Synthesis and Characterization of Broccoli-like Ag/Cu2O Nanostructures on ZnO Nanowires Using the Plasma–Liquid Interaction Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Decorating Ag NPs and Cu2O NPs on ZnO NWs via the Plasma–Liquid Interaction Method
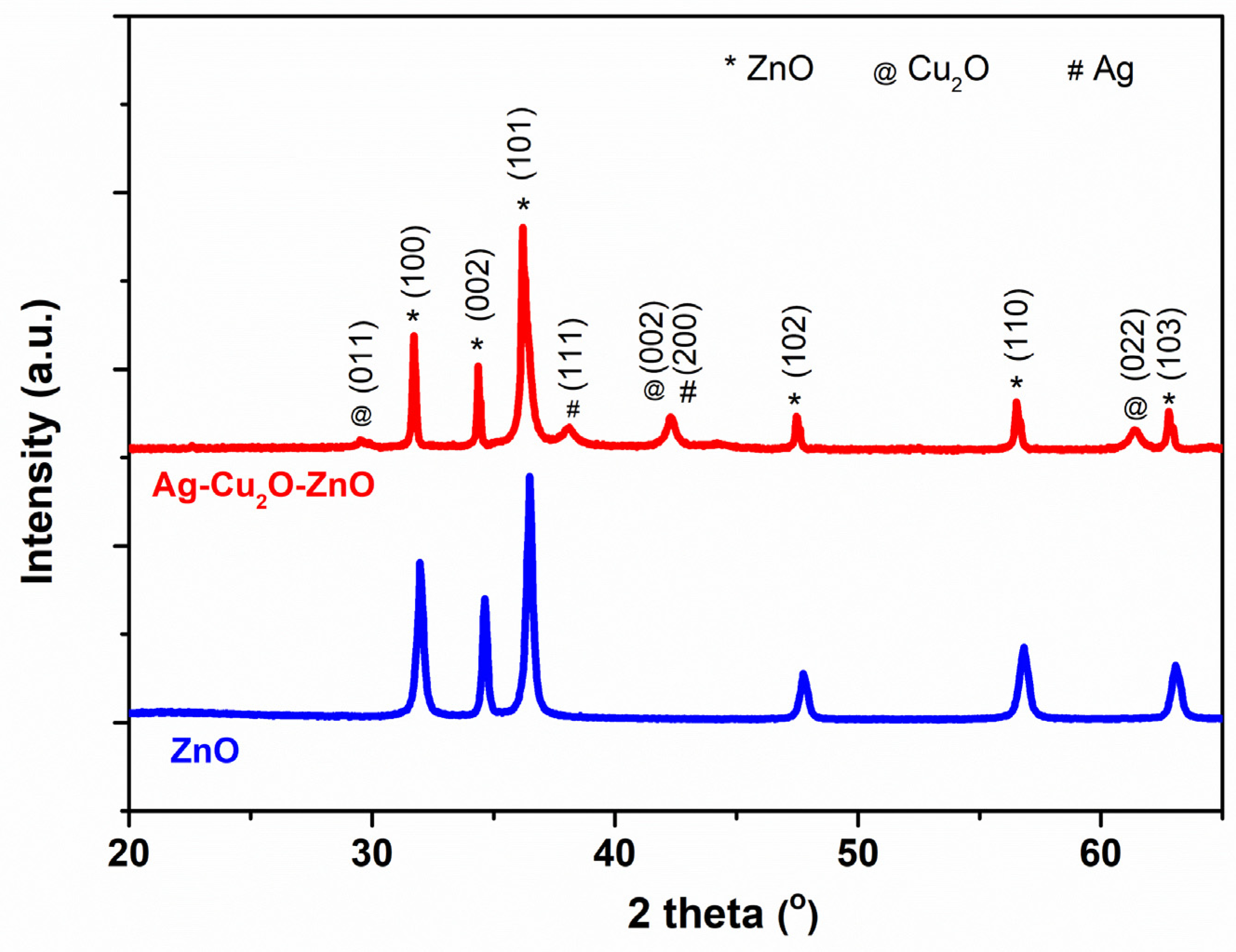
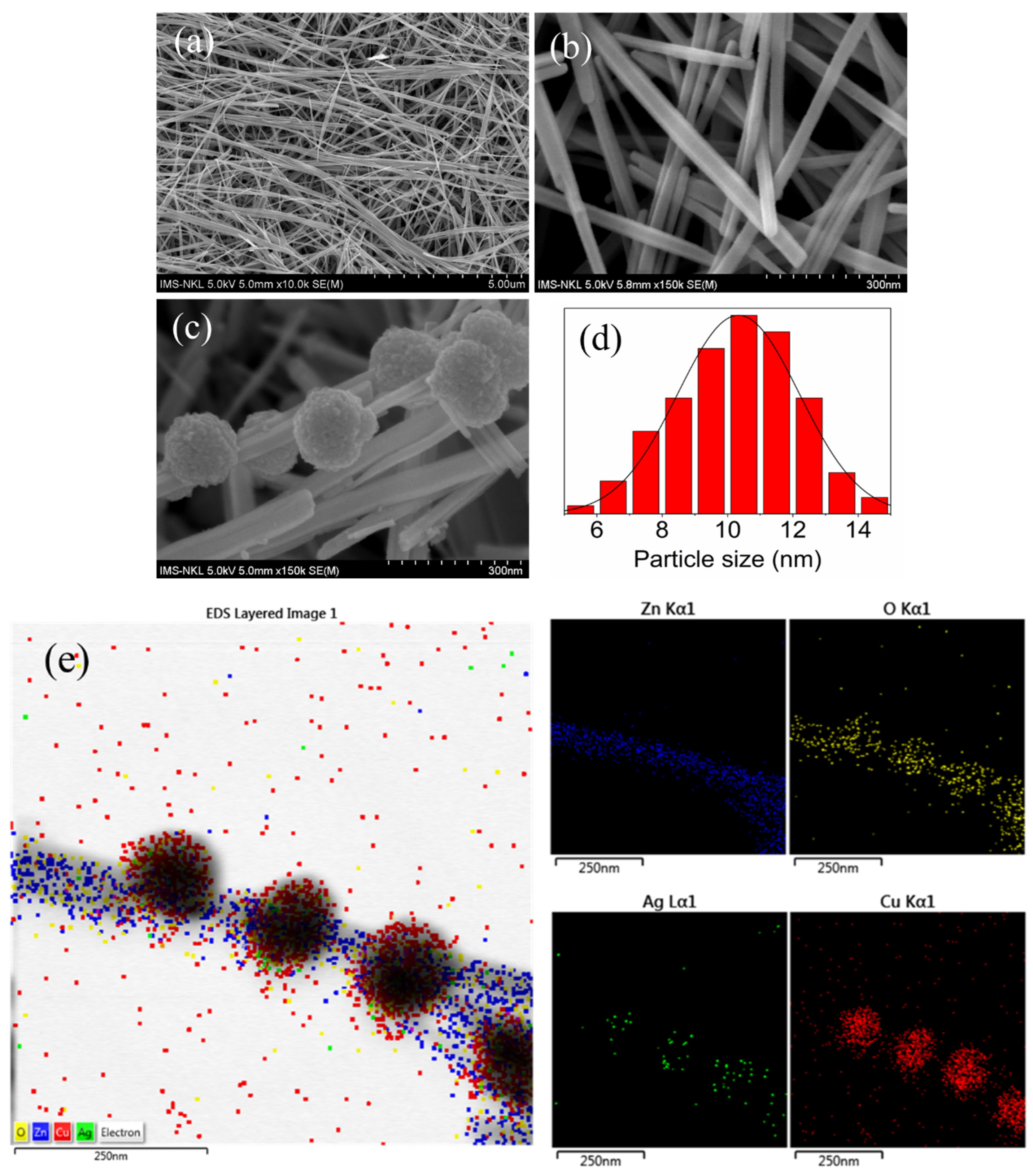
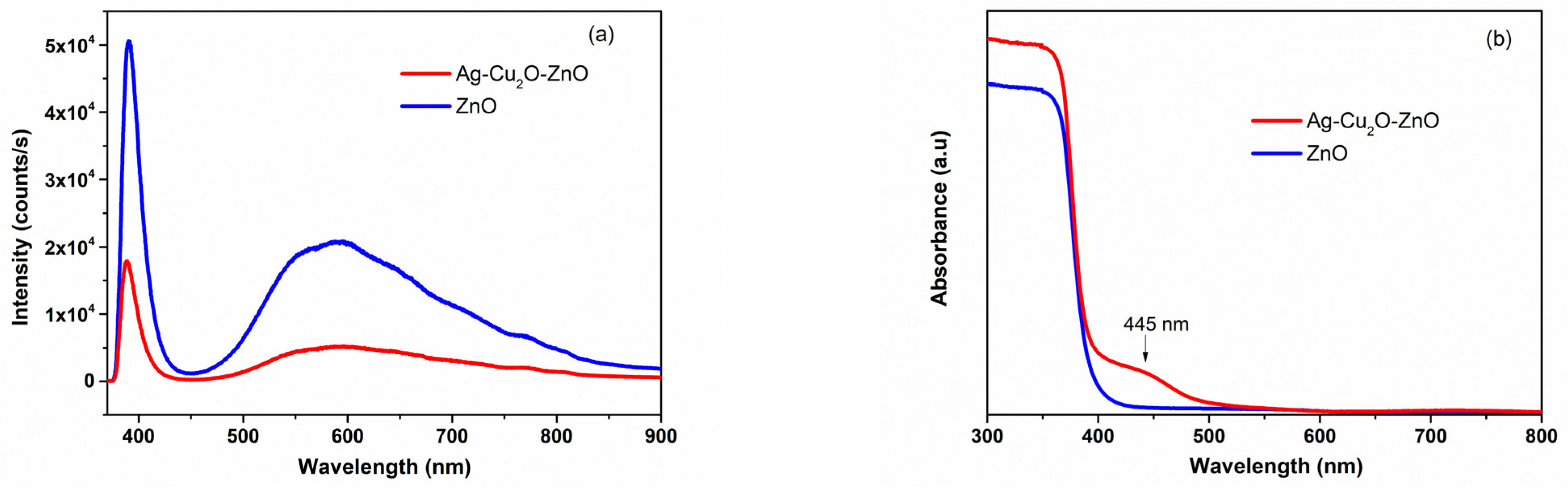
2.2. Structural and Morphological Characteristics
