Synthesis, Luminescence and Energy Transfer Properties of Ce3+/Mn2+ Co-Doped Calcium Carbodiimide Phosphors
Abstract
1. Introduction
2. Results and Discussion
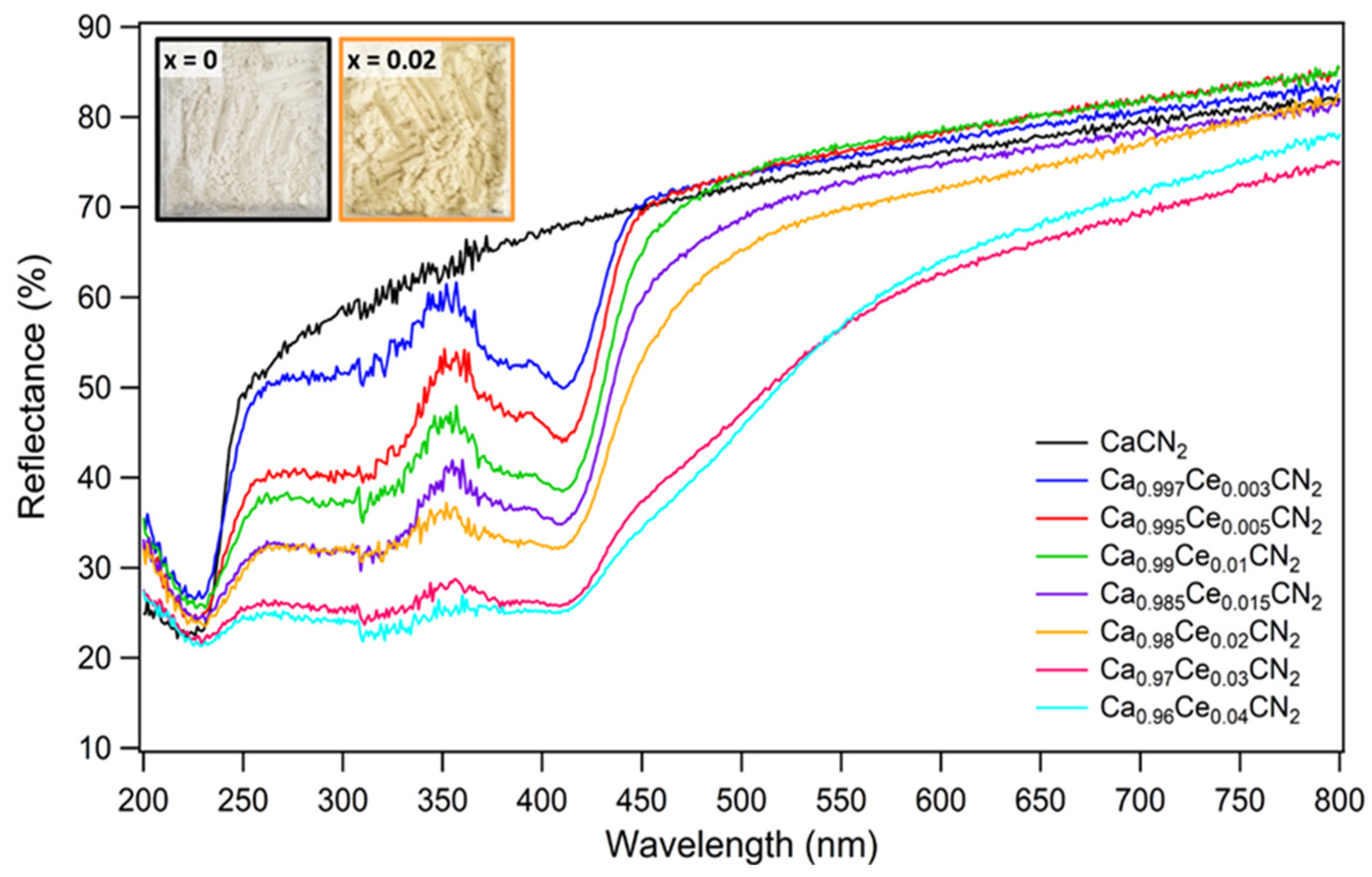
2.1. Materials Characterization and Optical Properties of Ca1−xCexCN2 (0 ≤ x ≤ 0.04)
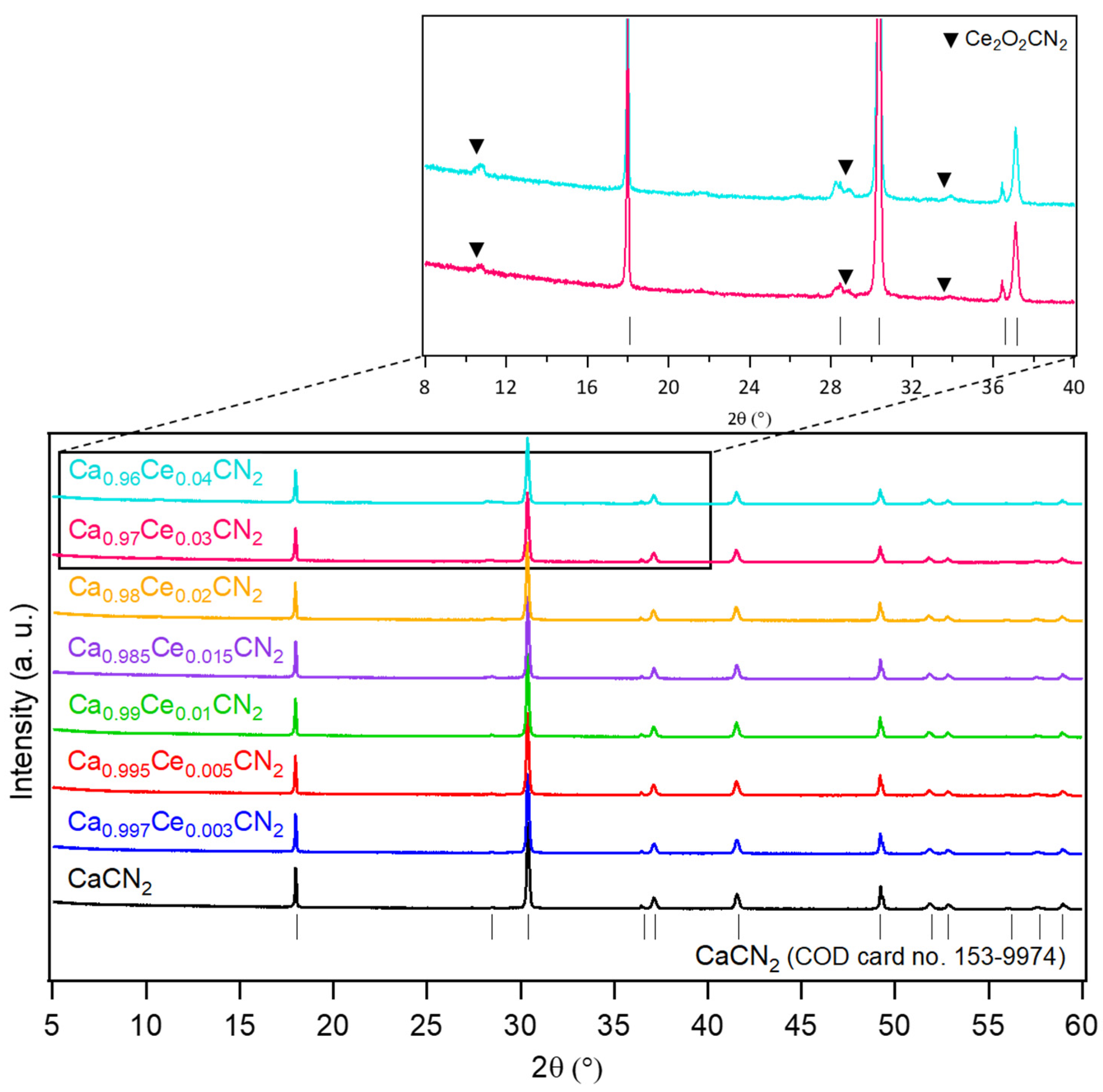
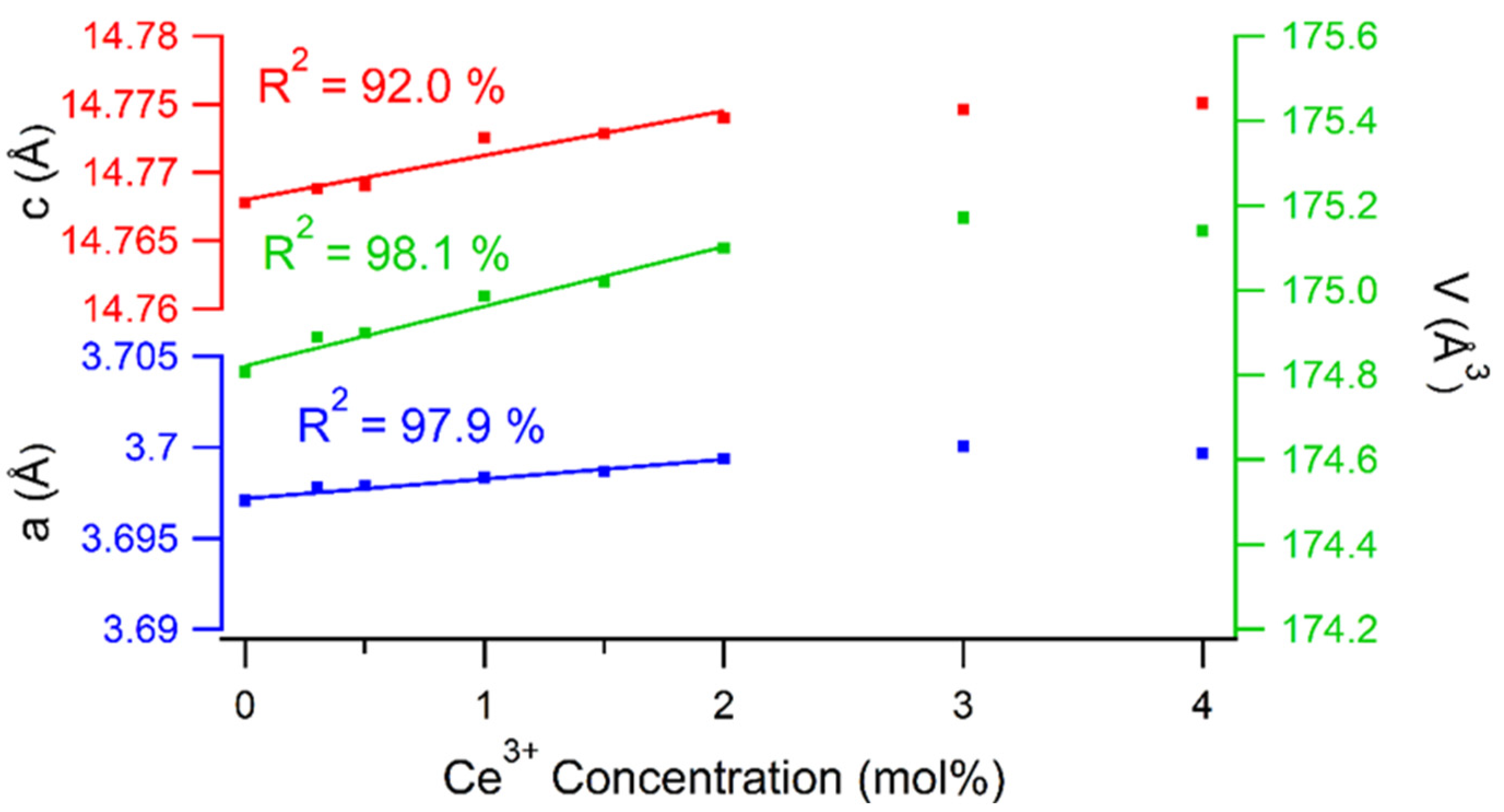
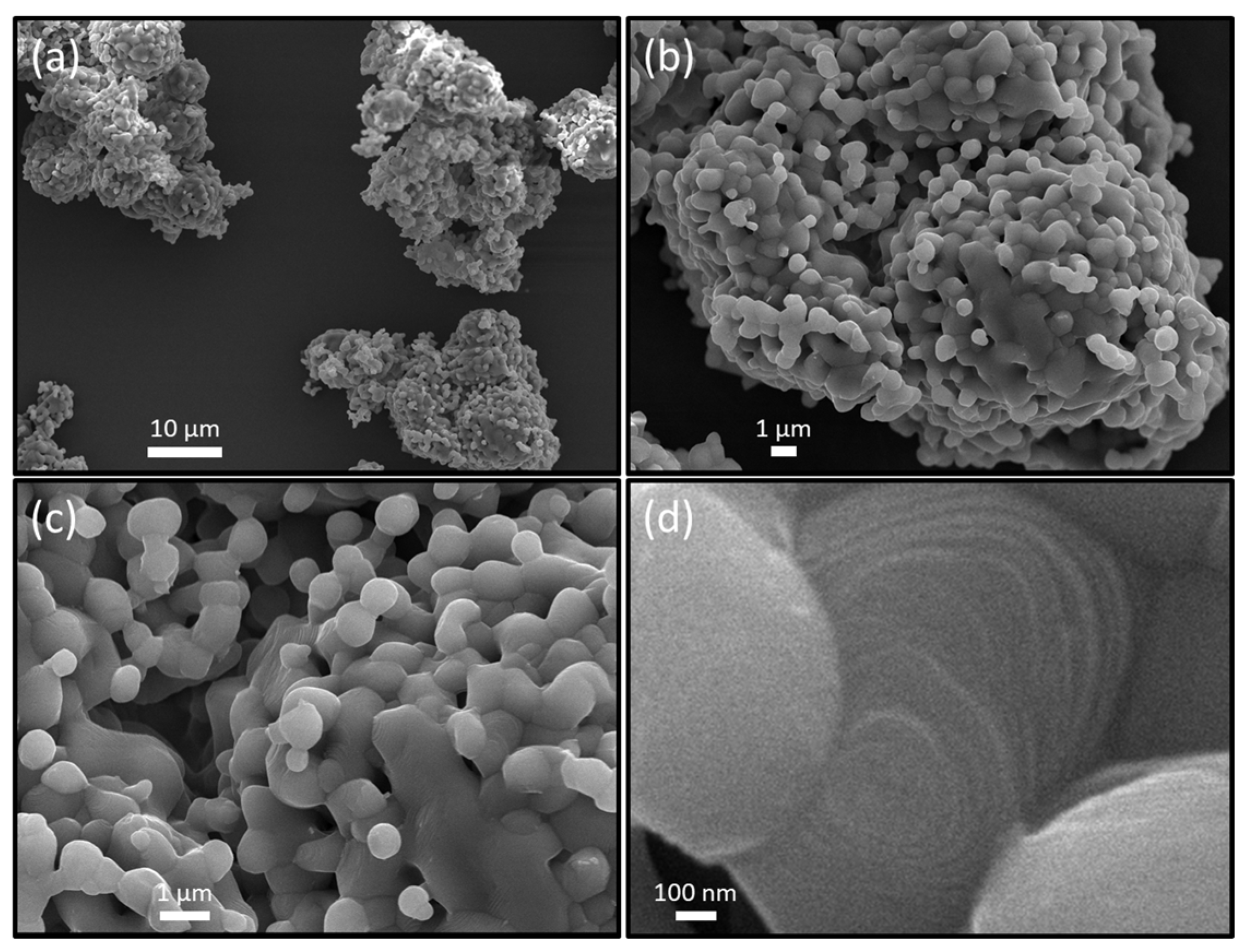
2.1.1. Materials Characterization
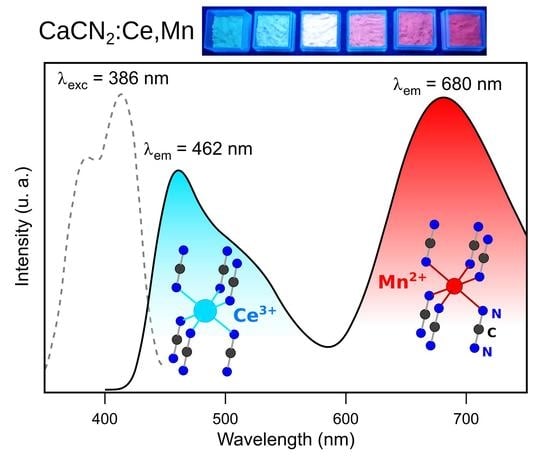
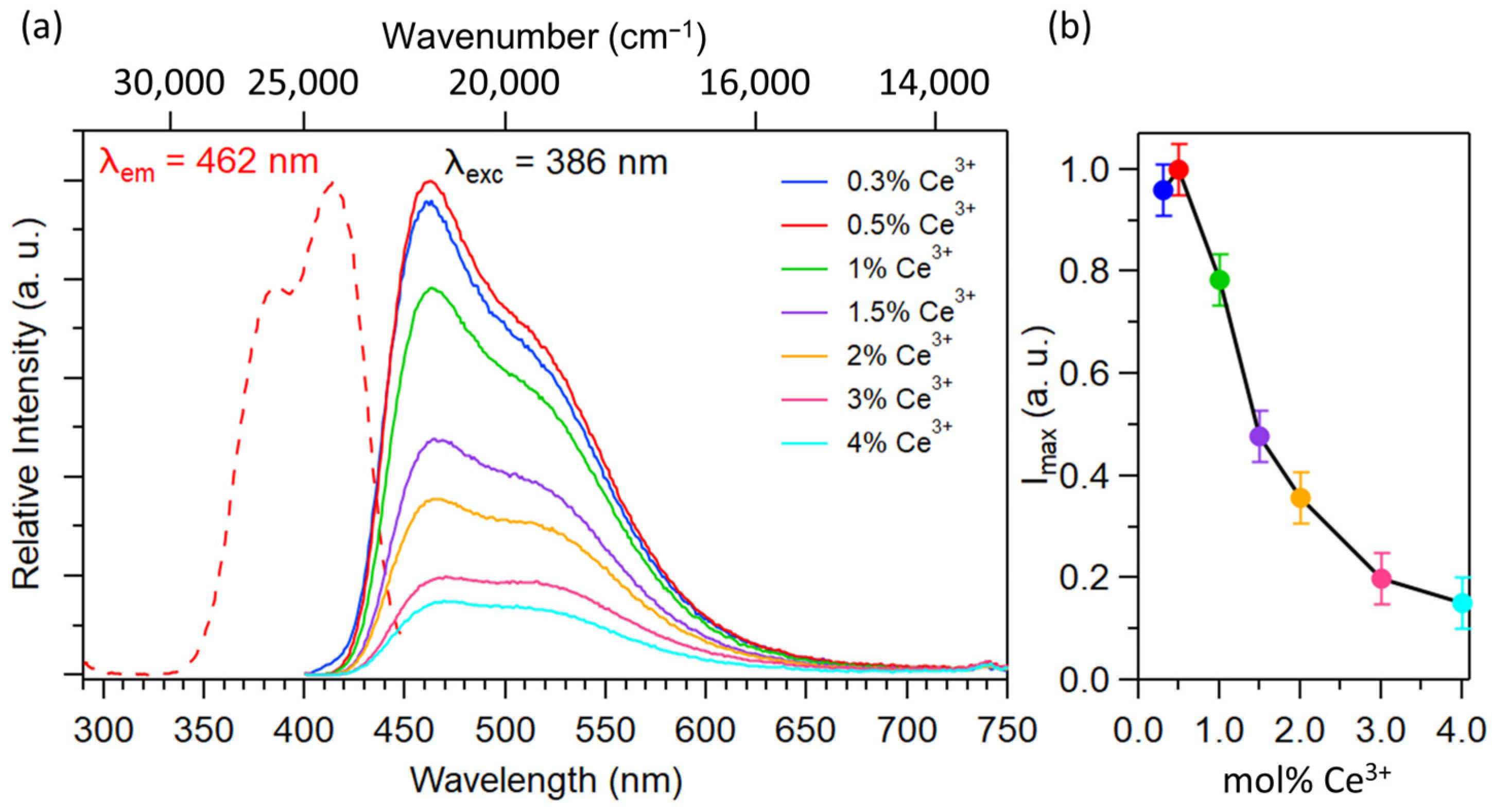
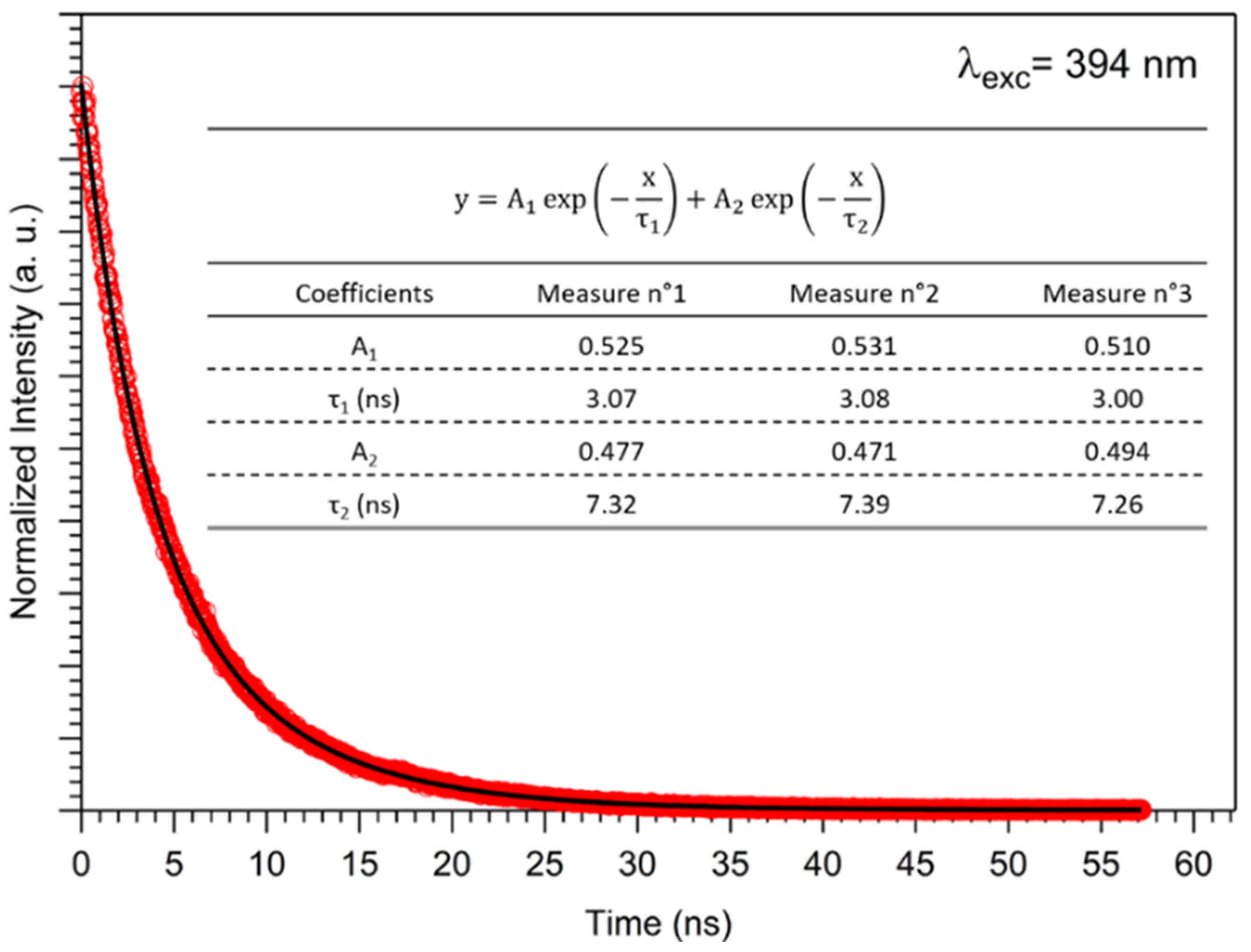
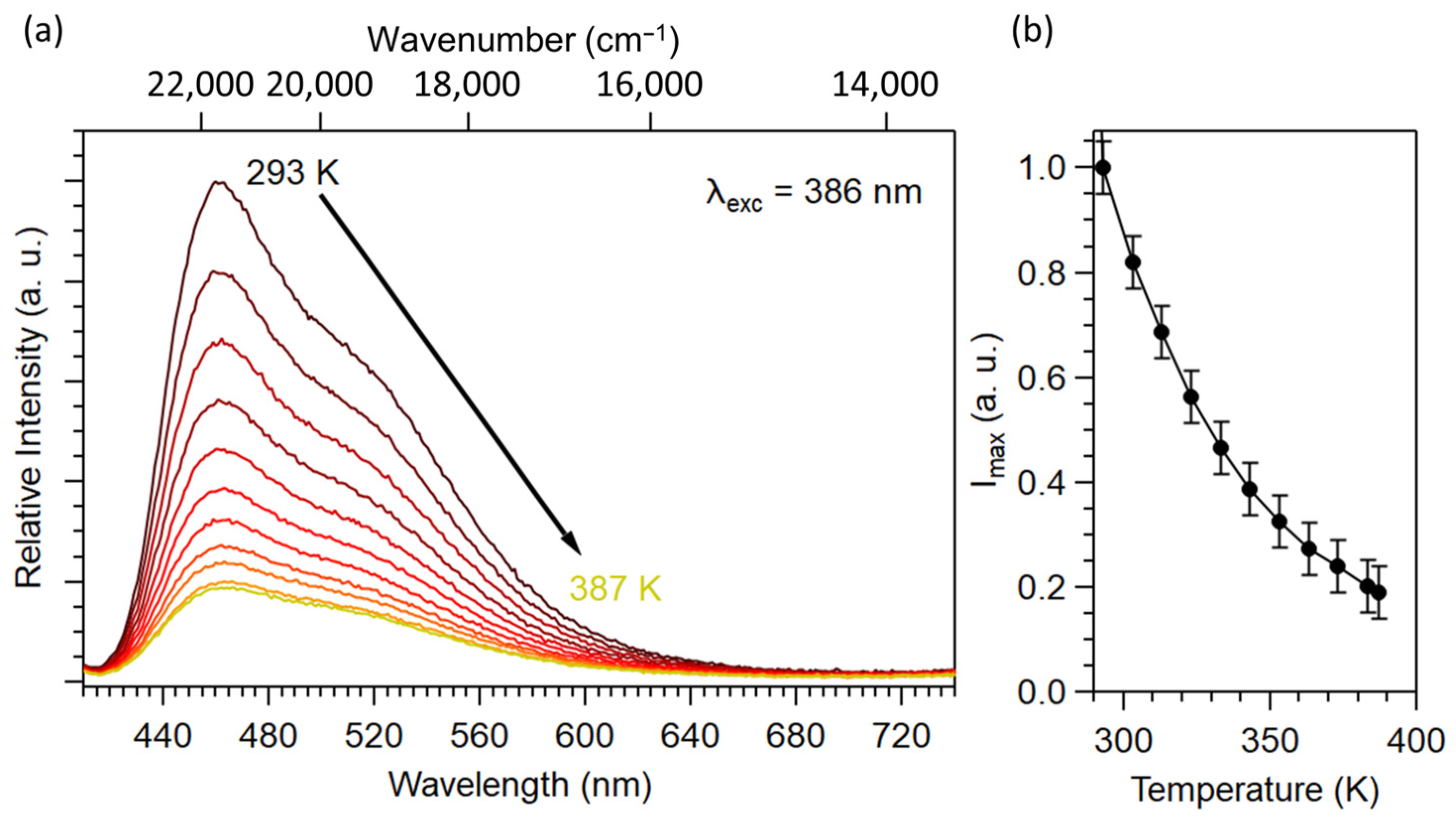
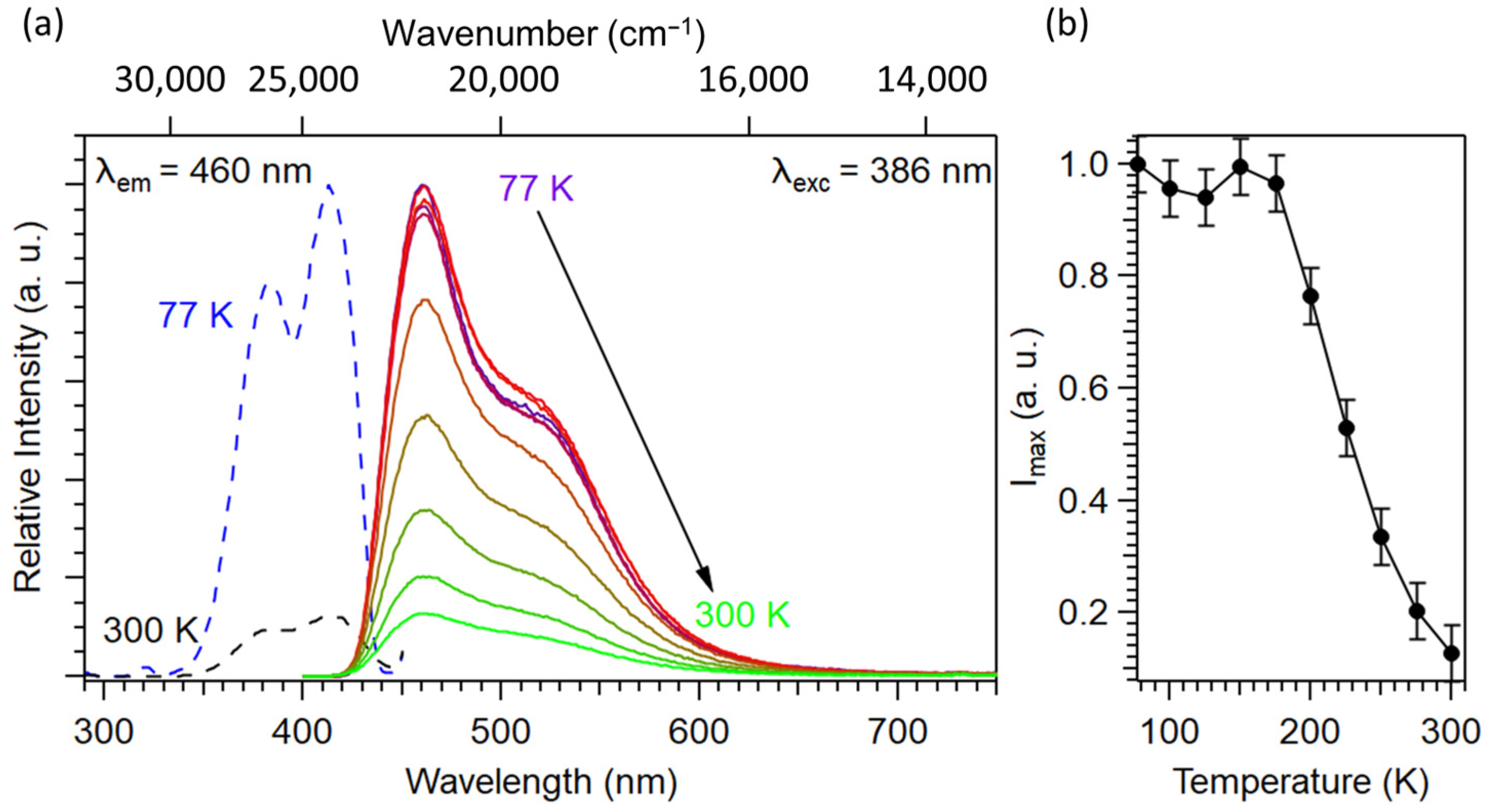
2.1.2. Optical Properties
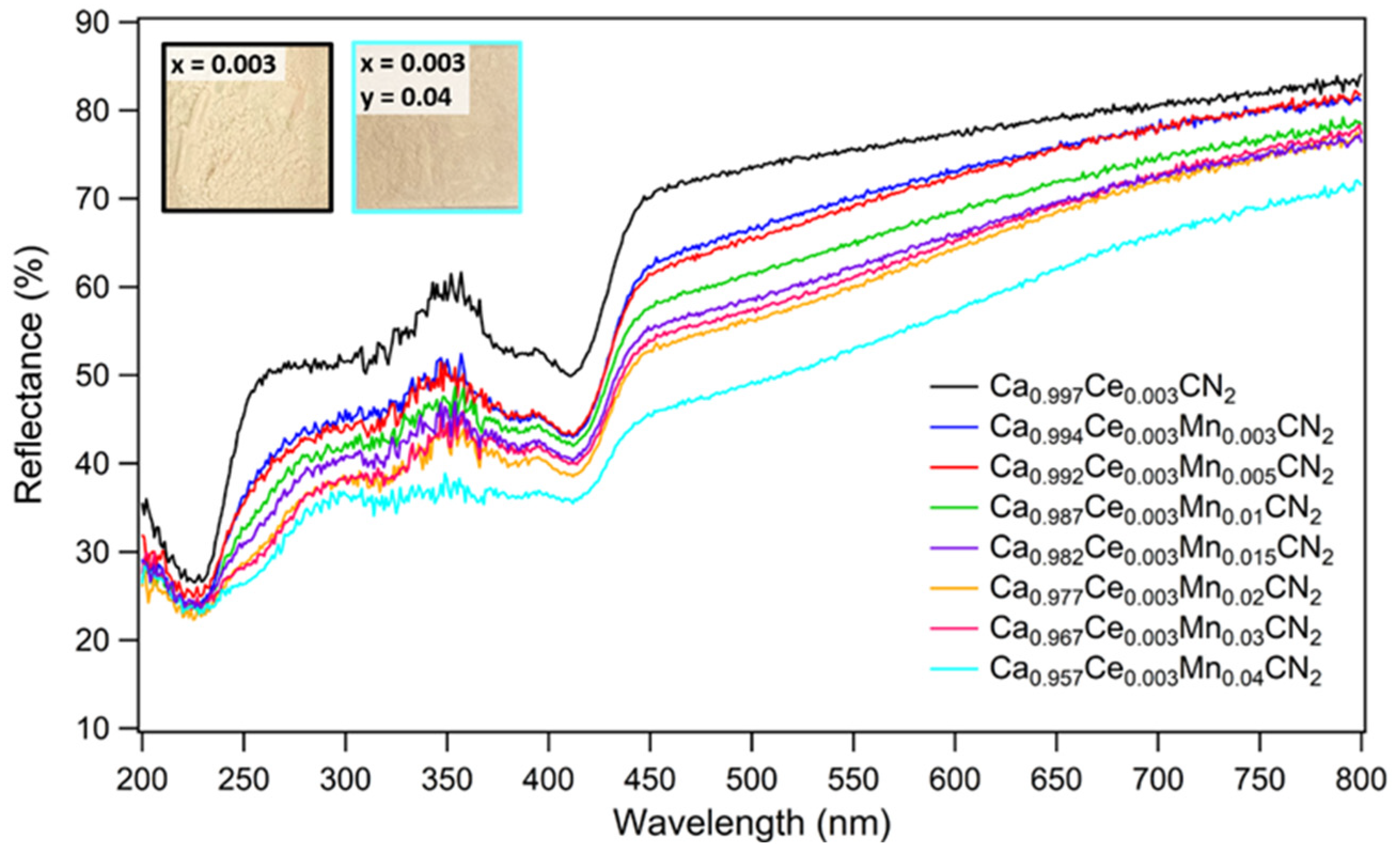
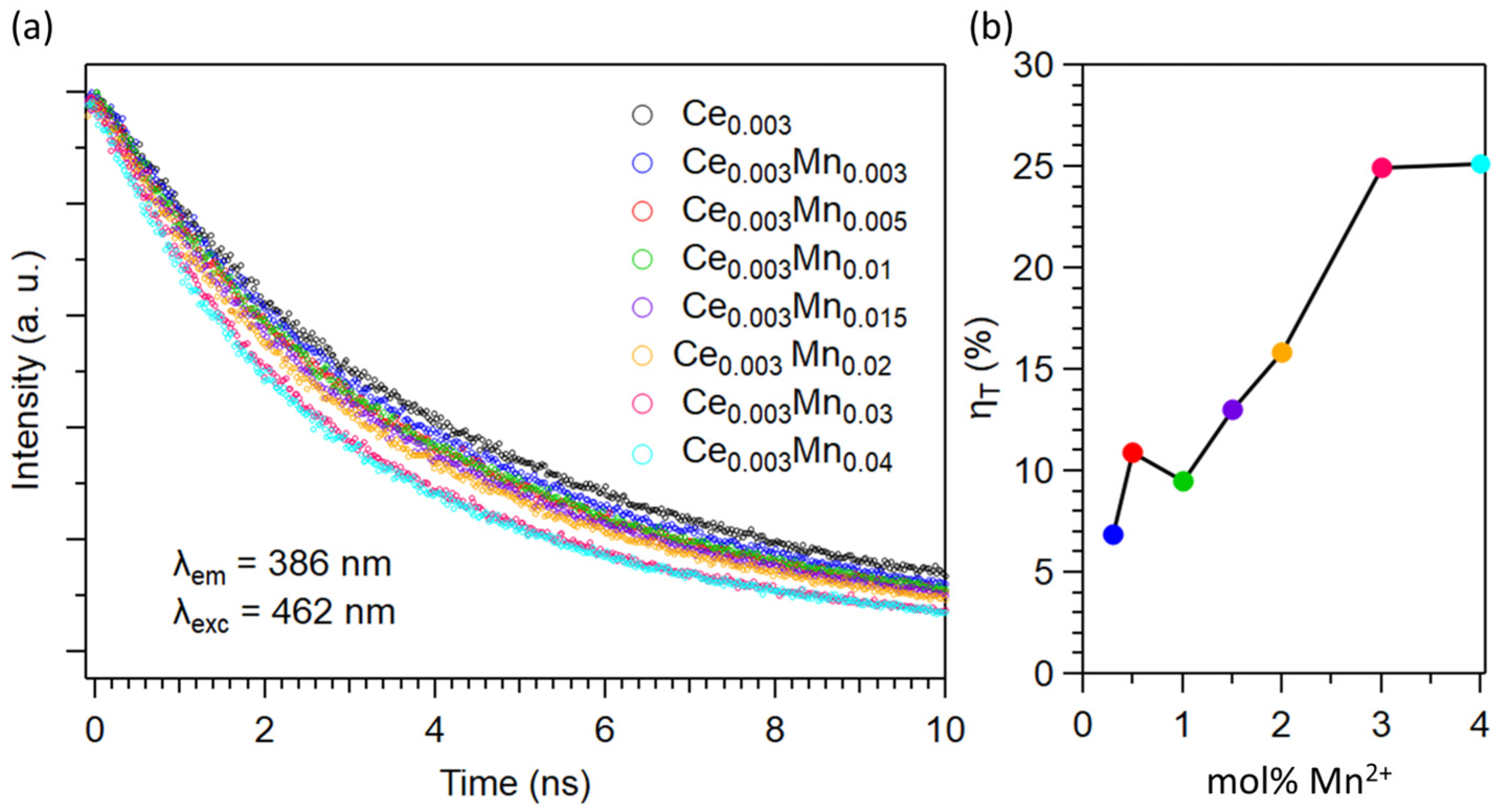
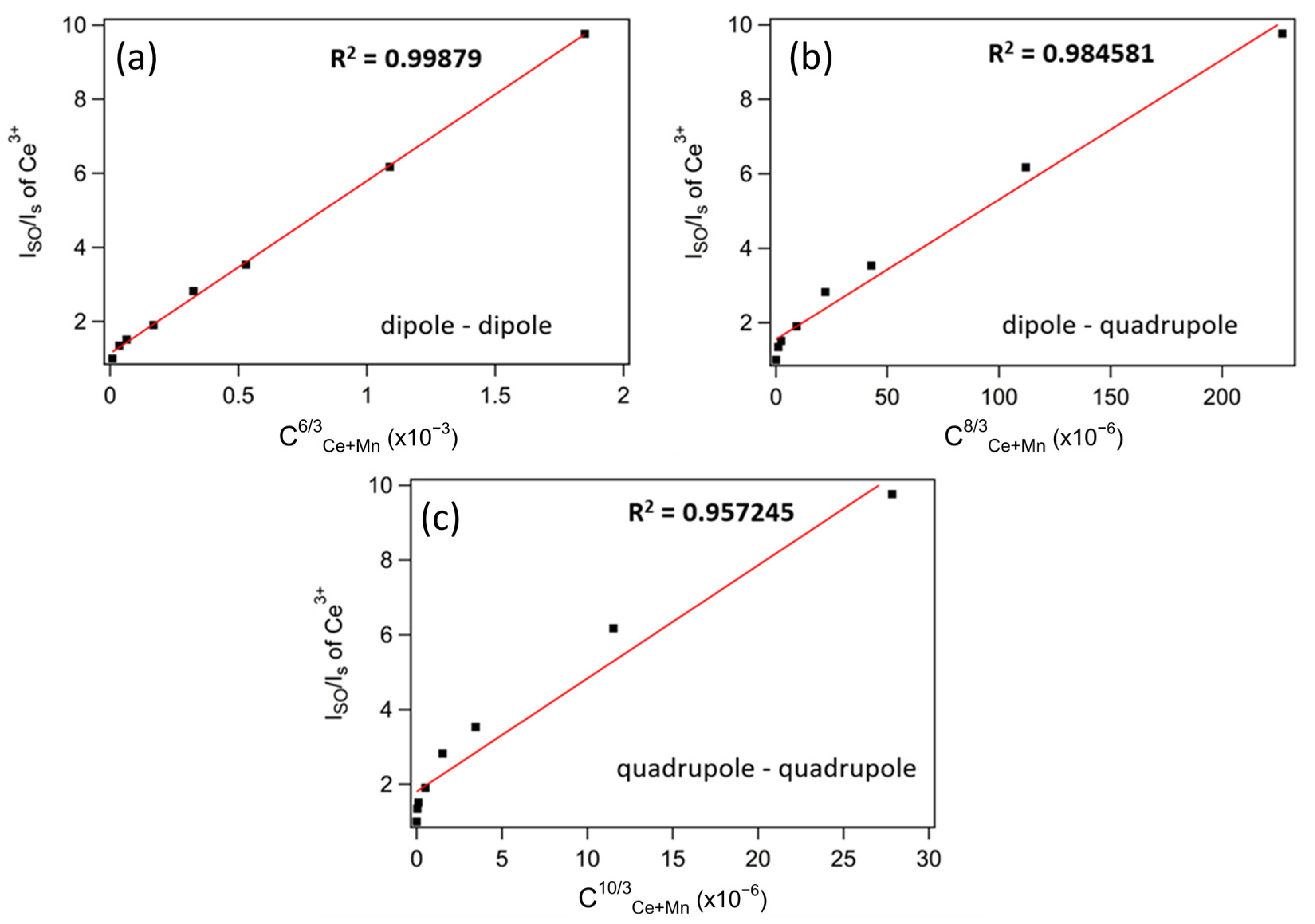
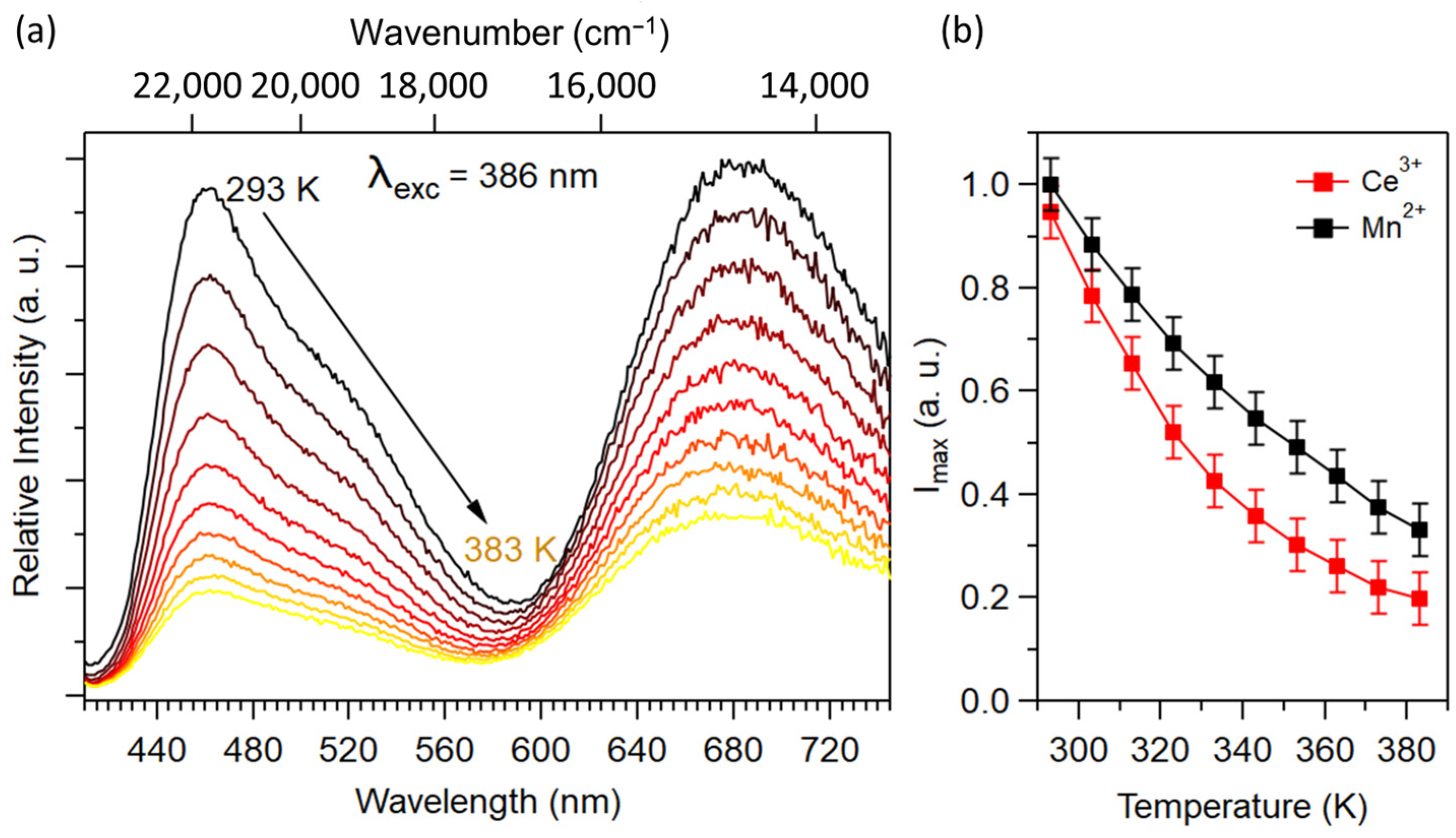
2.2. Optical Properties of Ca0.997−yCe0.003MnyCN2 (0.003 ≤ y ≤ 0.04)
3. Materials and Methods
3.1. Preparation of Ce3+, Mn2+ Co-Doped Calcium Carbodiimides
3.2. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Shang, M.; Lian, H.; Lin, J. Recent Development in Phosphors with Different Emitting Colors via Energy Transfer. J. Mater. Chem. C 2016, 4, 5507–5530. [Google Scholar] [CrossRef]
- Liang, C.; You, H.; Fu, Y.; Teng, X.; Liu, K.; He, J. A Novel Tunable Blue-Green-Emitting CaGdGaAl2O7:Ce3+,Tb3+ Phosphor via Energy Transfer for UV-Excited White LEDs. Dalton Trans. 2015, 44, 8100–8106. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K.M. Make Lighting Healthier. Nature 2018, 553, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Keller, P.R.; Busija, L.; Lawrenson, J.G.; Hull, C.C. Blue-light Filtering Spectacle Lenses for Visual Performance, Sleep, and Macular Health in Adults. Cochrane Database Syst. Rev. 2019, 1, 1–17. [Google Scholar] [CrossRef]
- Ci, Z.; Sun, Q.; Sun, M.; Jiang, X.; Qin, S.; Wang, Y. Structure, Photoluminescence and Thermal Properties of Ce3+, Mn2+ Co-Doped Phosphosilicate Sr7La3[(PO4)2.5(SiO4)3(BO4)0.5](BO2) Emission-Tunable Phosphor. J. Mater. Chem. C 2014, 2, 5850–5856. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Guo, Q.; Yang, Z. A Single-Phased Warm White-Light-Emitting Phosphor BaMg2(PO4)2:Eu2+,Mn2+,Tb3+ for White Light Emitting Diodes. Mater. Res. Bull. 2014, 52, 30–36. [Google Scholar] [CrossRef]
- Liu, W.-R.; Huang, C.-H.; Yeh, C.-W.; Tsai, J.-C.; Chiu, Y.-C.; Yeh, Y.-T.; Liu, R.-S. A Study on the Luminescence and Energy Transfer of Single-Phase and Color-Tunable KCaY(PO4)2:Eu2+,Mn2+ Phosphor for Application in White-Light LEDs. Inorg. Chem. 2012, 51, 9636–9641. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Qiu, Z.; Zhang, W.; Zhou, W.; Yu, L.; Lian, S. Site-Sensitive Energy Transfer Modes in Ca3Al2O6:Ce3+/Tb3+/Mn2+ Phosphors. Dalton Trans. 2014, 43, 18134–18145. [Google Scholar] [CrossRef]
