Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives
Abstract
1. Introduction
2. Results and Discussion
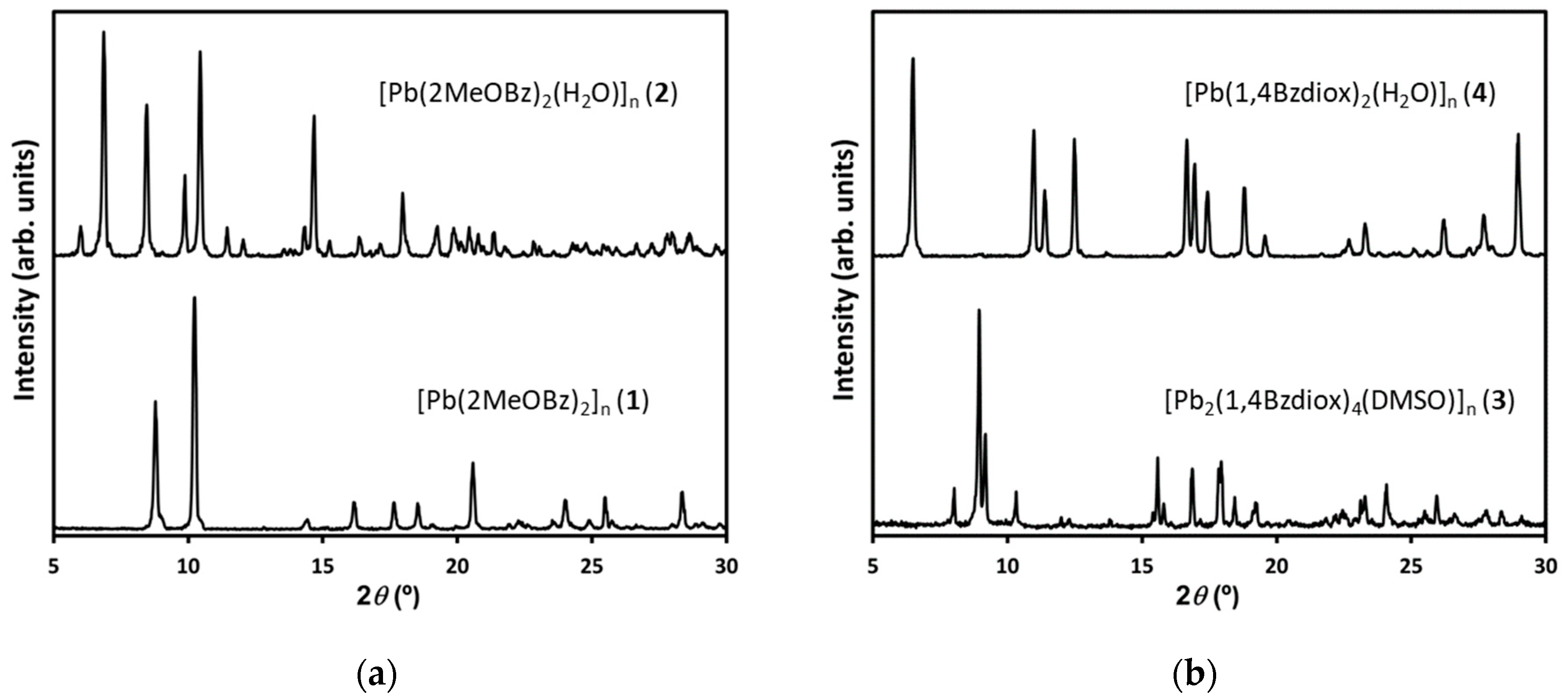
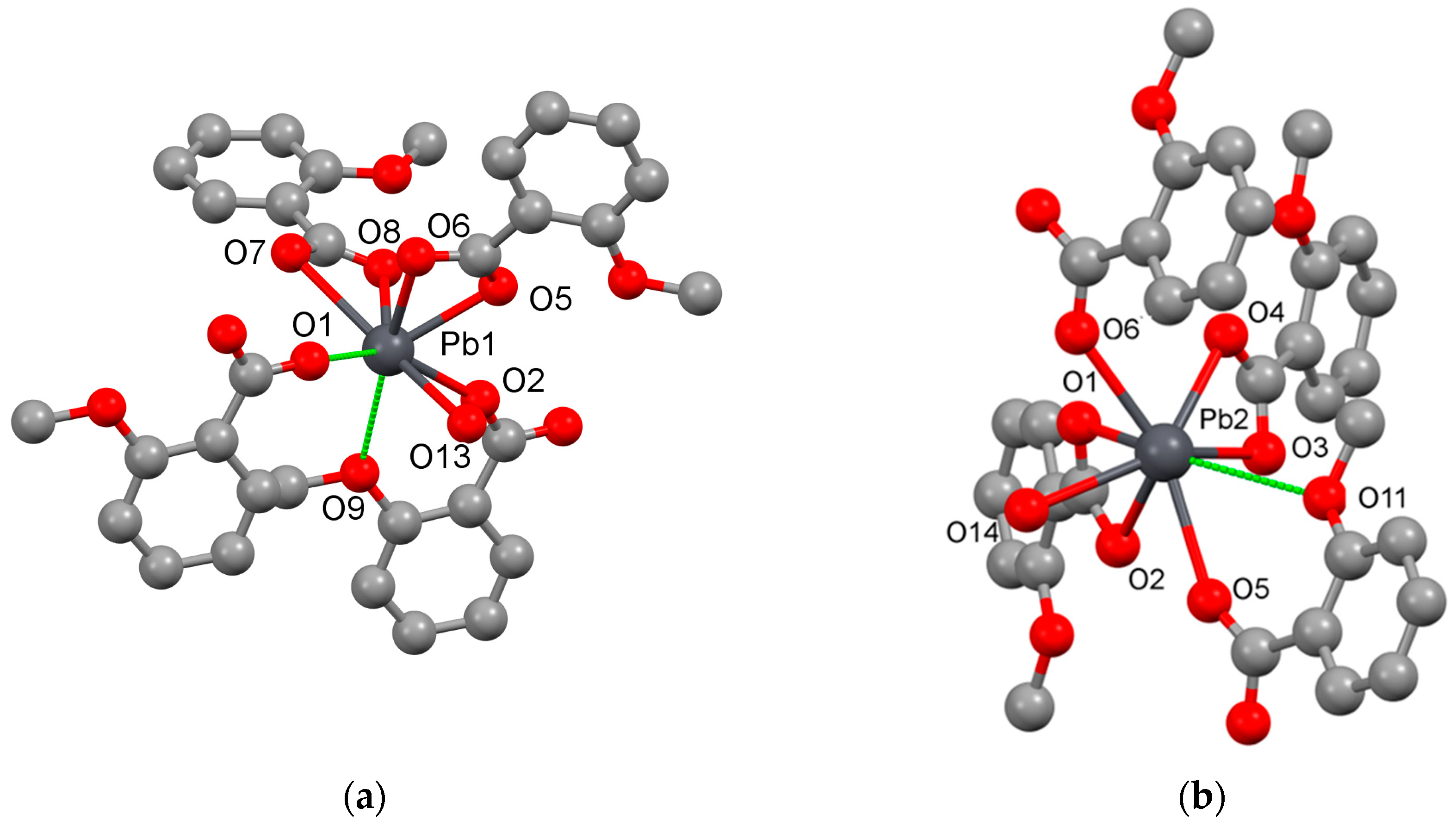
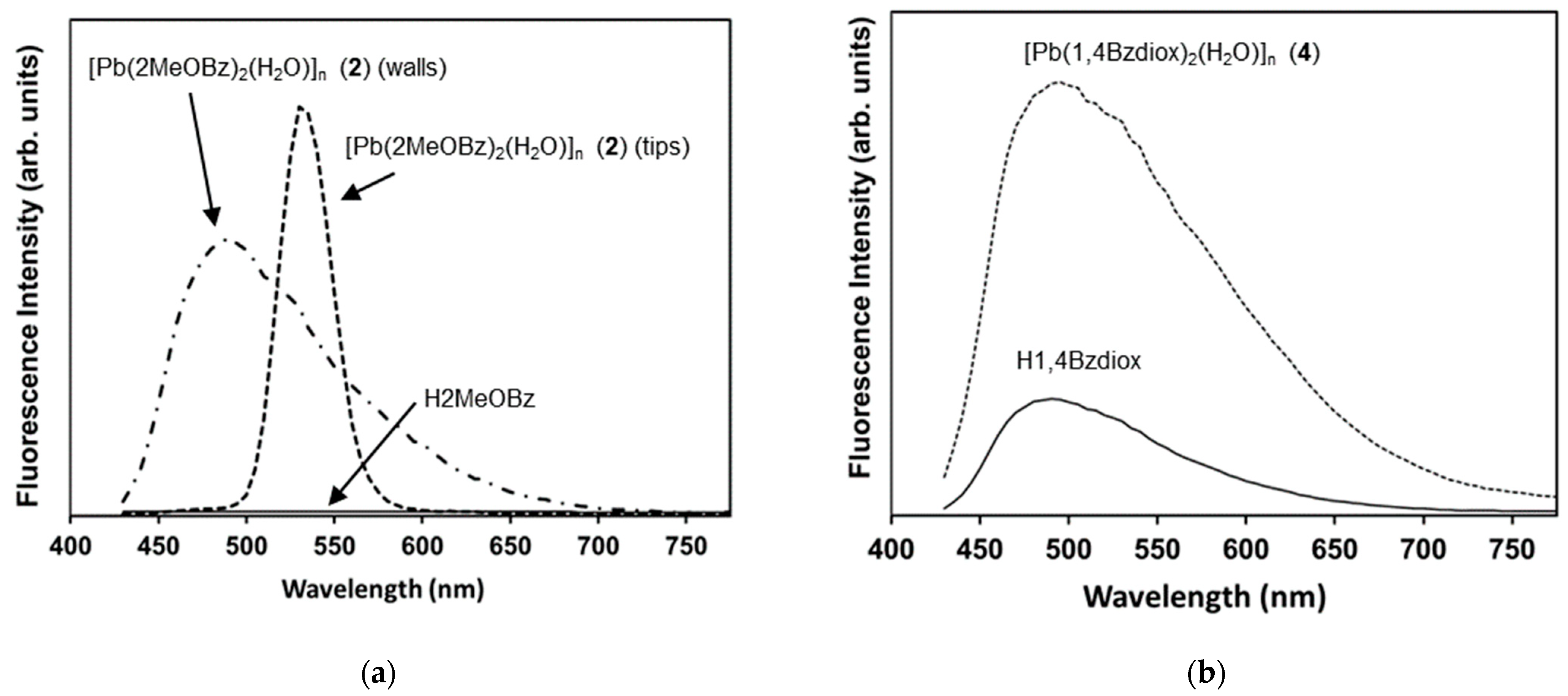
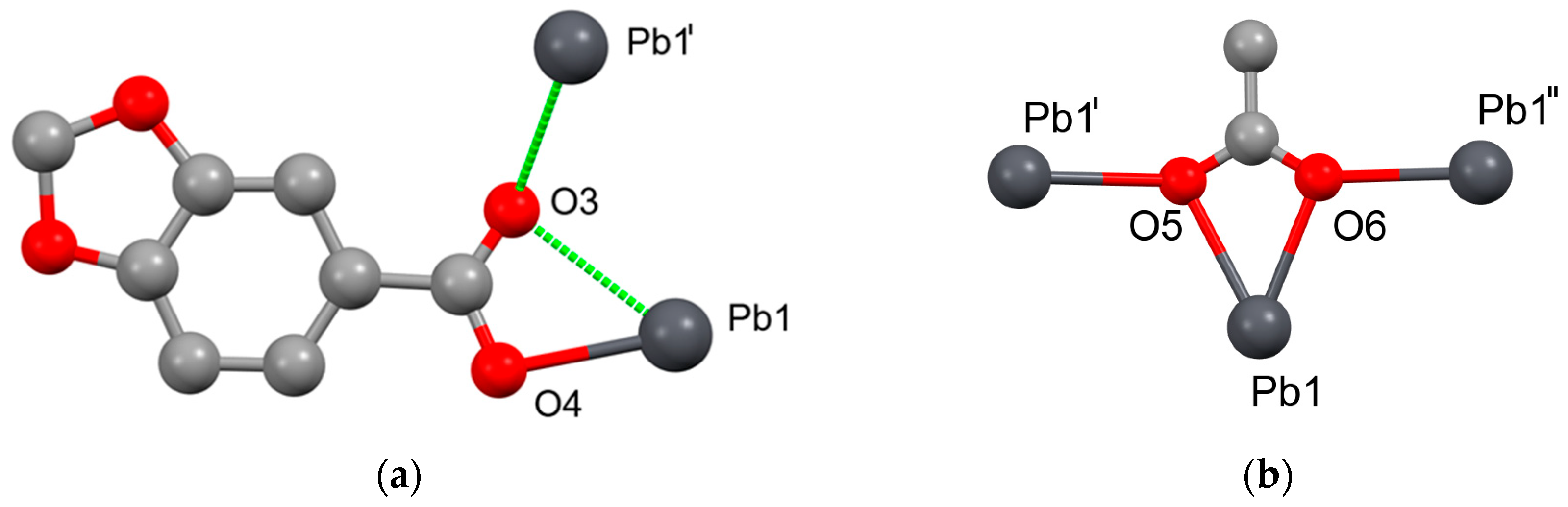
2.1. Pb(II) Complexes Containing 2MeOBz or 1,4Bzdiox Ligands
2.2. Pb(II) Complexes Containing Piperonylate Ligand
3. Experimental
3.1. Materials and Methods
3.2. Synthesis and Basic Characterization
3.3. X-ray Crystallographic Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magyar, J.S.; Weng, T.-C.; Stern, C.M.; Dye, D.F.; Rous, B.W.; Payne, J.C.; Bridgewater, B.M.; Mijovilovich, A.; Parkin, G.; Zaleski, J.M.; et al. Reexamination of Lead(II) Coordination Preferences in Sulfur-Rich Sites: Implications for a Critical Mechanism of Lead Poisoning. J. Am. Chem. Soc. 2005, 127, 9495–9505. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.-F.; Liu, Y.-Y.; Ma, J.-C.; Batten, S.R. A Series of Lead(II) Complexes with Π−π Stackings: Structural Diversities by Varying the Ligands. Cryst. Growth Des. 2009, 9, 1894–1911. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Z.-A.; Wu, Z.-M.; Lin, Z.-Q.; Liao, W.-M.; He, J. Hydrated Proton Conduction and Luminescence of a Carboxylate and Sulfonate-Included Lead(II) Coordination Polymer. J. Solid State Chem. 2020, 287, 121325. [Google Scholar] [CrossRef]
- Qi, H.-X.; Jo, H.; Ok, K.M. Pb[NC5H3(CO2)2]: A White Light Emitting Single Component Coordination Polymer Revealing High Quantum Efficiency and Thermal Stability. Inorg. Chem. Front. 2018, 5, 1273–1276. [Google Scholar] [CrossRef]