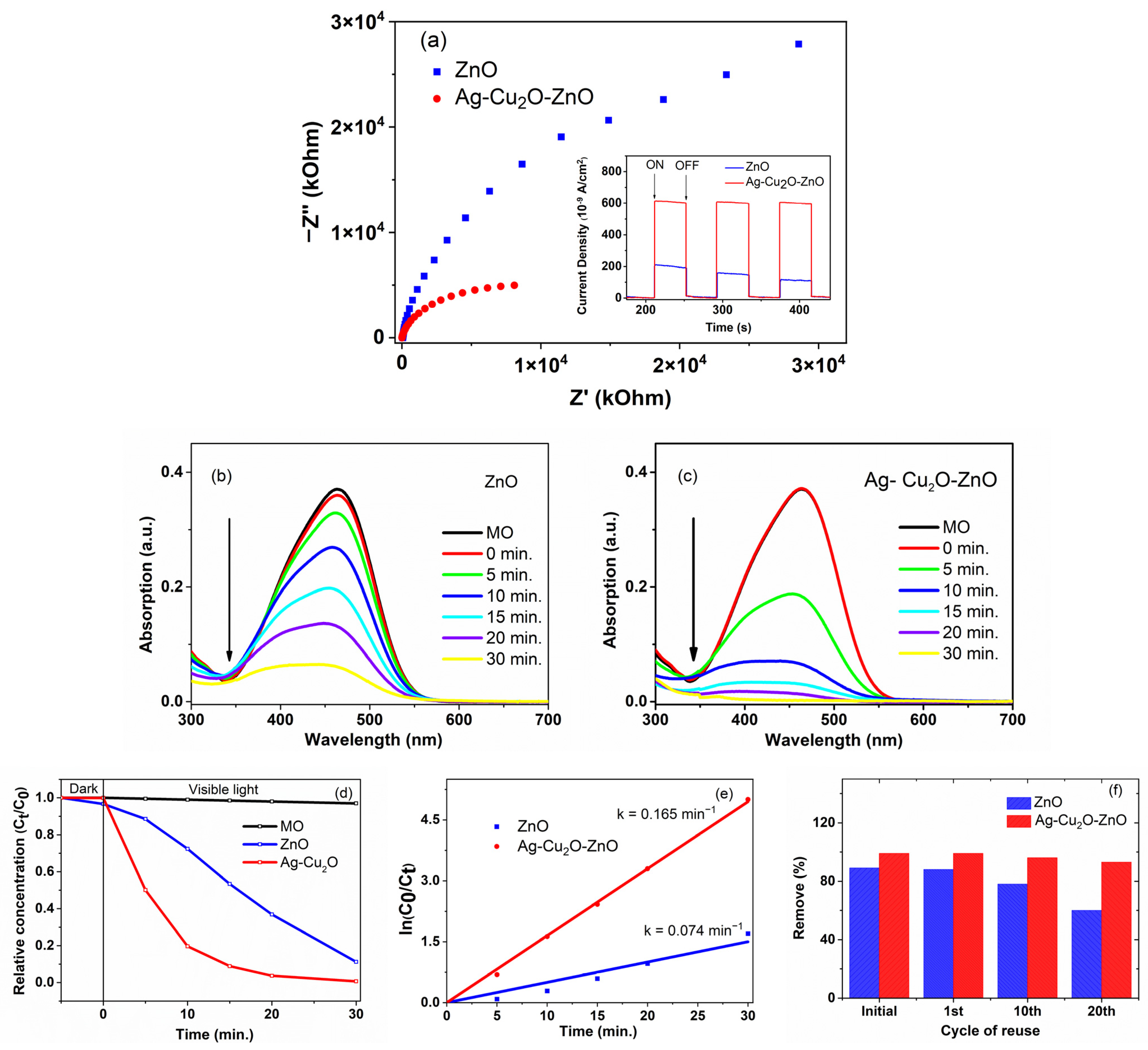
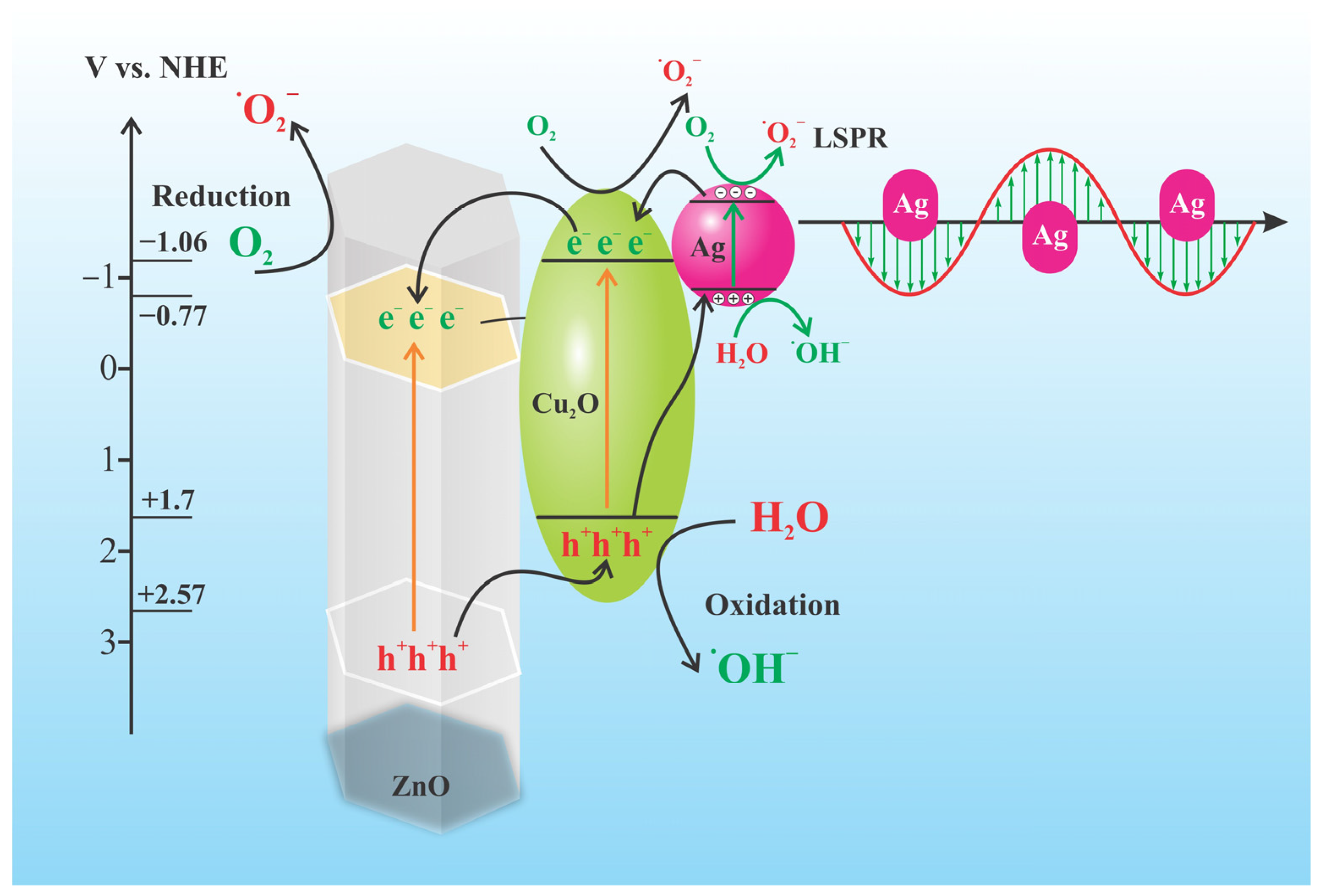
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Synthesis of ZnO Nanowires
3.2. Decoration of Silver and Copper(I) Oxide on ZnO NWs Using the Plasma–Liquid Interaction Technique
- Preparation of two precursor solutions:
- Setting up the plasma–liquid system:
- The as-prepared ZnO NW powder was uniformly dispersed in distilled water within a 50 mL beaker using ultrasonic equipment.
