A Study on the Volume Expansion of Vanadium-Based Alloy Powders and Compacts During Hydrogen Sorption
Abstract
1. Introduction
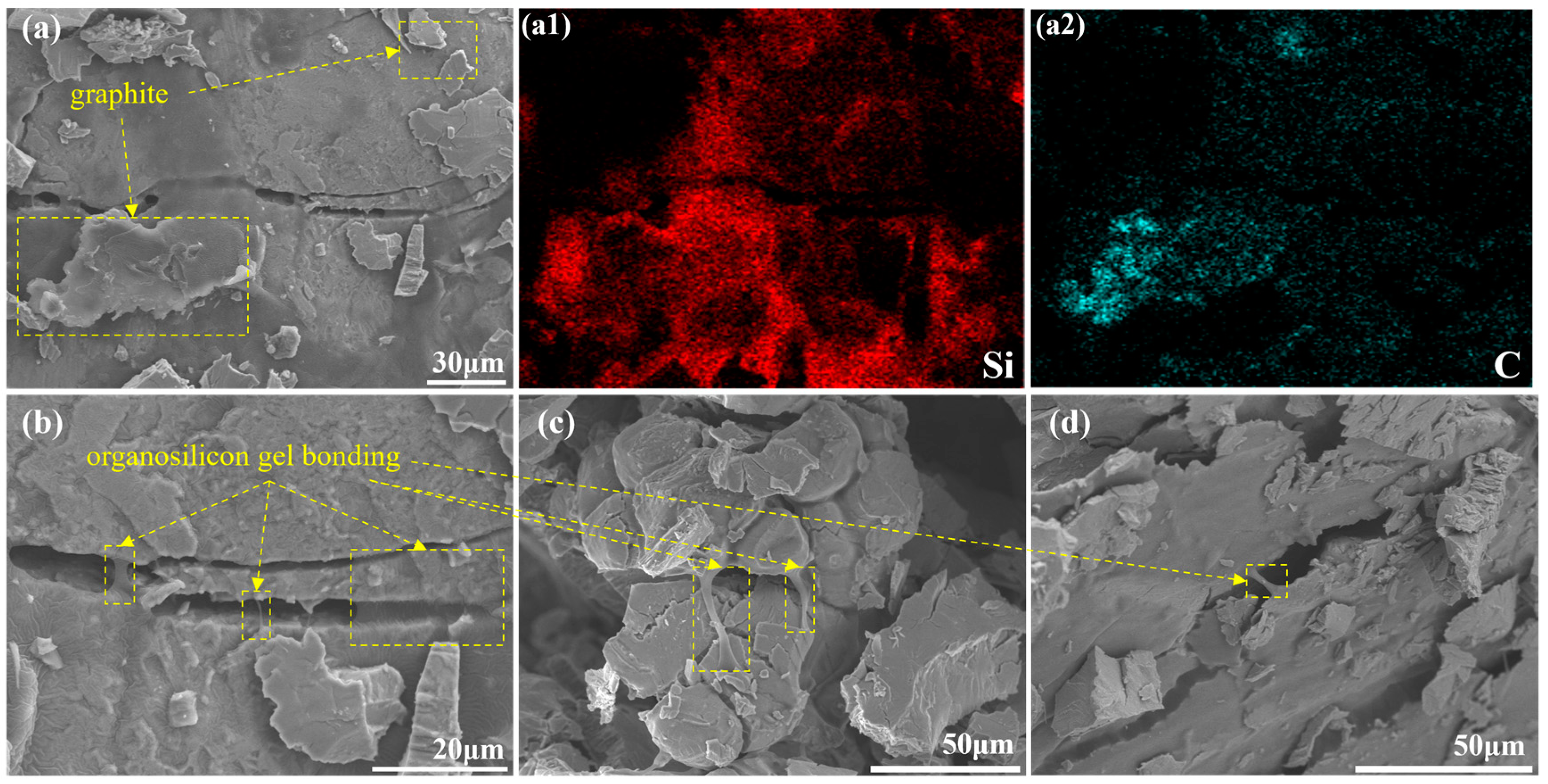
2. Results and Discussions
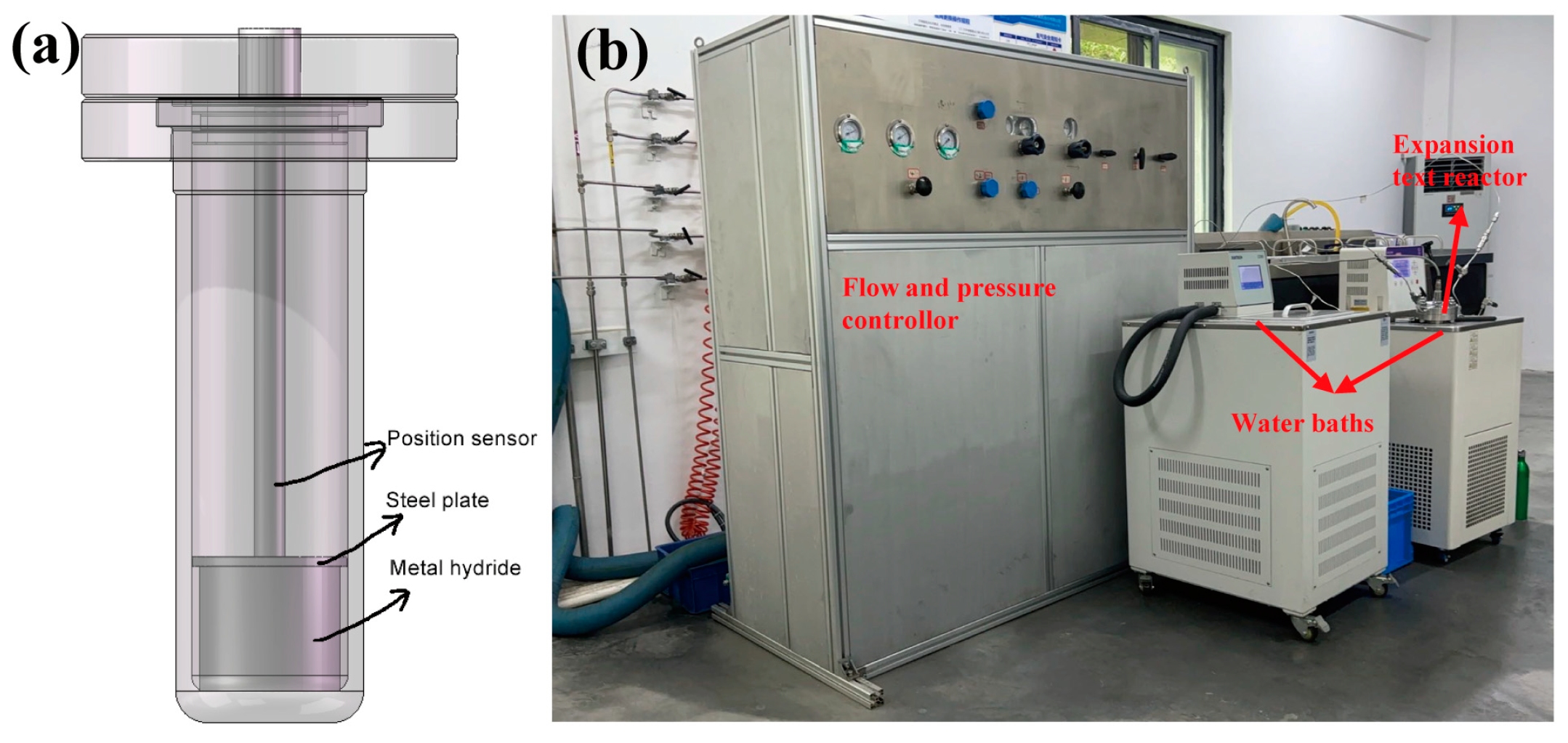
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chin. J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Dieterich, M.; Pohlmann, C.; Bürger, I.; Linder, M.; Röntzsch, L. Long-term cycle stability of metal hydride-graphite composites. Int. J. Hydrogen Energy 2015, 40, 16375–16382. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Kong, H.Y.; Xie, Q.F.; Wu, C.L.; Wang, Y.; Chen, Y.G.; Li, H.W.; Yan, Y.G. Vanadium-based alloy for hydrogen storage: A review. Rare Met. 2024, 6201–6232. [Google Scholar] [CrossRef]
- Matsushita, M.; Monde, M.; Mitsutake, Y. Experimental formula for estimating porosity in a metal hydride packed bed. Int. J. Hydrogen Energy 2013, 38, 7056–7064. [Google Scholar] [CrossRef]
