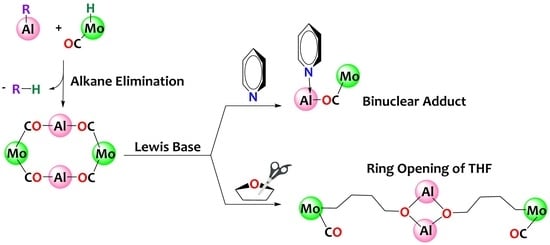
Alkane Elimination Preparation of Heterobimetallic MoAl Tetranuclear and Binuclear Complexes Promoting THF Ring Opening
Abstract
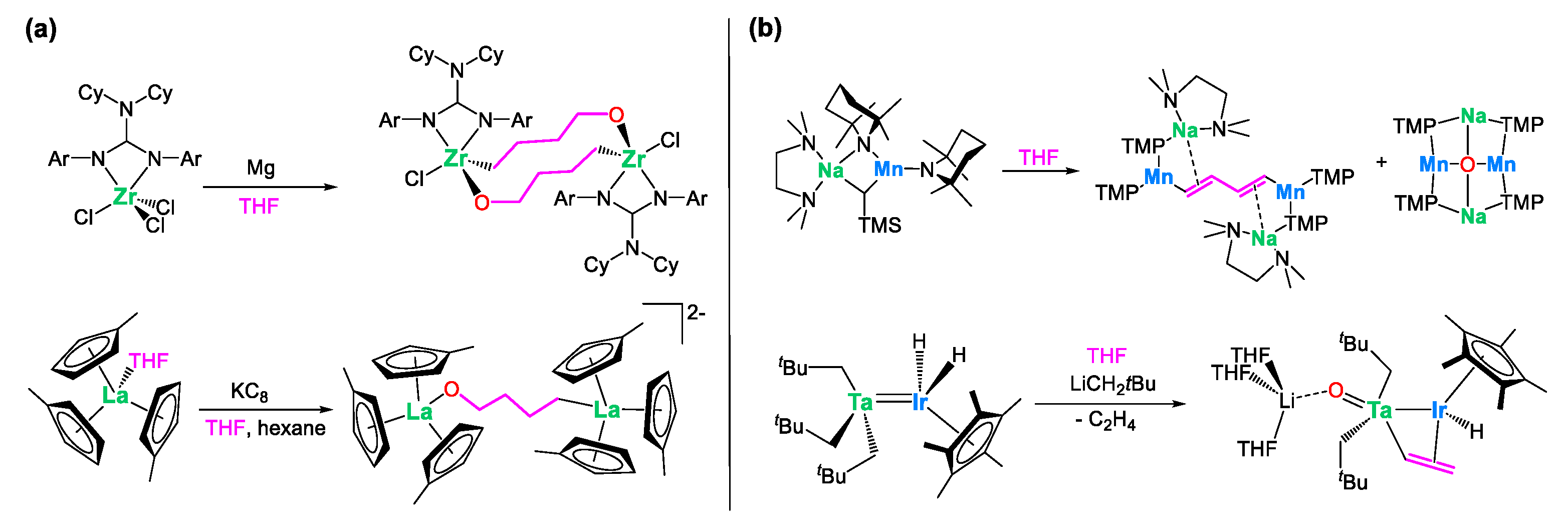
1. Introduction
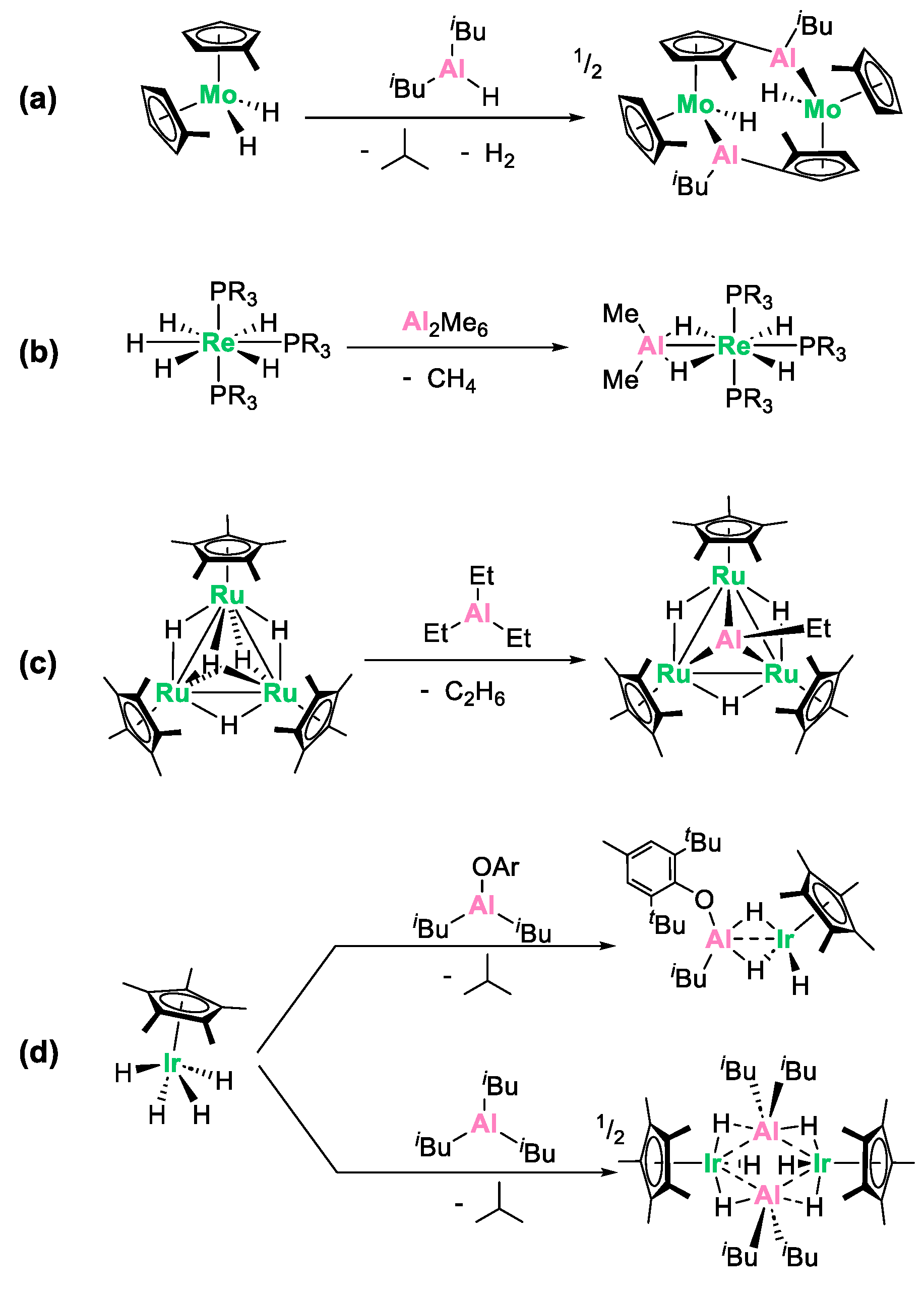
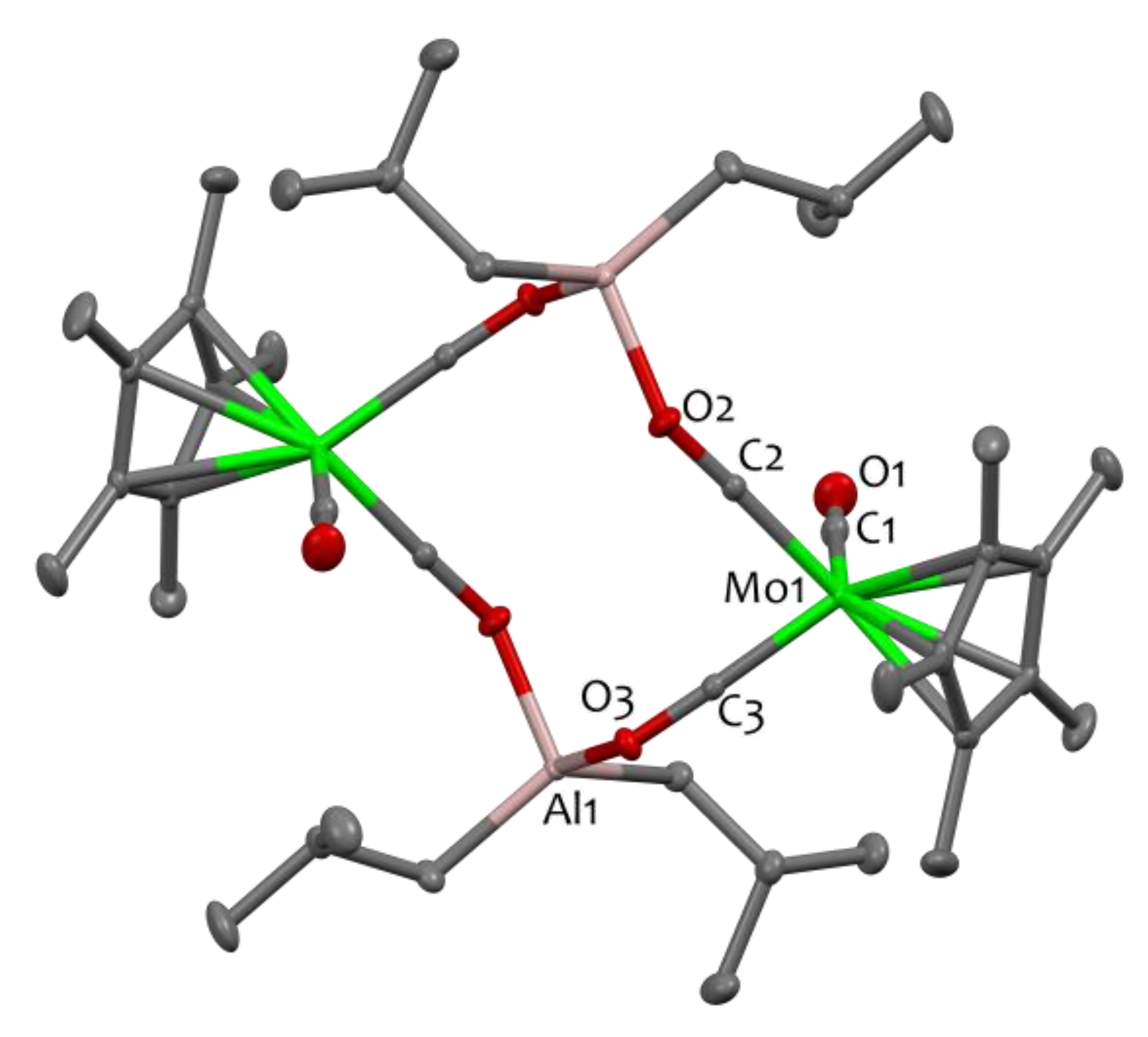
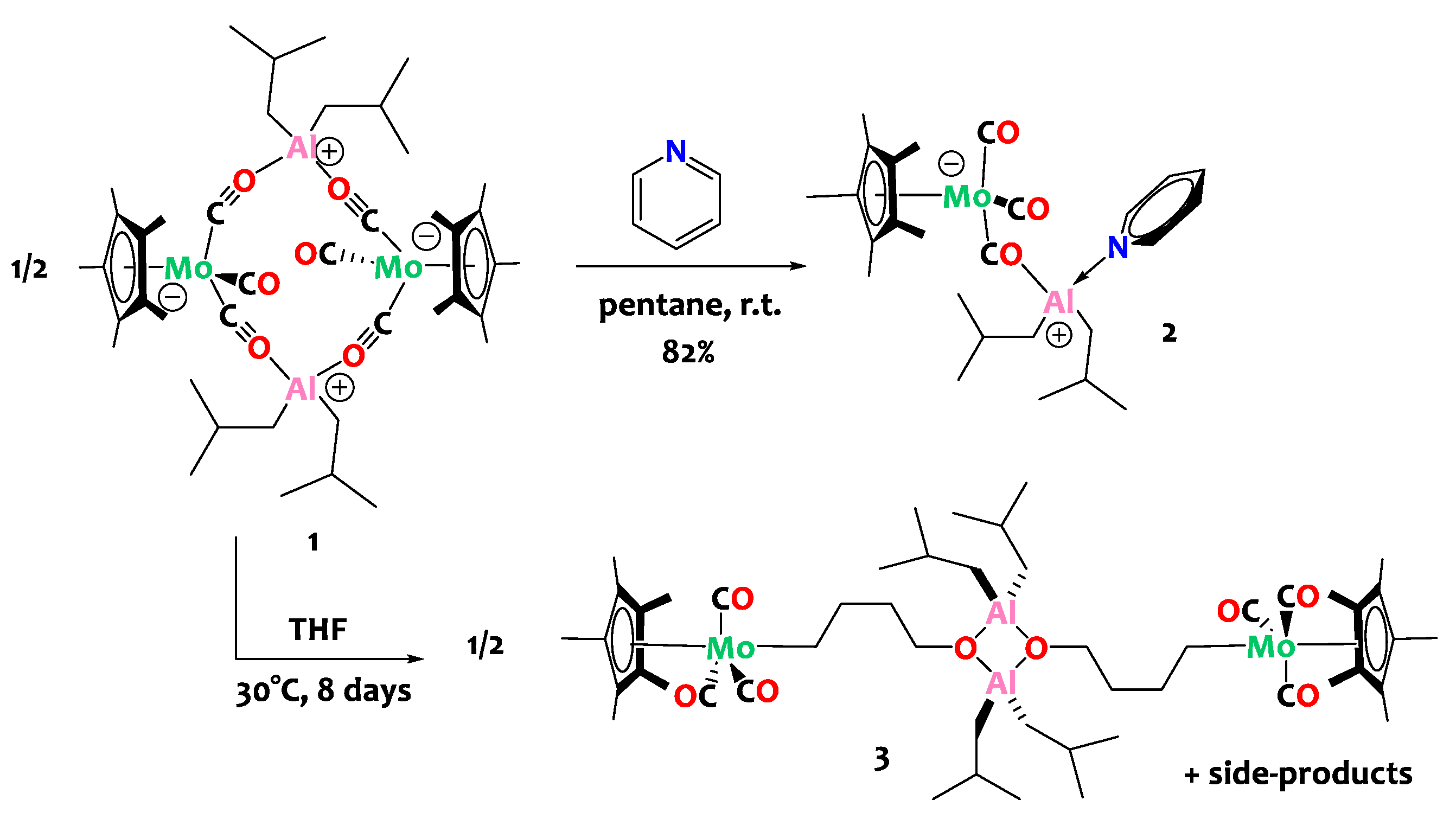
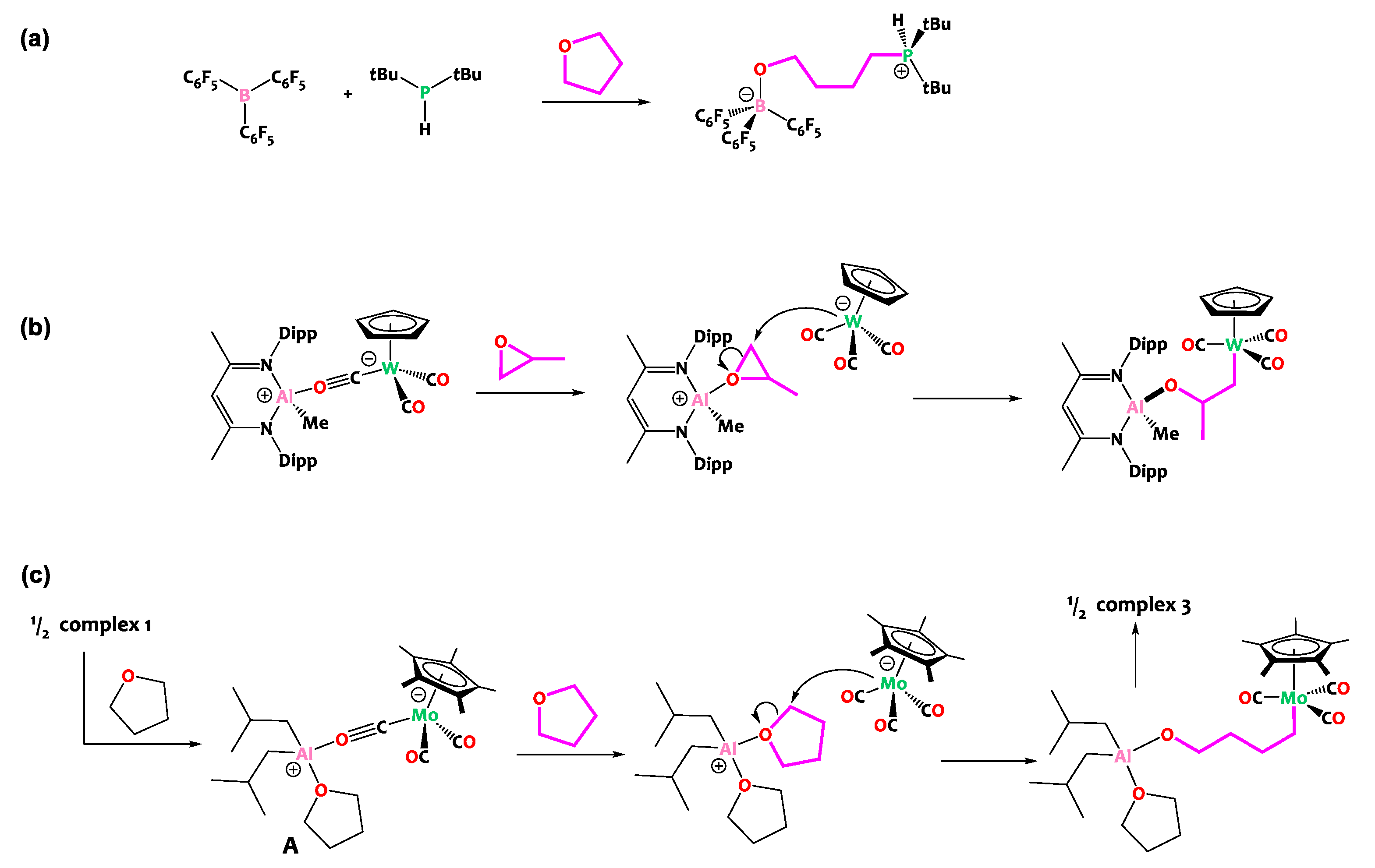
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Analytical Methods
3.3. Syntheses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mears, K.L.; Stennett, C.R.; Taskinen, E.K.; Knapp, C.E.; Carmalt, C.J.; Tuononen, H.M.; Power, P.P. Molecular Complexes Featuring Unsupported Dispersion-Enhanced Aluminum-Copper and Gallium-Copper Bonds. J. Am. Chem. Soc. 2020, 142, 19874–19878. [Google Scholar] [CrossRef]
- McManus, C.; Hicks, J.; Cui, X.; Zhao, L.; Frenking, G.; Goicoechea, J.M.; Aldridge, S. Coinage Metal Aluminyl Complexes: Probing Regiochemistry and Mechanism in the Insertion and Reduction of Carbon Dioxide. Chem. Sci. 2021, 12, 13458–13468. [Google Scholar] [CrossRef]
- Fernández, S.; Fernando, S.; Planas, O. Cooperation towards Nobility: Equipping First-Row Transition Metals with an Aluminium Sword. Dalton Trans. 2023, 52, 14259–14286. [Google Scholar] [CrossRef]
- Lachguar, A.; Pichugov, A.V.; Neumann, T.; Dubrawski, Z.; Camp, C. Cooperative Activation of Carbon-Hydrogen Bonds by Heterobimetallic Systems. Dalton Trans. 2023, 53, 1393–1409. [Google Scholar] [CrossRef]
- Weiss, J.; Stetzkamp, D.; Nuber, B.; Fischer, R.A.; Boehme, C.; Frenking, G. [(η5-C5Me5)Al-Fe(CO)4] Synthesis, Structure, and Bonding. Angew. Chem. Int. Ed. 1997, 36, 70–72. [Google Scholar] [CrossRef]
- Gorgas, N.; Stadler, B.; White, A.J.P.; Crimmin, M.R. Vinylic C–H Activation of Styrenes by an Iron–Aluminum Complex. J. Am. Chem. Soc. 2024, 146, 4252–4259. [Google Scholar] [CrossRef]
- Gorgas, N.; White, A.J.P.; Crimmin, M.R. Cooperative C-H Bond Activation by a Low-Spin D6Iron-Aluminum Complex. J. Am. Chem. Soc. 2022, 144, 8770–8777. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Schwamm, R.J.; Hill, M.S.; Mahon, M.F.; McMullin, C.L.; Rajabi, N.A. Ambiphilic Al−Cu Bonding. Angew. Chem. Int. Ed. 2021, 60, 14390–14393. [Google Scholar] [CrossRef] [PubMed]