- Shang, M.; Li, C.; Lin, J. How to Produce White Light in a Single-Phase Host? Chem. Soc. Rev. 2014, 5, 1372–1386. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, N.Z.; Sohail, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Xu, X.; Zhu, J.; Agathopoulos, S. Modern Aspects of Strategies for Developing Single-Phase Broadly Tunable White Light-Emitting Phosphors. J. Mater. Chem. C 2021, 9, 13041–13071. [Google Scholar] [CrossRef]
- Glaser, J.; Unverfehrt, L.; Bettentrup, H.; Heymann, G.; Huppertz, H.; Jüstel, T.; Meyer, H.-J. Crystal Structures, Phase-Transition, and Photoluminescence of Rare Earth Carbodiimides. Inorg. Chem. 2008, 47, 10455–10460. [Google Scholar] [CrossRef]
- Dutczak, D.; Ströbele, M.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Eu2(CN2)3 and KEu[Si(CN2)4]: Missing Members of the Rare Earth Metal Carbodiimide and Tetracyanamidosilicate Series. Eur. J. Inorg. Chem. 2016, 2016, 4011–4016. [Google Scholar] [CrossRef]
- Dolabdjian, K.; Schedel, C.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Synthesis, Luminescence and Nonlinear Optical Properties of Homoleptic Tetracyanamidogermanates ARE[Ge(CN2)4] (A = K, Cs, and RE = La, Ce, Pr, Nd, Sm, Eu, Gd). Z. Anorg. Allg. Chem. 2017, 643, 488–494. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Y.; Chevire, F.; Tessier, F.; Zhang, X.; Chen, G. Photoluminescence of Eu2+-Doped Strontium Cyanamide: A Novel Host Lattice for Eu2+. J. Am. Ceram. Soc. 2010, 93, 3052–3055. [Google Scholar] [CrossRef]
