Thermoelectric Characteristics of β-Ag2Se1+x Prepared via a Combined Rapid Mechano-Thermal Approach
Abstract
1. Introduction
2. Results and Discussion
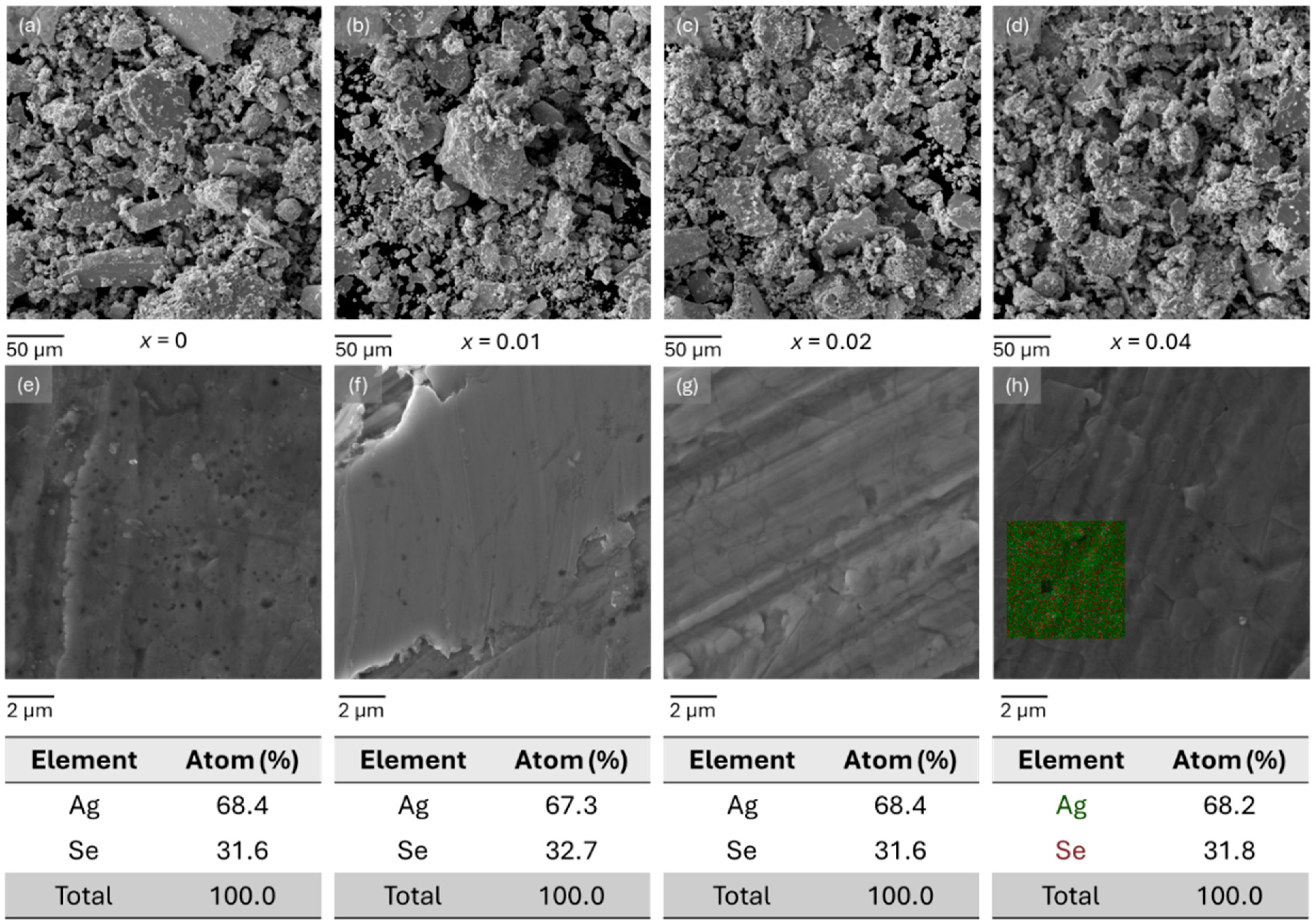
2.1. Phase Composition and Microstructure Analysis
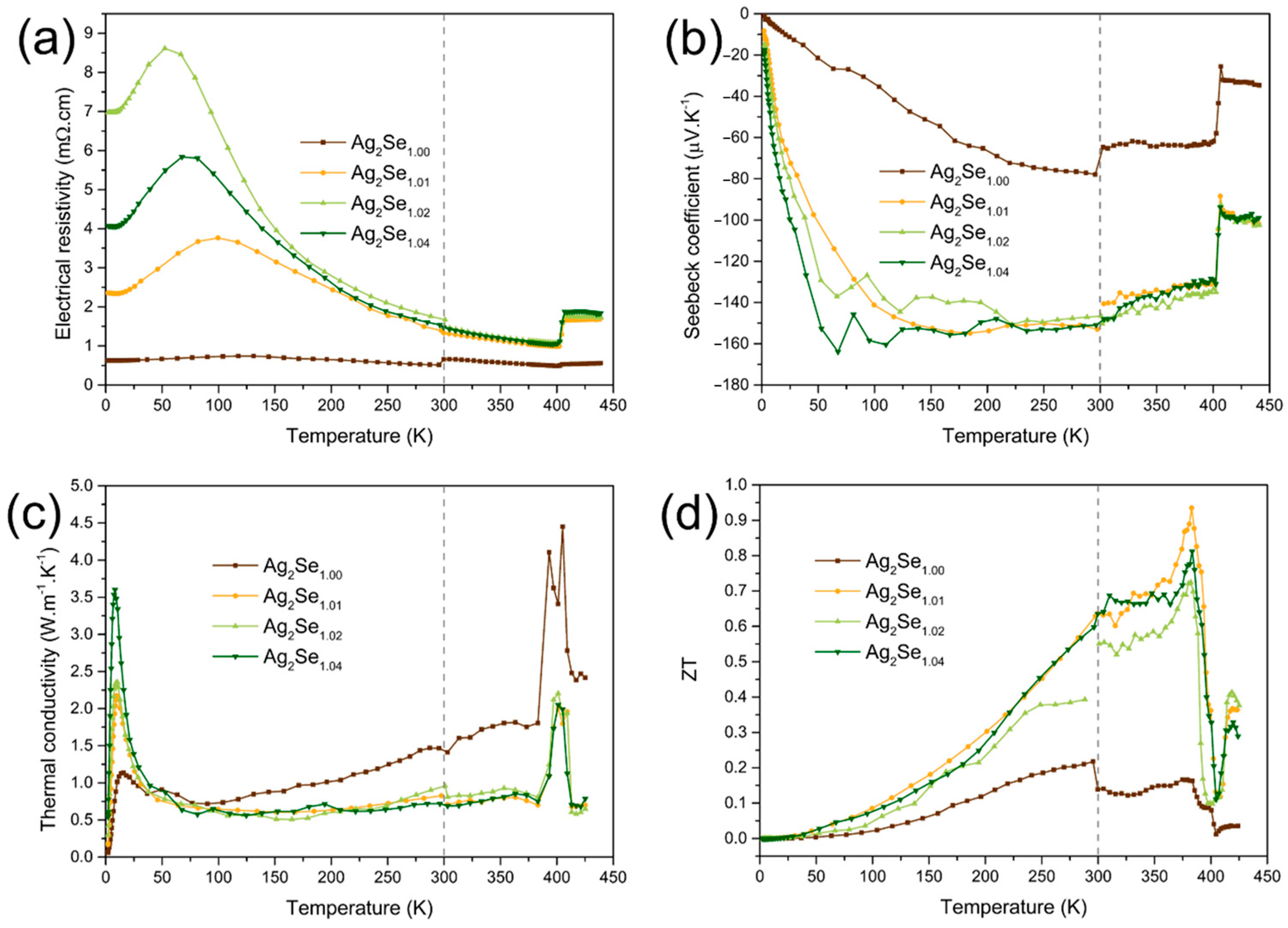
2.2. Thermoelectric Properties
3. Materials and Methods
3.1. Mechanochemical Synthesis
3.2. Pellets for the Measurements of Thermoelectric Properties
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, G.; Zhao, L.-D.; Kanatzidis, M.G. Rationally Designing High-Performance Bulk Thermoelectric Materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Zhang, S.; Pei, Y. Ag2Se as a Tougher Alternative to n-Type Bi2Te3 Thermoelectrics. Nat. Commun. 2024, 15, 6580. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Qi, R.; Chen, M.; Chen, H.; Xing, C.; Sui, F.; Gu, L.; He, W.; Zhang, Y.; Baba, T.; et al. Microstructurally Tailored Thin β-Ag2Se Films toward Commercial Flexible Thermoelectrics. Adv. Mater. 2022, 34, 2104786. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tritt, T.M. Advances in Thermoelectric Materials Research: Looking Back and Moving Forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, L.; Wei, J.; Yang, S.; Zhang, M.; Wang, X.; Yang, F. Doping Effect on Cu2Se Thermoelectric Performance: A Review. Materials 2020, 13, 5704. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Xiao, C.; Xu, J.; Li, K.; Feng, J.; Yang, J.; Xie, Y. Superionic Phase Transition in Silver Chalcogenide Nanocrystals Realizing Optimized Thermoelectric Performance. J. Am. Chem. Soc. 2012, 134, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Perez-Taborda, J.A.; Caballero-Calero, O.; Vera-Londono, L.; Briones, F.; Martin-Gonzalez, M. High Thermoelectric zT in n-Type Silver Selenide Films at Room Temperature. Adv. Energy Mater. 2018, 8, 1702024. [Google Scholar] [CrossRef]