- Escomel, L.; Del Rosal, I.; Maron, L.; Jeanneau, E.; Veyre, L.; Thieuleux, C.; Camp, C. Strongly Polarized Iridiumδ−-Aluminumδ+Pairs: Unconventional Reactivity Patterns Including CO2 Cooperative Reductive Cleavage. J. Am. Chem. Soc. 2021, 143, 4844–4856. [Google Scholar] [CrossRef] [PubMed]
- Escomel, L.; Soulé, N.; Robin, E.; Del Rosal, I.; Maron, L.; Jeanneau, E.; Thieuleux, C.; Camp, C. Rational Preparation of Well-Defined Multinuclear Iridium-Aluminum Polyhydride Clusters and Comparative Reactivity. Inorg. Chem. 2022, 61, 5715–5730. [Google Scholar] [CrossRef] [PubMed]
- Sinhababu, S.; Radzhabov, M.R.; Telser, J.; Mankad, N.P. Cooperative Activation of CO2 and Epoxide by a Heterobinuclear Al-Fe Complex via Radical Pair Mechanisms. J. Am. Chem. Soc. 2022, 144, 3210–3221. [Google Scholar] [CrossRef]
- Hicks, J.; Mansikkamäki, A.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. A Nucleophilic Gold Complex. Nat. Chem. 2019, 11, 237–241. [Google Scholar] [CrossRef]
- Lai, Q.; Bhuvanesh, N.; Ozerov, O.V. Unexpected B/Al Transelementation within a Rh Pincer Complex. J. Am. Chem. Soc. 2020, 142, 20920–20923. [Google Scholar] [CrossRef]
- Cammarota, R.C.; Lu, C.C. Tuning Nickel with Lewis Acidic Group 13 Metalloligands for Catalytic Olefin Hydrogenation. J. Am. Chem. Soc. 2015, 137, 12486–12489. [Google Scholar] [CrossRef]
- Butler, M.J.; Crimmin, M.R. Magnesium, Zinc, Aluminium and Gallium Hydride Complexes of the Transition Metals. Chem. Commun. 2017, 53, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Iwasawa, N. Synthesis, Structure, and Catalysis of Palladium Complexes Bearing a Group 13 Metalloligand: Remarkable Effect of an Aluminum-Metalloligand in Hydrosilylation of CO2. J. Am. Chem. Soc. 2017, 139, 6074–6077. [Google Scholar] [CrossRef] [PubMed]
- Morisako, S.; Watanabe, S.; Ikemoto, S.; Muratsugu, S.; Tada, M.; Yamashita, M. Synthesis of A Pincer-IrV Complex with A Base-Free Alumanyl Ligand and Its Application toward the Dehydrogenation of Alkanes. Angew. Chem.-Int. Ed. 2019, 58, 15031–15035. [Google Scholar] [CrossRef]
- Hara, N.; Aso, K.; Li, Q.Z.; Sakaki, S.; Nakao, Y. C2-Selective Alkylation of Pyridines by Rhodium–Aluminum Complexes. Tetrahedron 2021, 95, 132339. [Google Scholar] [CrossRef]
