Reactivity of [3+1+1] Uranyl-DGA Complex as Lewis-Acid Catalyst in Nucleophilic Acyl Substitution of Acid Anhydrides
Abstract
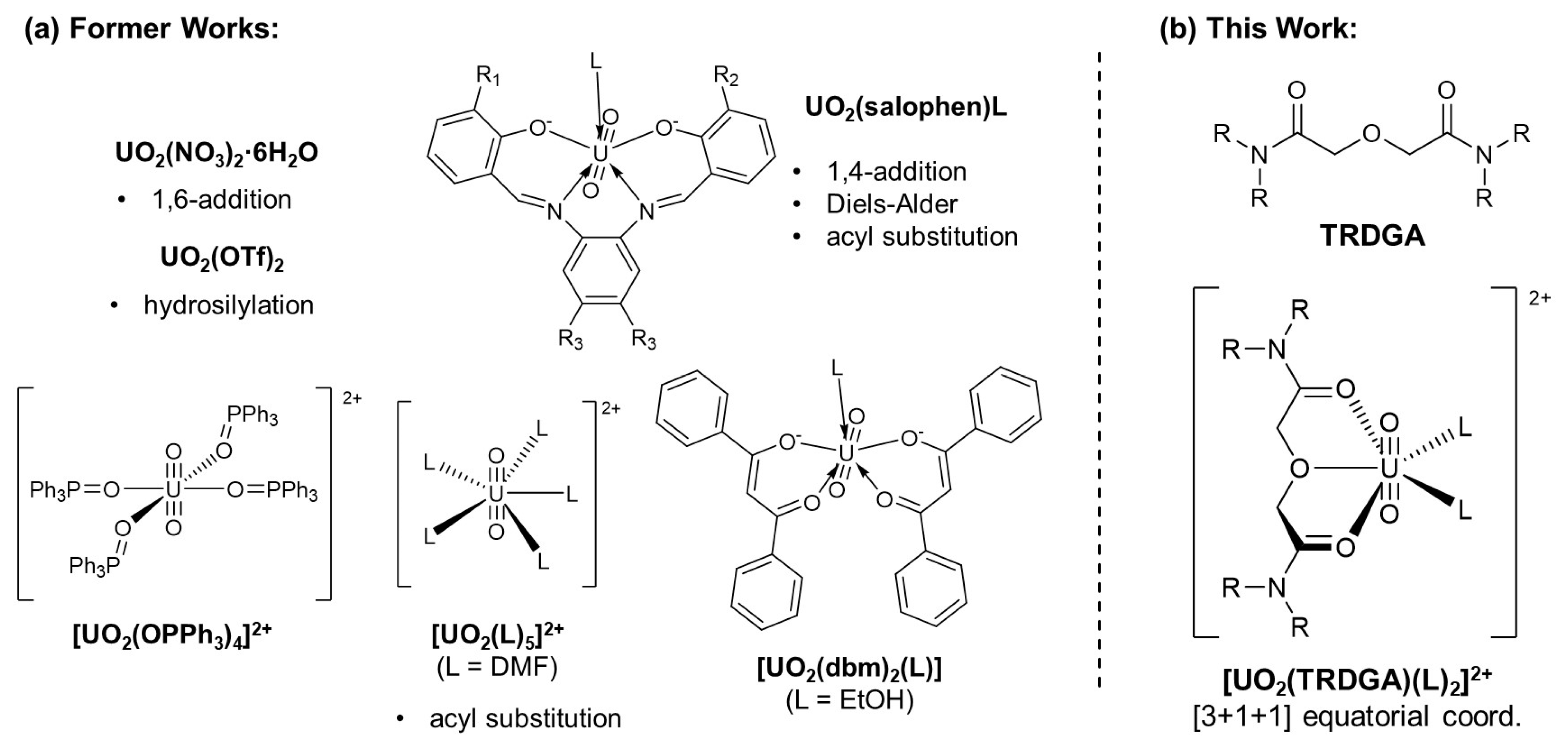
1. Introduction
2. Results and Discussion
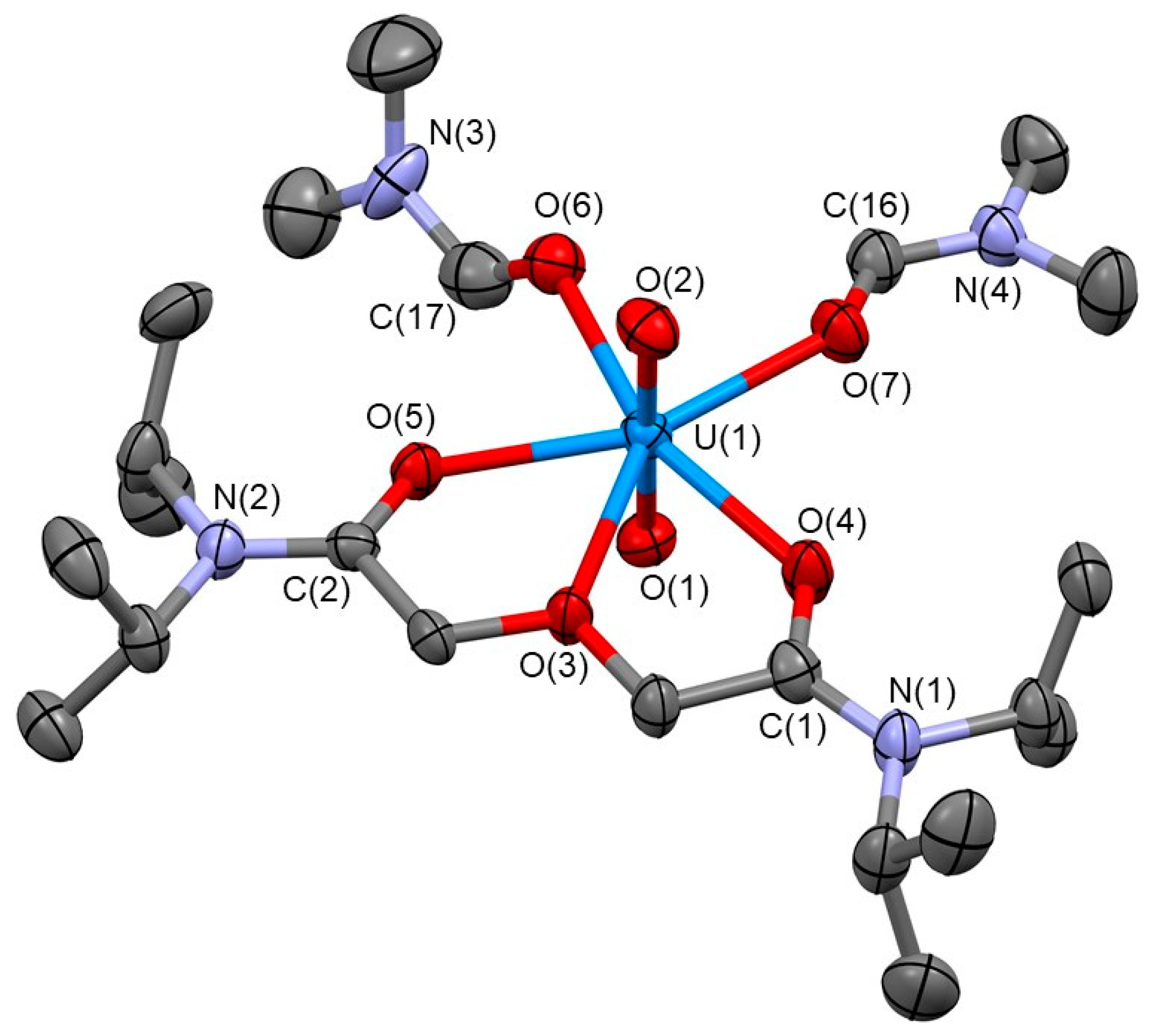
2.1. Synthesis and Characterisation of UO22+-Diglycolamide Complex
2.2. Nucleophilic Acyl Substitution Reactions Catalysed by [UO2(TiPDGA)(DMF)2]2+
2.3. Reaction Kinetics and Catalysis Mechanism
log βsub
log β20
log β11
3. Materials and Methods
3.1. Materials
3.2. Synthesis of N, N, N′, N′-Tetraisoprolyldiglycolamide (TiPDGA)
3.3. Synthesis of [UO2(TiPDGA)(DMF)2](ClO4)2·CH2Cl2
3.3.1. Characterisation of [UO2(TiPDGA)(DMF)2](ClO4)2·CH2Cl2
3.3.2. Characterisation of [UO2(TiPDGA)(DMF)(H2O)](ClO4)2⋅H2O
3.4. Methods
3.5. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takao, K. How Does Chemistry Contribute to Circular Economy in Nuclear Energy Systems to Make Them More Sustainable and Ecological? Dalton Trans. 2023, 52, 9866–9881. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Levi, H.; Pigford, T. Nuclear Chemical Engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Nuclear Energy Agency. Advanced Nuclear Fuel Cycles and Radioactive Waste Management; OECD-NEA: Paris, France, 2006. [Google Scholar]
- OECD Nuclear Energy Agency. Actinide and Fission Product Partitioning and Transmutation, Status and Assessment Report; OECD-NEA: Paris, France, 1999. [Google Scholar]
- OECD Nuclear Energy Agency. Actinide and Fission Product Partitioning and Transmutation, Eleventh Information Exchange Meeting; OECD-NEA: Paris, France, 2012. [Google Scholar]
- Fujita, R.; Kawashima, M.; Ozawa, M.; Matsuzaki, T. Reduction and Resource Recycling of High-level Radioactive Wastes through Nuclear Transmutation—Overview and Current Progress—. JPS Conf. Proc. 2020, 32, 010098. [Google Scholar] [CrossRef]
