Guardians of the Genome: Iron–Sulfur Proteins in the Nucleus
Abstract
1. Introduction
| Participates In | Name | Consolidated Localization | Fe-S Cluster Type |
|---|---|---|---|
| DNA replication/DNA repair | PRIM2 | Nucleus | [4Fe-4S] (Cys)4 [8,9] |
| POLA1 | Nucleus, Cytosol [10] | [4Fe-4S] (Cys)4 [11] | |
| POLD1 | Nucleus, Cytosol [12] | [4Fe-4S] (Cys)4 [11] | |
| POLE | Nucleus | [4Fe-4S] (Cys)4 [11,13] | |
| REV3L | Nucleus, Cytosol [14] | [4Fe-4S] (Cys)4 [11] | |
| FANCJ | Nucleus, Cytosol [15,16] | [4Fe-4S] (Cys)4 [4,17] | |
| DDX11 | Nucleus [18] | [4Fe-4S] (Cys)4 [19] | |
| RTEL1 | Nucleus [16] | [4Fe-4S] (Cys)4 [20] | |
| XPD | Nucleus, Cytosol [16,21] | [4Fe-4S] (Cys)4 [4] | |
| DNA2 | Nucleus, Mitochondria [22] | [4Fe-4S] (Cys)4 [23,24] | |
| EXO5 | Nucleus, Cytosol [25] | [4Fe-4S] (Cys)4 [25] | |
| MUTYH | Nucleus, Mitochondria [26] | [4Fe-4S] (Cys)4 [27,28] | |
| NTHL1 | Nucleus, Mitochondria [29] | [4Fe-4S] (Cys)4 [30,31] | |
| RNA transactions | ELP3 | Nucleus, Cytosol [32] | [4Fe-4S] (Cys)3 SAM [33,34] |
| RPC6 | Nucleus [35] | [4Fe-4S] (Cys)4 [36,37] | |
| CPSF4 | Nucleus [38] | [2Fe-2S] (Cys)3 (His)? [39,40] | |
| TYW1 | Nucleus, Cytosol | [4Fe-4S] (Cys)3 SAM [34,41] | |
| TYW1B | Nucleus | [4Fe-4S] (Cys)3 SAM [34,41] | |
| CDK5RAP1 | Nucleus, Mitochondria, Cytosol [42] | [4Fe-4S] (Cys)4, [4Fe-4S] (Cys)3 SAM [34,43] | |
| Mitosis | KIF4A | Nucleus [44] | [4Fe-4S] (Cys)4? [45] |
| KIF4B | Nucleus [44] | [4Fe-4S] (Cys)4? [45] | |
| Iron metabolism | NCOA4 | Nucleus, Cytosol [46] | [3Fe-4S] (Cys)4? [47,48] |
| FBXL5 | Nucleus, Perinuclear region [49] | [2Fe-2S] (Cys)4 [50] | |
| Post-translational modifications | DPH1 | Nucleus, Cytosol [51] | [4Fe-4S] (Cys)3 SAM [52] |
| DPH2 | Nucleus, Cytosol | [4Fe-4S] (Cys)3 SAM [52] | |
| ATE1 | Nucleus, Cytosol [53] | [4Fe-4S] (Cys)4 [54] | |
| Respiration | SDHB | Mitochondria, Nucleus [55] | [2Fe-2S] (Cys)4, [3Fe-4S] (Cys)3, [4Fe-4S] (Cys)4 [55] |
| Unknown | RFESD | Nucleus | [2Fe-2S] (Cys)2 (His)2 |
| CIA | CIAPIN1 | Nucleus, Mitochondria, Cytosol [56] | [2Fe-2S] (Cys)4, [4Fe-4S] (Cys)4? [57,58] |
| BOLA2 | Nucleus, Cytosol [59] | [2Fe-2S] * Ligands shared with GLRX3 [60,61] | |
| GLRX3 | Nucleus, Cytosol [62] | [2Fe-2S] * Ligands shared with BOLA2 [60,61] | |
| NUBP2 | Nucleus, Cytosol [63] | [4Fe-4S] * Ligands shared with NUBP1 [64,65] | |
| ISC | NFU1 | Nucleus, Mitochondria, Cytosol [66] | [4Fe-4S] * Ligands shared between dimers [66,67] |
| NFS1 | Nucleus, Mitochondria, Cytosol [68] | [2Fe-2S] * Ligands shared with ISCU [69,70] | |
| GLRX2 | Nucleus, Mitochondria [71] | [2Fe-2S] * Ligands shared between dimers [72] |
2. Metallation of Nuclear Fe-S Cluster Proteins
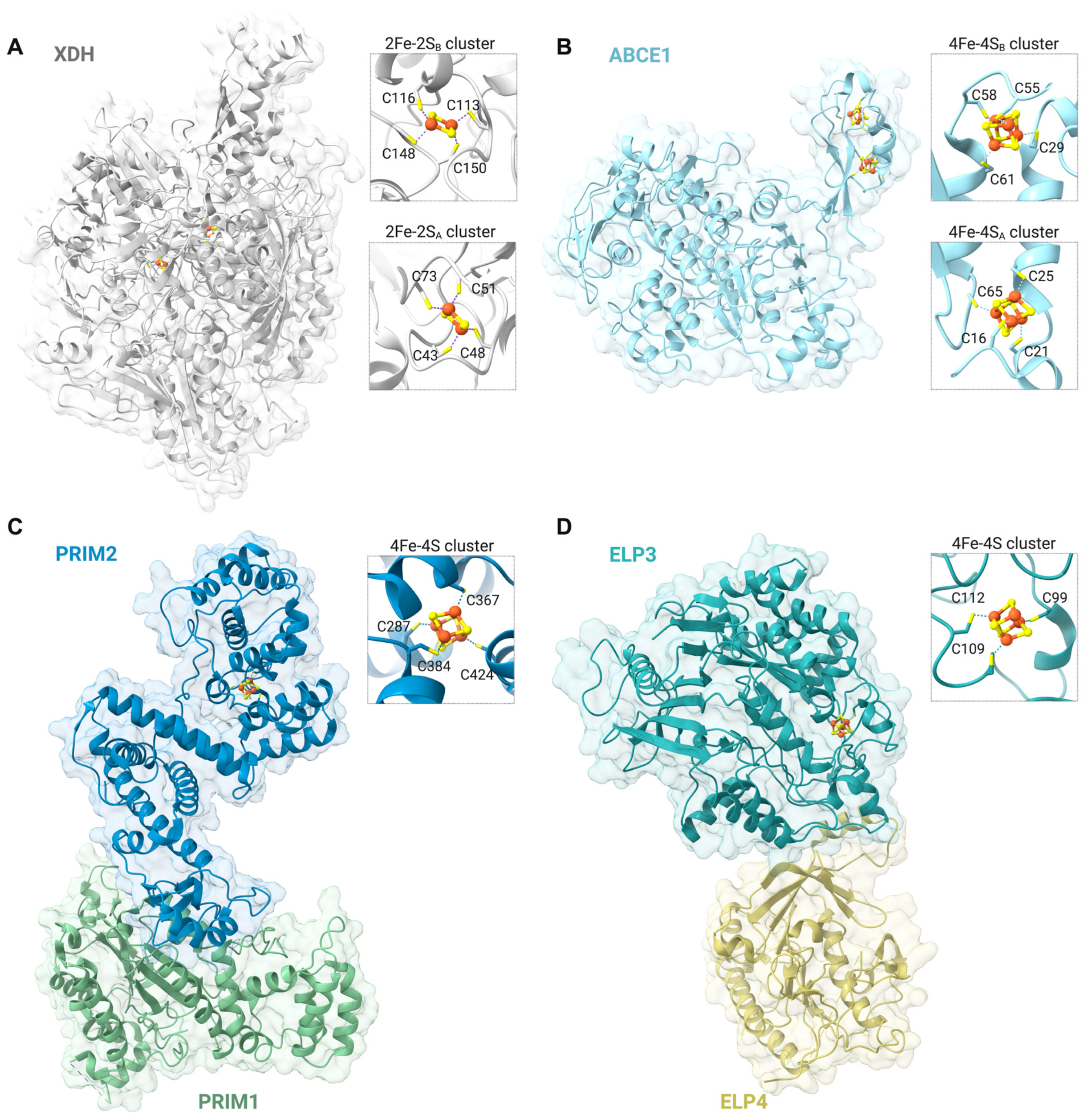
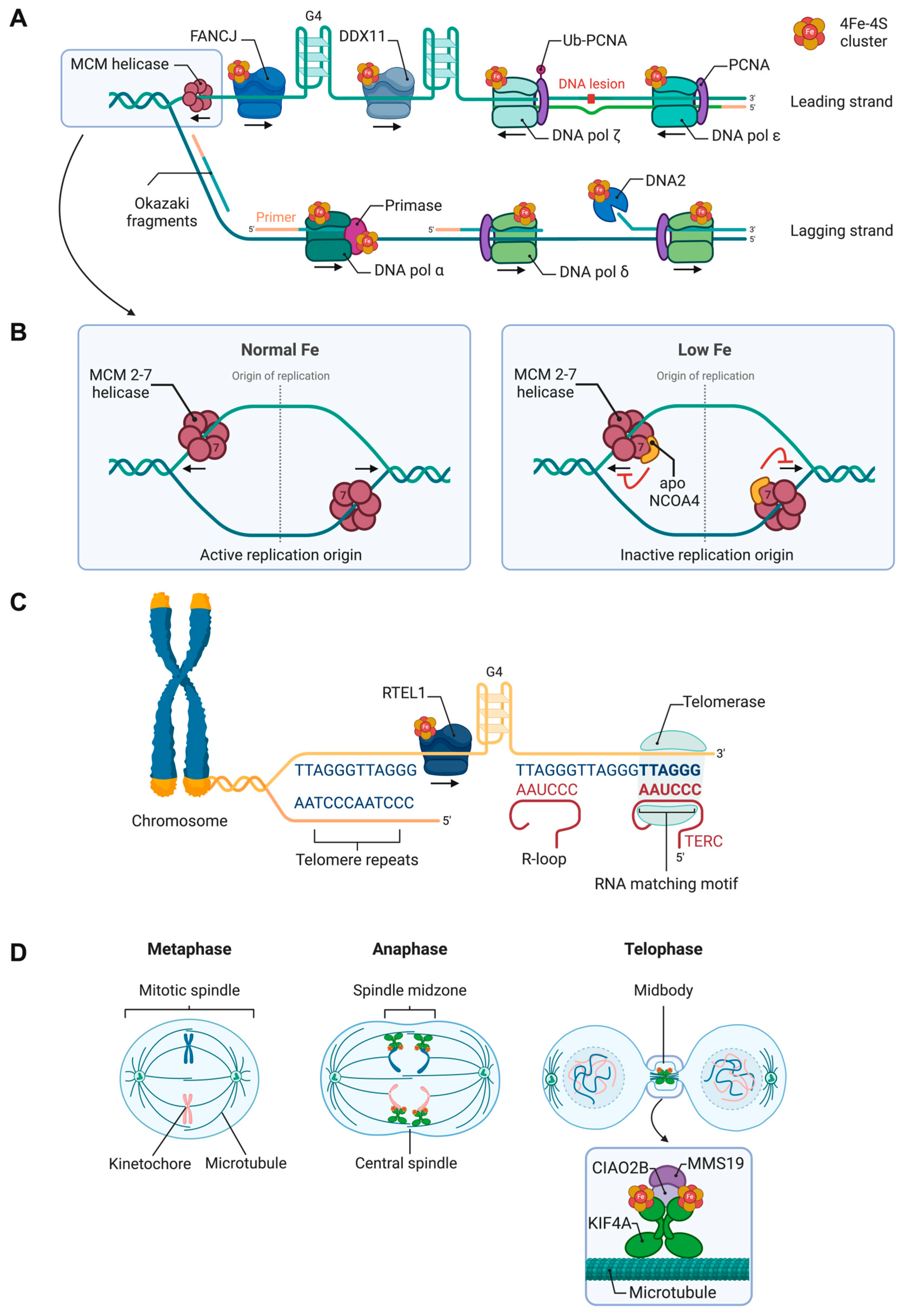
3. Fe-S Cluster Proteins Involved in Nuclear DNA Transactions
3.1. Fe-S Clusters and DNA Replication
3.1.1. DNA Polymerases
3.1.2. DNA Primase
3.1.3. Fe-S Clusters and DNA Replication Origins
3.1.4. Fe-S Clusters in Helicase Activity
3.1.5. Fe-S Clusters and Telomere Maintenance
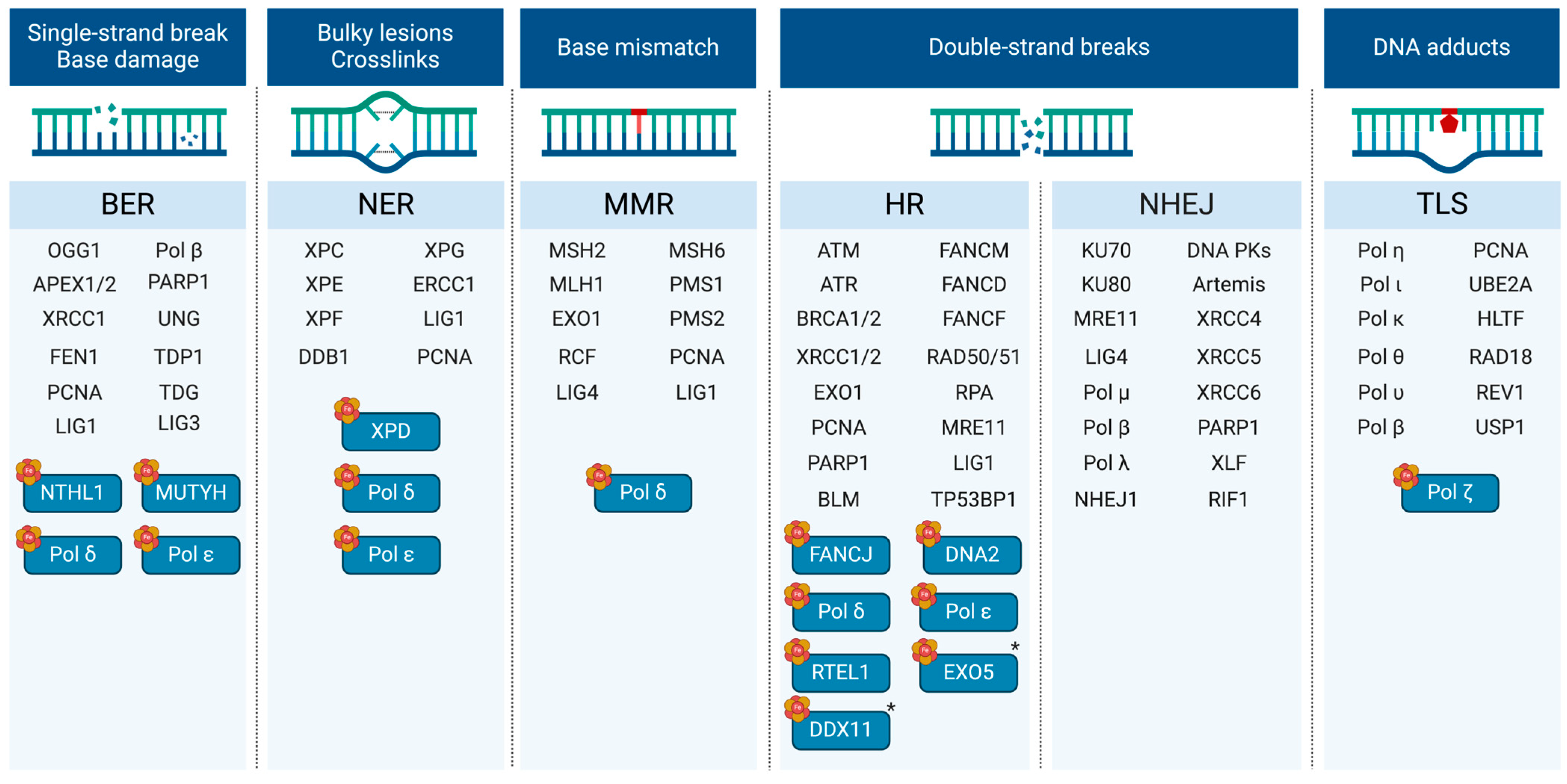
3.2. Fe-S Clusters and DNA Repair
3.3. Fe-S Clusters, Transcription, and Nuclear RNA Transactions
3.3.1. Fe-S Cluster-Binding Fe-Dependent Transcriptional Regulators
3.3.2. RNA Polymerase III
3.3.3. Transcription Factor IIH
3.3.4. RNA-Modifying Proteins
4. Fe-S Cluster Proteins in Mitosis
5. Other Fe-S Cluster Proteins in the Nucleus
6. Conclusions and Open Questions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lill, R.; Broderick, J.B.; Dean, D.R. Special issue on iron-sulfur proteins: Structure, function, biogenesis and diseases. Biochim. Biophys. Acta 2015, 1853, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.W.; Weerapana, E. Proteomic strategies to interrogate the Fe-S proteome. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119791. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Makrantoni, V.; Ingledew, W.J.; Stark, M.J.; White, M.F. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol. Cell 2006, 23, 801–808. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Binder, J.X.; Pletscher-Frankild, S.; Tsafou, K.; Stolte, C.; O’Donoghue, S.I.; Schneider, R.; Jensen, L.J. COMPARTMENTS: Unification and visualization of protein subcellular localization evidence. Database 2014, 2014, bau012. [Google Scholar] [CrossRef]
- Klinge, S.; Hirst, J.; Maman, J.D.; Krude, T.; Pellegrini, L. An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat. Struct. Mol. Biol. 2007, 14, 875–877. [Google Scholar] [CrossRef]
- Weiner, B.E.; Huang, H.; Dattilo, B.M.; Nilges, M.J.; Fanning, E.; Chazin, W.J. An iron-sulfur cluster in the C-terminal domain of the p58 subunit of human DNA primase. J. Biol. Chem. 2007, 282, 33444–33451. [Google Scholar] [CrossRef]
- Starokadomskyy, P.; Gemelli, T.; Rios, J.J.; Xing, C.; Wang, R.C.; Li, H.; Pokatayev, V.; Dozmorov, I.; Khan, S.; Miyata, N. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA: DNA synthesis. Nat. Immunol. 2016, 17, 495–504. [Google Scholar] [CrossRef]
- Netz, D.J.; Stith, C.M.; Stümpfig, M.; Köpf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2012, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chea, J.; Zhang, S.; Zhao, H.; Zhang, Z.; Lee, E.Y.; Darzynkiewicz, Z.; Lee, M.Y. Spatiotemporal recruitment of human DNA polymerase delta to sites of UV damage. Cell Cycle 2012, 11, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Roske, J.J.; Yeeles, J.T. Structural basis for processive daughter-strand synthesis and proofreading by the human leading-strand DNA polymerase Pol ε. Nat. Struct. Mol. Biol. 2024, 1–11. [Google Scholar] [CrossRef]
- Ræder, S.B.; Nepal, A.; Bjørås, K.Ø.; Seelinger, M.; Kolve, R.S.; Nedal, A.; Müller, R.; Otterlei, M. APIM-mediated REV3L–PCNA interaction important for error free TLS Over UV-induced DNA lesions in human cells. Int. J. Mol. Sci. 2018, 20, 100. [Google Scholar] [CrossRef]
- Cantor, S.B.; Bell, D.W.; Ganesan, S.; Kass, E.M.; Drapkin, R.; Grossman, S.; Wahrer, D.C.; Sgroi, D.C.; Lane, W.S.; Haber, D.A. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 2001, 105, 149–160. [Google Scholar] [CrossRef]
- Seki, M.; Takeda, Y.; Iwai, K.; Tanaka, K. IOP1 protein is an external component of the human cytosolic iron-sulfur cluster assembly (CIA) machinery and functions in the MMS19 protein-dependent CIA pathway. J. Biol. Chem. 2013, 288, 16680–16689. [Google Scholar] [CrossRef]
- Wu, Y.; Sommers, J.A.; Suhasini, A.N.; Leonard, T.; Deakyne, J.S.; Mazin, A.V.; Shin-Ya, K.; Kitao, H.; Brosz, R.M., Jr. Fanconi anemia group J mutation abolishes its DNA repair function by uncoupling DNA translocation from helicase activity or disruption of protein-DNA complexes. Blood J. Am. Soc. Hematol. 2010, 116, 3780–3791. [Google Scholar] [CrossRef]