- Lin, S.; Guo, L.; Wang, X.; Liu, Y.; Wu, Y.; Li, R.; Shao, H.; Jin, M. Revealing the Promising Near-Room-Temperature Thermoelectric Performance in Ag2Se Single Crystals. J. Materiomics 2023, 9, 754–761. [Google Scholar] [CrossRef]
- Hu, Q.-X.; Liu, W.-D.; Zhang, L.; Sun, W.; Gao, H.; Shi, X.-L.; Yang, Y.-L.; Liu, Q.; Chen, Z.-G. SWCNTs/Ag2Se Film with Superior Bending Resistance and Enhanced Thermoelectric Performance via in Situ Compositing. Chem. Eng. J. 2023, 457, 141024. [Google Scholar] [CrossRef]
- Byeon, D.; Sobota, R.; Delime-Codrin, K.; Choi, S.; Hirata, K.; Adachi, M.; Kiyama, M.; Matsuura, T.; Yamamoto, Y.; Matsunami, M.; et al. Discovery of Colossal Seebeck Effect in Metallic Cu2Se. Nat. Commun. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High Performance N-Type Ag2Se Film on Nylon Membrane for Flexible Thermoelectric Power Generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Mi, W.; Qiu, P.; Zhang, T.; Lv, Y.; Shi, X.; Chen, L. Thermoelectric Transport of Se-Rich Ag2Se in Normal Phases and Phase Transitions. Appl. Phys. Lett. 2014, 104, 133903. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Q.; Bao, D.; Liu, T.; Liu, W.-D.; Liu, C.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z.-G. Hierarchical Structures Advance Thermoelectric Properties of Porous N-Type β-Ag2Se. ACS Appl. Mater. Interfaces 2020, 12, 51523–51529. [Google Scholar] [CrossRef]
- Jood, P.; Chetty, R.; Ohta, M. Structural Stability Enables High Thermoelectric Performance in Room Temperature Ag2Se. J. Mater. Chem. A 2020, 8, 13024–13037. [Google Scholar] [CrossRef]
- Missen, O.P.; Ram, R.; Mills, S.J.; Etschmann, B.; Reith, F.; Shuster, J.; Smith, D.J.; Brugger, J. Love Is in the Earth: A Review of Tellurium (Bio)Geochemistry in Surface Environments. Earth-Sci. Rev. 2020, 204, 103150. [Google Scholar] [CrossRef]
- Ferhat, M.; Nagao, J. Thermoelectric and Transport Properties of β-Ag2Se Compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Conn, J.B.; Taylor, R.C. Thermoelectric and Crystallographic Properties of Ag2Se. J. Electrochem. Soc. 1960, 107, 977. [Google Scholar] [CrossRef]