- Morss, L.R.; Edelstein, N.M.; Fuger, J. (Eds.) The Chemistry of the Actinide and Transactinide Elements; Springer: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Barluzzi, L.; Giblin, S.R.; Mansikkamaki, A.; Layfield, R.A. Identification of Oxidation State +1 in a Molecular Uranium Complex. J. Am. Chem. Soc. 2022, 144, 18229–18233. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Fieser, M.E.; Bates, J.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Identification of the +2 oxidation state for uranium in a crystalline molecular complex, [K(2.2.2-cryptand)][(C5H4SiMe3)3U]. J. Am. Chem. Soc. 2013, 135, 13310–13313. [Google Scholar] [CrossRef]
- Billow, B.S.; Livesay, B.N.; Mokhtarzadeh, C.C.; McCracken, J.; Shores, M.P.; Boncella, J.M.; Odom, A.L. Synthesis and Characterization of a Neutral U(II) Arene Sandwich Complex. J. Am. Chem. Soc. 2018, 140, 17369–17373. [Google Scholar] [CrossRef] [PubMed]
- La Pierre, H.S.; Scheurer, A.; Heinemann, F.W.; Hieringer, W.; Meyer, K. Synthesis and Characterization of a Uranium(II) Monoarene Complex Supported by Delta Backbonding. Angew. Chem. Int. Ed. 2014, 53, 7158–7162. [Google Scholar] [CrossRef] [PubMed]
- Barnea, E.; Eisen, M. Organoactinides in catalysis. Coord. Chem. Rev. 2006, 250, 855–899. [Google Scholar] [CrossRef]
- Liu, H.; Ghatak, T.; Eisen, M.S. Organ actinides in catalytic transformations: Scope, mechanisms and Quo Vadis. Chem. Commun. 2017, 53, 11278–11297. [Google Scholar] [CrossRef] [PubMed]
- Cowie, B.E.; Purkis, J.M.; Austin, J.; Love, J.B.; Arnold, P.L. Thermal and Photochemical Reduction and Functionalization Chemistry of the Uranyl Dication, [UVIO2]2+. Chem. Rev. 2019, 119, 10595–10637. [Google Scholar] [CrossRef]
- Behera, N.; Sethi, S. Unprecedented Catalytic Behavior of Uranyl(VI) Compounds in Chemical Reactions. Eur. J. Inorg. Chem. 2021, 2021, 95–111. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Takao, K.; Takao, S.; Ikeda, Y.; Bernhard, G.; Hennig, C. Uranyl-Halide Complexation in N,N-Dimethylformamide: Halide Coordination Trend Manifests Hardness of [UO2]2+. Dalton Trans. 