Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview
Abstract
1. Introduction
2. Naturally Occurring Boron-Containing Complexes: From Prebiotic Chemistry to Biological Life
2.1. Prebiotic Chemistry
2.2. Biological Life
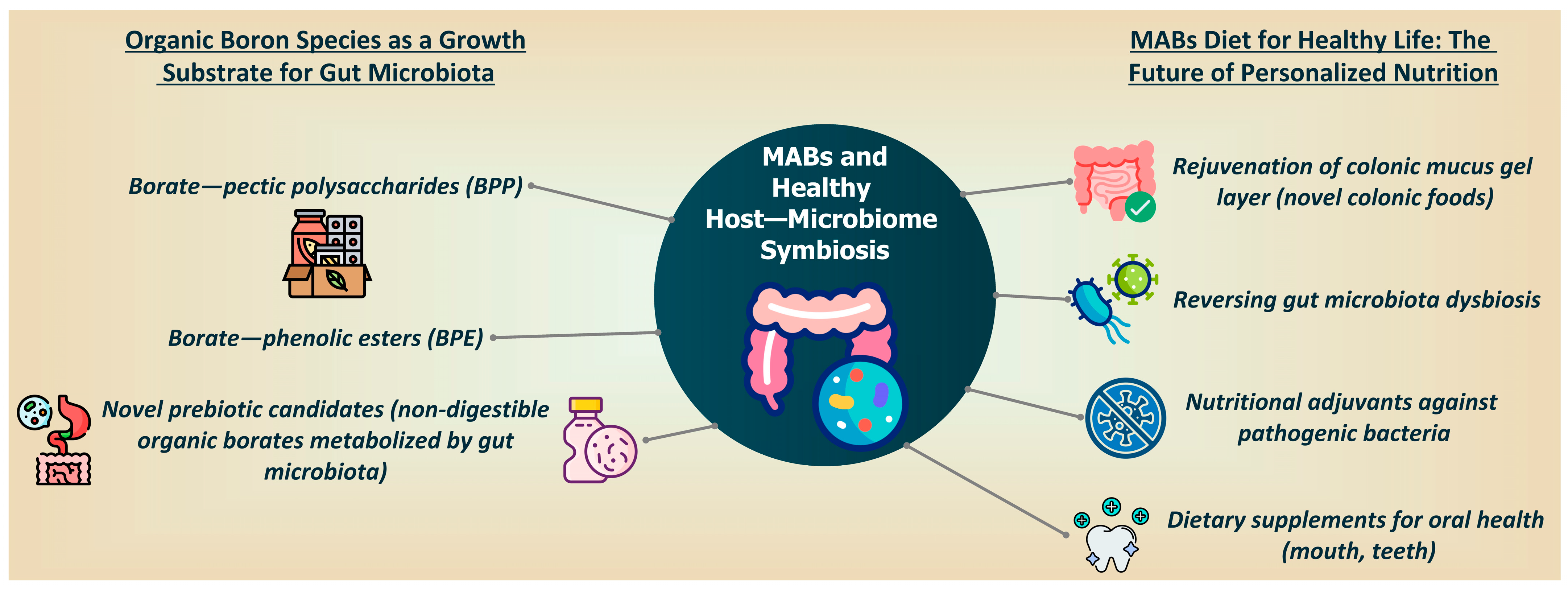
3. MABs and Healthy Host–Microbiome Symbiosis
4. Feeding Microbiome with Boron: Key Factor for Healthy Symbiosis Between Microbiota and Human/Animal Host
4.1. Nutrients Requiring Dietary Restriction
4.1.1. Low Sulfur-Containing Foods
4.1.2. Low Iron-Containing Foods
4.1.3. Reduced Gluten Foods
4.2. Nutrients That Need to Be High in the Food
4.2.1. Prebiotic Boron-Rich Foods
4.2.2. High Omega-3 Fatty Acid Foods
4.2.3. Foods Rich in SCFAs and MCFAs
4.2.4. Polyphenol-Rich Foods
- (i)
- Blueberries (656 mg polyphenols/100 g) [93].