- Cuscueta, D.J.; Silin, N.; Melnichuk, M. Stress reduction in a hydride container by the addition of a glidant agent. Int. J. Hydrogen Energy 2020, 45, 27452–27456. [Google Scholar] [CrossRef]
- Luo, L.; Wu, C.; Yang, S.; Zhou, J.; Chen, Y.; Yang, F.; Xu, Y.; Liu, P. Decaying behaviors of V40(TiCr)51Fe8Mn hydrogen storage alloys with different particle sizes. J. Alloys Compd. 2015, 645, S178–S183. [Google Scholar] [CrossRef]
- Herbrig, K.; Pohlmann, C.; Gondek, Ł.; Figiel, H.; Kardjilov, N.; Hilger, A.; Manke, I.; Banhart, J.; Kieback, B.; Röntzsch, L. Investigations of the structural stability of metal hydride composites by in-situ neutron imaging. J. Power Sources 2015, 293, 109–118. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, C.; Yan, Y.; Wang, Y.; Tang, X.; Chen, Y.; Li, J.; Wang, M.; Xie, Q.; Chen, Y.; et al. Effect of dehydrogenation depth on cyclic hydrogen desorp tion properties of V40Ti25.5Cr26.5Fe8 alloy. J. Alloys Compd. 2023, 955, 170036. [Google Scholar] [CrossRef]