- Parish, J.L.; Rosa, J.; Wang, X.; Lahti, J.M.; Doxsey, S.J.; Androphy, E.J. The DNA helicase ChlR1 is required for sister chromatid cohesion in mammalian cells. J. Cell Sci. 2006, 119, 4857–4865. [Google Scholar] [CrossRef]
- Simon, A.K.; Kummer, S.; Wild, S.; Lezaja, A.; Teloni, F.; Jozwiakowski, S.K.; Altmeyer, M.; Gari, K. The iron–sulfur helicase DDX11 promotes the generation of single-stranded DNA for CHK1 activation. Life Sci. Alliance 2020, 3. [Google Scholar] [CrossRef]
- Landry, A.P.; Ding, H. The N-Terminal Domain of Human DNA Helicase Rtel1 Contains a Redox Active Iron-Sulfur Cluster. BioMed Res. Int. 2014, 2014, 285791. [Google Scholar] [CrossRef]
- Ito, S.; Tan, L.J.; Andoh, D.; Narita, T.; Seki, M.; Hirano, Y.; Narita, K.; Kuraoka, I.; Hiraoka, Y.; Tanaka, K. MMXD, a TFIIH-independent XPD-MMS19 protein complex involved in chromosome segregation. Mol. Cell 2010, 39, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Duxin, J.P.; Dao, B.; Martinsson, P.; Rajala, N.; Guittat, L.; Campbell, J.L.; Spelbrink, J.N.; Stewart, S.A. Human Dna2 is a nuclear and mitochondrial DNA maintenance protein. Mol. Cell. Biol. 2009, 29, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Campbell, J.L. Cross talk between the nuclease and helicase activities of Dna2: Role of an essential iron–sulfur cluster domain. Nucleic Acids Res. 2012, 40, 7821–7830. [Google Scholar] [CrossRef]
- Mariotti, L.; Wild, S.; Brunoldi, G.; Piceni, A.; Ceppi, I.; Kummer, S.; Lutz, R.E.; Cejka, P.; Gari, K. The iron–sulphur cluster in human DNA2 is required for all biochemical activities of DNA2. Commun. Biol. 2020, 3, 322. [Google Scholar] [CrossRef]
- Sparks, J.L.; Kumar, R.; Singh, M.; Wold, M.S.; Pandita, T.K.; Burgers, P.M. Human exonuclease 5 is a novel sliding exonuclease required for genome stability. J. Biol. Chem. 2012, 287, 42773–42783. [Google Scholar] [CrossRef]
- Komine, K.; Shimodaira, H.; Takao, M.; Soeda, H.; Zhang, X.; Takahashi, M.; Ishioka, C. Functional complementation assay for 47 MUTYH variants in a MutY-disrupted Escherichia coli strain. Hum. Mutat. 2015, 36, 704–711. [Google Scholar] [CrossRef]
- Chepanoske, C.L.; Golinelli, M.-P.; Williams, S.D.; David, S.S. Positively Charged Residues within the Iron–Sulfur Cluster Loop of E. coli MutY Participate in Damage Recognition and Removal. Arch. Biochem. Biophys. 2000, 380, 11–19. [Google Scholar] [CrossRef]
- Nuñez, N.N.; Majumdar, C.; Lay, K.T.; David, S.S. Fe–S clusters and MutY base excision repair glycosylases: Purification, kinetics, and DNA affinity measurements. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 599, pp. 21–68. [Google Scholar]
- Ikeda, S.; Kohmoto, T.; Tabata, R.; Seki, Y. Differential intracellular localization of the human and mouse endonuclease III homologs and analysis of the sorting signals. DNA Repair. 2002, 1, 847–854. [Google Scholar] [CrossRef]
- Kuo, C.-F.; McRee, D.E.; Fisher, C.L.; O’Handley, S.F.; Cunningham, R.P.; Tainer, J.A. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science 1992, 258, 434–440. [Google Scholar] [CrossRef]
- Carroll, B.L.; Zahn, K.E.; Hanley, J.P.; Wallace, S.S.; Dragon, J.A.; Doublié, S. Caught in motion: Human NTHL1 undergoes interdomain rearrangement necessary for catalysis. Nucleic Acids Res. 2021, 49, 13165–13178. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lane, W.S.; Reinberg, D. Human Elongator facilitates RNA polymerase II transcription through chromatin. Proc. Natl. Acad. Sci. USA 2002, 99, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, C.; Fairhurst, S.A.; Lowe, D.J.; Brick, P.; Onesti, S. The elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 2006, 59, 795–806. [Google Scholar] [CrossRef]
- Kimura, S.; Suzuki, T. Iron-sulfur proteins responsible for RNA modifications. Biochim. Biophys. Acta 2015, 1853, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.P.; Abascal-Palacios, G.; Daiß, J.L.; King, H.; Gouge, J.; Pilsl, M.; Beuron, F.; Morris, E.; Gunkel, P.; Engel, C. Structure of human RNA polymerase III. Nat. Commun. 2020, 11, 6409. [Google Scholar] [CrossRef] [PubMed]
- Blombach, F.; Salvadori, E.; Fouqueau, T.; Yan, J.; Reimann, J.; Sheppard, C.; Smollett, K.L.; Albers, S.V.; Kay, C.W.; Thalassinos, K. Archaeal TFEα/β is a hybrid of TFIIE and the RNA polymerase III subcomplex hRPC62/39. Elife 2015, 4, e08378. [Google Scholar] [CrossRef]
- Li, L.; Yu, Z.; Zhao, D.; Ren, Y.; Hou, H.; Xu, Y. Structure of human RNA polymerase III elongation complex. Cell Res. 2021, 31, 791–800. [Google Scholar] [CrossRef]
- Chen, W.; Qin, L.; Wang, S.; Li, M.; Shi, D.; Tian, Y.; Wang, J.; Fu, L.; Li, Z.; Guo, W. CPSF4 activates telomerase reverse transcriptase and predicts poor prognosis in human lung adenocarcinomas. Mol. Oncol. 2014, 8, 704–716. [Google Scholar] [CrossRef]
- Shimberg, G.D.; Michalek, J.L.; Oluyadi, A.A.; Rodrigues, A.V.; Zucconi, B.E.; Neu, H.M.; Ghosh, S.; Sureschandra, K.; Wilson, G.M.; Stemmler, T.L.; et al. Cleavage and polyadenylation specificity factor 30: An RNA-binding zinc-finger protein with an unexpected 2Fe-2S cluster. Proc. Natl. Acad. Sci. USA 2016, 113, 4700–4705. [Google Scholar] [CrossRef]
- Pritts, J.D.; Hursey, M.S.; Michalek, J.L.; Batelu, S.; Stemmler, T.L.; Michel, S.L. Unraveling the RNA Binding Properties of the Iron–Sulfur Zinc Finger Protein CPSF30. Biochemistry 2020, 59, 970–982. [Google Scholar] [CrossRef]
- Young, A.P.; Bandarian, V. TYW1: A radical SAM enzyme involved in the biosynthesis of wybutosine bases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 606, pp. 119–153. [Google Scholar]
- Reiter, V.; Matschkal, D.M.; Wagner, M.; Globisch, D.; Kneuttinger, A.C.; Muller, M.; Carell, T. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012, 40, 6235–6240. [Google Scholar] [CrossRef]
- Pierrel, F.; Douki, T.; Fontecave, M.; Atta, M. MiaB protein is a bifunctional radical-S-adenosylmethionine enzyme involved in thiolation and methylation of tRNA. J. Biol. Chem. 2004, 279, 47555–47563. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, S.; Lee, E.; Shin, H.; Hahn, H.; Choi, W.; Kim, W. Human kinesin superfamily member 4 is dominantly localized in the nuclear matrix and is associated with chromosomes during mitosis. Biochem. J. 2001, 360, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimon, L.; Paul, V.D.; David-Kadoch, G.; Volpe, M.; Stümpfig, M.; Bill, E.; Mühlenhoff, U.; Lill, R.; Ben-Aroya, S. Fe-S cluster coordination of the chromokinesin KIF4A alters its subcellular localization during mitosis. J. Cell Sci. 2018, 131, jcs211433. [Google Scholar] [CrossRef]
- Bellelli, R.; Castellone, M.D.; Guida, T.; Limongello, R.; Dathan, N.A.; Merolla, F.; Cirafici, A.M.; Affuso, A.; Masai, H.; Costanzo, V. NCOA4 transcriptional coactivator inhibits activation of DNA replication origins. Mol. Cell 2014, 55, 123–137. [Google Scholar] [CrossRef]