- Yang, F.; Xiong, S.; Xia, Z.; Liu, F.; Han, C.; Zhang, D. Two-Step Synthesis of Silver Selenide Semiconductor with a Linear Magnetoresistance Effect. Semicond. Sci. Technol. 2012, 27, 125017. [Google Scholar] [CrossRef]
- Ali, H.M.; Khudayer, I.H. Study Structure and Optical Properties of Ag2Se, Ag2Se0.8Te0.2 and Ag2Se0.8S0.2 Thin Films. J. Ovonic Res. 2022, 18, 675–680. [Google Scholar] [CrossRef]
- Wang, P.; Chen, J.-L.; Zhou, Q.; Liao, Y.T.; Peng, Y.; Liang, J.S.; Miao, L. Enhancing the Thermoelectric Performance of Ag2Se by Non-Stoichiometric Defects. Appl. Phys. Lett. 2022, 120, 193902. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Luo, K.; Yin, X.; Lin, X.; Zhu, C. Biomimetic Synthesis of Ag2Se Quantum Dots with Enhanced Photothermal Properties and as “Gatekeepers” to Cap Mesoporous Silica Nanoparticles for Chemo–Photothermal Therapy. Chem. Asian J. 2019, 14, 155–161. [Google Scholar] [CrossRef]
- Duan, H.Z.; Li, Y.L.; Zhao, K.P.; Qiu, P.F.; Shi, X.; Chen, L.D. Ultra-Fast Synthesis for Ag2Se and CuAgSe Thermoelectric Materials. JOM 2016, 68, 2659–2665. [Google Scholar] [CrossRef]
- Karmakar, G.; Tyagi, A.; Shah, A.Y.; Nigam, S.; Wadawale, A.P.; Kedarnath, G.; Vats, B.G.; Naveen Kumar, N.; Singh, V. Facile One Pot Synthesis of Highly Photoresponsive Coinage Metal Selenides (Cu1.8Se and Ag2Se) Achieved through Novel Cu and Ag Pyridylselenolates as Molecular Precursors. Dalton Trans. 2022, 51, 12670–12685. [Google Scholar] [CrossRef]
- Tee, S.Y.; Tan, X.Y.; Wang, X.; Lee, C.J.J.; Win, K.Y.; Ni, X.P.; Teo, S.L.; Seng, D.H.L.; Tanaka, Y.; Han, M.-Y. Aqueous Synthesis, Doping, and Processing of n-Type Ag2Se for High Thermoelectric Performance at Near-Room-Temperature. Inorg. Chem. 2022, 61, 6451–6458. [Google Scholar] [CrossRef]