- Heubner, F.; Pohlmann, C.; Mauermann, S.; Kieback, B.; Röntzsch, L. Mechanical stresses originating from metal hydride composites during cyclic hydrogenation. Int. J. Hydrogen Energy 2015, 40, 10123–10130. [Google Scholar] [CrossRef]
- Charlas, B.; Gillia, O.; Doremus, P.; Imbault, D. Experimental investigation of the swelling/shrinkage of a hydride bed in a cell during hydrogen absorption/desorption cycles. Int. J. Hydrogen Energy 2012, 37, 16031–16041. [Google Scholar] [CrossRef]
- Wu, K.; Cai, B.; Fan, L.; Qin, L.; Chen, D.; Huang, Y. Stress measurement of MlNi4.5Cr0·45Mn0.05 alloy during hydrogen absorption-desorption process in a cylindrical reactor. Int. J. Hydrogen Energy 2020, 45, 28175–28182. [Google Scholar] [CrossRef]
- Gattia, D.M.; Jangir, M.; Jain, I.P. Behavior of Compacted Magnesium-Based Powders for Energy-Storage Applications. Inorganics 2020, 8, 54. [Google Scholar] [CrossRef]
- Duan, W.; Du, J.; Wang, Z.; Niu, Y.; Huang, T.; Li, Z.; Pu, C.; Wu, Z. Strain variation on the reaction tank of high hydrogen content during hydrogen absorptiondesorption cycles. Int. J. Hydrogen Energy 2013, 38, 2347–2351. [Google Scholar] [CrossRef]
- Gabriel, R.A.N.; Cesar, A.G.B.; Daniel, R.L.; Luiz, A.P. Polyetherimide-LaNi5 composite films for hydrogen storage applications. Int. J. Hydrogen Energy 2021, 46, 32767–32778. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Tavakoli, O. Investigation of graphene-based systems for hydrogen storage. Renew. Sustain. Energy Rev. 2017, 74, 104–109. [Google Scholar] [CrossRef]
- Kazuya, K.; Yoshinori, K.; Hideaki, I. Development of large MH tank system for renewable energy storage. Int. J. Hydrogen Energy 2017, 42, 22475–22479. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.A.; Fursikov, P.V. Composite materials with 2D graphene structures: Applications for hydrogen energetics and catalysis with hydrogen participation. J. Struct. Chem. 2018, 59, 830–838. [Google Scholar] [CrossRef]