- (ii)
- Strawberries are rich in anthocyanidins (289.20 mg/100 g) and especially flavonols (fisetin) [94].
- (iii)
- Apples are rich in phytochemicals; mainly, they are abundant in polyphenols, such as rutin, CA, catechin, epicatechin, proanthocyanidin, and vitamin B2 [95].
- (iv)
- (v)
- Tea is the most widely consumed beverage in the world after water. The polyphenol content in tea varies based on the fermentation type (61.86 mg/100 mL for green tea infusion; 104.48 mg/100 mL for black tea infusion); green tea primarily consists of catechins, while black tea contains a significant amount of tannins. Evidence suggest that polyphenols in green tea confer actions against accelerated ultraviolet (UV)-induced skin aging with anti-wrinkle, anti-melanogenic, antioxidant, and anti-inflammatory effects [98,99,100,101].
4.2.5. Functional Probiotic Foods Produced by Fermentation
5. MAB Diet for Healthy Life: The Future of Personalized Nutrition
- (i)
- For fruits, if we have an average of 0.5 mg B/100 g of fruits, at an average of 40 calories/100 g, BNDT: 12.5 mg B/1000 calories and BNDMA: 1.25 mg B/1000 calories.
- (ii)
- For vegetables, if we have an average of 0.3 mg B/100 g of vegetables, at an average of 30 calories/100 g, BNDT: 10 mg B/1000 calories and BNDMA: 1.0 mg B/1000 calories.
- (iii)
- For seeds, if we have an average of 1.5 mg B/100 g of seeds, at an average of 600 calories/100 g, BNDT: 2.5 mg B/1000 calories and BNDMA: 0.25 mg B/1000 calories.
- (iv)
- For fermented foods, with an average of 0.15 mg B/100 g, at an average of 70 calories/100 g, BNDT: 2 mg B/1000 calories and BNDMA: 0.2 mg B/1000 calories.
- (v)
- For marine fish, with an average of 0.1 mg B/100 g, at an average of 150 calories/100 g, BNDT: 0.8 mg B/1000 calories and BNDMA: 0/08 mg B/1000 calories.
6. Conclusions
- (i)
- Colonic mucus gel rejuvenation with far-reaching effects on the musculoskeletal system (bones, cartilages, and muscles) and the immune system (the brain, heart, and thyroid). The colonic mucus gel rejuvenation has a major meaning in the concept of healthy longevity. The MoA of MABs succeeds in rejuvenating the colonic mucus gel by stimulating BPB, stopping the growth of pathogenic bacteria, and strengthening the gel layer of the colonic mucus.
- (ii)
- Reversing DYS through MAB nutrition by reversing an aged microbiome into a younger one and the potential to provide healthy longevity to the aged host by supporting immunity and restoring HS in the GM system. Subsequently, reversing DYS slows aging.
- (iii)
- Essential nutritional adjuvants in antibiotic treatment applied in acute infections: stimulate commensal bacteria and stop the proliferation of pathogenic bacteria, thus regulating the GM during antibiotic treatment.
- (iv)
- Adjunct to periodontal therapy: MABs can be used effectively as an active ingredient in toothpastes and mouthwashes, as well as an adjuvant in the treatment of periodontal diseases (gingivitis and periodontitis).
Author Contributions
Funding
Conflicts of Interest
References
- Díaz-Díaz, L.M.; Rodríguez-Villafañe, A.; García-Arrarás, J.E. The Role of the Microbiota in Regeneration-Associated Processes. Front. Cell Dev. Biol. 2022, 9, 768783. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Haran, J. The human gut microbiome and aging. Gut Microbes 2024, 16, 2359677. [Google Scholar] [CrossRef] [PubMed]
- Scorei, R. Is Boron a Prebiotic Element? A Mini-review of the Essentiality of Boron for the Appearance of Life on Earth. Orig. Life Evol. Biosph. 2012, 42, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Furukawa, Y.; Kakegawa, T.; Bita, A.; Scorei, R.; Benner, S.A. Evaporite Borate-Containing Mineral Ensembles Make Phosphate Available and Regiospecifically Phosphorylate Ribonucleosides: Borate as a Multifaceted Problem Solver in Prebiotic Chemistry. Angew. Chem. 2016, 55, 15816–15820. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A. Boron Applications in Prevention, Diagnosis and Therapy for High Global Burden Diseases. Inorganics 2023, 11, 358. [Google Scholar] [CrossRef]
- Stogniy, M.Y. Fifth Element: The Current State of Boron Chemistry. Inorganics 2024, 12, 10. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Scorei, I.R.; Bita, A.; Dinca, L.; Mogosanu, G.D.; Rangavaila, N. Borate Complexes of Chlorogenic Acid and Uses Thereof. International Patent Application WO 2023/070074 A1, 27 April 2023. Available online: https://patents.google.com/patent/WO2023070074A1/en (accessed on 3 October 2024).