- Barszcz, B.; Masternak, J.; Kowalik, M. Structural Insights into Coordination Polymers Based on 6s2 Pb(II) and Bi(III) Centres Connected via Heteroaromatic Carboxylate Linkers and Their Potential Applications. Coord. Chem. Rev. 2021, 443, 213935. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photonics 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Sui, B.; Zhao, W.; Ma, G.; Okamura, T.; Fan, J.; Li, Y.-Z.; Tang, S.-H.; Sun, W.-Y.; Ueyama, N. Novel Pb(Ii) Coordination Frameworks: Synthesis, Crystal Structures and Unusual Third-Order Nonlinear Optical Properties. J. Mater. Chem. 2004, 14, 1631–1639. [Google Scholar] [CrossRef]
- Saah, S.A.; Boadi, N.O.; Adu-Poku, D.; Wilkins, C. Lead Ethyl Dithiocarbamates: Efficient Single-Source Precursors to PbS Nanocubes. R. Soc. Open Sci. 2019, 6, 190943. [Google Scholar] [CrossRef]
- Ezenwa, T.E.; McNaughter, P.D.; Raftery, J.; Lewis, D.J.; O’Brien, P. Full Compositional Control of PbS x Se 1−x Thin Films by the Use of Acylchalcogourato Lead(ii) Complexes as Precursors for AACVD. Dalton Trans. 2018, 47, 16938–16943. [Google Scholar] [CrossRef]
- Claudio, E.S.; Godwin, H.A.; Magyar, J.S. Fundamental Coordination Chemistry, Environmental Chemistry, and Biochemistry of Lead(II). In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; Volume 51, pp. 1–144. ISBN 978-0-471-26534-4. [Google Scholar]
- Davidovich, R.L.; Stavila, V.; Marinin, D.V.; Voit, E.I.; Whitmire, K.H. Stereochemistry of Lead(II) Complexes with Oxygen Donor Ligands. Coord. Chem. Rev. 2009, 253, 1316–1352. [Google Scholar] [CrossRef]