- Kuno, S.; Iwai, K. Oxygen modulates iron homeostasis by switching iron sensing of NCOA4. J. Biol. Chem. 2023, 299. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Y.; Zhang, J.; Sun, Z.; Cheng, C.; Liu, Y.; Wu, L.; Zhang, M.; He, W.; Hao, S. NCOA4 requires a [3Fe-4S] to sense and maintain the iron homeostasis. J. Biol. Chem. 2024, 300, 105612. [Google Scholar] [CrossRef]
- Vinas-Castells, R.; Frias, A.; Robles-Lanuza, E.; Zhang, K.; Longmore, G.D.; Garcia de Herreros, A.; Diaz, V.M. Nuclear ubiquitination by FBXL5 modulates Snail1 DNA binding and stability. Nucleic Acids Res. 2013, 42, 1079–1094. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Rajan, M.; Canarie, E.R.; Hong, S.; Simoneschi, D.; Pagano, M.; Bush, M.F.; Stoll, S.; Leibold, E.A. FBXL5 regulates IRP2 stability in iron homeostasis via an oxygen-responsive [2Fe2S] cluster. Mol. Cell 2020, 78, 31–41.e35. [Google Scholar] [CrossRef]
- Bruening, W.; Prowse, A.H.; Schultz, D.C.; Holgado-Madruga, M.; Wong, A.; Godwin, A.K. Expression of OVCA1, a candidate tumor suppressor, is reduced in tumors and inhibits growth of ovarian cancer cells. Cancer Res. 1999, 59, 4973–4983. [Google Scholar]
- Zhang, Y.; Zhu, X.; Torelli, A.T.; Lee, M.; Dzikovski, B.; Koralewski, R.M.; Wang, E.; Freed, J.; Krebs, C.; Ealick, S.E. Diphthamide biosynthesis requires an organic radical generated by an iron–sulphur enzyme. Nature 2010, 465, 891–896. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Kashina, A.S.; Varshavsky, A. Alternative splicing results in differential expression, activity, and localization of the two forms of arginyl-tRNA-protein transferase, a component of the N-end rule pathway. Mol. Cell. Biol. 1999, 19, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Van, V.; Brown, J.B.; O’Shea, C.R.; Rosenbach, H.; Mohamed, I.; Ejimogu, N.-E.; Bui, T.S.; Szalai, V.A.; Chacón, K.N.; Span, I. Iron-sulfur clusters are involved in post-translational arginylation. Nat. Commun. 2023, 14, 458. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, X.; Lai, Y.; Xu, J.; Zhang, Y.; Zhou, S.; Feng, Z.; Yu, L.; Tang, Y.; Wang, W. Structure of the human respiratory complex II. Proc. Natl. Acad. Sci. USA 2023, 120, e2216713120. [Google Scholar] [CrossRef]
- Hao, Z.; Li, X.; Qiao, T.; Du, R.; Zhang, G.; Fan, D. Subcellular localization of CIAPIN1. J. Histochem. Cytochem. 2006, 54, 1437–1444. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Boscaro, F.; Chatzi, A.; Mikolajczyk, M.; Tokatlidis, K.; Winkelmann, J. Anamorsin is a [2Fe-2S] cluster-containing substrate of the Mia40-dependent mitochondrial protein trapping machinery. Chem. Biol. 2011, 18, 794–804. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Dancis, A.; Nakamaru-Ogiso, E. EPR studies of wild type and mutant Dre2 identify essential [2Fe–-2S] and [4Fe–-4S] clusters and their cysteine ligands. J. Biochem. 2016, 161, 67–78. [Google Scholar] [CrossRef]
- Willems, P.; Wanschers, B.F.; Esseling, J.; Szklarczyk, R.; Kudla, U.; Duarte, I.; Forkink, M.; Nooteboom, M.; Swarts, H.; Gloerich, J. BOLA1 is an aerobic protein that prevents mitochondrial morphology changes induced by glutathione depletion. Antioxid. Redox Signal. 2013, 18, 129–138. [Google Scholar] [CrossRef]
- Li, H.; Mapolelo, D.T.; Randeniya, S.; Johnson, M.K.; Outten, C.E. Human glutaredoxin 3 forms [2Fe-2S]-bridged complexes with human BolA2. Biochemistry 2012, 51, 1687–1696. [Google Scholar] [CrossRef]
- Frey, A.G.; Palenchar, D.J.; Wildemann, J.D.; Philpott, C.C. A Glutaredoxin· BolA Complex Serves as an Iron-Sulfur Cluster Chaperone for the Cytosolic Cluster Assembly Machinery. J. Biol. Chem. 2016, 291, 22344–22356. [Google Scholar] [CrossRef]
- Pandya, P.; Braiman, A.; Isakov, N. PICOT (GLRX3) is a positive regulator of stress-induced DNA-damage response. Cell. Signal. 2019, 62, 109340. [Google Scholar] [CrossRef]
- Okuno, T.; Yamabayashi, H.; Kogure, K. Comparison of intracellular localization of Nubp1 and Nubp2 using GFP fusion proteins. Mol. Biol. Rep. 2010, 37, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Netz, D.J.; Pierik, A.J.; Stümpfig, M.; Mühlenhoff, U.; Lill, R. The Cfd1–Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 2007, 3, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Stehling, O.; Netz, D.J.; Niggemeyer, B.; Rösser, R.; Eisenstein, R.S.; Puccio, H.; Pierik, A.J.; Lill, R. Human Nbp35 is essential for both cytosolic iron-sulfur protein assembly and iron homeostasis. Mol. Cell. Biol. 2008, 28, 5517–5528. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.-H.; Jameson, G.N.; Huynh, B.H.; Rouault, T.A. Subcellular compartmentalization of human Nfu, an iron–sulfur cluster scaffold protein, and its ability to assemble a [4Fe–4S] cluster. Proc. Natl. Acad. Sci. USA 2003, 100, 9762–9767. [Google Scholar] [CrossRef]
- Cai, K.; Liu, G.; Frederick, R.O.; Xiao, R.; Montelione, G.T.; Markley, J.L. Structural/functional properties of human NFU1, an intermediate [4Fe-4S] carrier in human mitochondrial iron-sulfur cluster biogenesis. Structure 2016, 24, 2080–2091. [Google Scholar] [CrossRef]
- Land, T.; Rouault, T.A. Targeting of a human iron–sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol. Cell 1998, 2, 807–815. [Google Scholar] [CrossRef]
- Fox, N.G.; Yu, X.; Feng, X.; Bailey, H.J.; Martelli, A.; Nabhan, J.F.; Strain-Damerell, C.; Bulawa, C.; Yue, W.W.; Han, S. Structure of the human frataxin-bound iron-sulfur cluster assembly complex provides insight into its activation mechanism. Nat. Commun. 2019, 10, 2210. [Google Scholar] [CrossRef]
- Marelja, Z.; Stöcklein, W.; Nimtz, M.; Leimkühler, S. A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J. Biol. Chem. 2008, 283, 25178–25185. [Google Scholar] [CrossRef]
- Lundberg, M.; Johansson, C.; Chandra, J.; Enoksson, M.; Jacobsson, G.; Ljung, J.; Johansson, M.; Holmgren, A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J. Biol. Chem. 2001, 276, 26269–26275. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Vergnolle, O.; Lönn, M.E.; Hudemann, C.; Bill, E.; Holmgren, A. Characterization of human glutaredoxin 2 as iron–sulfur protein: A possible role as redox sensor. Proc. Natl. Acad. Sci. USA 2005, 102, 8168–8173. [Google Scholar] [CrossRef]
- Kispal, G.; Csere, P.; Prohl, C.; Lill, R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999, 18, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Netz, D.J.; Mascarenhas, J.; Stehling, O.; Pierik, A.J.; Lill, R. Maturation of cytosolic and nuclear iron–sulfur proteins. Trends Cell Biol. 2014, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lee, F.S. A role for IOP1 in mammalian cytosolic iron-sulfur protein biogenesis. J. Biol. Chem. 2008, 283, 9231–9238. [Google Scholar] [CrossRef] [PubMed]