- Hara, N.; Saito, T.; Semba, K.; Kuriakose, N.; Zheng, H.; Sakaki, S.; Nakao, Y. Rhodium Complexes Bearing PAlP Pincer Ligands. J. Am. Chem. Soc. 2018, 140, 7070–7073. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Hara, N.; Semba, K.; Nakao, Y.; Sakaki, S. Rh Complex with Unique Rh–Al Direct Bond: Theoretical Insight into Its Characteristic Features and Application to Catalytic Reaction via σ-Bond Activation. Top. Catal. 2022, 65, 392–417. [Google Scholar] [CrossRef]
- Hicks, J.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. The Aluminyl Anion: A New Generation of Aluminium Nucleophile. Angew. Chem. Int. Ed. 2021, 60, 1702–1713. [Google Scholar] [CrossRef]
- Coles, M.P.; Evans, M.J. The Emerging Chemistry of the Aluminyl Anion. Chem. Commun. 2023, 59, 503–519. [Google Scholar] [CrossRef]
- Kong, R.Y.; Crimmin, M.R. 1strow Transition Metal Aluminylene Complexes: Preparation, Properties and Bonding Analysis. Dalton Trans. 2021, 50, 7810–7817. [Google Scholar] [CrossRef]
- Butovskii, M.V.; Döring, C.; Bezugly, V.; Wagner, F.R.; Grin, Y.; Kempe, R. Molecules Containing Rare-Earth Atoms Solely Bonded by Transition Metals. Nat. Chem. 2010, 2, 741–744. [Google Scholar] [CrossRef]
- Sobaczynski, A.P.; Obenauf, J.; Kempe, R. Alkane Elimination Reactions between Yttrium Alkyls and Tungsten Hydrides. Eur. J. Inorg. Chem. 2014, 2014, 1211–1217. [Google Scholar] [CrossRef]
- Lassalle, S.; Jabbour, R.; Schiltz, P.; Berruyer, P.; Todorova, T.K.; Veyre, L.; Gajan, D.; Lesage, A.; Thieuleux, C.; Camp, C. Metal–Metal Synergy in Well-Defined Surface Tantalum–Iridium Heterobimetallic Catalysts for H/D Exchange Reactions. J. Am. Chem. Soc. 2019, 141, 19321–19335. [Google Scholar] [CrossRef]
- Lassalle, S.; Petit, J.; Falconer, R.L.; Hérault, V.; Jeanneau, E.; Thieuleux, C.; Camp, C. Reactivity of Tantalum/Iridium and Hafnium/Iridium Alkyl Hydrides with Alkyl Lithium Reagents: Nucleophilic Addition, Alpha-H Abstraction, or Hydride Deprotonation? Organometallics 2022, 41, 1675–1687. [Google Scholar] [CrossRef]