- Sentürk, N.B.; Kasapoglu, B.; Sahin, E.; Ozcan, O.; Ozansoy, M.; Ozansoy, M.B.; Siyah, P.; Sezerman, U.; Sahin, F. The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study. Pharmaceuticals 2024, 17, 1334. [Google Scholar] [CrossRef]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate Minerals Stabilize Ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef]
- Scorei, R.; Cimpoiaşu, V.M. Boron Enhances the Thermostability of Carbohydrates. Orig. Life Evol. Biosph. 2006, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sumie, Y.; Sato, K.; Kakegawa, T.; Furukawa, Y. Boron-assisted abiotic polypeptide synthesis. Commun. Chem. 2023, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; da Silva, J.A.L. Boron in Prebiological Evolution. Angew. Chem. 2021, 60, 10458–10468. [Google Scholar] [CrossRef] [PubMed]
- Biţă, C.E.; Scorei, I.R.; Vreju, A.F.; Muşetescu, A.E.; Mogoşanu, G.D.; Biţă, A.; Dinescu, V.C.; Dinescu, Ş.C.; Criveanu, C.; Bărbulescu, A.L.; et al. Microbiota-Accessible Boron-Containing Compounds in Complex Regional Pain Syndrome. Medicina 2023, 59, 1965. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Chapter 19—Manganese, molybdenum, boron, silicon, and other trace elements. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 485–500. [Google Scholar] [CrossRef]
- Cleaves, H.J., II. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331–345. [Google Scholar] [CrossRef]
- Klein, H.P.; Horowitz, N.H.; Levin, G.V.; Oyama, V.I.; Lederberg, J.; Rich, A.; Hubbard, J.S.; Hobby, G.L.; Straat, P.A.; Berdahl, B.J.; et al. The Viking Biological Investigation: Preliminary Results. Science 1976, 194, 99–105. [Google Scholar] [CrossRef]
- Levin, G.V.; Straat, P.A. The Case for Extant Life on Mars and Its Possible Detection by the Viking Labeled Release Experiment. Astrobiology 2016, 16, 798–810. [Google Scholar] [CrossRef]
- McKay, D.S.; Gibson, E.K., Jr.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.; Maechling, C.R.; Zare, R.N. Search for Past Life on Mars: Possible Relic Biogenic Activity in Martian Meteorite ALH84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef]
- Ananthaswamy, A. Interview: Chris MacKay: The man who found Mars on Earth. New Sci. 2008, 198, 44–45. [Google Scholar] [CrossRef]
- Stephenson, J.D.; Hallis, L.J.; Nagashima, K.; Freeland, S.J. Boron Enrichment in Martian Clay. PLoS ONE 2013, 8, e64624. [Google Scholar] [CrossRef]
- Gasda, P.J.; Haldeman, E.B.; Wiens, R.C.; Rapin, W.; Bristow, T.F.; Bridges, J.C.; Schwenzer, S.P.; Clark, B.; Herkenhoff, K.; Frydenvang, J.; et al. In situ detection of boron by ChemCam on Mars. Geophys. Res. Lett. 2017, 44, 8739–8748. [Google Scholar] [CrossRef]
- Furukawa, Y.; Horiuchi, M.; Kakegawa, T. Selective Stabilization of Ribose by Borate. Orig. Life Evol. Biosph. 2013, 43, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gasda, P.J.; Comellas, J.; Essunfeld, A.; Das, D.; Bryk, A.B.; Dehouck, E.; Schwenzer, S.P.; Crossey, L.; Herkenhoff, K.; Johnson, J.R.; et al. Overview of the Morphology and Chemistry of Diagenetic Features in the Clay-Rich Glen Torridon Unit of Gale Crater, Mars. J. Geophys. Res. Planets 2022, 127, e2021JE007097. [Google Scholar] [CrossRef]
