Reduction of Triple Bond in [B12H11NCR]− Anions by Lithium Aluminum Hydride: A Novel Approach to the Synthesis of N-Monoalkylammonio-Substituted closo-Dodecaborates
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. IR Spectra
3.2. 1H NMR, 11B NMR, and 13C NMR
3.3. Electrospray Ionization Mass Spectrometry (ESI-MS)
3.4. Preparative HPLC
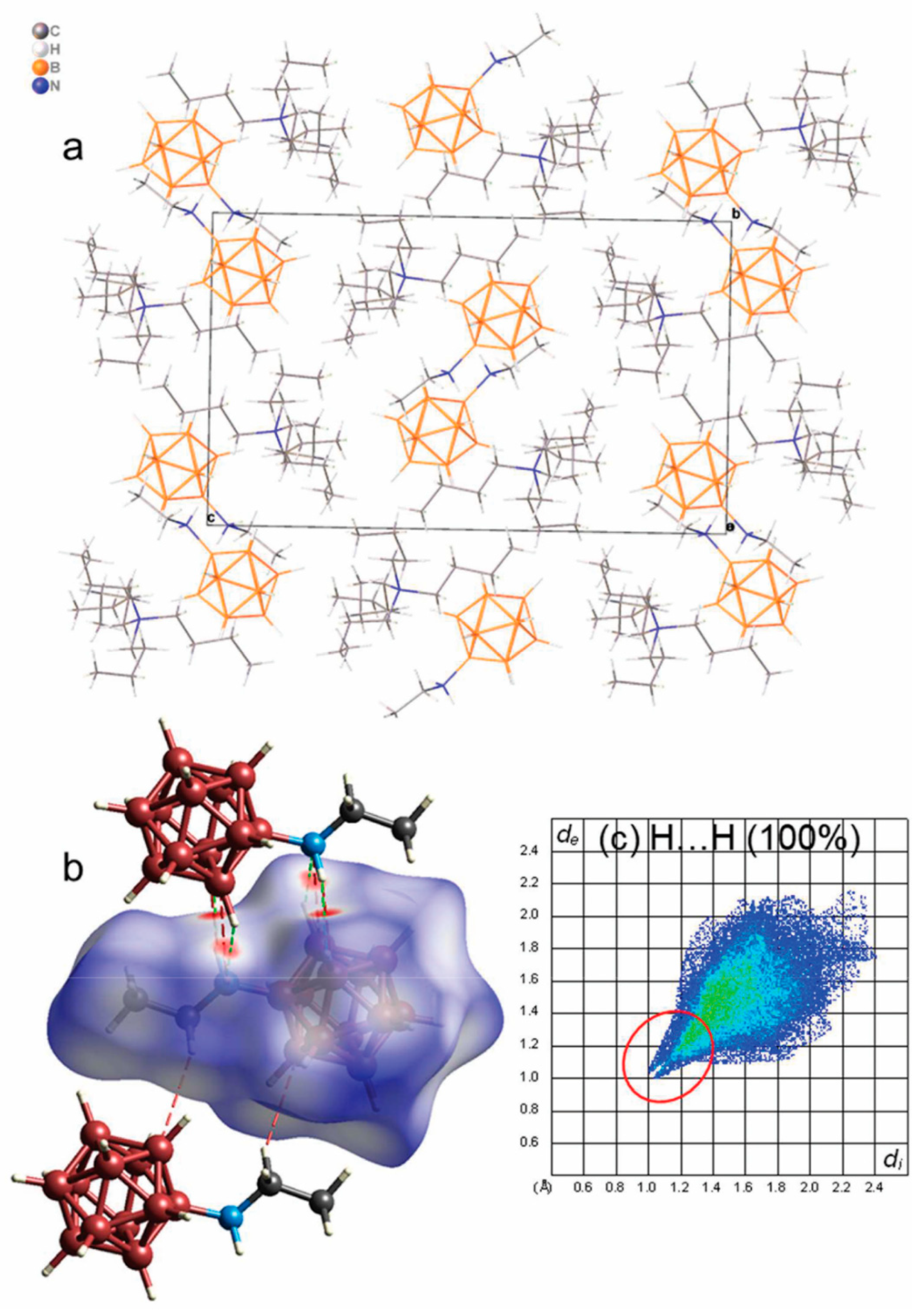
3.5. Single-Crystal X-ray Diffraction
3.6. Computational Details
3.7. Synthesis of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, H.; Kirihata, M. Boron Compounds: New Candidates for Boron Carriers in BNCT. In Neutron Capture Therapy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–116. [Google Scholar]
- Pitto-Barry, A. Polymers and Boron Neutron Capture Therapy (BNCT): A Potent Combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Novopashina, D.S.; Vorobyeva, M.A.; Venyaminova, A. Recent Advances in the Synthesis of High Boron-Loaded Nucleic Acids for BNCT. Front. Chem. 2021, 9, 619052. [Google Scholar] [CrossRef]
- Tjarks, W. The Use of Boron Clusters in the Rational Design of Boronated Nucleosides for Neutron Capture Therapy of Cancer. J. Organomet. Chem. 2000, 614–615, 37–47. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. What Are the Current Challenges with the Application of Boron Clusters to Drug Design? Expert. Opin. Drug Discov. 2021, 16, 481–483. [Google Scholar] [CrossRef]
- Gentil, S.; Crespo, E.; Rojo, I.; Friang, A.; Viñas, C.; Teixidor, F.; Grüner, B.; Gabel, D. Polypyrrole Materials Doped with Weakly Coordinating Anions: Influence of Substituents and the Fate of the Doping Anion during the Overoxidation Process. Polymer 2005, 46, 12218–12225. [Google Scholar] [CrossRef]
- Axtell, J.C.; Saleh, L.M.A.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. [Google Scholar] [CrossRef]
- Jenne, C.; Wegener, B. Silver Salts of the Weakly Coordinating Anion [Me3NB12Cl11]−. Z. Anorg. Allg. Chem. 2018, 644, 1123–1132. [Google Scholar] [CrossRef]
- Nieuwenhuyzen, M.; Seddon, K.R.; Teixidor, F.; Puga, A.V.; Viñas, C. Ionic Liquids Containing Boron Cluster Anions. Inorg. Chem. 2009, 48, 889–901. [Google Scholar] [CrossRef]
- Spokoyny, A.M. New Ligand Platforms Featuring Boron-Rich Clusters as Organomimetic Substituents. Pure Appl. Chem. 2013, 85, 903–919. [Google Scholar] [CrossRef]
- Grimes, R.N. Boron-Carbon Ring Ligands in Organometallic Synthesis. Chem. Rev. 1992, 92, 251–268. [Google Scholar] [CrossRef]
- Kirlikovali, K.O.; Axtell, J.C.; Gonzalez, A.; Phung, A.C.; Khan, S.I.; Spokoyny, A.M. Luminescent Metal Complexes Featuring Photophysically Innocent Boron Cluster Ligands. Chem. Sci. 2016, 7, 5132–5138. [Google Scholar] [CrossRef]