- Kumar, A.; Mehta, N.; Dahshan, A. A New Approach for Nano-Structuring of Glassy Selenium (g-Se) Using Silver Nanoparticles (AgNPs) as Precursor. Mater. Today Commun. 2021, 26, 101719. [Google Scholar] [CrossRef]
- Huang, S.; Wei, T.-R.; Chen, H.; Xiao, J.; Zhu, M.; Zhao, K.; Shi, X. Thermoelectric Ag2Se: Imperfection, Homogeneity, and Reproducibility. ACS Appl. Mater. Interfaces 2021, 13, 60192–60199. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, H.; Zhu, Y.-K.; Zheng, K.; Ge, Z.-H.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z.-G. Ternary Ag2Se1–xTex: A Near-Room-Temperature Thermoelectric Material with a Potentially High Figure of Merit. Inorg. Chem. 2021, 60, 14165–14173. [Google Scholar] [CrossRef]
- Achimovičová, M.; Gáborová, K.; Girman, V.; Dutková, E.; Briančin, J.; Levinský, P.; Puchý, V. Simple Mechanochemical Synthesis, Characterization, Optical and Thermoelectric Properties of a Nanostructured Silver (I) Selenide Semiconductor. Appl. Res. 2024, 3, e202300076. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, W.; Zhang, L.; Gao, H.; Wang, D.; Wu, T.; Shi, X.; Li, M.; Liu, Q.; Yang, Y.; et al. Carrier Separation Boosts Thermoelectric Performance of Flexible n-Type Ag2Se-Based Films. Adv. Energy Mater. 2024, 2401890. [Google Scholar] [CrossRef]
- Wu, H.; Shi, X.; Duan, J.; Liu, Q.; Chen, Z.-G. Advances in Ag2Se-Based Thermoelectrics from Materials to Applications. Energy Environ. Sci. 2023, 16, 1870–1906. [Google Scholar] [CrossRef]
- Wei, T.; Qiu, P.; Zhao, K.; Shi, X.; Chen, L. Ag2Q-Based (Q = S, Se, Te) Silver Chalcogenide Thermoelectric Materials. Adv. Mater. 2023, 35, 2110236. [Google Scholar] [CrossRef]
- Liang, J.; Qiu, P.; Zhu, Y.; Huang, H.; Gao, Z.; Zhang, Z.; Shi, X.; Chen, L. Crystalline Structure-Dependent Mechanical and Thermoelectric Performance in Ag2Se1-xSx System. Research 2020, 2020, 6591981. [Google Scholar] [CrossRef]