- Netz, D.J.; Pierik, A.J.; Stümpfig, M.; Bill, E.; Sharma, A.K.; Pallesen, L.J.; Walden, W.E.; Lill, R. A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron-sulfur protein maturation. J. Biol. Chem. 2012, 287, 12365–12378. [Google Scholar] [CrossRef]
- Stehling, O.; Vashisht, A.A.; Mascarenhas, J.; Jonsson, Z.O.; Sharma, T.; Netz, D.J.; Pierik, A.J.; Wohlschlegel, J.A.; Lill, R. MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 2012, 337, 195–199. [Google Scholar] [CrossRef]
- Gari, K.; León Ortiz, A.M.; Borel, V.; Flynn, H.; Skehel, J.M.; Boulton, S.J. MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 2012, 337, 243–245. [Google Scholar] [CrossRef]
- Balk, J.; Pierik, A.J.; Netz, D.J.A.; Mühlenhoff, U.; Lill, R. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron–sulphur proteins. EMBO J. 2004, 23, 2105–2115. [Google Scholar] [CrossRef]
- SantaMaria, A.M.; Rouault, T.A. Regulatory and Sensing Iron–Sulfur Clusters: New Insights and Unanswered Questions. Inorganics 2024, 12, 101. [Google Scholar] [CrossRef]
- Querci, L.; Piccioli, M.; Ciofi-Baffoni, S.; Banci, L. Structural aspects of iron-sulfur protein biogenesis: An NMR view. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119786. [Google Scholar] [CrossRef]
- Maio, N.; Singh, A.; Uhrigshardt, H.; Saxena, N.; Tong, W.-H.; Rouault, T.A. Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab. 2014, 19, 445–457. [Google Scholar] [CrossRef]
- Li, K.; Tong, W.-H.; Hughes, R.M.; Rouault, T.A. Roles of the mammalian cytosolic cysteine desulfurase, ISCS, and scaffold protein, ISCU, in iron-sulfur cluster assembly. J. Biol. Chem. 2006, 281, 12344–12351. [Google Scholar] [CrossRef]
- Kim, K.S.; Maio, N.; Singh, A.; Rouault, T.A. Cytosolic HSC20 integrates de novo iron–sulfur cluster biogenesis with the CIAO1-mediated transfer to recipients. Hum. Mol. Genet. 2018, 27, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Bencze, K.Z.; Stemmler, T.L.; Philpott, C.C. A cytosolic iron chaperone that delivers iron to ferritin. Science 2008, 320, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C. Coming into view: Eukaryotic iron chaperones and intracellular iron delivery. J. Biol. Chem. 2012, 287, 13518–13523. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Ryu, M.-S.; Frey, A.; Patel, S. Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 2017, 292, 12764–12771. [Google Scholar] [CrossRef]
- Philpott, C.C.; Patel, S.J.; Protchenko, O. Management versus miscues in the cytosolic labile iron pool: The varied functions of iron chaperones. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118830. [Google Scholar] [CrossRef]
- Patel, S.J.; Frey, A.G.; Palenchar, D.J.; Achar, S.; Bullough, K.Z.; Vashisht, A.; Wohlschlegel, J.A.; Philpott, C.C. A PCBP1–BolA2 chaperone complex delivers iron for cytosolic [2Fe–2S] cluster assembly. Nat. Chem. Biol. 2019, 15, 872–881. [Google Scholar] [CrossRef]
- Adamec, J.; Rusnak, F.; Owen, W.G.; Naylor, S.; Benson, L.M.; Gacy, A.M.; Isaya, G. Iron-dependent self-assembly of recombinant yeast frataxin: Implications for Friedreich ataxia. Am. J. Hum. Genet. 2000, 67, 549–562. [Google Scholar] [CrossRef]
- Bulteau, A.-L.; O’Neill, H.A.; Kennedy, M.C.; Ikeda-Saito, M.; Isaya, G.; Szweda, L.I. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science 2004, 305, 242–245. [Google Scholar] [CrossRef]
- Cavadini, P.; O’Neill, H.A.; Benada, O.; Isaya, G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum. Mol. Genet. 2002, 11, 217–227. [Google Scholar] [CrossRef]
- Bencze, K.Z.; Kondapalli, K.C.; Cook, J.D.; McMahon, S.; Millán-Pacheco, C.; Pastor, N.; Stemmler, T.L. The structure and function of frataxin. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.; Elduque, X.; Cornu, D.; Belot, L.; Le Caer, J.-P.; Grandas, A.; Toledano, M.B.; D’autréaux, B. Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat. Commun. 2015, 6, 5686. [Google Scholar] [CrossRef] [PubMed]
- Bridwell-Rabb, J.; Fox, N.G.; Tsai, C.-L.; Winn, A.M.; Barondeau, D.P. Human frataxin activates Fe–S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 2014, 53, 4904–4913. [Google Scholar] [CrossRef]
- Balk, J.; Aguilar Netz, D.J.; Tepper, K.; Pierik, A.J.; Lill, R. The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly. Mol. Cell. Biol. 2005, 25, 10833–10841. [Google Scholar] [CrossRef]
- Stehling, O.; Mascarenhas, J.; Vashisht, A.A.; Sheftel, A.D.; Niggemeyer, B.; Rösser, R.; Pierik, A.J.; Wohlschlegel, J.A.; Lill, R. Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab. 2013, 18, 187–198. [Google Scholar] [CrossRef]
- Marquez, M.D.; Greth, C.; Buzuk, A.; Liu, Y.; Blinn, C.M.; Beller, S.; Leiskau, L.; Hushka, A.; Wu, K.; Nur, K. Cytosolic iron–sulfur protein assembly system identifies clients by a C-terminal tripeptide. Proc. Natl. Acad. Sci. USA 2023, 120, e2311057120. [Google Scholar] [CrossRef]
- Paul, V.D.; Mühlenhoff, U.; Stümpfig, M.; Seebacher, J.; Kugler, K.G.; Renicke, C.; Taxis, C.; Gavin, A.-C.; Pierik, A.J.; Lill, R. The deca-GX3 proteins Yae1-Lto1 function as adaptors recruiting the ABC protein Rli1 for iron-sulfur cluster insertion. Elife 2015, 4, e08231. [Google Scholar] [CrossRef]
- Odermatt, D.C.; Gari, K. The CIA Targeting Complex Is Highly Regulated and Provides Two Distinct Binding Sites for Client Iron-Sulfur Proteins. Cell Rep. 2017, 18, 1434–1443. [Google Scholar] [CrossRef]
- Kassube, S.A.; Thomä, N.H. Structural insights into Fe–S protein biogenesis by the CIA targeting complex. Nat. Struct. Mol. Biol. 2020, 27, 735–742. [Google Scholar] [CrossRef]
- Alhebshi, A.; Sideri, T.C.; Holland, S.L.; Avery, S.V. The essential iron-sulfur protein Rli1 is an important target accounting for inhibition of cell growth by reactive oxygen species. Mol. Biol. Cell 2012, 23, 3582–3590. [Google Scholar] [CrossRef]
- Honarmand Ebrahimi, K.; Ciofi-Baffoni, S.; Hagedoorn, P.-L.; Nicolet, Y.; Le Brun, N.E.; Hagen, W.R.; Armstrong, F.A. Iron–sulfur clusters as inhibitors and catalysts of viral replication. Nat. Chem. 2022, 14, 253–266. [Google Scholar] [CrossRef]
- Jang, S.; Imlay, J.A. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007, 282, 929–937. [Google Scholar] [CrossRef]
- Mettert, E.L.; Kiley, P.J. Fe–S proteins that regulate gene expression. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef]
- Mettert, E.L.; Kiley, P.J. How is Fe-S cluster formation regulated? Annu. Rev. Microbiol. 2015, 69, 505–526. [Google Scholar] [CrossRef]
- Lauble, H.; Kennedy, M.; Beinert, H.; Stout, C. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry 1992, 31, 2735–2748. [Google Scholar] [CrossRef]
- Federico, G.; Carrillo, F.; Dapporto, F.; Chiariello, M.; Santoro, M.; Bellelli, R.; Carlomagno, F. NCOA4 links iron bioavailability to DNA metabolism. Cell Rep. 2022, 40, 111207. [Google Scholar] [CrossRef]