- Bolze, F.; Hayek, A.; Sun, X.H.; Baldeck, P.L.; Bourgogne, C.; Nicoud, J.F. New Insight in Boron Chemistry: Application in Two-Photon Absorption. Opt. Mater. 2011, 33, 1453–1458. [Google Scholar] [CrossRef]
- Omidvar, A. Nonlinear Optical Response of Teetotum Boron Clusters. Comput. Theor. Chem. 2021, 1198, 113178. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, G. Oxo Boron Clusters and Their Open Frameworks. Eur. J. Inorg. Chem. 2011, 2011, 3857–3867. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Boron Clusters in Luminescent Materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef]
- Allis, D.G.; Spencer, J.T. Polyhedral-Based Nonlinear Optical Materials. 2.1 Theoretical Investigation of Some New High Nonlinear Optical Response Compounds Involving Polyhedral Bridges with Charged Aromatic Donors and Acceptors. Inorg. Chem. 2001, 40, 3373–3380. [Google Scholar] [CrossRef]
- Scheifers, J.P.; Zhang, Y.; Fokwa, B.P.T. Boron: Enabling Exciting Metal-Rich Structures and Magnetic Properties. Acc. Chem. Res. 2017, 50, 2317–2325. [Google Scholar] [CrossRef]
- Oleshkevich, E.; Teixidor, F.; Rosell, A.; Viñas, C. Merging Icosahedral Boron Clusters and Magnetic Nanoparticles: Aiming toward Multifunctional Nanohybrid Materials. Inorg. Chem. 2018, 57, 462–470. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Polyhedral Boron Clusters in Materials Science. New J. Chem. 2011, 35, 1955. [Google Scholar] [CrossRef]
- Mori, T. Thermoelectric and Magnetic Properties of Rare Earth Borides: Boron Cluster and Layered Compounds. J. Solid. State Chem. 2019, 275, 70–82. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C.; Li, X.; Wang, D.; Sun, L.; Chen, B.; Liu, W.; Zhang, H.; Zhou, X. Self-Assembled Magnetic Gold Catalysts from Dual-Functional Boron Clusters. ChemCatChem 2018, 10, 2285–2290. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Ma, X.; Liu, Y.; Zhang, H. Direct Conversion of Methane into Methanol and Ethanol via Spherical Au@Cs2[Closo-B12H12] and Pd@Cs2[Closo-B12H12] Nanoparticles. Int. J. Hydrogen Energy 2021, 46, 30750–30761. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Duttwyler, S.; Zhang, Y. Efficient Adsorption Separation of Methane from CO2 and C2–C3 Hydrocarbons in a Microporous Closo-Dodecaborate [B12H12]2− Pillared Metal–Organic Framework. J. Solid. State Chem. 2021, 299, 122167. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhang, H.; Zhou, X. Cubic Platinum Nanoparticles Capped with Cs2[Closo-B12H12] as an Effective Oxidation Catalyst for Converting Methane to Ethanol. J. Colloid. Interface Sci. 2020, 566, 135–142. [Google Scholar] [CrossRef]
- Raghuwanshi, M.; Lanterne, A.; le Perchec, J.; Pareige, P.; Cadel, E.; Gall, S.; Duguay, S. Influence of Boron Clustering on the Emitter Quality of Implanted Silicon Solar Cells: An Atom Probe Tomography Study. Prog. Photovolt. Res. Appl. 2015, 23, 1724–1733. [Google Scholar] [CrossRef]