- Plasma was ignited for three minutes.
- To form the ternary structure of Ag-Cu2O-ZnO NWs, 5 mL of Cu-precursor solution was added, followed by five minutes of plasma treatment. Next, 80 μL of 50 mM Ag-precursor solution was added, and the plasma ignition process was continued for an additional five minutes.
- The Ag-Cu2O-ZnO powder was isolated using centrifugation. These samples were dried in a vacuum oven for 24 h at 50 °C and stored in sealed vessels.
3.3. Sample Characterization
3.4. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A review on Cu2O-based composites in photocatalysis: Synthesis, modification and applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, M.H. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 2010, 5, 106–116. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef]
- Kandjani, A.E.; Sabri, Y.M.; Periasamy, S.R.; Zohora, N.; Amin, M.H.; Nafady, A.; Bhargava, S.K. Controlling core/shell formation of nanocubic p-Cu2O/n-ZnO Toward Enhanced Photocatalytic Performance. Langmuir 2015, 31, 10922–10930. [Google Scholar] [CrossRef]
- Chen, X.; Lin, P.; Yan, X.; Bai, Z.; Yuan, H.; Shen, Y.; Liu, Y.; Zhang, G.; Zhang, Z.; Zhang, Y. Three-dimensional ordered ZnO/Cu2O nanoheterojunctions for efficient metal–oxide solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3216–3223. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, J.W.; Sung, Y.M. Enhanced photoelectrochemical properties of Zscheme ZnO/p-n Cu2O PV-PEC cells. J. Alloys Compd. 2019, 771, 869–876. [Google Scholar] [CrossRef]
- Jiang, T.; Xie, T.; Chen, L.; Fu, Z.; Wang, D. Carrier concentration-dependent electron transfer in Cu2O/ZnO nanorod arrays and their photocatalytic performance. Nanoscale 2013, 5, 2938–2944. [Google Scholar] [CrossRef]
- Zou, X.; Tian, H.; Yan, S. Syntheis of Cu2O/ZnO hetero-nanorod arrays with enhanced visible light-driven photocatalytic activity. Cryst. Eng. Comm 2014, 16, 1149–1156. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zhang, Y.-D.; Wang, Q.-C.; Dong, B.; Wang, Y.-J.; Feng, W. Photocatalytic activity of Cu2O/ZnO nanocomposite for the decomposition of methyl orange under visible light irreadiation. Sci. Eng. Compos. Mater. 2019, 26, 104–113. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Duan, X.; Ng, D.H.L.; Tang, H.; Yang, Y.; Kong, M.; Wu, Z.; Cai, W.; Wang, G. Ag nanoparticle decorated nanoporous ZnO microrods and their enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2012, 4, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In situ charge transfer at the Ag@ZnO photoelectrochemical interface toward the high photocatalytic performance of H2 evolution and RhB degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Thinh, V.D.; Lam, V.D.; Bach, T.N.; Van, N.D.; Manh, D.H.; Tung, D.H.; Lien, N.T.H.; Thuy, U.T.D.; Anh, T.X.; Tung, N.T.; et al. Enhanced optical and photocatalytic properties of Au/Ag nanoparticle-decorated ZnO films. J. Electron. Mater. 2020, 49, 2626–2632. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carrier and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.D.; Zimbone, M.; Scuderi, M.; Nicotra, G.; Fragalà, M.E.; Impellizzeri, G. Effect of Pt nanoparticles on the photocatalytic activity of ZnO nanofibers. Nanoscale Res. Lett. 2015, 10, 484. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Z.; Kang, L.; Li, M.; Gao, Z. The facile preparation of Ag decorated TiO2/ZnO nanotube and their potent photocatalytic degradation efficiency. RSC Adv. 2017, 7, 50064–50071. [Google Scholar] [CrossRef]