- Ye, C.Z.; Del Rosal, I.; Boreen, M.A.; Ouellette, E.T.; Russo, D.R.; Maron, L.; Arnold, J.; Camp, C. A Versatile Strategy for the Formation of Hydride-Bridged Actinide-Iridium Multimetallics. Chem. Sci. 2022, 14, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Stender, M.; Oesen, H.; Blaurock, S.; Hey-Hawkins, E. Synthese und Molekülstruktur von [{Cp’(μ-η1:η5-C5H3Me)Mo(μ-AlRH)}2] (Cp’ = C5H4Me, R = iBu, Et). Z. Anorg. Allg. Chem. 2001, 627, 980–984. [Google Scholar] [CrossRef]
- Skupiński, W.A.; Huffman, J.C.; Bruno, J.W.; Caulton, K.G. Dinuclear Elimination from Rhenium Hydrides and AlMe3: Rhenium/Aluminum Polyhydrides. J. Am. Chem. Soc. 1984, 106, 8128–8136. [Google Scholar] [CrossRef]
- Ohashi, M.; Matsubara, K.; Iizuka, T.; Suzuki, H. Trinuclear Ruthenium Polyhydride Complexes with a Triply Bridging Ligand: [{(η5-C5Me5)Ru}3(μ3-M)(μ-H)3(μ3-H)] (M = Li, MgiPr, and ZnEt) and [{(H5-C5Me5)Ru}3 (μ3-M)(μ-H)3] (M = AlEt and GaMe). Angew. Chem.-Int. Ed. 2003, 42, 937–940. [Google Scholar] [CrossRef]
- Golden, J.T.; Peterson, T.H.; Holland, P.L.; Bergman, R.G.; Andersen, R.A. Adduct Formation and Single and Double Deprotonation of Cp*(PMe3)Ir(H)2 with Main Group Metal Alkyls and Aryls: Synthesis and Structure of Three Novel Ir-Al and Ir-Mg Heterobimetallics. J. Am. Chem. Soc. 1998, 120, 223–224. [Google Scholar] [CrossRef]
- Isaac, C.J.; Miloserdov, F.M.; Pécharman, A.F.; Lowe, J.P.; McMullin, C.L.; Whittlesey, M.K. Structure and Reactivity of [Ru-Al] and [Ru-Sn] Heterobimetallic PPh3-Based Complexes. Organometallics 2022, 41, 2716–2730. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Quadrelli, E.A.; Camp, C. Lability of Ta-NHC Adducts as a Synthetic Route towards Heterobimetallic Ta/Rh Complexes. Dalton Trans. 2020, 49, 3120–3128. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Moneuse, R.; Petit, J.; Pavard, P.-A.A.; Dardun, V.; Rivat, M.; Schiltz, P.; Solari, M.; Jeanneau, E.; Veyre, L.; et al. Early/Late Heterobimetallic Tantalum/Rhodium Species Assembled Through a Novel Bifunctional NHC-OH Ligand. Chem.-Eur. J. 2018, 24, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, S.; Jabbour, R.; Del Rosal, I.; Maron, L.; Fonda, E.; Veyre, L.; Gajan, D.; Lesage, A.; Thieuleux, C.; Camp, C. Stepwise Construction of Silica-Supported Tantalum/Iridium Heteropolymetallic Catalysts Using Surface Organometallic Chemistry. J. Catal. 2020, 392, 287–301. [Google Scholar] [CrossRef]