- Palaporn, D.; Kurosaki, K.; Pinitsoontorn, S. Effect of Sintering Temperature on the Thermoelectric Properties of Ag2Se Fabricated by Spark Plasma Sintering with High Compression. Adv. Energy Sustain. Res. 2023, 4, 2300082. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.H.; Li, J.M.; Zhang, J.; Qin, X.Y. High Thermoelectric Performance for an Ag2Se-Based Material Prepared by a Wet Chemical Method. Mater. Chem. Front. 2020, 4, 875–880. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Meng, F.; Wang, S.; Yan, Y.; Yang, J.; He, J.; Zhang, Q.; Uher, C.; Kanatzidis, M.G.; et al. Facile Room Temperature Solventless Synthesis of High Thermoelectric Performance Ag2Se via a Dissociative Adsorption Reaction. J. Mater. Chem. A 2017, 5, 23243–23251. [Google Scholar] [CrossRef]
- Jin, M.; Liang, J.; Qiu, P.; Huang, H.; Yue, Z.; Zhou, L.; Li, R.; Chen, L.; Shi, X. Investigation on Low-Temperature Thermoelectric Properties of Ag2Se Polycrystal Fabricated by Using Zone-Melting Method. J. Phys. Chem. Lett. 2021, 12, 8246–8255. [Google Scholar] [CrossRef]
- Lim, K.H.; Wong, K.W.; Liu, Y.; Zhang, Y.; Cadavid, D.; Cabot, A.; Ng, K.M. Critical Role of Nanoinclusions in Silver Selenide Nanocomposites as a Promising Room Temperature Thermoelectric Material. J. Mater. Chem. C 2019, 7, 2646–2652. [Google Scholar] [CrossRef]
- Grientschnig, D.; Sitte, W. Interpretation of Ionic Transport Properties of Some Silver Chalcogenides. J. Phys. Chem. Solids 1991, 52, 805–820. [Google Scholar] [CrossRef]
- Pasternak, M.; Benczer-Koller, N.; Yang, T.; Ruel, R.; Herber, R.H. Impurity-Induced Local Disorder in the Ordered State of the Superionic Conductor β-Ag2Se. Phys. Rev. B 1983, 27, 2055–2058. [Google Scholar] [CrossRef]
- Yu, T.; Ning, S.; Liu, Q.; Zhang, T.; Chen, X.; Qi, N.; Su, X.; Tang, X.; Chen, Z. Balanced High Thermoelectric Performance in N-Type and p-Type CuAgSe Realized through Vacancy Manipulation. ACS Appl. Mater. Interfaces 2023, 15, 40781–40791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, X.; Yang, D.; Zhang, Q.; Tang, X. Boosting Thermoelectric Properties of AgBi3(Sey S1–y)5 Solid Solution via Entropy Engineering. ACS Appl. Mater. Interfaces 2021, 13, 4185–4191. [Google Scholar] [CrossRef]