- Krishnan, S.; Senthilkumar, K. Opto-Electronic Properties of Quasi-Planar Boron Clusters—A DFT Investigation. Chem. Phys. Lett. 2022, 804, 139914. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Liu, B.X. Boron-Doped Diamond-like Amorphous Carbon as Photovoltaic Films in Solar Cell. Sol. Energy Mater. Sol. Cells 2001, 69, 339–344. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of Closo-Dodecaborate Anion [B12H12]2−: A Review. Collect. Czechoslov. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Geis, V.; Guttsche, K.; Knapp, C.; Scherer, H.; Uzun, R. Synthesis and Characterization of Synthetically Useful Salts of the Weakly-Coordinating Dianion [B12Cl12]2−. Dalton Trans. 2009, 15, 2684–2687. [Google Scholar] [CrossRef]
- Peryshkov, D.V.; Popov, A.A.; Strauss, S.H. Direct Perfluorination of K2B12H12 in Acetonitrile Occurs at the Gas Bubble−Solution Interface and Is Inhibited by HF. Experimental and DFT Study of Inhibition by Protic Acids and Soft, Polarizable Anions. J. Am. Chem. Soc. 2009, 131, 18393–18403. [Google Scholar] [CrossRef]
- Pluntze, A.M.; Bukovsky, E.V.; Lacroix, M.R.; Newell, B.S.; Rithner, C.D.; Strauss, S.H. Deca-B-Fluorination of Diammonioboranes. Structures and NMR Characterization of 1,2-, 1,7-, and 1,12-B12H10(NH3)2 and 1,2-, 1,7-, and 1,12-B12F10(NH3)2. J. Fluor. Chem. 2018, 209, 33–42. [Google Scholar] [CrossRef]
- Tiritiris, I.; Schleid, T. The Crystal Structure of Solvent-Free Silver Dodecachloro-Closo-dodecaborate Ag2[B12Cl12] from Aqueous Solution. Z. Anorg. Allg. Chem. 2003, 629, 581–583. [Google Scholar] [CrossRef]
- Bayer, M.J.; Hawthorne, M.F. An Improved Method for the Synthesis of [Closo-B12(OH)12]−2. Inorg. Chem. 2004, 43, 2018–2020. [Google Scholar] [CrossRef]
- Bondarev, O.; Khan, A.A.; Tu, X.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis of [Closo-B12(OH)11NH3]−: A New Heterobifunctional Dodecaborane Scaffold for Drug Delivery Applications. J. Am. Chem. Soc. 2013, 135, 13204–13211. [Google Scholar] [CrossRef]
- Knoth, W.H.; Sauer, J.C.; Balthis, J.H.; Miller, H.C.; Muetterties, E.L. Boranes. XXX. Carbonyl derivatives of B10H102− and B12H122−. J. Am. Chem. Soc. 1967, 3973, 4842–4850. [Google Scholar] [CrossRef]
- Srebny, H.-G.; Preetz, W.; Marsmann, H.C. Darstellung, 11B-NMR- Und Schwingungsspektren Isomerenreiner Halogenhydrododecaborate XnB12H12-n2−; X = Cl, n = 1–3, X = Br, n =1,2; X = I, n =1. Z. Naturforschung B 1984, 39, 189–196. [Google Scholar] [CrossRef]
- Knoth, W.H.; Sauer, J.C.; England, D.C.; Hertler, W.R.; Muetterties, E.L. Chemistry of Boranes. XIX. 1 Derivative Chemistry of B10H10−2 and B12H12−2. J. Am. Chem. Soc. 1964, 86, 3973–3983. [Google Scholar] [CrossRef]