- Hu, M.-L.; Morsali, A.; Aboutorabi, L. Lead(II) Carboxylate Supramolecular Compounds: Coordination Modes, Structures and Nano-Structures Aspects. Coord. Chem. Rev. 2011, 255, 2821–2859. [Google Scholar] [CrossRef]
- Rajakannu, P.; Kaleeswaran, D.; Banerjee, S.; Butcher, R.J.; Murugavel, R. Effect of Benzoic Acid Substituents and Additional Functional Groups of Ancillary Ligands in Modulating the Nuclearity and Aggregation Behavior of Transition Metal Carboxylates. Inorg. Chim. Acta 2019, 486, 283–293. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kalyakina, A.S.; Solodukhin, N.N.; Aslandukov, A.N. On the Structural Features of Substituted Lanthanide Benzoates. Eur. J. Inorg. Chem. 2019, 2019, 2320–2332. [Google Scholar] [CrossRef]
- Sarma, R.; Baruah, J.B. Solvent Coordination in Changing Dimensionality of Lead Benzoate Coordination Polymers. Inorganica Chim. Acta 2009, 362, 4977–4984. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.; An, X. catena-Poly[[aqua(3-methylbenzoato-κ2O, O′)lead(II)]-μ-3-methylbenzoato-κ4O:O, O′:O′]. Acta Crystallogr. E Struct. Rep. Online 2009, 65, m709–m710. [Google Scholar] [CrossRef]
- Baruah, J.B. CCDC 673854: Experimental Crystal Structure Determination; CCDC: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Usubaliev, B.T.; Amirov, A.S.; Amiraslanov, I.R.; Mamedov, K.S. Crystal and molecular structure of di(para-nitrobenzoato)diaquolead. J. Struct. Chem. 1989, 30, 851–853. [Google Scholar] [CrossRef]