- Mayank, A.K.; Pandey, V.; Vashisht, A.A.; Barshop, W.D.; Rayatpisheh, S.; Sharma, T.; Haque, T.; Powers, D.N.; Wohlschlegel, J.A. An oxygen-dependent interaction between FBXL5 and the CIA-targeting complex regulates iron homeostasis. Mol. Cell 2019, 75, 382–393.e385. [Google Scholar] [CrossRef]
- van Wietmarschen, N.; Moradian, A.; Morin, G.B.; Lansdorp, P.M.; Uringa, E.-J. The mammalian proteins MMS19, MIP18, and ANT2 are involved in cytoplasmic iron-sulfur cluster protein assembly. J. Biol. Chem. 2012, 287, 43351–43358. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.; Martínez-Pastor, M. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Troadec, M.-B.; Loréal, O.; Brissot, P. The interaction of iron and the genome: For better and for worse. Mutat. Res./Rev. Mutat. Res. 2017, 774, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Veatch, J.R.; McMurray, M.A.; Nelson, Z.W.; Gottschling, D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009, 137, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Hou, W.; Wang, Z.-Q.; Xu, X. Biogenesis of iron–sulfur clusters and their role in DNA metabolism. Front. Cell Dev. Biol. 2021, 9, 735678. [Google Scholar] [CrossRef]
- Loeb, L.A.; Monnat, R.J., Jr. DNA polymerases and human disease. Nat. Rev. Genet. 2008, 9, 594–604. [Google Scholar] [CrossRef]
- Ter Beek, J.; Parkash, V.; Bylund, G.O.; Osterman, P.; Sauer-Eriksson, A.E.; Johansson, E. Structural evidence for an essential Fe–S cluster in the catalytic core domain of DNA polymerase ϵ. Nucleic Acids Res. 2019, 47, 5712–5722. [Google Scholar] [CrossRef]
- Jain, R.; Vanamee, E.S.; Dzikovski, B.G.; Buku, A.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. An iron–sulfur cluster in the polymerase domain of yeast DNA polymerase ε. J. Mol. Biol. 2014, 426, 301–308. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Lada, A.G.; Siebler, H.M.; Zhang, Y.; Pavlov, Y.I.; Tahirov, T.H. DNA polymerase δ and ζ switch by sharing accessory subunits of DNA polymerase δ. J. Biol. Chem. 2012, 287, 17281–17287. [Google Scholar] [CrossRef]
- Lisova, A.E.; Baranovskiy, A.G.; Morstadt, L.M.; Babayeva, N.D.; Stepchenkova, E.I.; Tahirov, T.H. The iron-sulfur cluster is essential for DNA binding by human DNA polymerase ε. Sci. Rep. 2022, 12, 17436. [Google Scholar] [CrossRef]
- Bartels, P.L.; Stodola, J.L.; Burgers, P.M.; Barton, J.K. A redox role for the [4Fe4S] cluster of yeast DNA polymerase δ. J. Am. Chem. Soc. 2017, 139, 18339–18348. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Babayeva, N.D.; Zhang, Y.; Gu, J.; Suwa, Y.; Pavlov, Y.I.; Tahirov, T.H. Mechanism of concerted RNA-DNA primer synthesis by the human primosome. J. Biol. Chem. 2016, 291, 10006–10020. [Google Scholar] [CrossRef]
- Suwa, Y.; Gu, J.; Baranovskiy, A.G.; Babayeva, N.D.; Pavlov, Y.I.; Tahirov, T.H. Crystal structure of the human Pol α B subunit in complex with the C-terminal domain of the catalytic subunit. J. Biol. Chem. 2015, 290, 14328–14337. [Google Scholar] [CrossRef] [PubMed]
- Klinge, S.; Núñez-Ramírez, R.; Llorca, O.; Pellegrini, L. 3D architecture of DNA Pol α reveals the functional core of multi-subunit replicative polymerases. EMBO J. 2009, 28, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Baranovskiy, A.G.; Tahirov, T.H.; Pavlov, Y.I. The C-terminal domain of the DNA polymerase catalytic subunit regulates the primase and polymerase activities of the human DNA polymerase α-primase complex. J. Biol. Chem. 2014, 289, 22021–22034. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Tahirov, T.H. Elaborated action of the human primosome. Genes 2017, 8, 62. [Google Scholar] [CrossRef]
- Pritts, J.D.; Michel, S.L. Fe-S clusters masquerading as zinc finger proteins. J. Inorg. Biochem. 2022, 230, 111756. [Google Scholar] [CrossRef]
- Maio, N.; Raza, M.K.; Li, Y.; Zhang, D.-L.; Bollinger, J.M., Jr.; Krebs, C.; Rouault, T.A. An iron–sulfur cluster in the zinc-binding domain of the SARS-CoV-2 helicase modulates its RNA-binding and-unwinding activities. Proc. Natl. Acad. Sci. USA 2023, 120, e2303860120. [Google Scholar] [CrossRef]
- Maio, N.; Lafont, B.A.; Sil, D.; Li, Y.; Bollinger, J.M., Jr.; Krebs, C.; Pierson, T.C.; Linehan, W.M.; Rouault, T.A. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science 2021, 373, 236–241. [Google Scholar] [CrossRef]
- Conlan, A.R.; Axelrod, H.L.; Cohen, A.E.; Abresch, E.C.; Zuris, J.; Yee, D.; Nechushtai, R.; Jennings, P.A.; Paddock, M.L. Crystal structure of Miner1: The redox-active 2Fe-2S protein causative in Wolfram Syndrome 2. J. Mol. Biol. 2009, 392, 143–153. [Google Scholar] [CrossRef]
- Cutone, A.; Howes, B.D.; Miele, A.E.; Miele, R.; Giorgi, A.; Battistoni, A.; Smulevich, G.; Musci, G.; Di Patti, M.C.B. Pichia pastoris Fep1 is a [2Fe-2S] protein with a Zn finger that displays an unusual oxygen-dependent role in cluster binding. Sci. Rep. 2016, 6, 31872. [Google Scholar] [CrossRef]
- Liu, L.; Huang, M. Essential role of the iron-sulfur cluster binding domain of the primase regulatory subunit Pri2 in DNA replication initiation. Protein Cell 2015, 6, 194–210. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Zhang, Y.; Suwa, Y.; Babayeva, N.D.; Gu, J.; Pavlov, Y.I.; Tahirov, T.H. Crystal structure of the human primase. J. Biol. Chem. 2015, 290, 5635–5646. [Google Scholar] [CrossRef] [PubMed]
- Sauguet, L.; Klinge, S.; Perera, R.L.; Maman, J.D.; Pellegrini, L. Shared active site architecture between the large subunit of eukaryotic primase and DNA photolyase. PLoS ONE 2010, 5, e10083. [Google Scholar] [CrossRef] [PubMed]
- Agarkar, V.B.; Babayeva, N.D.; Pavlov, Y.I.; Tahirov, T.H. Crystal structure of the C-terminal domain of human DNA primase large subunit: Implications for the mechanism of the primase-polymerase α switch. Cell Cycle 2011, 10, 926–931. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Holt, M.E.; Thompson, M.K.; Salay, L.E.; Ehlinger, A.C.; Chazin, W.J.; Barton, J.K. The [4Fe4S] cluster of human DNA primase functions as a redox switch using DNA charge transport. Science 2017, 355, eaag1789. [Google Scholar] [CrossRef]
- O’Brien, E.; Salay, L.E.; Epum, E.A.; Friedman, K.L.; Chazin, W.J.; Barton, J.K. Yeast require redox switching in DNA primase. Proc. Natl. Acad. Sci. USA 2018, 115, 13186–13191. [Google Scholar] [CrossRef]
- Amin, M.; Brooks, B.R. The oxidation of the [4Fe-4S] cluster of DNA primase alters the binding energies with DNA and RNA primers. Biophys. J. 2024, 123, 1648–1653. [Google Scholar] [CrossRef]
- O’Brien, E.; Holt, M.E.; Salay, L.E.; Chazin, W.J.; Barton, J.K. Substrate binding regulates redox signaling in human DNA primase. J. Am. Chem. Soc. 2018, 140, 17153–17162. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef]
- Singleton, M.R.; Dillingham, M.S.; Wigley, D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef]