- Mulkhopadhyay, S.; Maiti, D.; Chatterjee, S.; Devi, P.S.; Kumar, G.S. Design and application of Au decorated ZnO/TiO2 as a stable photocatalyst for wide spectral coverage. Phys. Chem. Chem. Phys. 2016, 18, 31622–31633. [Google Scholar] [CrossRef]
- Yin, H.; Yu, K.; Song, C.; Huang, R.; Zhu, Z. Synthesis of Au-decorated V2O5@ZnO heteronanostructures and enhanced plasmonic photocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 14851–14860. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Yeh, S.-M.; Chen, C.-H.; Lin, H.-N. Flexible photocatalytic paper with Cu2O and Ag nanoparticle-decorated ZnO nanorods for visible light photodegradation of organic dye. Nanoscale Res. Lett. 2019, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Wang, B.; Zhang, H.; Ding, P.; Wang, Q. Sandwiched ZnO@Au@Cu2O Nanorod Films as Efficient Visible-Light-Driven Plasmonic Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. ZnS–Ag–ZnO as an excellent UV-light-active photocatalyst for the degradation of AV 7, AB 1, RR 120, and RY 84 dyes: Synthesis, characterization, and catalytic applications. Ind. Eng. Chem. Res. 2014, 53, 12953–12963. [Google Scholar] [CrossRef]
- Gavade, N.L.; Babar, S.B.; Kadam, A.N.; Gophane, A.; Garadka, K.M. Fabrication of M@CuxO/ZnO (M = Ag, Au) heterostructured nanocomposite with enhanced photocatalytic performance under sunlight. Ind. Eng. Chem. Res. 2017, 56, 14489–14501. [Google Scholar] [CrossRef]
- Yuan, X.; Pei, F.; Luo, X.; Hu, H.; Qian, H.; Wen, P.; Miao, K.; Wang, W.; Feng, G. Fabrication of ZnO/Au@Cu2O heterojunction towards deeply oxidative photodegradation of organic dyes. Sep. Purif. Technol. 2021, 262, 118301. [Google Scholar] [CrossRef]
- Thu, P.T.; Thinh, V.D.; Lam, V.D.; Bach, T.N.; Phong, L.T.H.; Tung, D.H.; Manh, D.H.; Van Khien, N.; Anh, T.X.; Le, N.T.H. Decorating of Ag and CuO on ZnO nanowires by plasma electrolyte oxidation method for enhanced photocatalytic efficiency. Catalysts 2022, 12, 801. [Google Scholar] [CrossRef]
- Prete, P.; Lovergine, N.; Tapfer, L. Nanostructure size evolution during Au-catalysed growth by carbo-thermal evaporation of well-aligned ZnO nanowires on (100)Si. Appl. Phys. A 2007, 88, 21–26. [Google Scholar] [CrossRef]
- Yatsui, T.; Lim, J.; Nakamata, T.; Kitamura, K.; Ohtsu, M.; Yi, G.-C. Low-temperature (∼270 °C) growth of vertically aligned ZnO nanorods using photoinduced metal organic vapour phase epitaxy. Nanotechnology 2007, 18, 065606. [Google Scholar] [CrossRef]
- Liang, H.W.; Lu, Y.M.; Shen, D.Z.; Li, B.H.; Zhang, Z.Z.; Shan, C.X.; Zhang, J.Y.; Fan, X.W.; Du, G.T. Growth of vertically aligned single crystal ZnO nanotubes by plasma-molecular beam epitaxy. Solid State Commun. 2006, 137, 182–186. [Google Scholar] [CrossRef]
- Fufa, P.A.; Feysia, G.B.; Gultom, N.S.; Kuo, D.-H.; Chen, X.; Kabtamu, D.M.; Zelekew, O.A. Visible light-driven photocata-lytic activity of Cu2O/ZnO/Kaolinite-based composite catalyst for the degradation of organic pollutant. Nanotechnology 2022, 33, 315601. [Google Scholar] [CrossRef] [PubMed]