- Carsten, P.; Lars, R.; Siarhei, K.; Thomas, H.; Bernd, K. Magnesium alloy-graphite composites with tailored heat conduction properties for hydrogen storage applications. Int. J. Hydrogen Energy 2010, 35, 12829–12836. [Google Scholar] [CrossRef]
- Strugova, D.V.; Zadorozhnyy, M.Y.; Berdonosova, E.A.; Yablokova, M.Y.; Konik, P.A.; Zheleznyi, M.V.; Semenov, D.V.; Milovzorov, G.S.; Padaki, M.; Kaloshkin, S.D.; et al. Novel process for preparation of metal-polymer composite membranes for hydrogen separation. Int. J. Hydrogen Energy 2018, 43, 12146–12152. [Google Scholar] [CrossRef]
- Zheng, X.; Kong, H.Y.; Chu, D.S.; Hu, F.P.; Wang, Y.; Yan, Y.G.; Wu, C.L. Stress Reduction of a V-Based BCC Metal Hydride Bed Using Silicone Oil as a Glidant. Inorganics 2022, 10, 167. [Google Scholar] [CrossRef]
- Marc, P.W.; Georgette, B.; Salieb, B.; Patrick, H. PDMS with designer functionalities-properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Warfsmann, J.; Puszkiel, J.A.; Passing, M.; Krause, P.S.; Wienken, E.; Taube, K.; Klassen, T.; Pistidda, C.; Jepsen, J. Applying wash coating techniques for swelling-induced stress reduction and thermal improvement in metal hydrides. J. Alloys Compd. 