- Levin, G.V.; Straat, P.A. Life on Mars? The Viking labeled release experiment. Biosystems 1977, 9, 165–174. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic Chemistry and the Origin of the RNA World. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [CrossRef]
- Pressman, A.; Blanco, C.; Chen, I.A. The RNA World as a Model System to Study the Origin of Life. Curr. Biol. 2015, 25, R953–R963. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kakegawa, T. Borate and the Origin of RNA: A Model for the Precursors to Life. Elements 2017, 13, 261–265. [Google Scholar] [CrossRef]
- Benner, S.A.; Bell, E.A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.-J.; Mojzsis, S.J.; Omran, A.; Pasek, M.A.; Trail, D. When Did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem 2020, 2, e1900035. [Google Scholar] [CrossRef]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Kim, H.J.; Ricardo, A.; Illangkoon, H.I.; Kim, M.J.; Carrigan, M.A.; Frye, F.; Benner, S.A. Synthesis of Carbohydrates in Mineral-Guided Prebiotic Cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Benner, S.A. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. Proc. Natl. Acad. Sci. USA 2017, 114, 11315–11320. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Benner, S.A. A Direct Prebiotic Synthesis of Nicotinamide Nucleotide. Chemistry 2018, 24, 581–584. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.J.; Biondi, E. Mineral–Organic Interactions in Prebiotic Synthesis. In Prebiotic Chemistry and Chemical Evolution of Nucleic Acids, 1st ed.; Menor-Salván, C., Ed.; Book Series: Nucleic Acids and Molecular Biology (NUCLEIC); Springer Nature: Cham, Switzerland, 2018; Volume 35, pp. 31–83. [Google Scholar] [CrossRef]
- Harrison, P.M.; Zheng, D.; Zhang, Z.; Carriero, N.; Gerstein, M. Transcribed processed pseudogenes in the human genome: An intermediate form of expressed retrosequence lacking protein-coding ability. Nucleic Acids Res. 2005, 33, 2374–2383. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef] [PubMed]
- Peretó, J.; Bada, J.L.; Lazcano, A. Charles Darwin and the Origin of Life. Orig. Life Evol. Biosph. 2009, 39, 395–406. [Google Scholar] [CrossRef]
- Funakawa, H.; Miwa, K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015, 6, 223. [Google Scholar] [CrossRef]
- Miller, E.P.; Wu, Y.; Carrano, C.J. Boron uptake, localization, and speciation in marine brown algae. Metallomics 2016, 8, 161–169. [Google Scholar] [CrossRef]
- Ali, I.; Ali, A.; Guo, L.; Burki, S.; Rehman, J.U.; Fazal, M.; Ahmad, N.; Khan, S.; Toloza, C.A.T.; Shah, M.R. Synthesis of calix (4) resorcinarene based amphiphilic macrocycle as an efficient nanocarrier for Amphotericin-B to enhance its oral bioavailability. Colloids Surf. B Biointerfaces 2024, 238, 113918. [Google Scholar] [CrossRef]
- Dinca, L.; Scorei, R. Boron in Human Nutrition and its Regulations Use. J. Nutr. Ther. 2013, 2, 22–29. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally Occurring Boron Containing Compounds and Their Biological Activities. J. Nat. Prod. Resour. 2017, 3, 147–154. Available online: https://www.jacsdirectory.com/journal-of-natural-products-and-resources/articleview.php?id=40 (accessed on 5 October 2024).