- Shubha, J.P.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Khan, A. Facile fabrication of a ZnO/Eu2O3/NiO-based ternary heterostruc-ture nanophotocatalyst and its application for the degradation of methylene Blue. ACS Omega 2021, 6, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Do, H.T.; Kim, J.H. Microplasma-assisted synthesis of TiO2–Au hybrid nanoparticles and their photocatalytic mechanism for degradation of methylene blue dye under ultraviolet and visible light irradiation. Appl. Surf. Sci. 2022, 573, 151383. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Tung, D.H.; Manh, L.H.; Kim, J.H.; Kudryashov, S.I.; Minh, P.H.; Hien, N.T. Plasma enhanced wet chemical sur-face sctivation of TiO2 for the synthesis of high-performance photocatalytic Au/TiO2 nanocomposites. Appl. Sci. 2020, 10, 3345. [Google Scholar] [CrossRef]
- Fatemeh, R.; Patrick, V.; Anton, N.; Rino, M.; Nathalie, D.G. Applications of plasma-liquid systems: A review. Materials 2019, 12, 2751. [Google Scholar]
- Qiang, C.; Junshuai, L.; Yongfeng, L. A review of plasma–liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar]
- Hu, H.; Huang, X.; Deng, C.; Chen, X.; Qian, Y. Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater. Chem. Phys. 2007, 106, 58–62. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Li, Y.; Ma, H.; Wang, R.; Liu, Y.; Suzuki, N.; Terashima, C.; Ohtani, B.; Ochiai, T.; et al. Enhanced solar photothermal catalysis over solution plasma activated TiO2. Adv. Sci. 2020, 7, 2000204. [Google Scholar] [CrossRef]
- Qiang, C.; Toshiro, K.; Rikizo, H. Reductants in Gold Nanoparticle Synthesis Using Gas–Liquid Interfacial Discharge Plasmas. Appl. Phys. Express 2012, 5, 086201. [Google Scholar]
- Li, Y.; Wang, Y.; Lin, J.; Shi, Y.; Zhu, K.; Xing, Y.; Li, X.; Jia, Y.; Zhang, X. Solution-plasma-induced oxygen vacancy enhances MoOx/Pt electrocatalytic counter electrode for bifacial dye-sensitizes solar cells. Front. Energy Res. 2022, 10, 924515. [Google Scholar] [CrossRef]
- Pitchaimuthu, S.; Honda, K.; Suzuki Shoki Naito, A.; Suzuki, N.; Katsumata, K.; Nakata, K.; Ishida, N.; Kitamura, N.; Idemoto, Y.; Kondo, T.; et al. Solution plasma process-derived defect-induced heterophase anatase/brookite TiO2 nanocrystals for enhanced gaseous photocatalytic performance. ACS Omega 2018, 3, 898–905. [Google Scholar] [CrossRef]
- Kondeti, V.S.; Gangal, U.; Yatom, S.; Bruggeman, P.J. Ag+ reduction and silver nanoparticle at the plasma-liquid interface by an RF driven atmospheric pressure plasma jet: Mechanisms and the effect of surfactant. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 061302. [Google Scholar] [CrossRef]
- Leonardo, V.; Ignacio, C.; Javier, E.; Nancy, B.; Dinesh, P.S. Ascorbic acid based controlled growth of various Cu and Cu2O nanostructures. Mater. Res. Express 2019, 6, 065033. [Google Scholar]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma-liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process. Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.; Wang, X.; Zhan, R.; Chen, H.; Deng, S.; Xu, N.; Chen, J. Mechanism of photoluminescence quenching in visible and ultraviolet emissions of ZnO nanowires decorated with gold nanoparticles. Jpn. J. Appl. Phys. 2019, 58, 051005. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Jian, W.-J.; Dang, H.-F.; Zhao, X.-F.; Zhang, L.-Z.; Li, J.-H. Hierarchical Ag-ZnO Microspheres with Enhanced Photocatalytic Degradation Activities. Pol. J. Environ. Stud. 2017, 26, 871–880. [Google Scholar] [CrossRef]