- Hertler, W.R.; Raasch, M.S. Chemistry of Boranes. XIV. Amination of B10H10−2 and B12H12−2 with Hydroxylamine-O-Sulfonic Acid. J. Am. Chem. Soc. 1964, 86, 3661–3668. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in Three-Dimensional Aromatic-like Closo-Dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Semioshkin, A.A.; Brellochs, B.; Sjoberg, S.; Bregadze, V.I. Synthesis of Oxonium Derivatives of the Dodecahydro-closo-dodecaborate anion [B12H12]2−. Polyhedron. 2000, 19, 627–632. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; et al. Synthesis and in vitro Evaluation of Thiododecaborated α, α-Cycloalkylamino Acids for the Treatment of Malignant Brain Tumors by Boron Neutron Capture Therapy. Amino Acids 2014, 46, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Musah, R.A. Evaluation of the Potential of 2-Amino-3-(1,7-Dicarba- Closo-Dodecaboranyl-1-Thio)Propanoic Acid as a Boron Neutron Capture Therapy Agent. ACS Omega 2019, 4, 3820–3826. [Google Scholar] [CrossRef]
- Nagasawa, K.; Narisada, M. Synthesis of Polyhedral Borane Derivatives Having a Carboxy Group. Tetrahedron Lett. 1990, 31, 4029–4032. [Google Scholar] [CrossRef]
- Nagasawa, K.; Ikenishi, Y.; Nakagawa, Y. Oxidation Products of Cesium Monomercaptoundecahydro-Closo-Dodecaborate(2−). J. Organomet. Chem. 1990, 391, 139–146. [Google Scholar] [CrossRef]
- Goswami, L.N.; Ma, L.; Cai, Q.; Sarma, S.J.; Jalisatgi, S.S.; Frederick Hawthorne, M. CRGD Peptide-Conjugated Icosahedral Closo-B122− Core Carrying Multiple Gd3+-DOTA Chelates for Avβ3 Integrin-Targeted Tumor Imaging (MRI). Inorg. Chem. 2013, 52, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.N.; Chakravarty, S.; Cai, Q.-Y.; Shapiro, E.M.; Frederick Hawthorne, M.; Ma, L. Amphiphilic DTPA Multimer Assembled on Icosahedral Closo-Borane Motif as High-Performance MRI Blood Pool Contrast Agent. ACS Appl. Bio Mater. 2021, 4, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic Oxonium Derivatives of Polyhedral Boron Hydrides and Their Synthetic Applications. Dalton Trans. 2008, 11, 977–992. [Google Scholar] [CrossRef]
- Prikaznov, A.V.; Shmal’ko, A.V.; Sivaev, I.B.; Petrovskii, P.V.; Bragin, V.I.; Kisin, A.V.; Bregadze, V.V. Synthesis of Carboxylic Acids Based on the Closo-Decaborate Anion. Polyhedron 2011, 30, 1494–1501. [Google Scholar] [CrossRef]
- Himmelspach, A.; Finze, M.; Vöge, A.; Gabel, D. Cesium and Tetrabutylammonium Salt of the Ethynyl-Closo-Dodecaborate Dianion. Z. Anorg. Allg. Chem. 2012, 638, 512–519. [Google Scholar] [CrossRef]
- Peymann, T.; Knobler, C.B.; Hawthorne, M.F. Synthesis of Alkyl and Aryl Derivatives of Closo-B12H122− by the Palladium-Catalyzed Coupling of Closo-B12H11I2− with Grignard Reagents. Inorg. Chem. 1998, 37, 1544–1548. [Google Scholar] [CrossRef]