- Del Rosal, I.; Lassalle, S.; Dinoi, C.; Thieuleux, C.; Maron, L.; Camp, C. Mechanistic Investigations via DFT Support the Cooperative Heterobimetallic C-H and O-H Bond Activation across TaIr Multiple Bonds. Dalton Trans. 2021, 50, 504–510. [Google Scholar] [CrossRef]
- Weberg, R.T.; Norton, J.R. Kinetic and Thermodynamic Acidity of Hydrido Transition-Metal Complexes. 6. Interstitial Hydrides. J. Am. Chem. Soc. 1990, 112, 1105–1108. [Google Scholar] [CrossRef]
- Morris, R.H. Estimating the Acidity of Transition Metal Hydride and Dihydrogen Complexes by Adding Ligand Acidity Constants. J. Am. Chem. Soc. 2014, 136, 1948–1959. [Google Scholar] [CrossRef]
- Liddle, S.T.; Gardner, B.M. Reactions of [Y(BDI)(I)2(THF)] [BDI = {HC(CMeNAr)2}-, Ar = 2,6-Diisopropylphenyl] with Na[M(Cp)(CO)3] (M = Cr, W): X-Ray Crystal Structures of [{Y(BDI)[Cr(Cp)(CO)3]2(THF)}2] and [W(Cp)(CO)3][Na(THF)2]. J. Organomet. Chem. 2009, 694, 1581–1585. [Google Scholar] [CrossRef]
- Arleth, N.; Gamer, M.T.; Köppe, R.; Pushkarevsky, N.A.; Konchenko, S.N.; Fleischmann, M.; Bodensteiner, M.; Scheer, M.; Roesky, P.W. The Approach to 4d/4f-Polyphosphides. Chem. Sci. 2015, 6, 7179–7184. [Google Scholar] [CrossRef]
- Reinfandt, N.; Schoo, C.; Dütsch, L.; Köppe, R.; Konchenko, S.N.; Scheer, M.; Roesky, P.W. Synthesis of Unprecedented 4d/4f-Polypnictogens. Chem.-Eur. J. 2021, 27, 3974–3978. [Google Scholar] [CrossRef]
- Abdalla, J.A.B.; Riddlestone, I.M.; Tirfoin, R.; Phillips, N.; Bates, J.I.; Aldridge, S. Al-H σ-Bond Coordination: Expanded Ring Carbene Adducts of AlH3 as Neutral Bi- and Tri-Functional Donor Ligands. Chem. Commun. 2013, 49, 5547–5549. [Google Scholar] [CrossRef]
- Abdalla, J.A.B.; Riddlestone, I.M.; Turner, J.; Kaufman, P.A.; Tirfoin, R.; Phillips, N.; Aldridge, S. Coordination and Activation of Al-H and Ga-H Bonds. Chem.-Eur. J. 2014, 20, 17624–17634. [Google Scholar] [CrossRef]