- Wu, Y.; Brosh, R.M., Jr. DNA helicase and helicase–nuclease enzymes with a conserved iron–sulfur cluster. Nucleic Acids Res. 2012, 40, 4247–4260. [Google Scholar] [CrossRef] [PubMed]
- Yeeles, J.T.; Cammack, R.; Dillingham, M.S. An iron-sulfur cluster is essential for the binding of broken DNA by AddAB-type helicase-nucleases. J. Biol. Chem. 2009, 284, 7746–7755. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, M.; Guo, Z.; Lu, H.; Qian, L.; Dai, H.; Qiu, J.; Yakubovskaya, E.; Bogenhagen, D.F.; Demple, B. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol. Cell 2008, 32, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Estep, K.N.; Brosh, R.M., Jr. RecQ and Fe–S helicases have unique roles in DNA metabolism dictated by their unwinding directionality, substrate specificity, and protein interactions. Biochem. Soc. Trans. 2018, 46, 77–95. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Borel, V.; Adelman, C.A.; Schindler, D.; Boulton, S.J. FANCJ suppresses microsatellite instability and lymphomagenesis independent of the Fanconi anemia pathway. Genes Dev. 2015, 29, 2532–2546. [Google Scholar] [CrossRef]
- Capo-Chichi, J.M.; Bharti, S.K.; Sommers, J.A.; Yammine, T.; Chouery, E.; Patry, L.; Rouleau, G.A.; Samuels, M.E.; Hamdan, F.F.; Michaud, J.L. Identification and biochemical characterization of a novel mutation in DDX11 causing W arsaw breakage syndrome. Hum. Mutat. 2013, 34, 103–107. [Google Scholar] [CrossRef]
- Deng, Z.; Glousker, G.; Molczan, A.; Fox, A.J.; Lamm, N.; Dheekollu, J.; Weizman, O.-E.; Schertzer, M.; Wang, Z.; Vladimirova, O. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal–Hreidarsson syndrome. Proc. Natl. Acad. Sci. USA 2013, 110, E3408–E3416. [Google Scholar] [CrossRef]
- Falquet, B.; Ölmezer, G.; Enkner, F.; Klein, D.; Challa, K.; Appanah, R.; Gasser, S.M.; Rass, U. Disease-associated DNA2 nuclease–helicase protects cells from lethal chromosome under-replication. Nucleic Acids Res. 2020, 48, 7265–7278. [Google Scholar] [CrossRef]
- Sommers, J.A.; Rawtani, N.; Gupta, R.; Bugreev, D.V.; Mazin, A.V.; Cantor, S.B.; Brosh, R.M. FANCJ uses its motor ATPase to destabilize protein-DNA complexes, unwind triplexes, and inhibit RAD51 strand exchange. J. Biol. Chem. 2009, 284, 7505–7517. [Google Scholar] [CrossRef]
- Levran, O.; Attwooll, C.; Henry, R.T.; Milton, K.L.; Neveling, K.; Rio, P.; Batish, S.D.; Kalb, R.; Velleuer, E.; Barral, S. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat. Genet. 2005, 37, 931–933. [Google Scholar] [CrossRef]
- Odermatt, D.C.; Lee, W.T.C.; Wild, S.; Jozwiakowski, S.K.; Rothenberg, E.; Gari, K. Cancer-associated mutations in the iron-sulfur domain of FANCJ affect G-quadruplex metabolism. PLoS Genet. 2020, 16, e1008740. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Sommers, J.A.; George, F.; Kuper, J.; Hamon, F.; Shin-ya, K.; Teulade-Fichou, M.-P.; Kisker, C.; Brosh, R.M. Specialization among iron-sulfur cluster helicases to resolve G-quadruplex DNA structures that threaten genomic stability. J. Biol. Chem. 2013, 288, 28217–28229. [Google Scholar] [CrossRef] [PubMed]
- Wulfridge, P.; Sarma, K. Intertwining roles of R-loops and G-quadruplexes in DNA repair, transcription and genome organization. Nat. Cell Biol. 2024, 26, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Hänsel-Hertsch, R.; Di Antonio, M.; Balasubramanian, S. DNA G-quadruplexes in the human genome: Detection, functions and therapeutic potential. Nat. Rev. Mol. Cell Biol. 2017, 18, 279–284. [Google Scholar] [CrossRef]
- Wu, Y.; Shin-ya, K.; Brosh, R.M., Jr. FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol. Cell. Biol. 2008, 28, 4116–4128. [Google Scholar] [CrossRef]
- London, T.B.; Barber, L.J.; Mosedale, G.; Kelly, G.P.; Balasubramanian, S.; Hickson, I.D.; Boulton, S.J.; Hiom, K. FANCJ is a structure-specific DNA helicase associated with the maintenance of genomic G/C tracts. J. Biol. Chem. 2008, 283, 36132–36139. [Google Scholar] [CrossRef]
- Wu, C.G.; Spies, M. G-quadruplex recognition and remodeling by the FANCJ helicase. Nucleic Acids Res. 2016, 44, 8742–8753. [Google Scholar] [CrossRef]
- Vannier, J.-B.; Sandhu, S.; Petalcorin, M.I.; Wu, X.; Nabi, Z.; Ding, H.; Boulton, S.J. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science 2013, 342, 239–242. [Google Scholar] [CrossRef]
- Uringa, E.-J.; Youds, J.L.; Lisaingo, K.; Lansdorp, P.M.; Boulton, S.J. RTEL1: An essential helicase for telomere maintenance and the regulation of homologous recombination. Nucleic Acids Res. 2011, 39, 1647–1655. [Google Scholar] [CrossRef]
- Wu, W.; Bhowmick, R.; Vogel, I.; Özer, Ö.; Ghisays, F.; Thakur, R.S.; Sanchez de Leon, E.; Richter, P.H.; Ren, L.; Petrini, J.H. RTEL1 suppresses G-quadruplex-associated R-loops at difficult-to-replicate loci in the human genome. Nat. Struct. Mol. Biol. 2020, 27, 424–437. [Google Scholar] [CrossRef]
- Ghisays, F.; Garzia, A.; Wang, H.; Canasto-Chibuque, C.; Hohl, M.; Savage, S.A.; Tuschl, T.; Petrini, J.H. RTEL1 influences the abundance and localization of TERRA RNA. Nat. Commun. 2021, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sommers, J.A.; Khan, I.; de Winter, J.P.; Brosh, R.M. Biochemical characterization of Warsaw breakage syndrome helicase. J. Biol. Chem. 2012, 287, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- van Schie, J.J.; Faramarz, A.; Balk, J.A.; Stewart, G.S.; Cantelli, E.; Oostra, A.B.; Rooimans, M.A.; Parish, J.L.; de Almeida Estéves, C.; Dumic, K. Warsaw Breakage Syndrome associated DDX11 helicase resolves G-quadruplex structures to support sister chromatid cohesion. Nat. Commun. 2020, 11, 4287. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.T.; Vallur, A.C.; Eddy, J.; Maizels, N. G quadruplexes are genomewide targets of transcriptional helicases XPB and XPD. Nat. Chem. Biol. 2014, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Zhou, Y.; Gorospe, R.C.; Wang, Z. Mms19 protein functions in nucleotide excision repair by sustaining an adequate cellular concentration of the TFIIH component Rad3. Proc. Natl. Acad. Sci. USA 2008, 105, 15714–15719. [Google Scholar] [CrossRef]
- Seroz, T.; Winkler, G.S.; Auriol, J.; Verhage, R.A.; Vermeulen, W.; Smit, B.; Brouwer, J.; Eker, A.P.; Weeda, G.; Egly, J.-M. Cloning of a human homolog of the yeast nucleotide excision repair gene MMS19 and interaction with transcription repair factor TFIIH via the XPB and XPD helicases. Nucleic Acids Res. 2000, 28, 4506–4513. [Google Scholar] [CrossRef]
- Ding, H.; Schertzer, M.; Wu, X.; Gertsenstein, M.; Selig, S.; Kammori, M.; Pourvali, R.; Poon, S.; Vulto, I.; Chavez, E. Regulation of murine telomere length by Rtel: An essential gene encoding a helicase-like protein. Cell 2004, 117, 873–886. [Google Scholar] [CrossRef]