- Krings, M.; Montana, G.; Dronskowski, R.; Wickleder, C. α-SrNCN:Eu2+ − A Novel Efficient Orange-Emitting Phosphor. Chem. Mater. 2011, 23, 1694–1699. [Google Scholar] [CrossRef]
- Leysour de Rohello, E.; Suffren, Y.; Merdrignac-Conanec, O.; Guillou, O.; Cheviré, F. Effect of Cationic Substitutions on the Photoluminescence Properties of Eu2+ Doped SrCN2 Prepared by a Facile C3N4 Based Synthetic Approach. J. Eur. Ceram. Soc. 2020, 40, 6316–6321. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nishitani, S.; Hosono, A.; Kitagawa, Y.; Ueda, J.; Tanabe, S.; Yamane, H.; Higuchi, M.; Kikkawa, S. Red-Emission over a Wide Range of Wavelengths at Various Temperatures from Tetragonal BaCN2:Eu2+. J. Mater. Chem. C 2018, 6, 6370–6377. [Google Scholar] [CrossRef]
- Kubus, M.; Castro, C.; Enseling, D.; Jüstel, T. Room Temperature Red Emitting Carbodiimide Compound Ca(CN2):Mn2+. Opt. Mater. 2016, 59, 126–129. [Google Scholar] [CrossRef]
- Leysour de Rohello, E.; Bour, F.; Suffren, Y.; Merdrignac-Conanec, O.; Guillou, O.; Cheviré, F. Synthesis and Photoluminescence Properties of Mn2+ Doped ZnCN2 Phosphors. Open Ceram. 2021, 7, 100157. [Google Scholar] [CrossRef]
- Leysour de Rohello, E.; Suffren, Y.; Merdrignac-Conanec, O.; Guillou, O.; Calers, C.; Cheviré, F. Synthesis and Photoluminescence Properties of Mn2+ Doped Ca1−xSrxCN2 Phosphors Prepared by a Carbon Nitride Based Route. J. Solid State Chem. 2021, 300, 122240. [Google Scholar] [CrossRef]