- Dardun, V.; Escomel, L.; Jeanneau, E.; Camp, C. On the Alcoholysis of Alkyl-Aluminum(Iii) Alkoxy-NHC Derivatives: Reactivity of the Al-Carbene Lewis Pair versus Al-Alkyl. Dalton Trans. 2018, 47, 10429–10433. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.L.; Lukens, W.W.; Lu, C.C.; Arnold, J. Photochemical Route to Actinide-Transition Metal Bonds: Synthesis, Characterization and Reactivity of a Series of Thorium and Uranium Heterobimetallic Complexes. J. Am. Chem. Soc. 2014, 136, 3647–3654. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Schulten, C.; Nembenna, S.; Stasch, A. Synthesis and Crystal Structures of Bulky Guanidinato Zirconium(IV) and Hafnium(IV) Chloride Complexes. J. Chem. Crystallogr. 2012, 42, 866–870. [Google Scholar] [CrossRef]
- Woen, D.H.; Huh, D.N.; Ziller, J.W.; Evans, W.J. Reactivity of Ln(II) Complexes Supported by (C5H4Me)1- Ligands with THF and PhSiH3: Isolation of Ring-Opened, Bridging Alkoxyalkyl, Hydride, and Silyl Products. Organometallics 2018, 37, 3055–3063. [Google Scholar] [CrossRef]
- Mulvey, R.E.; Blair, V.L.; Clegg, W.; Kennedy, A.R.; Klett, J.; Russo, L. Cleave and Capture Chemistry Illustrated through Bimetallic-Induced Fragmentation of Tetrahydrofuran. Nat. Chem. 2010, 2, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Kronig, S.; Theuergarten, E.; Holschumacher, D.; Bannenberg, T.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Dihydrogen Activation by Frustrated Carbene-Borane Lewis Pairs: An Experimental and Theoretical Study of Carbene Variation. Inorg. Chem. 2011, 50, 7344–7359. [Google Scholar] [CrossRef] [PubMed]
- Holschumacher, D.; Bannenberg, T.; Hrib, C.G.; Jones, P.G.; Tamm, M. Heterolytic Dihydrogen Activation by a Frustrated Carbene–Borane Lewis Pair. Angew. Chem. Int. Ed. 2008, 47, 7428–7432. [Google Scholar] [CrossRef]
- Geier, S.J.; Stephan, D.W. Lutidine/B(C6F5)3: At the Boundary of Classical and Frustrated Lewis Pair Reactivity. J. Am. Chem. Soc. 2009, 131, 3476–3477. [Google Scholar] [CrossRef] [PubMed]
- Krachko, T.; Nicolas, E.; Ehlers, A.W.; Nieger, M.; Slootweg, J.C. Ring-Opening of Epoxides Mediated by Frustrated Lewis Pairs. Chem. Eur. J. 2018, 24, 12669–12677. [Google Scholar] [CrossRef] [PubMed]
- Birkmann, B.; Voss, T.; Geier, S.J.; Ullrich, M.; Kehr, G.; Erker, G.; Stephan, D.W. Frustrated Lewis Pairs and Ring-Opening of THF, Dioxane, and Thioxane. Organometallics 2010, 29, 5310–5319. [Google Scholar] [CrossRef]