- Hunter, J.M.; Nemzer, B.V.; Rangavajla, N.; Biţă, A.; Rogoveanu, O.C.; Neamţu, J.; Scorei, I.R.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; et al. The Fructoborates: Part of a Family of Naturally Occurring Sugar–Borate Complexes—Biochemistry, Physiology, and Impact on Human Health: A Review. Biol. Trace Elem. Res. 2019, 188, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.R.; Amin, S.A.; Küpper, F.C.; Green, D.H.; Carrano, C.J. Borate Binding to Siderophores: Structure and Stability. J. Am. Chem. Soc. 2007, 129, 12263–12271. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Şahin, E.; Orhan, C.; Erten, F.; Şahin, F.; Şahin, N.; Şahin, K. The effect of different boron compounds on nutrient digestibility, intestinal nutrient transporters, and liver lipid metabolism. Turk. J. Med. Sci. 2023, 53, 619–629. [Google Scholar] [CrossRef]
- Maffei, V.J.; Kim, S.; Blanchard, E., 4th; Luo, M.; Jazwinski, S.M.; Taylor, C.M.; Welsh, D.A. Biological Aging and the Human Gut Microbiota. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1474–1482. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Ye, C.; Li, Z.; Ye, C.; Yuan, L.; Wu, K.; Zhu, C. Association Between Gut Microbiota and Biological Aging: A Two-Sample Mendelian Randomization Study. Microorganisms 2024, 12, 370. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarães, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of Butyrate, a Gut Microbiota Derived Metabolite, in Cardiovascular Diseases: A comprehensive narrative review. Front. Pharmacol. 2022, 12, 837509. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Sun, Z.; Grimm, V.; Riedel, C.U. AI-2 to the rescue against antibiotic-induced intestinal dysbiosis? Trends Microbiol. 2015, 23, 327–328. [Google Scholar] [CrossRef]
- Hu, R.; Yang, T.; Ai, Q.; Shi, Y.; Ji, Y.; Sun, Q.; Tong, B.; Chen, J.; Wang, Z. Autoinducer-2 promotes the colonization of Lactobacillus rhamnosus GG to improve the intestinal barrier function in a neonatal mouse model of antibiotic-induced intestinal dysbiosis. J. Transl. Med. 2024, 22, 177. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Nielsen, F.H. Boron in Aging and Longevity. In Trace Elements and Minerals in Health and Longevity, 1st ed.; Malavolta, M., Mocchegiani, E., Eds.; Book Series: Healthy Ageing and Longevity; Springer Nature: Cham, Switzerland, 2018; Volume 8, pp. 163–177. [Google Scholar] [CrossRef]
- McKenney, E.S.; Kendall, M.M. Microbiota and pathogen ‘pas de deux’: Setting up and breaking down barriers to intestinal infection. Pathog. Dis. 2016, 74, ftw051. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Chia, N.; Nelson, H.; Segal, E.; Elinav, E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin. Proc. 2017, 92, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Sina, C.; Ferrario, P.G.; Daniel, H. Gut Microbiome Analysis for Personalized Nutrition: The State of Science. Mol. Nutr. Food Res. 2023, 67, e2200476. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous Hydrogen Sulfide Production is Essential for Dietary Restriction Benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Longo, V.D. A Protein Restriction-Dependent Sulfur Code for Longevity. Cell 2015, 160, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef]
- Dong, Z.; Sinha, R.; Richie, J.P., Jr. Disease prevention and delayed aging by dietary sulfur amino acid restriction: Translational implications. Ann. N. Y. Acad. Sci. 2018, 1418, 44–55. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef]
- Timmers, P.R.H.J.; Wilson, J.F.; Joshi, P.K.; Deelen, J. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat. Commun. 2020, 11, 3570. [Google Scholar] [CrossRef]
- Ahern, J.; Boyle, M.E.; Thompson, W.K.; Fan, C.C.; Loughnan, R. Dietary and Lifestyle Factors of Brain Iron Accumulation and Parkinson’s Disease Risk. medRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Ellervik, C.; Marott, J.L.; Tybjærg-Hansen, A.; Schnohr, P.; Nordestgaard, B.G. Total and Cause-Specific Mortality by Moderately and Markedly Increased Ferritin Concentrations: General Population Study and Metaanalysis. Clin. Chem. 2014, 60, 1419–1428. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Al Hasan, S.M.; Hassan, M.; Saha, S.; Islam, M.; Billah, M.; Islam, S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr. 2016, 2, 24. [Google Scholar] [CrossRef]