- Schädler, H.D.; Jäger, L.; Senf, I. Pseudoelement Compounds. V. Pseudochalcogens—An Attempt of an Empirical and Theoretical Characterization. Z. Anorg. Allg. Chem. 1993, 619, 1115–1120. [Google Scholar] [CrossRef]
- Pauling, L. The Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1967. [Google Scholar]
- Güthner, T.; Mertschenk, B. Cyanamides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Vannerberg, N.G. The Crystal Structure of Calcium Cyanamide. Acta Chem. Scand. 1962, 16, 2263–2266. [Google Scholar] [CrossRef]
- Zhu, Q.-Q.; Wang, L.; Hirosaki, N.; Hao, L.Y.; Xu, X.; Xie, R.-J. Extra-Broad Band Orange-Emitting Ce3+-Doped Y3Si5N9O Phosphor for Solid-State Lighting: Electronic, Crystal Structures and Luminescence Properties. Chem. Mater. 2016, 28, 4829–4839. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, J.; Li, Y.; Liao, S.; Zhang, X.; Yang, Z.; Wang, Z.; Qiu, Z.; Zhou, W.; Yu, L.; et al. Improving Quantum Efficiency and Thermal Stability in Blue-Emitting Ba2−xSrxSiO4:Ce3+ Phosphor via Solid Solution. Chem. Mater. 2018, 30, 5137–5147. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, H.; Liu, T.; Wang, C.; Liu, R.; Lü, W.; You, H. Luminescent Properties and Energy Transfer of CaO:Ce3+,Mn2+ Phosphors for White LED. J. Lumin. 2016, 177, 349–353. [Google Scholar] [CrossRef]