- Wang, W.; Shen, D.; Li, H.; Chen, C.; Chen, Y. Thermoelectric Optimization of N-Type AgBiSe2 via Se Vacancy Control and Transition-Metal Doping. ACS Appl. Energy Mater. 2023, 6, 9709–9715. [Google Scholar] [CrossRef]
- Wei, W.; Chang, C.; Yang, T.; Liu, J.; Tang, H.; Zhang, J.; Li, Y.; Xu, F.; Zhang, Z.; Li, J.-F.; et al. Achieving High Thermoelectric Figure of Merit in Polycrystalline SnSe via Introducing Sn Vacancies. J. Am. Chem. Soc. 2018, 140, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yun, J.H.; Kim, G.; Lee, J.E.; Park, S.-D.; Reith, H.; Schierning, G.; Nielsch, K.; Ko, W.; Li, A.-P.; et al. Synergetic Enhancement of Thermoelectric Performance by Selective Charge Anderson Localization–Delocalization Transition in n-Type Bi-Doped PbTe/Ag2 Te Nanocomposite. ACS Nano 2019, 13, 3806–3815. [Google Scholar] [CrossRef] [PubMed]
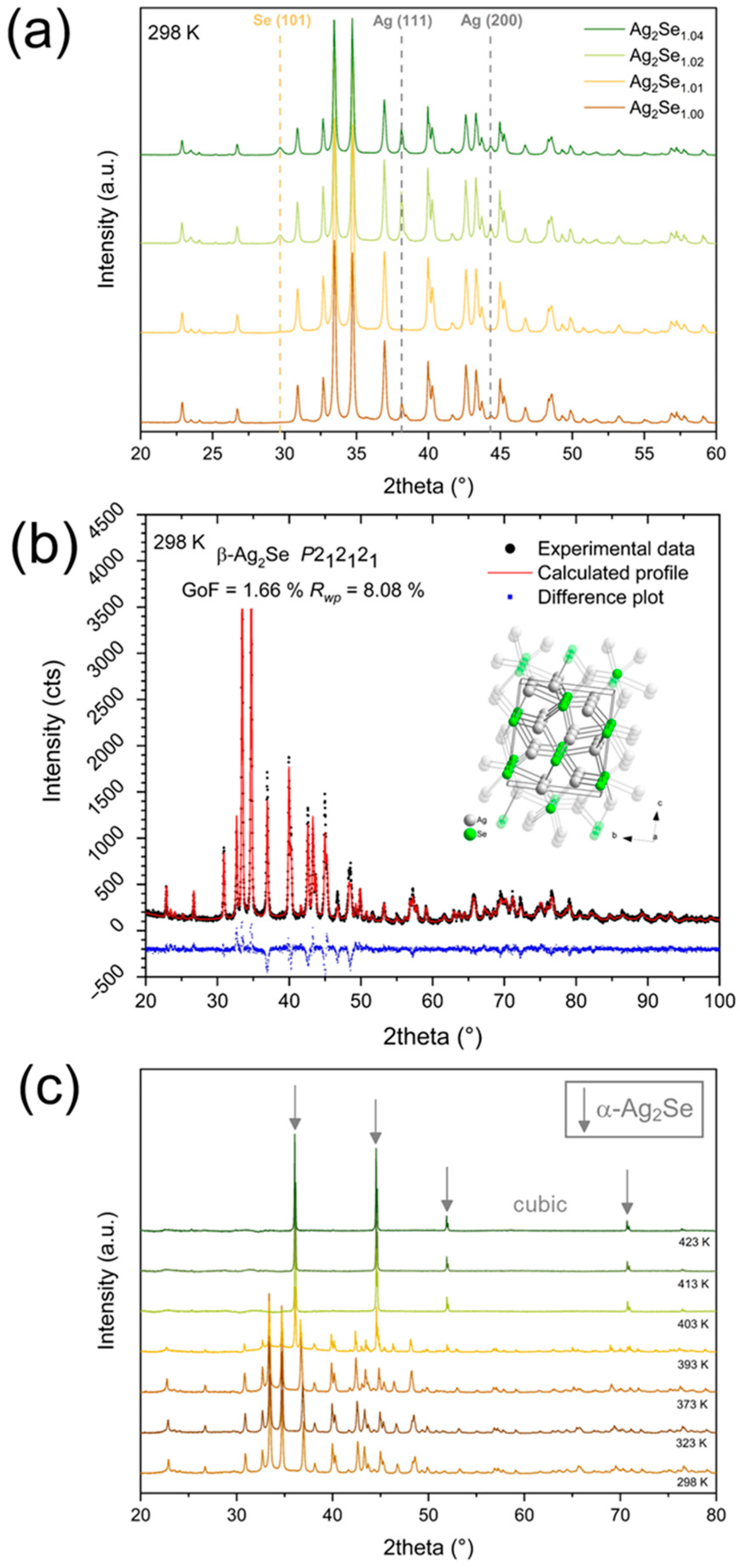
| Sample | Individual Phases (mol.%) | Lattice Parameters for Ag2Se1+x Phase (Å) | ||||
|---|---|---|---|---|---|---|
| Ag2Se | Ag | Se | a | b | c | |
| Ag2Se1.00 | 93.0 | 7.0 | 0.0 | 4.3352 ± 0.0002 | 7.0675 ± 0.0003 | 7.7723 ± 0.0003 |
| Ag2Se1.01 | 99.1 | 0.6 | 0.3 | 4.3347 ± 0.0001 | 7.0673 ± 0.0002 | 7.7725 ± 0.0002 |
| Ag2Se1.02 | 73.5 | 13.0 | 13.4 | 4.3350 ± 0.0001 | 7.0675 ± 0.0002 | 7.7726 ± 0.0002 |
| Ag2Se1.04 | 73.2 | 10.4 | 16.4 | 4.3350 ± 0.0001 | 7.0674 ± 0.0002 | 7.7723 ± 0.0002 |
| Ag2Se1.00 SPS | 100 | 0.0 | 0.0 | 4.3367 ± 0.0003 | 7.0695 ± 0.0004 | 7.7742 ± 0.0004 |
| Ag2Se1.01 SPS | 100 | 0.0 | 0.0 | 4.3356 ± 0.0002 | 7.0676 ± 0.0003 | 7.7697 ± 0.0003 |
| Ag2Se1.02 SPS | 100 | 0.0 | 0.0 | 4.3356 ± 0.0002 | 7.0678 ± 0.0003 | 7.7704 ± 0.0003 |
| Ag2Se1.04 SPS | 100 | 0.0 | 0.0 | 4.3346 ± 0.0002 | 7.0663 ± 0.0003 | 7.7680 ± 0.0003 |