- Rashtak, S.; Murray, J.A. Celiac Disease in the Elderly. Gastroenterol. Clin. N. Am. 2009, 38, 433–446. [Google Scholar] [CrossRef]
- Quarpong, W.; Card, T.R.; West, J.; Solaymani-Dodaran, M.; Logan, R.F.; Grainge, M.J. Mortality in people with coeliac disease: Long-term follow-up from a Scottish cohort. United Eur. Gastroenterol. J. 2019, 7, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.S.; Reshma, M.V.; Shanimol, P.S.; Krishnan, G.; Priya, S. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Naghii, M.R.; Wall, P.M.; Samman, S. The boron content of selected foods and the estimation of its daily intake among free-living subjects. J. Am. Coll. Nutr. 1996, 15, 614–619. [Google Scholar] [CrossRef]
- Choi, M.K.; Jun, Y.S. Analysis of Boron Content in Frequently Consumed Foods in Korea. Biol. Trace Elem. Res. 2008, 126, 13–26. [Google Scholar] [CrossRef]
- Yazbeck, C.; Kloppmann, W.; Cottier, R.; Sahuquillo, J.; Debotte, G.; Huel, G. Health Impact Evaluation of Boron in Drinking Water: A Geographical Risk Assessment in Northern France. Environ. Geochem. Health 2005, 27, 419–427. [Google Scholar] [CrossRef]
- Koletzko, B. Omega-3 fatty acid requirement. Am. J. Clin. Nutr. 1987, 46, 374. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.H.; Chen, Y.Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef]
- Jové, M.; Naudí, A.; Aledo, J.C.; Cabré, R.; Ayala, V.; Portero-Otin, M.; Barja, G.; Pamplona, R. Plasma long-chain free fatty acids predict mammalian longevity. Sci. Rep. 2013, 3, 3346. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.S.; Duffy, J.; Wang, M.C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 2021, 56, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.R.; Teixeira-Lemos, E.; Oliveira, J.; Teixeira-Lemos, L.P.; Monteiro, A.M.C.; Costa, J.M. Chapter 10. Nutritional and Health Profile of Goat Products: Focus on Health Benefits of Goat Milk. In Goat Science, 1st ed.; Kukovics, S., Ed.; InTech Open: London, UK, 2018; pp. 189–232. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Zhou, H.; Nie, J.; Cao, Y.; Diao, L.; Zhang, X.; Li, J.; Chen, S.; Zhang, X.; Chen, G.; Zhang, Z.; et al. Association of daily sitting time and coffee consumption with the risk of all-cause and cardiovascular disease mortality among US adults. BMC Public Health 2024, 24, 1069. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Patel, S. Blueberry as functional food and dietary supplement: The natural way to ensure holistic health. Mediterr. J. Nutr. Metab. 2014, 7, 133–143. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Urso, E.; D’Avino, M.; Campiglia, P.; Marinelli, L.; Novellino, E. Annurca (Malus pumila Miller cv. Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: Results of a randomized clinical trial. J. Sci. Food Agric. 2017, 97, 2107–2115. [Google Scholar] [CrossRef]
- Poonia, A.; Pandey, S. Bioactive compounds, nutritional benefits and food applications of black rice: A review. Nutr. Food Sci. 2022, 52, 466–482. [Google Scholar] [CrossRef]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Pastoriza de la Cueva, S.; Rufián-Henares, J.Á. Green and white teas as health-promoting foods. Food Funct. 2021, 12, 3799–3819. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; D’Angelo, S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef]
- Zhor, C.; Wafaa, L.; Ghzaiel, I.; Kessas, K.; Zarrouk, A.; Ksila, M.; Ghrairi, T.; Latruffe, N.; Masmoudi-Kouki, O.; El Midaoui, A.; et al. Effects of polyphenols and their metabolites on age-related diseases. Biochem. Pharmacol. 2023, 214, 115674. [Google Scholar] [CrossRef]
- Choudhary, P.; Kathuria, D.; Suri, S.; Bahndral, A.; Naveen, A.K. Probiotics—Its functions and influence on the ageing process: A comprehensive review. Food Biosci. 2023, 52, 102389. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001, 73, 374S–379S. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: Potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V.L., 3rd. Nutrient density: Principles and evaluation tools. Am. J. Clin. Nutr. 2014, 99, 1223S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Calories in Food: Calorie Chart Database. Available online: https://www.calories.info/ (accessed on 3 October 2024).