- Kaszyński, P.; Ringstrand, B. Functionalization of Closo-Borates via Iodonium Zwitterions. Angew. Chem.-Int. Ed. 2015, 54, 6576–6581. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Kaszyński, P.; Domagała, S.; Woźniak, K. The [Closo-B12H11-1-IAr]− Zwitterion as a Precursor to Monosubstituted Derivatives of [Closo-B12H12]2−. J. Organomet. Chem. 2015, 798, 70–79. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bruskin, A.B.; Nesterov, V.V.; Antipin, M.Y.; Bregadze, V.I.; Sjöberg, S. Synthesis of Schiff Bases Derived from the Ammoniaundecahydro-Closo-Dodecaborate(1−) Anion, [B12H11NHCHR]−, and Their Reduction into Monosubstituted Amines [B12H11NH2CH2R]−: A New Route to Water Soluble Agents for BNCT. Inorg. Chem. 1999, 38, 5887–5893. [Google Scholar] [CrossRef]
- Justus, E.; Vöge, A.; Gabel, D. N-Alkylation of Ammonioundecahydro-Closo-dodecaborate(1–) for the Preparation of Anions for Ionic Liquids. Eur. J. Inorg. Chem. 2008, 2008, 5245–5250. [Google Scholar] [CrossRef]
- Peymann, T.; Lork, E.; Schmidt, M.; Nöth, H.; Gabel, D. N-Alkylation of Ammine—Undecahydro-Closo-Dodecaborate(1−). Chem. Ber. 1997, 130, 795–799. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary Amine Nucleophilic Addition to Nitrilium Closo-Dodecaborate [B12H11NCCH3]−: A Simple and Effective Route to the New BNCT Drug Design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium Derivatives of Polyhedral Boron Compounds (Boranes, Carboranes, Metallocarboranes): Synthesis and Reactivity. Phosphorus Sulfur. Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Laskova, J.; Ananiev, I.; Kosenko, I.; Serdyukov, A.; Stogniy, M.; Sivaev, I.; Grin, M.; Semioshkin, A.; Bregadze, V.I. Nucleophilic Addition Reactions to Nitrilium Derivatives [B12H11NCCH3]− and [B12H11NCCH2CH3]−. Synthesis and Structures of Closo-Dodecaborate-Based Iminols, Amides and Amidines. Dalton Trans. 2022, 51, 3051–3059. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Selivanov, N.A.; Bykov, A.Y.; Kubasov, A.S.; Zhizhin, K.Y.; Kuznetsov, N.T. New Aspects of the Synthesis of Closo-Dodecaborate Nitrilium Derivatives [B12H11NCR]− (R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7): Experimental and Theoretical Studies. Inorganics 2022, 10, 196. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Voinova, V.V.; Klyukin, I.N.; Kubasov, A.S.; Zhizhin, K.Y.; Kuznetsov, N.T. New Synthesis Method of N-Monosubstituted Ammonium-Closo-Decaborates. J. Clust. Sci. 2019, 30, 1327–1333. [Google Scholar] [CrossRef]
- Ashby, E.C.; Dobbs, F.R.; Hopkins, H.P. Composition of Lithium Aluminum Hydride, Lithium Borohydride, and Their Alkoxy Derivatives in Ether Solvents as Determined by Molecular Association and Conductance Studies. J. Am. Chem. Soc. 1975, 97, 3158–3162. [Google Scholar] [CrossRef]