| Volume of the Milling Chamber: | 250 mL |
| Material of Milling Chamber and Medium: | WC |
| Loading of Milling Chamber: | 50 balls (⌀ 10 mm) |
| Ball-to-Powder Ratio (BPR): | 73:1 |
| Total Mass of Input Reactants: | 5 g |
| Milling Atmosphere: | Ar |
| Addition: | Methanol (<1 mL) |
| Rotation Speed: | 550 rpm |
| Milling Time: | 10 min |
| Sample Composition | Experimental Density of Pellet (g·cm−3) | Experimental Density of Pellet (%) |
|---|---|---|
| Ag2Se1.00 | 7.07 | 86.1 |
| Ag2Se1.01 | 6.94 | 84.4 |
| Ag2Se1.02 | 6.71 | 81.6 |
| Ag2Se1.04 | 6.95 | 84.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gáborová, K.; Hegedüs, M.; Levinský, P.; Mihok, F.; Matvija, M.; Knížek, K.; Milkovič, O.; Vatraľová, D.; Hejtmánek, J.; Saksl, K. Thermoelectric Characteristics of β-Ag2Se1+x Prepared via a Combined Rapid Mechano-Thermal Approach. Inorganics 2024, 12, 334. https://doi.org/10.3390/inorganics12120334
Gáborová K, Hegedüs M, Levinský P, Mihok F, Matvija M, Knížek K, Milkovič O, Vatraľová D, Hejtmánek J, Saksl K. Thermoelectric Characteristics of β-Ag2Se1+x Prepared via a Combined Rapid Mechano-Thermal Approach. Inorganics. 2024; 12(12):334. https://doi.org/10.3390/inorganics12120334
Chicago/Turabian StyleGáborová, Katarína, Michal Hegedüs, Petr Levinský, František Mihok, Miloš Matvija, Karel Knížek, Ondrej Milkovič, Dagmara Vatraľová, Jiří Hejtmánek, and Karel Saksl. 2024. "Thermoelectric Characteristics of β-Ag2Se1+x Prepared via a Combined Rapid Mechano-Thermal Approach" Inorganics 12, no. 12: 334. https://doi.org/10.3390/inorganics12120334
APA StyleGáborová, K., Hegedüs, M., Levinský, P., Mihok, F., Matvija, M., Knížek, K., Milkovič, O., Vatraľová, D., Hejtmánek, J., & Saksl, K. (2024). Thermoelectric Characteristics of β-Ag2Se1+x Prepared via a Combined Rapid Mechano-Thermal Approach. Inorganics, 12(12), 334. https://doi.org/10.3390/inorganics12120334