- The Top Boron-Rich Food Sources. Available online: https://www.algaecal.com/algaecal-ingredients/boron/boron-sources/ (accessed on 3 October 2024).
- Eichholz, I.; Huyskens-Keil, S.; Kroh, L.W.; Rohn, S. Phenolic compounds, pectin and antioxidant activity in blueberries (Vaccinium corymbosum L.) influenced by boron and mulch cover. J. Appl. Bot. Food Qual. 2011, 84, 26–32. Available online: https://ojs.openagrar.de/index.php/JABFQ/article/view/1692 (accessed on 6 October 2024).
- Ispiryan, A.; Viškelis, J.; Viškelis, P.; Urbonavičienė, D.; Raudonė, L. Biochemical and Antioxidant Profiling of Raspberry Plant Parts for Sustainable Processing. Plants 2023, 12, 2424. [Google Scholar] [CrossRef] [PubMed]
- Boron: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Boron-HealthProfessional/#h3 (accessed on 3 October 2024).
- Top Foods High in Boron. Available online: https://www.webmd.com/diet/foods-high-in-boron (accessed on 3 October 2024).
- Investigation on Background Content of Boron in Major Aquatic Products in China (Overview). Available online: https://en.cfsa.net.cn/UpLoadFiles//news/upload/2021/2021-12/ad1112fe-284f-4c9a-be5c-23d97bd8bf06.pdf (accessed on 3 October 2024).
- Table 6–3: Boron Levels in Food. Toxicological Profile for Boron. Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA. 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599072/table/ch6.tab3/ (accessed on 3 October 2024).


| Food Category | Food Group | Source | Calories/100 g | B Content (mg/100 g) | BND | Refs. |
|---|---|---|---|---|---|---|
| Plant-based products | Fruits | Apricots | 44 | 2.11 | 47.95 | [107,108] |
| Avocado | 161 | 2.06 | 12.80 | [80,107] | ||
| Blueberries | 47 | 0.76 | 16.17 | [107,109] | ||
| Currants | 40 | 1.74 | 43.50 | [80,107] | ||
| Dates | 292 | 1.08 | 3.70 | [80,107,108] | ||
| Figs | 65 | 1.26 | 19.38 | [80,107] | ||
| Peaches | 39 | 0.52 | 13.33 | [80,107,108] | ||
| Prunes | 50 | 1.88 | 37.60 | [80,107,108] | ||
| Raisins | 310 | 4.51 | 14.55 | [80,107,108] | ||
| Raspberries | 43 | 1.74 | 40.47 | [107,110] | ||
| Plant-based products | Vegetables | Beet root (canned) | 46 | 0.32 | 6.96 | [80,107] |
| Broccoli | 34 | 0.31 | 9.12 | [107,108] | ||
| Carrot | 38 | 0.30 | 7.89 | [107,108] | ||
| Celery | 21 | 0.50 | 32.81 | [107,108] | ||
| Dill | 11 | 0.38 | 34.55 | [80,107] | ||
| Lentils | 333 | 0.74 | 2.22 | [80,107,108] | ||
| Mushrooms | 25 | 0.16 | 6.40 | [80,107] | ||
| Olive | 115 | 0.35 | 3.04 | [107,108] | ||
| Onion | 33 | 0.20 | 6.06 | [107,108] | ||
| Parsley | 36 | 0.59 | 16.39 | [80,107] | ||
| Potato (sweet) | 116 | 0.18 | 1.55 | [107,108] | ||
| Spinach | 22 | 0.13 | 5.90 | [107,111] | ||
| Plant-based products | Nuts, seeds, and cereals | Almond | 576 | 2.82 | 4.90 | [80,107,108] |