- Game, D.N.; Ingale, N.B.; Omanwar, S.K. Converted White Light Emitting Diodes from Ce3+ Doping of Alkali Earth Sulfide Phosphors. Mater. Discov. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Yu, R.; Wang, J.; Zhang, M.; Zhang, J.; Yuan, H.; Su, Q. A New Blue-Emitting Phosphor of Ce3+-Activated CaLaGa3S6O for White-Light-Emitting Diodes. Chem. Phys. Lett. 2008, 453, 197–201. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature Quenching of Yellow Ce3+ Luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Wang, Z.; Lou, S.; Li, P. Enhanced Orange–Red Emission of Sr3La(PO4)3:Ce3+,Mn2+ via Energy Transfer. J. Lumin. 2014, 156, 87–90. [Google Scholar] [CrossRef]
- Liu, H.; Liao, L.; Xia, Z. Structure, Luminescence Property and Energy Transfer Behavior of Color-Adjustable La5Si2BO13:Ce3+,Mn2+ Phosphors. RSC Adv. 2014, 4, 7288–7295. [Google Scholar] [CrossRef]
- Si, J.; Wang, L.; Liu, L.; Yi, W.; Cai, G.; Takeda, T.; Funahashi, S.; Hirosaki, N.; Xie, R.-J. Structure, Luminescence and Energy Transfer in Ce3+ and Mn2+ Codoped γ-AlON Phosphors. J. Mater. Chem. C 2019, 7, 733–742. [Google Scholar] [CrossRef]
- Paulose, P.I.; Jose, G.; Thomas, V.; Unnikrishnan, N.V.; Warrier, M.K.R. Sensitized Fluorescence of Ce3+/Mn2+ System in Phosphate Glass. J. Phys. Chem. Solids 2003, 64, 841–846. [Google Scholar] [CrossRef]
- Reisfeld, R.; Greenberg, E.; Velapoldi, R.; Barnett, B. Luminescence Quantum Efficiency of Gd and Tb in Borate Glasses and the Mechanism of Energy Transfer between Them. J. Chem. Phys. 2003, 56, 1698. [Google Scholar] [CrossRef]
