME | – | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafi, M.E.; Alsabi, H.A.; Almasoudi, S.H.; Mufti, F.A.M.; Alowaidi, S.A.; Alaswad, A.A. Catalytic Conversion of Jatropha curcas Oil to Biodiesel Using Mussel Shell-Derived Catalyst: Characterization, Stability, and Comparative Study. Inorganics 2024, 12, 109. https://doi.org/10.3390/inorganics12040109
Shafi ME, Alsabi HA, Almasoudi SH, Mufti FAM, Alowaidi SA, Alaswad AA. Catalytic Conversion of Jatropha curcas Oil to Biodiesel Using Mussel Shell-Derived Catalyst: Characterization, Stability, and Comparative Study. Inorganics. 2024; 12(4):109. https://doi.org/10.3390/inorganics12040109
Chicago/Turabian StyleShafi, Manal E., Halimah A. Alsabi, Suad H. Almasoudi, Faten A. M. Mufti, Safaa A. Alowaidi, and Alaa A. Alaswad. 2024. "Catalytic Conversion of Jatropha curcas Oil to Biodiesel Using Mussel Shell-Derived Catalyst: Characterization, Stability, and Comparative Study" Inorganics 12, no. 4: 109. https://doi.org/10.3390/inorganics12040109
APA StyleShafi, M. E., Alsabi, H. A., Almasoudi, S. H., Mufti, F. A. M., Alowaidi, S. A., & Alaswad, A. A. (2024). Catalytic Conversion of Jatropha curcas Oil to Biodiesel Using Mussel Shell-Derived Catalyst: Characterization, Stability, and Comparative Study. Inorganics, 12(4), 109. https://doi.org/10.3390/inorganics12040109






