Abstract
We reported herein the synthesis, structure determination and emission properties of a cubic molecular Cd(II) coordination cluster whose faces are composed of 12-MC-4 metallacrown units built up from Cd2+ and 2-methylmercaptobenzohydroxamic acid (LmmbHA), resulting in [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O. The polynuclear complex obtained was characterised by single crystal X-ray diffraction at 193 K. The bulk sample was also analysed by elemental analysis. UV-Vis and emission spectra of the complex were measured in chloroform, as well as the emission spectra of the ligand for comparison. The results of the emission studies revealed that both the ligand and the complex are weakly emissive.
1. Introduction
In recent years, an enormous amount of attention has been paid to the design of coordination-driven self-assembly of polymetallic supramolecular architectures with multi-topic organic ligands by crystal engineering [1,2,3,4,5,6,7]. The successful formation of supramolecular self-assemblies depends strongly on the ligand design in many cases, as the nature of the binding pocket and the metal–ligand interactions are the factors that play a key role in the control and self-orientation during the self-assembly of the complexes [6,7,8,9,10,11].
Among these, Metallacrown (MC) complexes, have been of great interest in various fields of research including molecular recognition, catalysis and magnetism and luminescence, since their first discovery in 1989 by Pecoraro [12,13,14]. MCs, like their organic crown ether analogues, contain oxygen donor atoms in their repeating unit bridged by metal ions and heteroatomic atoms in the ring. The most common types of MCs are 9-MC-3, 12-MC-4 and 15-MC-5. For example, in the case of 9-MC-3 unit the ring consists of nine atoms with three of them being donor atoms such as oxygen or nitrogen to bind a central guest metal ion. The first vanadium MC [(VVO)3(shi)3(CH3OH)3] and [Fe4(shi)3(MeOH)3(OAc)3] MC, where shi is salicylhydroximate, showing 9-MC-3 structural motif were reported by Pecoraro in 1989 [15]. The first vacant CoIII with 12-MC-4 structural motif was reported by some of us in 2015 and a CrIII4 complex with a 9-MC-3 structural motif in 2020 [14,16]. If the luminescence properties are to be investigated in MCs, they must be built up via 3d metal ions without the metal being able to quench the luminescence. MC based on Gallium (3p) as ring ions was reported with lanthanides as a guest metal ion to study the luminescence properties in 2019 [17]. MCs with metal ions containing filled d-orbitals such as Zn(II) were also reported to investigate the luminescence properties. In a next step, we now went down the group to Cd(II) in order to examine the MC obtained herewith for its luminescence [18,19]. In comparison to Zinc, the size of Cadmium and its completely filled 4d orbitals make it a potentially interesting candidate for luminescence.
Cadmium-based materials are also used in building quantum dots because of the broad UV excitation, narrow emission and high photostability [20,21]. The potential applications of Cadmium also extend to the usage in solar cells, batteries, alloys and battery storage [22,23,24]. Cadmium being a soft metal prefers softer donor atoms such as sulphur in comparison to oxygen, which is one of the common donors in the metallacrown synthesised with salicylhydroxamic acid. Some of us have previously reported a novel sulphur containing salicylhydroxamate LmmbHA ligand synthesized in a three-step reaction procedure, along with two copper 12-MC-4 metallacrowns based on the ligand [25]. Tong and co-workers in 2016 reported interesting single molecular magnetic behaviour in non-classical Cd containing 4d-4f MC [26].
We herein reported the synthesis, structural determination and emission properties of a cubic molecular Cd(II) coordination cluster of which faces were made-up of 12-MC-4 metallacrown units composed from Cd2+ and 2-methylmercaptobenzohydroxamic acid (LmmbHA) to form [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O. The emission studies showed that both the ligand and the complex were weakly emissive.