2023, 950, 169814. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]

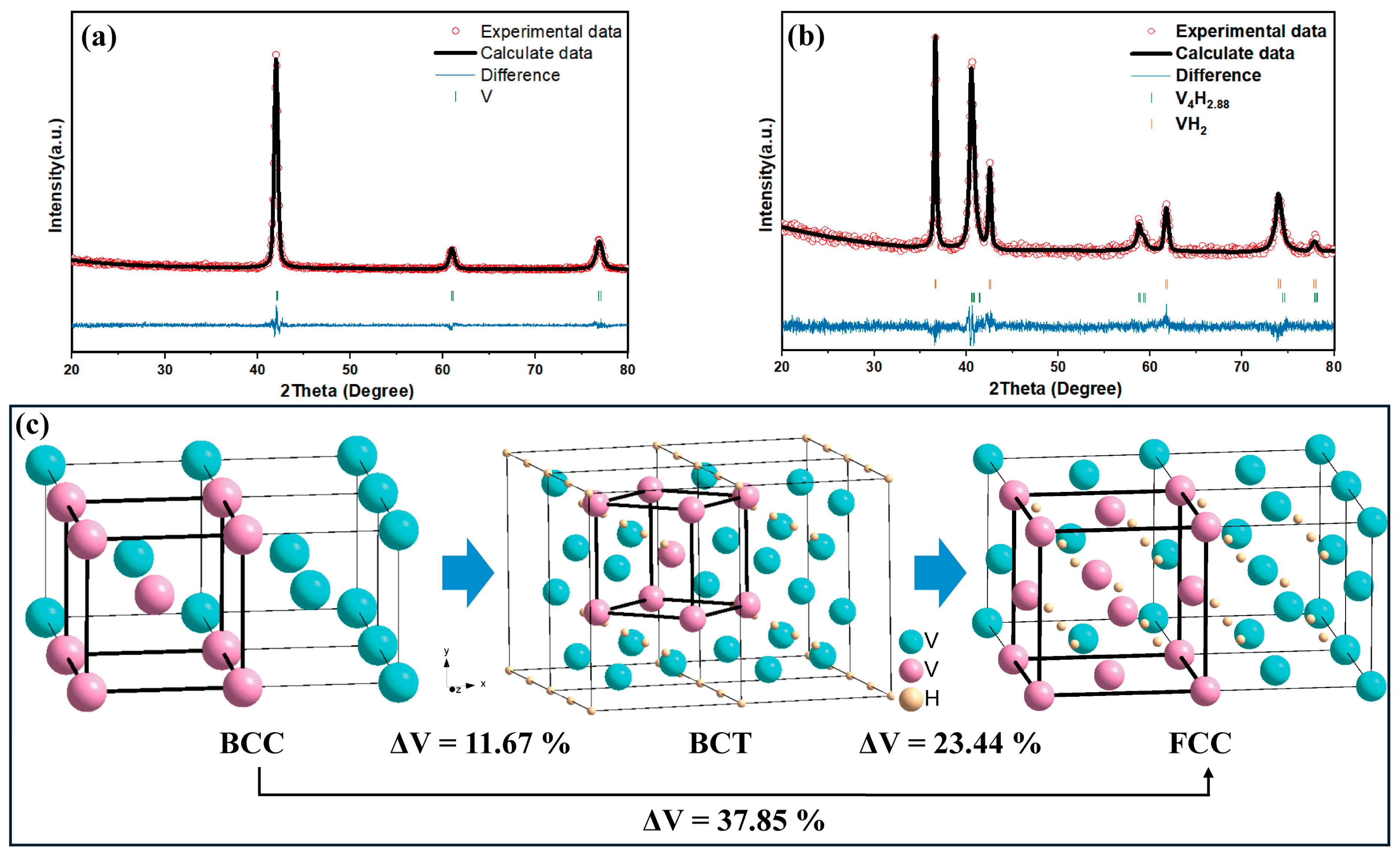
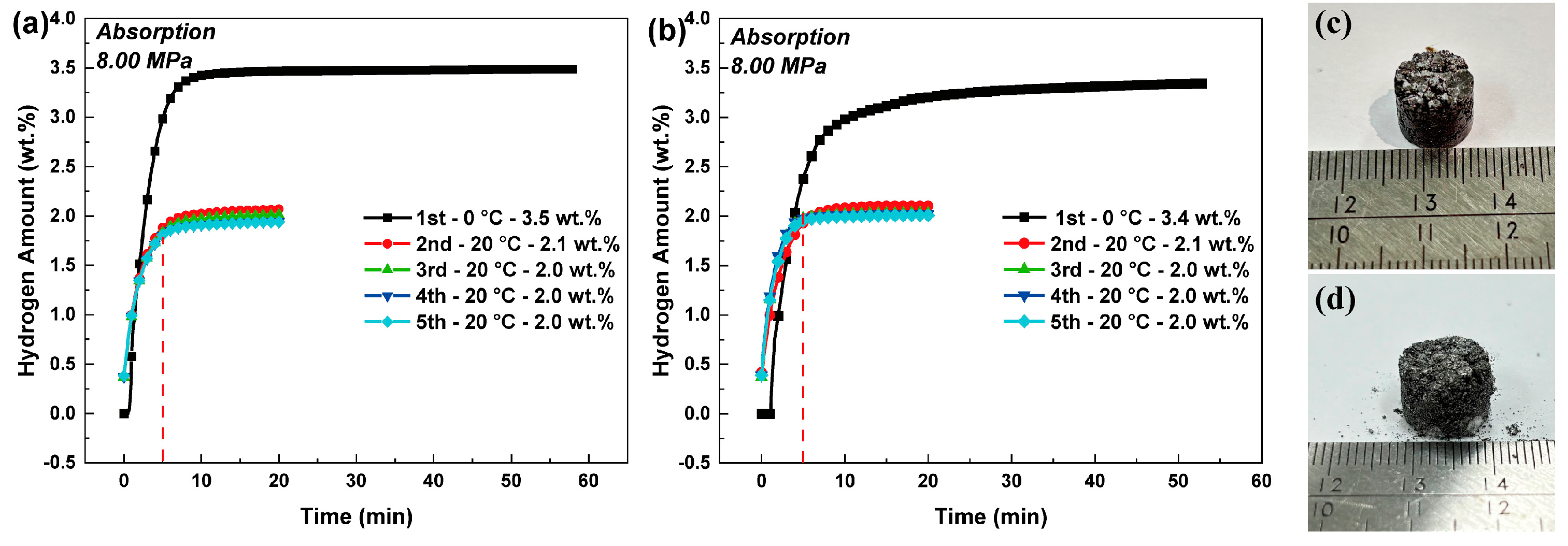
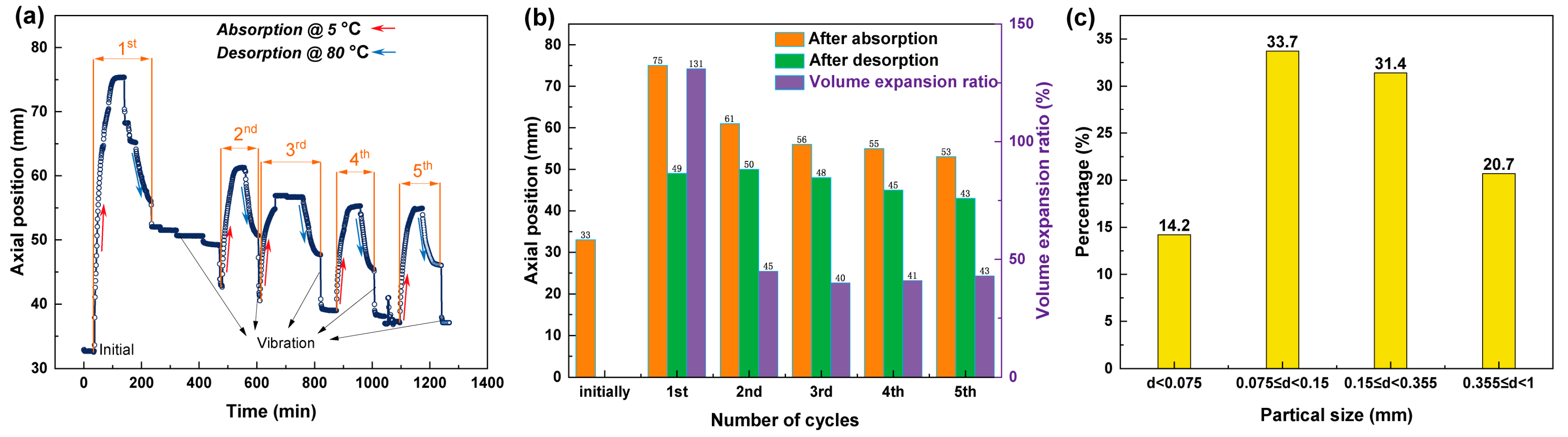
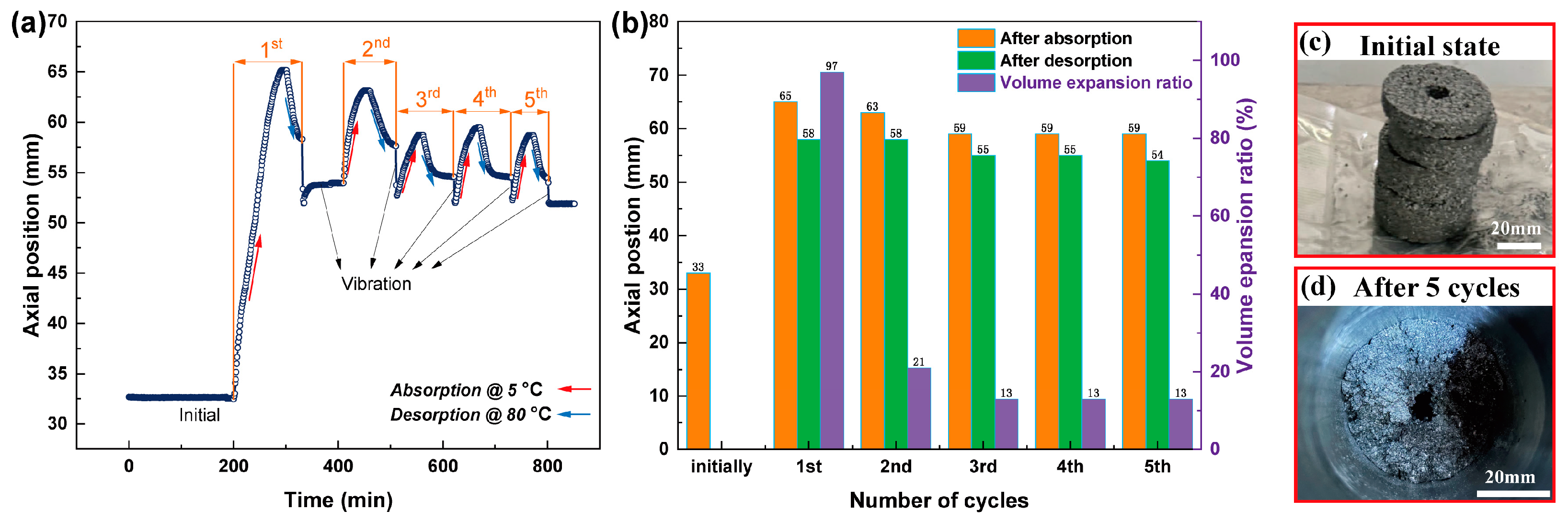
| Properties | Hydrogen Absorption | Hydrogen Desorption |
|---|---|---|
| ΔH (kJ/mol H2) | −31.18 | 36.74 |
| ΔS (J/mol H2) | −129.36 | 138.99 |
| Phase | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|
| BCC | 0.3031(1) | 0.3031(1) | 0.3031(1) | 0.02785 |
| BCT | 0.3143(0) | 0.3143(0) | 0.3148(3) | 0.03110 |
| FCC | 0.4250(3) | 0.4250(3) | 0.4250(3) | 0.07678 |
| Sensor Movement Distance Caused by Vibration (mm) | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Powder bed | 42.88 | 40.54 | 38.99 | 37.10 | 36.00 |