- Yolsal, U.; Horton, T.A.R.; Wang, M.; Shaver, M.P. Cyclic Ether Triggers for Polymeric Frustrated Lewis Pair Gels. J. Am. Chem. Soc. 2021, 143, 12980–12984. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.C.; Prieto, R.; Dureen, M.A.; Lough, A.J.; Labeodan, O.A.; Höltrichter-Rössmann, T.; Stephan, D.W. Reactions of Phosphines with Electron Deficient Boranes. Dalton Trans. 2009, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Wallach, C.; Geitner, F.S.; Fässler, T.F. FLP-Type Nitrile Activation and Cyclic Ether Ring-Opening by Halo-Borane Nonagermanide-Cluster Lewis Acid–Base Pairs. Chem. Sci. 2021, 12, 6969–6976. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Bresciani, G.; Marchetti, F.; Pampaloni, G.; Zacchini, S. Synthesis and Structural Characterization of Mixed Halide–N,N-Diethylcarbamates of Group 4 Metals, Including a Case of Unusual Tetrahydrofuran Activation. New J. Chem. 2017, 41, 1781–1789. [Google Scholar] [CrossRef]
- Liddle, S.T.; Arnold, P.L. Synthesis and Characterisation of Yttrium Complexes Supported by the Beta-Diketiminate Ligand {ArNC(CH3)CHC(CH3)NAr}- (Ar = 2,6-iPr2C6H3). Dalton Trans. 2007, 3305–3313. [Google Scholar] [CrossRef]
- Breen, T.L.; Stephan, D.W. Substitution or Nucleophilic Attack by Phosphines on ZrCl4(THF)2. Inorg. Chem. 1992, 31, 4019–4022. [Google Scholar] [CrossRef]
- Marchetti, F.; Pampaloni, G.; Zacchini, S. Reactivity of Niobium and Tantalum Pentahalides with Cyclic Ethers and the Isolation and Characterization of Intermediates in the Polymerization of Tetrahydrofuran. Inorg. Chem. 2008, 47, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Travia, N.E.; Monreal, M.J.; Scott, B.L.; Kiplinger, J.L. Thorium-Mediated Ring-Opening of Tetrahydrofuran and the Development of a New Thorium Starting Material: Preparation and Chemistry of ThI4(DME)2. Dalton Trans. 2012, 41, 14514–14523. [Google Scholar] [CrossRef] [PubMed]
- Avens, L.R.; Barnhart, D.M.; Burns, C.J.; Mckee, S.D. Uranium-Mediated Ring Opening of Tetrahydrofuran. Crystal Structure of UI2(OCH2CH2CH2CH2I)2(Ph3PO)2. Inorg. Chem. 1996, 35, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Sinhababu, S.; Singh, R.P.; Radzhabov, M.R.; Kumawat, J.; Ess, D.H.; Mankad, N.P. Coordination-Induced O-H/N-H Bond Weakening by a Redox Non-Innocent, Aluminum-Containing Radical. Nat. Commun. 2024, 15, 1315. [Google Scholar] [CrossRef] [PubMed]