2. Results and Discussion
2.1. Synthesis
The ligand LmmbHA used to prepare the metallacrown was synthesized in three steps as previously reported by us [25]. In the first step, the methyl 2-mercaptobenzoate was formed by esterification of 2-mercaptobenzoic acid in methanol under an inert atmosphere. In the second step, methyl 2-(methylthio)benzoate was formed by methylation of the thiol group with methyl iodide. Finally, in the last step, a commonly used substitution reaction with hydroxylamine gave the desired product in moderate yields. The LmmbHA ligand obtained was complexed with Cd(II) perchlorate salt in DMF in the presence of triethylamine as a base to yield colourless block shaped crystals in low yields. The complex obtained was characterized by single crystal X-ray crystallography, elemental analysis and IR spectroscopy. The single crystal X-ray structure was obtained from a single crystal carefully picked up from the mother liquor whereas the elemental analysis and IR were measured from the bulk sample which was filtered, washed with diethyl ether and dried in air, as detailed in the complex synthesis section. During this process, the lattice DMF molecules present in the crystal lattice might have evaporated and the void spaces in the crystal lattice filled with water molecules from the atmosphere. This is described more often in the literature [27,28,29,30,31].
2.2. Crystallographic Discussion
The reaction of the LmmbHA ligand with Cadmium(II) perchlorate in the presence of triethylamine base in dimethylformamide afforded colourless block-shaped crystalline [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O in low yields after fourteen days. A structural determination at 193 K shows that the complex crystallized in the P21/c space group in a monoclinic crystal system (Table 1). One unique unit of a single MC structure is shown in Figure 1. It is evident from Figure 1 that the non-coordinating sulphur atoms in ortho-position of the ring in LmmbHA led to a lack of pre-orientation, which is the reason why the metallacrown ring was not built up regularly by four [M-N-O] repeating units, but instead resulted in an irregular N-O arrangement (Figure 1). One N-O bridge (Cd2-O6-N4-Cd7) is oriented opposite to the main direction. Interestingly, twelve ligand molecules are involved to form the polynuclear coordination complex, as shown in Figure 2. In the 12-MC-4 unit is formed by four ring-forming Cd(II) ions, each of these Cd(II) ions is also part of three different MCs and, thus, represents the edges of a face-centred cube. The central core of the complex was, thus, formed by a face-centred cube of 14 Cd(II) ions. The inner cavity of this cube is occupied by an oxo (μ6−O2−) ligand coordinating the central metal ion of each MC. Two non-coordinating perchlorate counterions in the lattice constitute to the overall charge balance. The coordination sphere of the Cd(II) ions is fulfilled by ten co-ordinating dimethylformamide molecules which helped to form the cube structure.

Table 1.
Crystallography data of [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O complex.
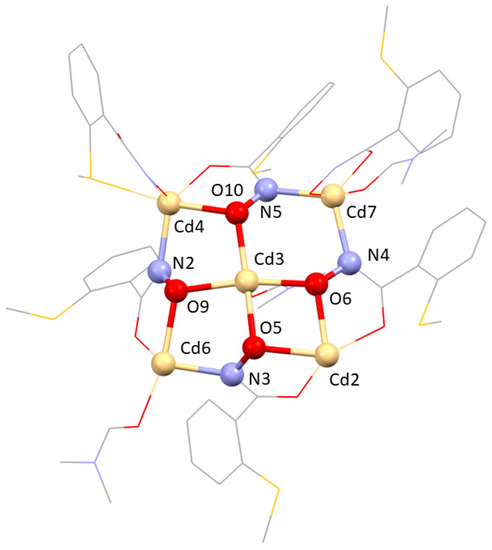
Figure 1.
Excerpt of the cube structure of [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O. One single 12-MC-4 motif highlighting the irregular [M-N-O] repetition units. Colour code: Pale yellow—Cd(II), red—oxygen, blue—nitrogen, yellow—sulphur, grey—carbon. Hydrogen atoms have been omitted for clarity.