2013, 42, 13101–13111. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Liu, K.; Yuan, L.; Zhao, Y.; Chai, Z.; Mei, L. Facile Construction of Diverse Diarylmethane Scaffolds via Uranyl-Catalyzed 1,6-Addition Reaction. Tetrahedron Lett. 2020, 61, 152076. [Google Scholar] [CrossRef]
- Van Axel Castelli, V.; Cort, A.D.; Mandolini, L.; Reinhoudt, D.N. Supramolecular Catalysis of 1,4-Thiol Addition by Salophen−Uranyl Complexes. J. Am. Chem. Soc. 1998, 120, 12688–12689. [Google Scholar] [CrossRef]
- Van Axel Castelli, V.; Dalla Cort, A.; Mandolini, L.; Reinhoudt, D.N.; Schiaffino, L. Catalysis fo the Addition of Benzenethiol to 2-Cyclohexene-1-Ones by Uranyl-Salophen Complexes: A Catalytic Metalloclreft with High Substrate Specificity. Chem. Eur. J. 2000, 6, 1193–1198. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Mandolini, L.; Schiaffino, L. Exclusive Transition State Stabilization in the Supramolecular Catalysis of Diels—Alder Reaction by a Uranyl Salophen Complex. Chem. Commun. 2005, 3867–3869. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Akashi, S. Exploring the Catalytic Activity of Lewis-Acidic Uranyl Complexes in the Nucleophilic Acyl Substitution of Acid Anhydrides. RSC Adv. 2017, 7, 12201–12207. [Google Scholar] [CrossRef]
- Monsigny, L.; Thuéry, P.; Berthet, J.-C.; Cantat, T. Breaking C–O Bonds with Uranium: Uranyl Complexes as Selective Catalysts in the Hydrosilylation of Aldehydes. ACS Catal. 2019, 9, 9025–9033. [Google Scholar] [CrossRef]
- Yu, J.; Chen, S.; Liu, K.; Yuan, L.; Mei, L.; Chai, Z.; Shi, W. Uranyl-Catalyzed Hydrosilylation of Para-Quinone Methides: Access to Diarylmethane Derivatives. Org. Biomol. Chem. 2021, 19, 1575–1579. [Google Scholar] [CrossRef]
- Sasaki, Y.; Choppin, G.R. Solvent Extraction of Eu, Th, U, Np and Am with N,N′-Dimethyl-N,N′-Dihexyl-3-Oxapentanediamide and Its Analogous Compounds. Anal. Sci. 1996, 12, 225–230. [Google Scholar] [CrossRef]