- Campos, J. Dihydrogen and Acetylene Activation by a Gold(I)/Platinum(0) Transition Metal Only Frustrated Lewis Pair. J. Am. Chem. Soc. 2017, 139, 2944–2947. [Google Scholar] [CrossRef]
- Bauer, J.; Braunschweig, H.; Dewhurst, R.D. Metal-Only Lewis Pairs with Transition Metal Lewis Bases. Chem. Rev. 2012, 112, 4329–4346. [Google Scholar] [CrossRef]
- Navarro, M.; Campos, J. Bimetallic Frustrated Lewis Pairs. Adv. Organomet. Chem. 2021, 75, 95–148. [Google Scholar] [CrossRef]
- Sinhababu, S.; Mankad, N.P. Diverse Thermal and Photochemical Reactivity of an Al-Fe Bonded Heterobimetallic Complex. Organometallics 2022, 41, 1917–1921. [Google Scholar] [CrossRef]
- Nolan, S.P.; Hoff, C.D.; Landrum, J.T. Synthesis and Thermochemistry of HMo(CO)3C5Me5; Comparison of Cyclopentadienyl and Pentamethylcyclopentadienyl Ligands. J. Organomet. Chem. 1985, 282, 357–362. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Rigaku Corporation: Oxford, UK, 2019. [Google Scholar]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
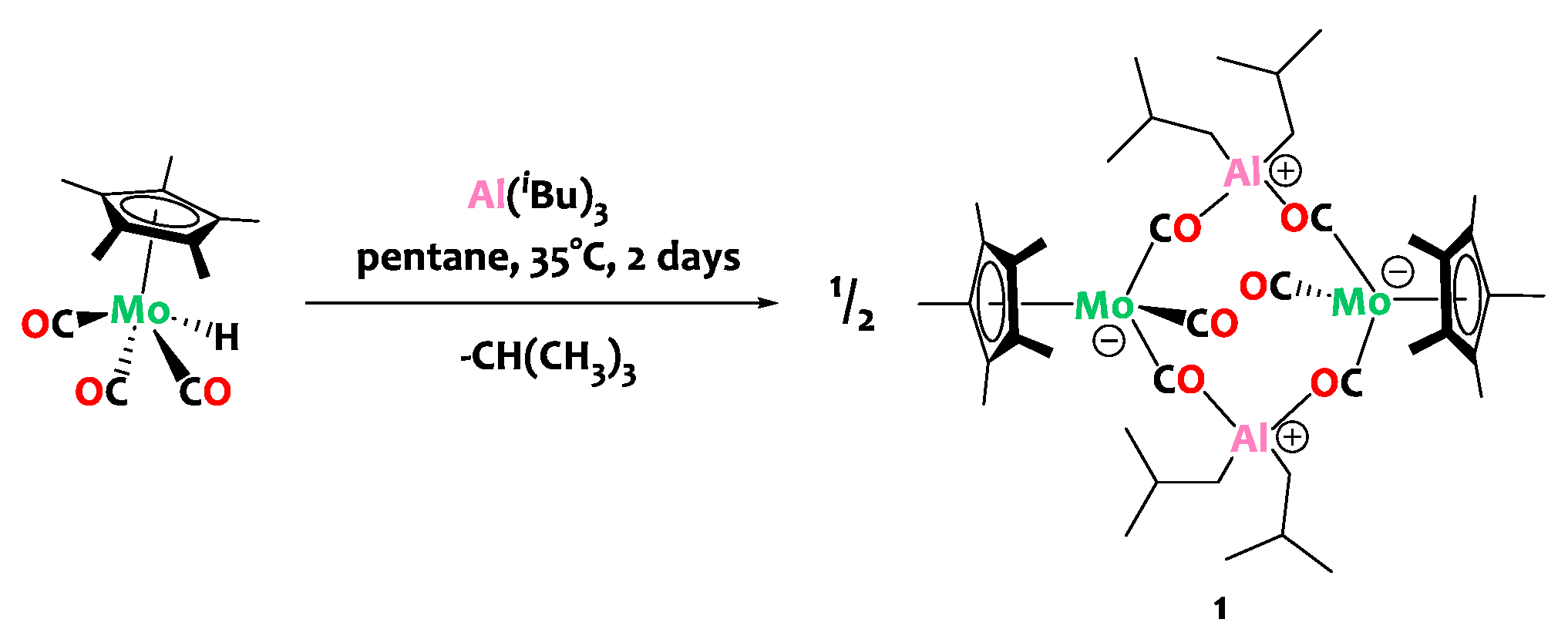

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escomel, L.; Jeanneau, E.; Thieuleux, C.; Camp, C. Alkane Elimination Preparation of Heterobimetallic MoAl Tetranuclear and Binuclear Complexes Promoting THF Ring Opening. Inorganics 2024, 12, 72. https://doi.org/10.3390/inorganics12030072
Escomel L, Jeanneau E, Thieuleux C, Camp C. Alkane Elimination Preparation of Heterobimetallic MoAl Tetranuclear and Binuclear Complexes Promoting THF Ring Opening. Inorganics. 2024; 12(3):72. https://doi.org/10.3390/inorganics12030072
Chicago/Turabian StyleEscomel, Léon, Erwann Jeanneau, Chloé Thieuleux, and Clément Camp. 2024. "Alkane Elimination Preparation of Heterobimetallic MoAl Tetranuclear and Binuclear Complexes Promoting THF Ring Opening" Inorganics 12, no. 3: 72. https://doi.org/10.3390/inorganics12030072
APA StyleEscomel, L., Jeanneau, E., Thieuleux, C., & Camp, C. (2024). Alkane Elimination Preparation of Heterobimetallic MoAl Tetranuclear and Binuclear Complexes Promoting THF Ring Opening. Inorganics, 12(3), 72. https://doi.org/10.3390/inorganics12030072