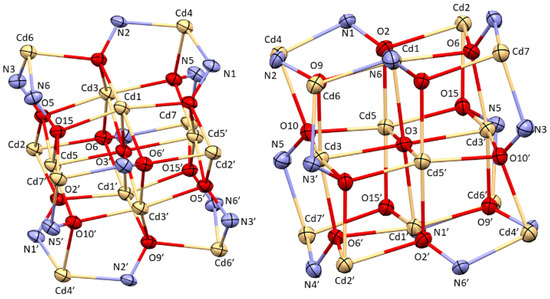
Figure 2.
Two different perspectives on the N-O bridged face-centred Cd cube (left and right). Colour code: Pale yellow—Cd(II), red—oxygen, blue—nitrogen, yellow—sulphur, grey—carbon, green—chlorine. ORTEP representation with atomic displacement parameters at 50% level of probability.
The results of the Continuous Shape Measures (CShM) on each Cadmium ion are tabulated in Table 2. The CShM shows that Cd1, Cd3 and Cd5 are all in a slightly distorted octahedral geometry with six coordinating oxygen atoms. On the other hand, Cd2 exists in a trigonal prismatic polyhedron. In the case of Cd6 and Cd7, they are surrounded by three oxygen and two nitrogen atoms, forming a trigonal bipyramidal environment. Cd4 also possesses the same geometry with two nitrogen donor atoms (N1; N2) and one oxygen donor atom (O11) in the horizontal plane; additionally, an oxygen atom (O10) and sulphur atom (S1) coordinated from the axial position.

Table 2.
SHAPE 2.1 results of each of the seven crystallographically independent Cadmium(II) ions of Cd(II) 12-MC.
The crystal structure can also be described as a penetrated 18-MC-6 following the [M-N-O] units of Cd4, Cd6 and Cd7 (Figure 3 and Figure 4). The cores of the MCs were filled by two cubes sharing one corner. The cube structure was built by Cd1, Cd2, Cd3, Cd5 and oxygen atoms. The ring-building Cadmium ions are all in the trigonal bipyramidal coordination geometry (TBPY-5) as confirmed by CShM analysis. The central building block of the cubes is a cadmium octahedron in which each Cd ion is coordinated by six oxygen atoms (OC-6). This octahedron of metal ions is capped by two Cd2 ions, which is the only cadmium showing a trigonal prismatic (TPR-6) coordination environment.
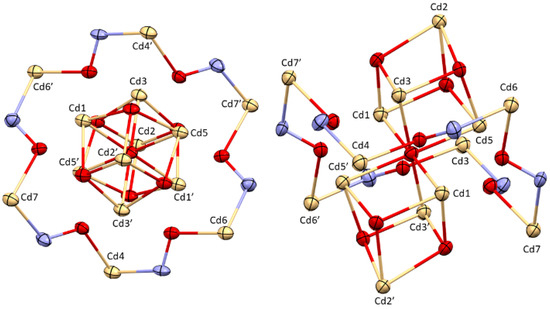
Figure 3.
Ring motif of an 18-MC-6 structure in [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O (left), with penetrating cubes formed from eight Cd ions (right). Colour code: Pale yellow—Cd(II), red—oxygen, blue—nitrogen, yellow—sulphur, grey—carbon. ORTEP representation with atomic displacement parameters at 50% level of probability.
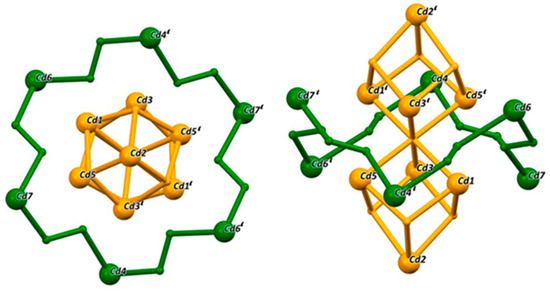
Figure 4.
18-MC-6 representation of [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O. The cubic core is coloured in yellow; the MC ring is coloured in green.