- Kannan, S.; Moody, M.A.; Barnes, C.L.; Duval, P.B. Lanthanum(III) and Uranyl(VI) Diglycolamide Complexes: Synthetic Precursors and Structural Studies Involving Nitrate Complexation. Inorg. Chem. 2008, 47, 4691–4695. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, K.; Kurahashi, K.; Naganawa, H. Extraction Behavior of Lanthanides Using a Diglycolamide Derivative TODGA in Ionic Liquids. Dalton Trans. 2008, 5083–5088. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Okumura, S.; Sasaki, Y.; Ikeda, Y. Crystal Structures of Ln(III) (Ln = La, Pr, Nd, Sm, Eu, and Gd) Complexes with N,N,N′,N′-Tetraethyldiglycolamide Associated with Homoleptic [Ln(NO3)6)]3−. Bull. Chem. Soc. Jpn. 2014, 87, 294–300. [Google Scholar] [CrossRef]
- Tian, G.; Rao, L.; Teat, S.J.; Liu, G. Quest for Environmentally Benign Ligands for Actinide Separations: Thermodynamic, Spectroscopic, and Structural Characterization of U(VI) Complexes with Oxa-Diamide and Related Ligands. Chem. Eur. J. 2009, 15, 4172–4181. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Wadawale, A.P.; Verboom, W.; Mohapatra, P.K. Isolation of Single Crystals of a Homoleptic UO22+-Diglycolamide Complex from a Room Temperature Ionic Liquid: X-ray Crystallography and Complexation Studies. New J. Chem. 2022, 46, 950–954. [Google Scholar] [CrossRef]
- Linert, W.; Fukuda, Y.; Camard, A. Chromotropism of Coordination Compounds and Its Applications in Solution. Coord. Chem. Rev. 2001, 218, 113–152. [Google Scholar] [CrossRef]
- Deshayes, L.; Keller, N.; Lance, M.; Nierlich, M.; Vigner, D. Structure of Pentakis (N,N-dimethylformamide) Dioxouranium(VI) Tetrafluoroborate. Acta Crystallogr. 1992, C48, 1660–1661. [Google Scholar] [CrossRef]
- Takao, K.; Ikeda, Y. Structural Characterization and Reactivity of UO2(salophen)L and [UO2(salophen)]2: Dimerization of UO2(salophen) Fragments in Noncoordinating Solvents (salophen = N,N′-disalicylidene-o-phenylenediaminate, L = N,N-dimethylformamide, dimethyl sulfoxide). Inorg. Chem. 2007, 46, 1550–1562. [Google Scholar] [CrossRef]