- Guo, Z.; Sindelar, R.D. A New Preparation of Esters from Carbonyl Compounds Following Lithium Aluminum Hydride Reduction. Synth. Commun. 1998, 28, 1031–1039. [Google Scholar] [CrossRef]
- Brown, H.C.; Weissman, P.M.; Yoon, N.M. Selective Reductions. IX. Reaction of Lithium Aluminum Hydride with Selected Organic Compounds Containing Representative Functional Groups1. J. Am. Chem. Soc. 1966, 88, 1458–1463. [Google Scholar] [CrossRef]
- Newman, M.S.; Fukunaga, T. The Reduction of Amides to Amines via Nitriles by Lithium Aluminum Hydride1. J. Am. Chem. Soc. 1960, 82, 693–696. [Google Scholar] [CrossRef]
- Sruthi, P.R.; Anas, S. An Overview of Synthetic Modification of Nitrile Group in Polymers and Applications. J. Polym. Sci. 2020, 58, 1039–1061. [Google Scholar] [CrossRef]
- Moshnenko, N.; Kazantsev, A.; Chupakhin, E.; Bakulina, O.; Dar’in, D. Synthetic Routes to Approved Drugs Containing a Spirocycle. Molecules 2023, 28, 4209. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Belsito, E.L.; Leggio, A.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. Reduction of Amide Carbonyl Group and Formation of Modified Amino Acids and Dipeptides. Tetrahedron Lett. 2015, 56, 2062–2066. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Lithium Aluminum Hydride as Reducing Agent for Chemically Reduced Graphene Oxides. Chem. Mater. 2012, 24, 2292–2298. [Google Scholar] [CrossRef]
- Amundsen, L.H.; Nelson, L.S. Reduction of Nitriles to Primary Amines with Lithium Aluminum Hydride1. J. Am. Chem. Soc. 1951, 73, 242–244. [Google Scholar] [CrossRef]
- Glaser, R.; Ulmer, L.; Coyle, S. Mechanistic Models for LAH Reductions of Acetonitrile and Malononitrile. Aggregation Effects of Li+ and AlH3 on Imide–Enamide Equilibria. J. Org. Chem. 2013, 78, 1113–1126. [Google Scholar] [CrossRef]
- Málek, J. Reductions by Metal Alkoxyaluminum Hydrides. In Organic Reactions; Wiley: Hoboken, NJ, USA, 1985; pp. 1–317. [Google Scholar]
- Li, H.; Zhang, Y.; Liu, L.; Jiao, N.; Meng, X.; Zhang, S. Amino Functionalized [B12H12]2− Salts as Hypergolic Fuels. New J. Chem. 2018, 42, 3568–3573. [Google Scholar] [CrossRef]
- Gainsford, G.J.; Bowden, M.E. Propylamine–Borane. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o1395. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.-Y.; Watson, W.H.; Kelly, H.C. Molecular and Crystal Structure of Ethylenediamine-Bisborane, C2H14B2N3. Inorg. Chem. 1972, 11, 374–377. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic Addition of Amino Acid Esters to Nitrilium Derivatives of Closo-Decaborate Anion. Mendeleev Commun. 2021, 31, 201–203. [Google Scholar] [CrossRef]