The obtained complex structure is a close analogue of the face-centred cubes with Ni(II) and Co(II) reported by Zhuang et al. [32]. This also clearly indicates that the non-crucial role of the sulphur donor atoms in the ligand for the formation of MC, as in the case of the Ni(II) and Co(II) reported by Zhuang et al., the face capped cubes is formed by 4-bromo-benzohydroximic acid, which did not possess a donor atom at the ortho position of the ligand.
2.3. Infrared Spectroscopy
The three characteristic bands at 1677, 1561 and 1311 cm−1 can be assigned to the carbonyl stretching vibrations of the salicylhydroxamic acid and confirmed the formation of the desired ligand (Figure 5; left). The respective shifts to 1642, 1568 and 1348 cm−1 together with the stretching vibration of the coordinated DMF at 1677 cm−1 and a strong perchlorate counterion stretching vibration at 1064 cm−1 confirms the formation of the desired complex (Figure 5; right).
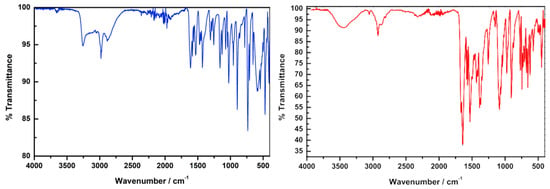
Figure 5.
(Left): FT-Infrared spectra of LmmbHA (blue). (Right): complex (red). [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O.
2.4. Luminescence
The luminescence spectra of MC and Ligand (Figure 6) were recorded at ambient conditions in chloroform at a concentration of 1 × 10−5 M. The photoluminescence of MC is ligand-based and structured with a broad tail in the visible range. The emission maximum of the free ligand is 360 nm and that of MC is slightly red-shifted with an emission maximum at 377 nm.
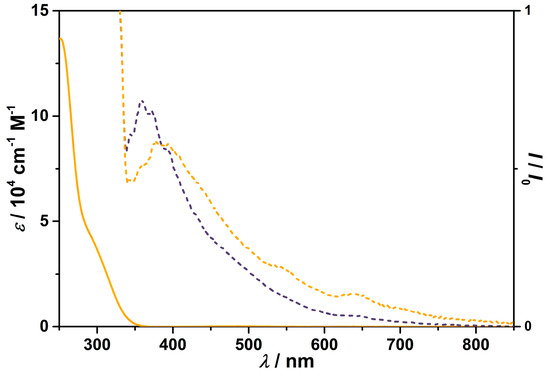
Figure 6.
Absorption spectrum of [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O (solid line) and luminescence spectra of [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O (λexc = 300 nm, orange dotted lines) and LmmbHA (λexc = 300 nm, purple dotted lines).
3. Materials and Methods
All chemicals were used without further purification and were purchased from Alfa Aesar, Acros Organics and Fisher Chemicals. The C, H and N elemental analyses were carried out on a Foss Heraeus Vario EL (Elementar Analysensysteme GmbH, Langenselbold, Germany) at the microanalytical laboratories of the department of chemistry at the Johannes Gutenberg University Mainz. The infrared absorption spectra were collected at room temperature in a range of 4000–400 cm−1 on a Thermo Fischer NICOLET Nexus FT/IR-5700 spectrometer (Thermo Fischer Scientific, Waltham, MA, USA) equipped with Smart Orbit ATR Diamond cell. X-ray diffraction data for the structure analysis were collected from suitable single crystals at 173 K with a STOE IPDS 2T (STOE, Darmstadt, Germany) at the Johannes Gutenberg University Mainz. The structures were solved with ShelXT [33] and refined with ShelXL [34,35] implemented in the program Olex2 [36]. The UV-Vis spectra was recorded using a JASCO V-770 (JASCO, Yokohama, Japan) in a single beam mode with a data interval of 0.2 nm and a scanning speed of 400 nm/min in chloroform and emission spectra were recorded with a FLS1000 spectrometer from Edinburgh Instruments (Livingston, UK) equipped with the photomultiplier detector PMT-980. A xenon arc lamp Xe2 (450 W) was used for excitation in chloroform. The emission and UV-Vis data were plotted in Origin V7.5.