- Askree, S.H.; Yehuda, T.; Smolikov, S.; Gurevich, R.; Hawk, J.; Coker, C.; Krauskopf, A.; Kupiec, M.; McEachern, M.J. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 2004, 101, 8658–8663. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D. DNA Repair and Mutagenesis; American Society for Microbiology Press: Washington, DC, USA, 2005. [Google Scholar]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 2018, 23, 239–254.e236. [Google Scholar] [CrossRef]
- Fuss, J.O.; Tsai, C.-L.; Ishida, J.P.; Tainer, J.A. Emerging critical roles of Fe–S clusters in DNA replication and repair. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Asahara, H.; Bank, J.F.; Scholes, C.P.; Salerno, J.C.; Surerus, K.; Munck, E.; McCracken, J.; Peisach, J.; Emptage, M.H. Endonuclease III is an iron-sulfur protein. Biochemistry 1989, 28, 4450–4455. [Google Scholar] [CrossRef] [PubMed]
- Hinks, J.A.; Evans, M.C.; De Miguel, Y.; Sartori, A.A.; Jiricny, J.; Pearl, L.H. An iron-sulfur cluster in the family 4 uracil-DNA glycosylases. J. Biol. Chem. 2002, 277, 16936–16940. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Gu, Y.; Lu, A.-L. Purification and characterization of a mammalian homolog of Escherichia coli MutY mismatch repair protein from calf liver mitochondria. Nucleic Acids Res. 2000, 28, 3206–3215. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.L.; Schär, P. DNA glycosylases: In DNA repair and beyond. Chromosoma 2012, 121, 1–20. [Google Scholar] [CrossRef]
- McGoldrick, J.P.; Yeh, Y.-C.; Solomon, M.; Essigmann, J.M.; Lu, A.-L. Characterization of a mammalian homolog of the Escherichia coli MutY mismatch repair protein. Mol. Cell. Biol. 1995, 15, 989–996. [Google Scholar] [CrossRef]
- Aspinwall, R.; Rothwell, D.G.; Roldan-Arjona, T.; Anselmino, C.; Ward, C.J.; Cheadle, J.P.; Sampson, J.R.; Lindahl, T.; Harris, P.C.; Hickson, I.D. Cloning and characterization of a functional human homolog of Escherichia coli endonuclease III. Proc. Natl. Acad. Sci. USA 1997, 94, 109–114. [Google Scholar] [CrossRef]
- Lukianova, O.A.; David, S.S. A role for iron–sulfur clusters in DNA repair. Curr. Opin. Chem. Biol. 2005, 9, 145–151. [Google Scholar] [CrossRef]
- Trasviña-Arenas, C.H.; Lopez-Castillo, L.M.; Sanchez-Sandoval, E.; Brieba, L.G. Dispensability of the [4Fe-4S] cluster in novel homologues of adenine glycosylase MutY. FEBS J. 2016, 283, 521–540. [Google Scholar] [CrossRef]
- Guan, Y.; Manuel, R.C.; Arvai, A.S.; Parikh, S.S.; Mol, C.D.; Miller, J.H.; Lloyd, R.S.; Tainer, J.A. MutY catalytic core, mutant and bound adenine structures define specificity for DNA repair enzyme superfamily. Nat. Struct. Biol. 1998, 5, 1058–1064. [Google Scholar] [CrossRef]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef]
- Romano, C.A.; Sontz, P.A.; Barton, J.K. Mutants of the base excision repair glycosylase, endonuclease III: DNA charge transport as a first step in lesion detection. Biochemistry 2011, 50, 6133–6145. [Google Scholar] [CrossRef] [PubMed]
- Porello, S.L.; Cannon, M.J.; David, S.S. A substrate recognition role for the [4Fe-4S] 2+ cluster of the DNA repair glycosylase MutY. Biochemistry 1998, 37, 6465–6475. [Google Scholar] [CrossRef] [PubMed]
- Bartels, P.L.; Zhou, A.; Arnold, A.R.; Nuñez, N.N.; Crespilho, F.N.; David, S.S.; Barton, J.K. Electrochemistry of the [4Fe4S] cluster in base excision repair proteins: Tuning the redox potential with DNA. Langmuir 2017, 33, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.C.; Zwang, T.J.; Barton, J.K. The oxidation state of [4Fe4S] clusters modulates the DNA-binding affinity of DNA repair proteins. J. Am. Chem. Soc. 2017, 139, 12784–12792. [Google Scholar] [CrossRef]
- Bruner, S.D.; Norman, D.P.; Verdine, G.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [Google Scholar] [CrossRef]
- Poor, C.B.; Wegner, S.V.; Li, H.; Dlouhy, A.C.; Schuermann, J.P.; Sanishvili, R.; Hinshaw, J.R.; Riggs-Gelasco, P.J.; Outten, C.E.; He, C. Molecular mechanism and structure of the Saccharomyces cerevisiae iron regulator Aft2. Proc. Natl. Acad. Sci. USA 2014, 111, 4043–4048. [Google Scholar] [CrossRef]
- Ueta, R.; Fujiwara, N.; Iwai, K.; Yamaguchi-Iwai, Y. Iron-induced dissociation of the Aft1p transcriptional regulator from target gene promoters is an initial event in iron-dependent gene suppression. Mol. Cell Biol. 2012, 32, 4998–5008. [Google Scholar] [CrossRef]
- Li, H.; Mapolelo, D.T.; Dingra, N.N.; Naik, S.G.; Lees, N.S.; Hoffman, B.M.; Riggs-Gelasco, P.J.; Huynh, B.H.; Johnson, M.K.; Outten, C.E. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 2009, 48, 9569–9581. [Google Scholar] [CrossRef]
- Mercier, A.; Watt, S.; Bähler, J.; Labbé, S. Key function for the CCAAT-binding factor Php4 to regulate gene expression in response to iron deficiency in fission yeast. Eukaryot. Cell 2008, 7, 493–508. [Google Scholar] [CrossRef]
- Vachon, P.; Mercier, A.; Jbel, M.; Labbé, S. The monothiol glutaredoxin Grx4 exerts an iron-dependent inhibitory effect on Php4 function. Eukaryot. Cell 2012, 11, 806–819. [Google Scholar] [CrossRef]
- Dlouhy, A.C.; Beaudoin, J.; Labbé, S.; Outten, C.E. Schizosaccharomyces pombe Grx4 regulates the transcriptional repressor Php4 via [2Fe–2S] cluster binding. Metallomics 2017, 9, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Hati, D.; Brault, A.; Gupta, M.; Fletcher, K.; Jacques, J.F.; Labbe, S.; Outten, C.E. Iron homeostasis proteins Grx4 and Fra2 control activity of the Schizosaccharomyces pombe iron repressor Fep1 by facilitating [2Fe-2S] cluster removal. J. Biol. Chem. 2023, 299, 105419. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.F.; Mercier, A.; Brault, A.; Mourer, T.; Labbe, S. Fra2 is a co-regulator of Fep1 inhibition in response to iron starvation. PLoS ONE 2014, 9, e98959. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, B.; Trott, A.; Morano, K.A.; Labbé, S. Functional characterization of the iron-regulatory transcription factor Fep1 from Schizosaccharomyces pombe. J. Biol. Chem. 2005, 280, 25146–25161. [Google Scholar] [CrossRef]
- Ogunjimi, B.; Zhang, S.-Y.; Sørensen, K.B.; Skipper, K.A.; Carter-Timofte, M.; Kerner, G.; Luecke, S.; Prabakaran, T.; Cai, Y.; Meester, J. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J. Clin. Investig. 2017, 127, 3543–3556. [Google Scholar] [CrossRef]
- Girbig, M.; Misiaszek, A.D.; Vorländer, M.K.; Lafita, A.; Grötsch, H.; Baudin, F.; Bateman, A.; Müller, C.W. Cryo-EM structures of human RNA polymerase III in its unbound and transcribing states. Nat. Struct. Mol. Biol. 2021, 28, 210–219. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Wan, F.; Xu, Y.; Wu, Z.; Cao, M.; Lan, P.; Lei, M.; Wu, J. Structural insights into transcriptional regulation of human RNA polymerase III. Nat. Struct. Mol. Biol. 2021, 28, 220–227. [Google Scholar] [CrossRef]