- Sabatini, A.; Vacca, A.; Gans, P. Mathematical Algorithms and Computer Programs for the Determination of Equilibrium Constants from Potentiometric and Spectrophotometric Measurements. Coord. Chem. Rev. 1992, 120, 389–405. [Google Scholar] [CrossRef]
- Rossberg, A.; Reich, T.; Bernhard, G. Complexation of Uranium(VI) with Protocatechuic Acid-Application of Iterative Transformation Factor Analysis to EXAFS Spectroscopy. Anal. Bioanal. Chem. 2003, 376, 631–638. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]




| Entry | R b | R′ c | Yield (%) d |
|---|---|---|---|
| 1 | Me | 2-phenylethyl | 90 |
| 2 | Me | 2-phenylethyl | 9 e |
| 3 | Me | Me | 100 |
| 4 | Me | Et | 88 |
| 5 | Me | nBu | 88 |
| 6 | Me | Ph | 95 |
| 7 | Me | tBu | 78 |
| 8 | Et | 2-phenylethyl | 85 |
| 9 | iPr | 2-phenylethyl | 48 |
| 10 | tBu | 2-phenylethyl | 10 |
| 11 | Ph | 2-phenylethyl | 6 |
| 12 | tBu | Me | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akashi, S.; Takao, K. Reactivity of [3+1+1] Uranyl-DGA Complex as Lewis-Acid Catalyst in Nucleophilic Acyl Substitution of Acid Anhydrides. Inorganics 2024, 12, 324. https://doi.org/10.3390/inorganics12120324
Akashi S, Takao K. Reactivity of [3+1+1] Uranyl-DGA Complex as Lewis-Acid Catalyst in Nucleophilic Acyl Substitution of Acid Anhydrides. Inorganics. 2024; 12(12):324. https://doi.org/10.3390/inorganics12120324
Chicago/Turabian StyleAkashi, Shin, and Koichiro Takao. 2024. "Reactivity of [3+1+1] Uranyl-DGA Complex as Lewis-Acid Catalyst in Nucleophilic Acyl Substitution of Acid Anhydrides" Inorganics 12, no. 12: 324. https://doi.org/10.3390/inorganics12120324
APA StyleAkashi, S., & Takao, K. (2024). Reactivity of [3+1+1] Uranyl-DGA Complex as Lewis-Acid Catalyst in Nucleophilic Acyl Substitution of Acid Anhydrides. Inorganics, 12(12), 324. https://doi.org/10.3390/inorganics12120324