Complex Synthesis
To a 12 mL dimethylformamide, Cadmium perchlorate (94 mg, 0.3 mmol, 1 eq) and ligand LmmbHA (37 mg, 0.3 mmol, 1 eq) were added as solids and dissolved. Triethylamine (75 μL, 0.6 mmol, 2 eq) was added to the colourless solution. The reaction mixture was stirred for 3 h. The resulting colourless mixture was filtered under aerobic conditions to remove any undissolved impurities. The filtrate was layered with diethyl ether to obtain colourless block shaped crystals after two weeks in low yields (11 mg, 24%), and the filtrate was then filtered, washed with diethyl ether and dried in air. IR (ATR, ν˜ (cm−1): 3440 (vw), 2923 (w), 1668 (m), 1642 (s), 1583 (m), 1568 (m), 1532 (m), 1495 (m), 1466 (w), 1432 (m), 1415 (m), 1386 (m), 1370 (m), 1348 (m), 1255 (w), 1151 (vw), 1085 (m), 1064 (m), 1039 (w), 974 (w), 906 (m), 872 (w), 772 (w), 745 (m), 728 (w), 705 (w), 678 (w), 655 (m), 622 (m), 574 (w), 500 (w), 481 (w), 449 (w), 415 (vw). Elemental Analysis Calculated for C126H154N22S12Cl2Cd14O43·3H2O (M = 4694 g mol−1): C, 31.87%, H, 3.40%, N, 6.49%. Found: C, 31.40%; H, 3.48%; N,6.37%.
4. Conclusions
We reported the successful synthesis of the [CdII14(LmmbHA)12(µ6−O)(DMF)10](ClO4)2·3H2O complex and the implementation of a softer sulphur donor atom to form LmmbHA into a well-studied salicylhydroxamic acid ligand for MCs allowed us to successfully isolate a 4d MC with Cd(II) ions. The necessary next step is the coordination of a 4f metal ion as a central guest ion to provoke improved luminescence properties of the reported Cd(II) MC complex. A mixed 4d–4f classical MC complex will also be interesting from the magnetic point of view.
Author Contributions
Synthesis, investigation, experimental, C.G., M.J.F.; methodology, C.G. and E.R.; spectroscopy and data analysis, S.S., M.J.F.; crystal structure analysis, L.M.C., C.G., S.S.; writing-original draft preparation, C.G. and S.S.; writing-review and editing, S.S. and E.R.; supervision, E.R.; funding acquisition, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors also thank DFG funding, INST 247/1018-1, for the funding. CG kindly acknowledges the Excellence Initiative by the Graduate School of Excellence Materials Science in Mainz, Germany (DFG/GSC 266). All the authors thank the Johannes Gutenberg University of Mainz for funding and support.
Data Availability Statement
The single crystal X-ray data presented in this manuscript is submitted to CCDC data base for access and the spectroscopy data presented are available on request from the corresponding author.