- Cui, J.; Ye, L.; Chen Xi Li, J.; Bian Yang Yang, M.; Yang, Q.; Yun, D.; Sun, S. Simultaneously promoting adsorption and charge separation in Z-scheme ZnO/Cu2O heterojunctions for efficient removal of tetracycline. Appl. Surf. Sci. 2023, 638, 158046. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-band ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef]
- Chaudhary, K.; Shaheen, N.; Zulfiqar, S.; Sarwar, M.I.; Suleman, M.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Binary WO3-ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue. Synth. Met. 2020, 269, 116526. [Google Scholar] [CrossRef]
- Tran Thi, V.H.; Pham, T.N.; Pham, T.T.; Le, M.C. Synergistic Adsorption and Photocatalytic Activity under Visible Irradiation Using Ag-ZnO/GO Nanoparticles Derived at Low Temperature. J. Chem. 2019, 2019, 2979517. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 29, 122983. [Google Scholar] [CrossRef]
- Ashiegbu, D.C.; Potgieter, H.J. ZnO-based heterojunction catalysts for the photocatalytic degradation of methyl orange dye. Heliyon 2023, 9, e20674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A Ternary Magnetic Recyclable ZnO/Fe3O4/g-C3N4 Composite Photocatalyst for Efficient Photodegradation of Monoazo Dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef]

| Structure | Method | Organic Dye | Kinetics k | Yield (%) | Time (min)/Ref. |
|---|---|---|---|---|---|
| Cu2O-ZnO/kaolinite | Co-precipitation | MB | 0.098 min−1 | 93 | 105 [32] |
| Cu2O-ZnO | Precipitation and calcination | MO | 1.30762 h−1 | 98 | 240 [13] |
| Ag-Cu2O-ZnO | Hydrothermal and photoreduction | RhB | 0.041 min−1 | 80 | 60 [23] |
| WO3-ZnO@rGO | Ultrasound | MB | 0.0278 min−1 | 94 | 90 [50] |
| TiO2-ZnO | Hydrothermal | RhB | 0.011 min−1 | 89 | 180 [51] |
| ZnO-CdO-CuO | Co-precipitation | MB | 0.027 min−1 | 94 | 100 [52] |
| ZnO-Au-Cu2O | Electrodeposition and sputtering | MO | 0.3495 h−1 | ~75 | 240 [24] |
| ZnO-WO3 | Sol–gel | MO | 0.0521 min−1 | 100 | 90 [53] |
| ZnO-Fe3O4-g-C3N4 | Sol–gel and annellation | MO | 0.0243 min−1 | ~97 | 140 [54] |
| Ag-Cu2O-ZnO NW | Hydrothermal and plasma–liquid | MO | 0.165 min−1 | 98 | 30 This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, P.T.; Bach, T.N.; Phong, L.T.H.; Tung, D.H.; Ky, V.H.; Tung, D.K.; Lam, V.D.; Manh, D.H.; Dan, N.H.; Anh, T.X.; et al. Synthesis and Characterization of Broccoli-like Ag/Cu2O Nanostructures on ZnO Nanowires Using the Plasma–Liquid Interaction Method. Inorganics 2024, 12, 80. https://doi.org/10.3390/inorganics12030080
Thu PT, Bach TN, Phong LTH, Tung DH, Ky VH, Tung DK, Lam VD, Manh DH, Dan NH, Anh TX, et al. Synthesis and Characterization of Broccoli-like Ag/Cu2O Nanostructures on ZnO Nanowires Using the Plasma–Liquid Interaction Method. Inorganics. 2024; 12(3):80. https://doi.org/10.3390/inorganics12030080
Chicago/Turabian StyleThu, Phung Thi, Ta Ngoc Bach, Le Thi Hong Phong, Do Hoang Tung, Vu Hong Ky, Do Khanh Tung, Vu Dinh Lam, Do Hung Manh, Nguyen Huy Dan, Trinh Xuan Anh, and et al. 2024. "Synthesis and Characterization of Broccoli-like Ag/Cu2O Nanostructures on ZnO Nanowires Using the Plasma–Liquid Interaction Method" Inorganics 12, no. 3: 80. https://doi.org/10.3390/inorganics12030080
APA StyleThu, P. T., Bach, T. N., Phong, L. T. H., Tung, D. H., Ky, V. H., Tung, D. K., Lam, V. D., Manh, D. H., Dan, N. H., Anh, T. X., & Le, N. T. H. (2024). Synthesis and Characterization of Broccoli-like Ag/Cu2O Nanostructures on ZnO Nanowires Using the Plasma–Liquid Interaction Method. Inorganics, 12(3), 80. https://doi.org/10.3390/inorganics12030080