- Easterday, C.C.; Dedon, L.R.; Zeller, M.; Oertel, C.M. Helical ∞ 1 [Pb2O] Chains in Polymorphs of Pb2O(C6H5COO)2. Cryst. Growth Des. 2014, 14, 2048–2055. [Google Scholar] [CrossRef]
- Liu, E.E.; Gang, C.; Zeller, M.; Fabini, D.H.; Oertel, C.M. Ligand-Induced Variations in Symmetry and Structural Dimensionality of Lead Oxide Carboxylates. Cryst. Growth Des. 2017, 17, 1574–1582. [Google Scholar] [CrossRef]
- Seth, S.K.; Bauzá, A.; Mahmoudi, G.; Stilinović, V.; López-Torres, E.; Zaragoza, G.; Keramidas, A.D.; Frontera, A. On the Importance of Pb⋯X (X = O, N, S, Br) Tetrel Bonding Interactions in a Series of Tetra- and Hexa-Coordinated Pb(II) Compounds. CrystEngComm 2018, 20, 5033–5044. [Google Scholar] [CrossRef]
- Bauzá, A.; Seth, S.K.; Frontera, A. Tetrel Bonding Interactions at Work: Impact on Tin and Lead Coordination Compounds. Coord. Chem. Rev. 2019, 384, 107–125. [Google Scholar] [CrossRef]
- Ejarque, D.; Sánchez-Férez, F.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Pons, J. Diverse Structures and Dimensionalities in Zn(II), Cd(II), and Hg(II) Metal Complexes with Piperonylic Acid. Cryst. Growth Des. 2020, 20, 383–400. [Google Scholar] [CrossRef]
- Guerrero, M.; Vázquez, S.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Pons, J. Zn(II) and Cd(II) Coordination Dimers Based on Mixed Benzodioxole-Carboxylate and N-Donor Ligands: Synthesis, Characterization, Crystal Structures and Photoluminescence Properties. ChemistrySelect 2017, 2, 632–639. [Google Scholar] [CrossRef]