- Wang, Z.; Roeder, R.G. Three human RNA polymerase III-specific subunits form a subcomplex with a selective function in specific transcription initiation. Genes Dev. 1997, 11, 1315–1326. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; MacMillan, J.B.; Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Tian, K.; Wang, R.; Huang, J.; Wang, H.; Ji, X. Subcellular localization shapes the fate of RNA polymerase III. Cell Rep. 2023, 42, 112941. [Google Scholar] [CrossRef]
- Zurita, M.; Merino, C. The transcriptional complexity of the TFIIH complex. TRENDS Genet. 2003, 19, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Giglia-Mari, G.; Coin, F.; Ranish, J.A.; Hoogstraten, D.; Theil, A.; Wijgers, N.; Jaspers, N.G.; Raams, A.; Argentini, M.; Van Der Spek, P. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat. Genet. 2004, 36, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Keriel, A.; Stary, A.; Sarasin, A.; Rochette-Egly, C.; Egly, J.-M. XPD mutations prevent TFIIH-dependent transactivation by nuclear receptors and phosphorylation of RARα. Cell 2002, 109, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dubaele, S.; De Santis, L.P.; Bienstock, R.J.; Keriel, A.; Stefanini, M.; Van Houten, B.; Egly, J.-M. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol. Cell 2003, 11, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Compe, E.; Drané, P.; Laurent, C.; Diderich, K.; Braun, C.; Hoeijmakers, J.H.; Egly, J.-M. Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mol. Cell. Biol. 2005, 25, 6065–6076. [Google Scholar] [CrossRef]
- Abbassi, N.-e.-H.; Jaciuk, M.; Scherf, D.; Böhnert, P.; Rau, A.; Hammermeister, A.; Rawski, M.; Indyka, P.; Wazny, G.; Chramiec-Głąbik, A. Cryo-EM structures of the human Elongator complex at work. Nat. Commun. 2024, 15, 4094. [Google Scholar] [CrossRef]
- Selvadurai, K.; Wang, P.; Seimetz, J.; Huang, R.H. Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism. Nat. Chem. Biol. 2014, 10, 810–812. [Google Scholar] [CrossRef]
- Dalwadi, U.; Yip, C.K. Structural insights into the function of Elongator. Cell. Mol. Life Sci. 2018, 75, 1613–1622. [Google Scholar] [CrossRef]
- Maio, N.; Orbach, R.; Zaharieva, I.T.; Töpf, A.; Donkervoort, S.; Munot, P.; Mueller, J.; Willis, T.; Verma, S.; Peric, S. CIAO1 loss of function causes a neuromuscular disorder with compromise of nucleocytoplasmic Fe-S enzymes. J. Clin. Investig. 2024, 134, e179559. [Google Scholar] [CrossRef]
- Romero, A.M.; Martinez-Pastor, M.T.; Puig, S. Iron in Translation: From the Beginning to the End. Microorganisms 2021, 9, 1058. [Google Scholar] [CrossRef]
- Sun, C.; Guo, R.; Ye, X.; Tang, S.; Chen, M.; Zhou, P.; Yang, M.; Liao, C.; Li, H.; Lin, B. Wybutosine hypomodification of tRNAphe activates HERVK and impairs neuronal differentiation. Iscience 2024, 27, 109748. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.C.; Silveira d’Almeida, G.; Alfonzo, J.D. The role of intracellular compartmentalization on tRNA processing and modification. RNA Biol. 2018, 15, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Zhou, B.; Suzuki, T.; Miyata, K.; Ujihara, Y.; Horiguchi, H.; Takahashi, N.; Xie, P.; Michiue, H.; Fujimura, A.; et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 2015, 21, 428–442. [Google Scholar] [CrossRef]
- Sheng, L.; Hao, S.-L.; Yang, W.-X.; Sun, Y. The multiple functions of kinesin-4 family motor protein KIF4 and its clinical potential. Gene 2018, 678, 90–99. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, W. Kinesin superfamily protein member 4 (KIF4) is localized to midzone and midbody in dividing cells. Exp. Mol. Med. 2004, 36, 93–97. [Google Scholar] [CrossRef]
- Mazumdar, M.; Sundareshan, S.; Misteli, T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 2004, 166, 613–620. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl. Acad. Sci. USA 2005, 102, 343–348. [Google Scholar] [CrossRef]
- Kurasawa, Y.; Earnshaw, W.C.; Mochizuki, Y.; Dohmae, N.; Todokoro, K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 2004, 23, 3237–3248. [Google Scholar] [CrossRef]
- Mazumdar, M.; Sung, M.-H.; Misteli, T. Chromatin maintenance by a molecular motor protein. Nucleus 2011, 2, 591–600. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, L.; Khidr, L.; Guo, X.E.; Kim, W.; Lee, Y.M.; Krasieva, T.; Chen, P.-L. A novel role of the chromokinesin Kif4A in DNA damage response. Cell Cycle 2008, 7, 2013–2020. [Google Scholar] [CrossRef]
- Kalantari, S.; Carlston, C.; Alsaleh, N.; Abdel-Salam, G.M.; Alkuraya, F.; Kato, M.; Matsumoto, N.; Miyatake, S.; Yamamoto, T.; Fares-Taie, L. Expanding the KIF4A-associated phenotype. Am. J. Med. Genet. Part A 2021, 185, 3728–3739. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.A.; Sugiyama, M.; Tsuge, M.; Wakae, K.; Fukano, K.; Oshima, M.; Sureau, C.; Watanabe, N.; Kato, T.; Murayama, A. The kinesin KIF4 mediates HBV/HDV entry through the regulation of surface NTCP localization and can be targeted by RXR agonists in vitro. PLoS Pathog. 2022, 18, e1009983. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, M.; Misteli, T. Chromokinesins: Multitalented players in mitosis. Trends Cell Biol. 2005, 15, 349–355. [Google Scholar] [CrossRef]
- Wu, G.; Chen, P.-L. Structural requirements of chromokinesin Kif4A for its proper function in mitosis. Biochem. Biophys. Res. Commun. 2008, 372, 454–458. [Google Scholar] [CrossRef]
- Barton, J.K.; Silva, R.M.; O’Brien, E. Redox chemistry in the genome: Emergence of the [4Fe4S] cofactor in repair and replication. Annu. Rev. Biochem. 2019, 88, 163–190. [Google Scholar] [CrossRef]
- Ueda, C.; Langton, M.; Chen, J.; Pandelia, M.-E. The HBx protein from hepatitis B virus coordinates a redox-active Fe-S cluster. J. Biol. Chem. 2022, 298, 101698. [Google Scholar] [CrossRef]
- Henkler, F.; Hoare, J.; Waseem, N.; Goldin, R.D.; McGarvey, M.J.; Koshy, R.; King, I.A. Intracellular localization of the hepatitis B virus HBx protein. J. Gen. Virol. 2001, 82, 871–882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoa-Aponte, L.; Leon-Torres, A.; Philpott, C.C. Guardians of the Genome: Iron–Sulfur Proteins in the Nucleus. Inorganics 2024, 12, 316. https://doi.org/10.3390/inorganics12120316
Novoa-Aponte L, Leon-Torres A, Philpott CC. Guardians of the Genome: Iron–Sulfur Proteins in the Nucleus. Inorganics. 2024; 12(12):316. https://doi.org/10.3390/inorganics12120316
Chicago/Turabian StyleNovoa-Aponte, Lorena, Andres Leon-Torres, and Caroline C. Philpott. 2024. "Guardians of the Genome: Iron–Sulfur Proteins in the Nucleus" Inorganics 12, no. 12: 316. https://doi.org/10.3390/inorganics12120316
APA StyleNovoa-Aponte, L., Leon-Torres, A., & Philpott, C. C. (2024). Guardians of the Genome: Iron–Sulfur Proteins in the Nucleus. Inorganics, 12(12), 316. https://doi.org/10.3390/inorganics12120316