Acknowledgments
The authors express their gratitude to Dieter Schollmeyer for valuable discussions and for performing the XRD studies. The authors also thank Robert Naumann and Katja Heinze for their valuable discussion and help with recording the emission spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardy, J.G. Metallosupramolecular Grid Complexes: Towards Nanostructured Materials with High-Tech Applications. Chem. Soc. Rev. 2013, 42, 7881. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Riddell, I.A.; Nitschke, J.R. Building on Architectural Principles for Three-Dimensional Metallosupramolecular Construction. Chem. Soc. Rev. 2013, 42, 1728. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Li, M.; Zhang, L.; Zhu, J. Bioinspired Supramolecular Photonic Composites: Construction and Emerging Applications. Macromol. Rapid Commun. 2022, 43, 2100867. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.Y.; Yam, V.W.W. Toward the Design and Construction of Supramolecular Functional Molecular Materials Based on Metal-Metal Interactions. J. Am. Chem. Soc. 2022, 144, 22805–22825. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.; Preuss, M.D.; van Basten, J.; Schoenmakers, S.M.C.; Spiering, A.J.H.; Vantomme, G.; Meijer, E.W. How Subtle Changes Can Make a Difference: Reproducibility in Complex Supramolecular Systems. Angew. Chem. 2022, 134, e202206738. [Google Scholar] [CrossRef]
- Constable, E.C. Stereogenic Metal Centres-from Werner to Supramolecular Chemistry. Chem. Soc. Rev. 2013, 42, 1637. [Google Scholar] [CrossRef]
- Constable, E.C. Expanded Ligands-An Assembly Principle for Supramolecular Chemistry. Coord. Chem. Rev. 2008, 252, 842–855. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Chemistry—Steps towards Complex Matter. Angew. Chem.—Int. Ed. 2013, 52, 2836–2850. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Chemistry—Aspects of Adaptive Chemistry and Materials. Angew. Chem.—Int. Ed. 2015, 54, 3276–3289. [Google Scholar] [CrossRef]
- Mezei, G.; Zaleski, C.M.; Pecoraro, V.L. Structural and Functional Evolution of Metallacrowns. Chem. Rev. 2007, 107, 4933–5003. [Google Scholar] [CrossRef] [PubMed]
- Bodwin, J.J.; Cutland, A.D.; Malkani, R.G.; Pecoraro, V.L. The Development of Chiral Metallacrowns into Anion Recognition Agents and Porous Materials. Coord. Chem. Rev. 2001, 216–217, 489–512. [Google Scholar] [CrossRef]
- Happ, P.; Plenk, C.; Rentschler, E. 12-MC-4 Metallacrowns as Versatile Tools for SMM Research. Coord. Chem. Rev. 2015, 289–290, 238–260. [Google Scholar] [CrossRef]
- Lah, M.S.; Pecoraro, V.L. Isolation and Characterization of {mnii[Mniii(Salicylhydroximate)]4(Acetate)2(Dmf)6}. 2dmf: An Inorganic Analogue of M2+(12-Crown-4). J. Am. Chem. Soc. 1989, 111, 7258–7259. [Google Scholar] [CrossRef]
- Lüpke, A.; Carrella, L.M.; Rentschler, E. Filling the Gap in the Metallacrown Family: The 9-MC-3 Chromium Metallacrown. Chem.—Eur. J. 2021, 27, 4283–4286. [Google Scholar] [CrossRef]
- Athanasopoulou, A.A.; Baldoví, J.J.; Carrella, L.M.; Rentschler, E. Field-Induced Slow Magnetic Relaxation in the First Dy(III)-Centered 12-Metallacrown-4 Double-Decker. Dalton Trans. 2019, 48, 15381–15385. [Google Scholar] [CrossRef]
- Xue, J.; Yang, F.; Jin, J.; Li, Y.; Wu, D.; Yang, G.-P.; Wang, Y.-Y. Design and Synthesis of Four Newly Water-Stable Pb-Based Heterometallic Organic Frameworks: How Do the Second Metals (Zn, Cd, Co, and Mn) Optimize Their Fluorescent and Catalytic Properties? Cryst. Growth Des. 2022, 14, 33. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kubota, E.; Fuyuhiro, A.; Kawata, S.; Harrowfield, J.M.; Kim, Y.; Hayami, S. Synthesis, structure and luminescence properties of Cu(II), Zn(II) and Cd(II) complexes with 4′-tetraphenylterpyridine. Dalton Trans. 2012, 41, 10825–10831. [Google Scholar] [CrossRef]