- Antipenko, B.M.; Batyaev, I.M.; Ermolaev, V.L.; Lyubimov, E.I.; Privalova, T.A. Radiationless Transfer of Electron Excitation Energy between Rare Earth Ions in POCl3-SnCl4. Opt. Spektrosk. 1970, 29, 335–338. [Google Scholar]
- Blasse, G. Energy Transfer in Oxidic Phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Bettinelli, M.; Karlsson, M. Unraveling the Mechanisms of Thermal Quenching of Luminescence in Ce3+-Doped Garnet Phosphors. Chem. Mater. 2019, 31, 3851–3862. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378, 118–123. [Google Scholar] [CrossRef]
- Kubelka, D.; Munk, L. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Wyszecki, G. Colorimetry. In Handbook of Optics; Driscoll, W.G., Vaughan, W., Eds.; MacGraw-Hill Book Company: New York, NY, USA, 1978; pp. 1–15. [Google Scholar]
- [CIE] International Commission on Illumination. Method of Measuring and Specifying Colour Rendering Properties of Light Sources; Publication No. CIE 13.3-1995; CIE: Vienna, Austria, 1995; 16p. [Google Scholar]



| Ca at% | Ce at% * | Na at% | Nexp wt% | Ncalc wt% | ΔN % ** | O wt% | |
|---|---|---|---|---|---|---|---|
| Ca0.997Ce0.003CN2 | 98.83 | 0.45 (0.45) | 0.72 | 34.45 | 34.83 | 1.09 | 0.51 |
| Ca0.995Ce0.005CN2 | 98.11 | 0.59 (0.60) | 1.30 | 34.19 | 34.75 | 1.64 | 0.41 |
| Ca0.99Ce0.01CN2 | 96.47 | 1.28 (1.31) | 2.25 | 35.34 | 34.53 | 2.34 | 0.78 |
| Ca0.985Ce0.015CN2 | 95.75 | 1.61 (1.65) | 2.64 | 34.35 | 34.32 | 0.09 | 0.55 |