- Rattle, H.W.E. NMR of Amino Acids, Peptides, and Proteins (1977–1979). In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 1981; Volume 11, pp. 1–64. [Google Scholar]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of AccuracyElectronic Supplementary Information (ESI) Available: [DETAILS]. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.; Legare, D. Properties of Atoms in Molecules: Structures and Reactivities of Boranes and Carboranes. Can. J. Chem. 1992, 70, 657–677. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]


| Compound | 2a |
|---|---|
| Empirical formula | C18H54B12N2 |
| Formula weight | 428.35 |
| Temperature/K | 150.00 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 10.292(12) |
| b/Å | 12.938(10) |
| c/Å | 21.49(3) |
| β/° | 90.15(6) |
| 90 | |
| Volume/Å3 | 2862(5) |
| Z | 4 |
| ρcalcg/cm3 | 0.994 |
| μ/mm−1 | 0.050 |
| F(000) | 944.0 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 3.674 to 51.994 |
| Reflections collected | 11,524 |
| Independent reflections | 5562 [Rint = 0.0379, Rsigma = 0.0722] |
| Data/restraints/parameters | 5562/7/295 |
| Goodness of fit on F2 | 1.057 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0783, wR2 = 0.2136 |
| Final R indexes [all data] | R1 = 0.1340, wR2 = 0.2438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelyubin, A.V.; Neumolotov, N.K.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Kubasov, A.S.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Reduction of Triple Bond in [B12H11NCR]− Anions by Lithium Aluminum Hydride: A Novel Approach to the Synthesis of N-Monoalkylammonio-Substituted closo-Dodecaborates. Inorganics 2024, 12, 2. https://doi.org/10.3390/inorganics12010002
Nelyubin AV, Neumolotov NK, Selivanov NA, Bykov AY, Klyukin IN, Novikov AS, Kubasov AS, Zhdanov AP, Zhizhin KY, Kuznetsov NT. Reduction of Triple Bond in [B12H11NCR]− Anions by Lithium Aluminum Hydride: A Novel Approach to the Synthesis of N-Monoalkylammonio-Substituted closo-Dodecaborates. Inorganics. 2024; 12(1):2. https://doi.org/10.3390/inorganics12010002
Chicago/Turabian StyleNelyubin, Alexey V., Nikolay K. Neumolotov, Nikita A. Selivanov, Alexander Yu. Bykov, Ilya N. Klyukin, Alexander S. Novikov, Alexey S. Kubasov, Andrey P. Zhdanov, Konstantin Yu. Zhizhin, and Nikolay T. Kuznetsov. 2024. "Reduction of Triple Bond in [B12H11NCR]− Anions by Lithium Aluminum Hydride: A Novel Approach to the Synthesis of N-Monoalkylammonio-Substituted closo-Dodecaborates" Inorganics 12, no. 1: 2. https://doi.org/10.3390/inorganics12010002
APA StyleNelyubin, A. V., Neumolotov, N. K., Selivanov, N. A., Bykov, A. Y., Klyukin, I. N., Novikov, A. S., Kubasov, A. S., Zhdanov, A. P., Zhizhin, K. Y., & Kuznetsov, N. T. (2024). Reduction of Triple Bond in [B12H11NCR]− Anions by Lithium Aluminum Hydride: A Novel Approach to the Synthesis of N-Monoalkylammonio-Substituted closo-Dodecaborates. Inorganics, 12(1), 2. https://doi.org/10.3390/inorganics12010002