- Werlin, R.; Priester, J.H.; Mielke, R.E.; Krämer, S.; Jackson, S.; Stoimenov, P.K.; Stucky, G.D.; Cherr, G.N.; Orias, E.; Holden, P.A. Biomagnification of Cadmium Selenide Quantum Dots in a Simple Experimental Microbial Food Chain. Nat. Nanotechnol. 2011, 6, 65–71. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Santra, P.K.; Kamat, P.V. Tandem-Layered Quantum Dot Solar Cells: Tuning the Photovoltaic Response with Luminescent Ternary Cadmium Chalcogenides The Influence of the Se:S Ratio in Tuning. J. Am. Chem. Soc. 2013, 135, 32. [Google Scholar] [CrossRef]
- Chu, T.L.; Chu, S.S. Recent Progress in Thin-Film Cadmium Telluride Solar Cells. Prog. Photovolt. Res. Appl. 1993, 1, 31–42. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Lin Zhong, Y.; Burn, P.L.; Khaja Nazeeruddin, M.; Shaw, P.E.; Batmunkh, M. Next-Generation Applications for Integrated Perovskite Solar Cells. Commun. Mater. 2023, 4, 2. [Google Scholar] [CrossRef]
- Gamer, C.; Sundaresan, S.; Carrella, L.M.; Rentschler, E. Single- vs Double-Decker Copper 12-MC-4 Metallacrown Using the Coordination Flexibility of a Soft Donor Ligand. Eur. J. Inorg. Chem. 2022, 2022, e202200261. [Google Scholar] [CrossRef]
- Li, Q.-W.; Wan, R.-C.; Chen, Y.-C.; Liu, J.-L.; Wang, L.-F.; Jia, J.-H.; Chilton, N.F.; Tong, M.-L. Unprecedented Hexagonal Bipyramidal Single-Ion Magnets Based on Metallacrowns. Chem. Commun. 2016, 52, 13365–13368. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Becker, J.-G.; Eppelsheimer, J.; Sedykh, A.E.; Carrella, L.; Müller-Buschbaum, K.; Rentschler, E. Synergetic Spin Singlet-Quintet Switching and Luminescence in Mononuclear Fe(II) 1,3,4-Oxadiazole Tetradentate Chelates with NCBH3 Co-Ligand. Dalton Trans. 2023; accepted manuscript. [Google Scholar] [CrossRef]
- Sundaresan, S.; Kiehl, J.; Carrella, L.M.; Rentschler, E. Effect of Methoxy Ligand-Substituent and NCE Coligands in Tuning T 1/2 of Coordinatively Elastic Fe(II) Spin Crossover Complexes. Cryst. Growth Des. 2023, 23, 1648–1655. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, Z.-B.; Luo, T.-K.; Peng, Y.; Liu, S.-J.; Anson, C.E.; Powell, A.K.; Wen, H.-R. Nine-Coordinate Ln III-Based One-Dimensional Complexes: Synthesis and Magnetic Properties. Cryst. Growth Des. 2023, 11, 43. [Google Scholar] [CrossRef]
- Sundaresan, S.; Diehl, M.; Carrella, L.M.; Rentschler, E. Triggering of Valence Tautomeric Transitions in Dioxolene-Based Cobalt Complexes Influenced by Ligand Substituents, Co-Ligands, and Anions. Magnetochemistry 2022, 8, 109. [Google Scholar] [CrossRef]
- Harris, M.M.; Kühne, I.A.; Kelly, C.T.; Jakobsen, V.B.; Jordan, R.; O’Brien, L.; Müller-Bunz, H.; Felton, S.; Morgan, G.G. Compressed and Expanded Lattices—Barriers to Spin-State Switching in Mn3+ Complexes. Cryst. Growth Des. 2023, 23, 3996–4012. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.C.; Liu, Y.J.; Zhu, D.R.; Zhuang, J.Z.; You, X.Z. Preparation, Crystal Structures and Magnetic Properties of 12-Metallacrown-4 Complexes with the Donors on the Organic Periphery of Molecule. Inorganica Chim. Acta 2000, 305, 135–142. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Foundations and Advances SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M.; Schneider, T.R. [16] SHELXL: High-Resolution Refinement. Methods Enzymol. 1997, 277, 319–343. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).