| Ca0.98Ce0.02CN2 | 95.19 | 2.17 (2.23) | 2.64 | 33.23 | 34.11 | 2.58 | 0.41 |
| Ca0.97Ce0.03CN2 | 94.86 | 3.35 (3.41) | 1.79 | 32.22 | 33.70 | 4.39 | 1.36 |
| Ca0.96Ce0.04CN2 | 91.86 | 4.76 (4.93) | 3.38 | 31.77 | 33.30 | 4.59 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leysour de Rohello, E.; Suffren, Y.; Gouttefangeas, F.; Merdrignac-Conanec, O.; Guillou, O.; Cheviré, F. Synthesis, Luminescence and Energy Transfer Properties of Ce3+/Mn2+ Co-Doped Calcium Carbodiimide Phosphors. Inorganics 2023, 11, 291. https://doi.org/10.3390/inorganics11070291
Leysour de Rohello E, Suffren Y, Gouttefangeas F, Merdrignac-Conanec O, Guillou O, Cheviré F. Synthesis, Luminescence and Energy Transfer Properties of Ce3+/Mn2+ Co-Doped Calcium Carbodiimide Phosphors. Inorganics. 2023; 11(7):291. https://doi.org/10.3390/inorganics11070291
Chicago/Turabian StyleLeysour de Rohello, Erwan, Yan Suffren, Francis Gouttefangeas, Odile Merdrignac-Conanec, Olivier Guillou, and François Cheviré. 2023. "Synthesis, Luminescence and Energy Transfer Properties of Ce3+/Mn2+ Co-Doped Calcium Carbodiimide Phosphors" Inorganics 11, no. 7: 291. https://doi.org/10.3390/inorganics11070291
APA StyleLeysour de Rohello, E., Suffren, Y., Gouttefangeas, F., Merdrignac-Conanec, O., Guillou, O., & Cheviré, F. (2023). Synthesis, Luminescence and Energy Transfer Properties of Ce3+/Mn2+ Co-Doped Calcium Carbodiimide Phosphors. Inorganics, 11(7), 291. https://doi.org/10.3390/inorganics11070291