- Guerrero, M.; Pou, R.; Bayés-García, L.; Font-Bardia, M.; Sort, J.; Pons, J.; Ayllón, J.A. Syntheses, Supramolecular Architectures and Photoluminescence Properties of Zn(II) Complexes Based on 3,5 dihydroxybenzoic and Pyridine/Pyrazole Derived Ligands. Inorg. Chem. Comm. 2018, 96, 34–38. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Pons, J. Synthesis, Crystal Structure Inorganic Chemistry Communications and Magnetic Properties of a Cu(II) Paddle-Wheel Complex with Mixed Bridges. Inorg. Chem. Comm. 2016, 71, 90–93. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Pou, R.; Bayés-García, L.; Font-Bardia, M.; Pons, J.; Ayllón, J.A. Benzoate Substituents Effects on the Structure of Zn(II) Complexes and 1D 4,4′-Bipyridine Derived Coordination Polymers. Inorg. Chim. Acta 2020, 500, 119218. [Google Scholar] [CrossRef]
- Cotton, F.A.; Francis, R.; Horrocks, W.D. Sulfoxides as ligands II. The infrared spectra of some dimethyl sulfoxide complexes. J. Phys. Chem. 1960, 64, 1534–1536. [Google Scholar] [CrossRef]
- Kowalik, M.; Masternak, J.; Brzeski, J.; Daszkiewicz, M.; Barszcz, B. Effect of a Lone Electron Pair and Tetrel Interactions on the Structure of Pb(II) CPs Constructed from Pyrimidine Carboxylates and Auxiliary Inorganic Ions. Polyhedron 2022, 219, 115818. [Google Scholar] [CrossRef]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 6th ed.; McGraw-Hill: London, UK, 2008; ISBN 978-0-07-711812-9. [Google Scholar]
- Juanhuix, J.; Gil-Ortiz, F.; Cuní, G.; Colldelram, C.; Nicolás, J.; Lidón, J.; Boter, E.; Ruget, C.; Ferrer, S.; Benach, J. Developments in Optics and Performance at BL13-XALOC, the Macromolecular Crystallography Beamline at the Alba Synchrotron. J. Synchrotron Rad. 2014, 21, 679–689. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Fauth, F.; Peral, I.; Popescu, C.; Knapp, M. The new Material Science Powder Diffraction beamline at ALBA Synchrotron. Powder Diffr. 2013, 28, S360–S370. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Vallcorba, O.; Rius, J.; Frontera, C.; Peral, I.; Miravitlles, C. DAJUST: A suite of computer programs for pattern matching, space-group determination and intensity extraction from powder diffraction data. J. Appl. Crystallogr. 2012, 45, 844–848. [Google Scholar] [CrossRef]
- Vallcorba, O.; Rius, J.; Frontera, C.; Miravitlles, C. TALP: A multisolution direct-space strategy for solving molecular crystals from powder diffraction data based on restrained least squares. J. Appl. Crystallogr. 2012, 45, 1270–1277. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayllón, J.A.; Vallcorba, O.; Domingo, C. Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives. Inorganics 2024, 12, 24. https://doi.org/10.3390/inorganics12010024
Ayllón JA, Vallcorba O, Domingo C. Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives. Inorganics. 2024; 12(1):24. https://doi.org/10.3390/inorganics12010024
Chicago/Turabian StyleAyllón, José A., Oriol Vallcorba, and Concepción Domingo. 2024. "Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives" Inorganics 12, no. 1: 24. https://doi.org/10.3390/inorganics12010024
APA StyleAyllón, J. A., Vallcorba, O., & Domingo, C. (2024). Solvent Influence in the Synthesis of Lead(II) Complexes Containing Benzoate Derivatives. Inorganics, 12(1), 24. https://doi.org/10.3390/inorganics12010024