| Amaranth seeds | 400 | 0.53 | 1.33 | [80,107,108] | ||
| Black rice | 362 | 0.32 | 0.88 | [80,107,108] | ||
| Brazil nuts | 656 | 1.72 | 2.62 | [80,107] | ||
| Cashew nuts | 553 | 1.15 | 2.08 | [80,107] | ||
| Hazelnuts | 628 | 2.77 | 4.41 | [107,108] | ||
| Oat | 350 | 0.35 | 1.00 | [80,107,108] | ||
| Peanuts | 567 | 1.49 | 2.63 | [107,112] | ||
| Pistachio nuts | 562 | 1.20 | 2.14 | [80,107] | ||
| Quinoa seeds | 366 | 0.85 | 2.32 | [80,107,108] | ||
| Walnuts | 654 | 1.63 | 2.49 | [80,107] | ||
| Dairy products | Functional probiotic foods produced by fermentation | Cottage cheese | 103 | 0.05 | 0.49 | [80,107] |
| Cow’s yogurt | 61 | 0.10 | 1.64 | [80,107,108] | ||
| Donkey’s cheese | 350 | 0.12 | 0.43 | [80,107,108] | ||
| Fermented soy (natto) | 212 | 0.10 | 0.47 | [80,107,108] | ||
| Goat’s cheese | 347 | 0.08 | 0.23 | [80,107,108] | ||
| Goat’s yogurt | 97 | 0.15 | 1.55 | [80,107,108] | ||
| Kefir | 52 | 0.10 | 1.92 | [80,107,108] | ||
| Sheep’s cheese | 288 | 0.05 | 0.17 | [80,107,108] | ||
| Skyr | 48 | 0.10 | 2.08 | [80,107,108] | ||
| Aquatic products | Marine fish | Barracuda | 211 | 0.10 | 0.47 | [107,113] |
| Salmon | 181 | 0.12 | 0.66 | [107,113] | ||
| Seafood | Clams | 89 | 0.10 | 1.12 | [107,113] | |
| Crabs | 97 | 0.13 | 1.34 | [107,113] | ||
| Oysters | 63 | 0.40 | 6.35 | [107,113] | ||
| Shrimps | 91 | 0.12 | 1.32 | [107,113] | ||
| Beverages | Natural beverages | Coffee | 2 | 0.03 | 15.00 | [107,114] |
| Green tea | 1 | 0.01 | 10.00 | [107,114] | ||
| Shiraz Cabernet wine | 83 | 0.86 | 10.36 | [107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biţă, A.; Scorei, I.R.; Mogoşanu, G.D.; Bejenaru, L.E.; Biţă, C.E.; Dinescu, V.C.; Rău, G.; Ciocîlteu, M.V.; Bejenaru, C.; Croitoru, O. Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview. Inorganics 2024, 12, 308. https://doi.org/10.3390/inorganics12120308
Biţă A, Scorei IR, Mogoşanu GD, Bejenaru LE, Biţă CE, Dinescu VC, Rău G, Ciocîlteu MV, Bejenaru C, Croitoru O. Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview. Inorganics. 2024; 12(12):308. https://doi.org/10.3390/inorganics12120308
Chicago/Turabian StyleBiţă, Andrei, Ion Romulus Scorei, George Dan Mogoşanu, Ludovic Everard Bejenaru, Cristina Elena Biţă, Venera Cristina Dinescu, Gabriela Rău, Maria Viorica Ciocîlteu, Cornelia Bejenaru, and Octavian Croitoru. 2024. "Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview" Inorganics 12, no. 12: 308. https://doi.org/10.3390/inorganics12120308
APA StyleBiţă, A., Scorei, I. R., Mogoşanu, G. D., Bejenaru, L. E., Biţă, C. E., Dinescu, V. C., Rău, G., Ciocîlteu, M. V., Bejenaru, C., & Croitoru, O. (2024). Naturally Occurring Microbiota-Accessible Borates: A Focused Minireview. Inorganics, 12(12), 308. https://doi.org/10.3390/inorganics12120308