| Compact bed | 53.95 | 52.73 | 51.99 | 52.27 | 51.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Hu, Y.; Kong, H.; Huang, Q.; Chen, Y.; Yan, Y. A Study on the Volume Expansion of Vanadium-Based Alloy Powders and Compacts During Hydrogen Sorption. Inorganics 2024, 12, 318. https://doi.org/10.3390/inorganics12120318
Li M, Hu Y, Kong H, Huang Q, Chen Y, Yan Y. A Study on the Volume Expansion of Vanadium-Based Alloy Powders and Compacts During Hydrogen Sorption. Inorganics. 2024; 12(12):318. https://doi.org/10.3390/inorganics12120318
Chicago/Turabian StyleLi, Mojia, Yunfeng Hu, Hanyang Kong, Qiuwei Huang, Yusong Chen, and Yigang Yan. 2024. "A Study on the Volume Expansion of Vanadium-Based Alloy Powders and Compacts During Hydrogen Sorption" Inorganics 12, no. 12: 318. https://doi.org/10.3390/inorganics12120318
APA StyleLi, M., Hu, Y., Kong, H., Huang, Q., Chen, Y., & Yan, Y. (2024). A Study on the Volume Expansion of Vanadium-Based Alloy Powders and Compacts During Hydrogen Sorption. Inorganics, 12(12), 318. https://doi.org/10.3390/inorganics12120318







