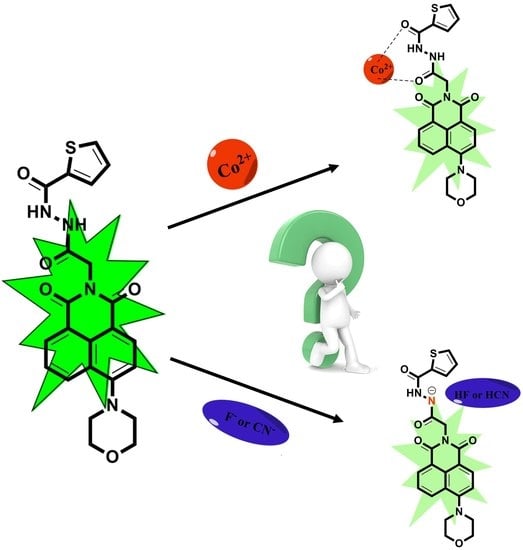
A Multifunctional Fluorescent Probe Based on 1,8-Naphthalimide for the Detection of Co2+, F−, and CN−
Abstract
1. Introduction
2. Results and Discussion
2.1. Fluorescence Spectral Characteristics Studies
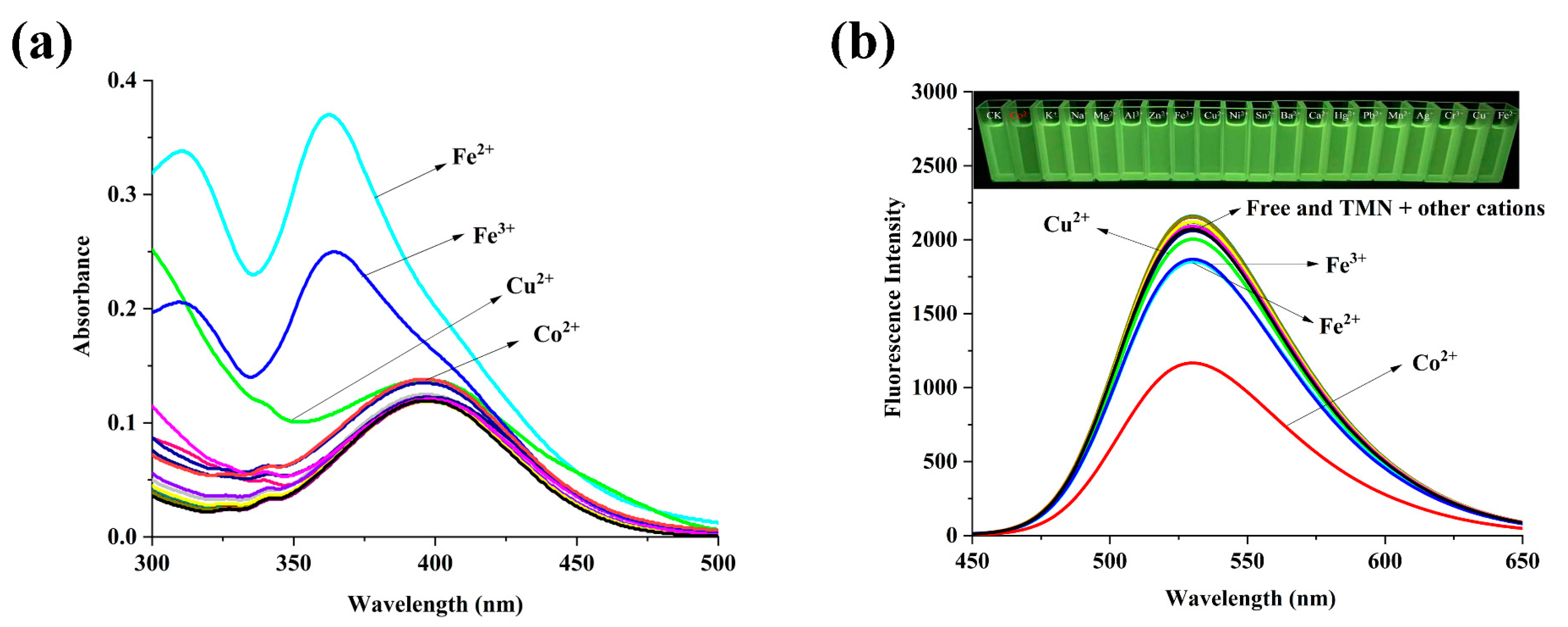
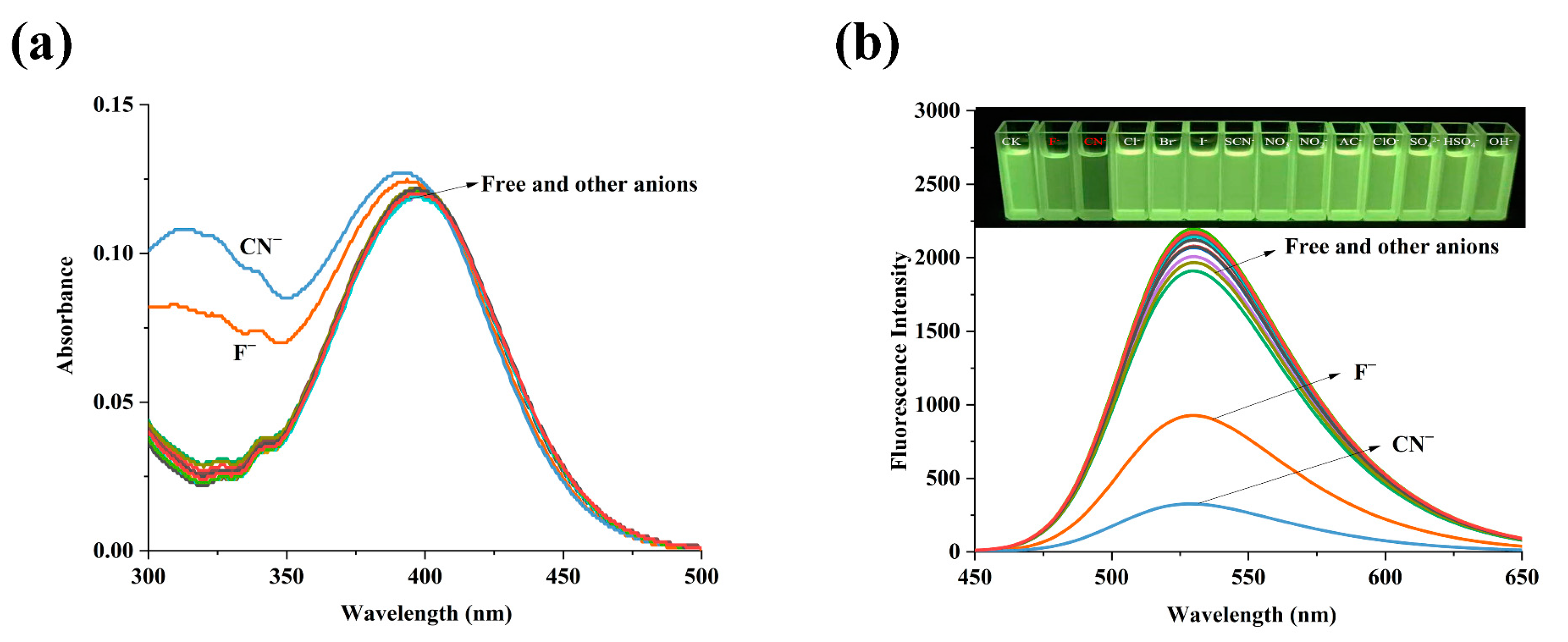
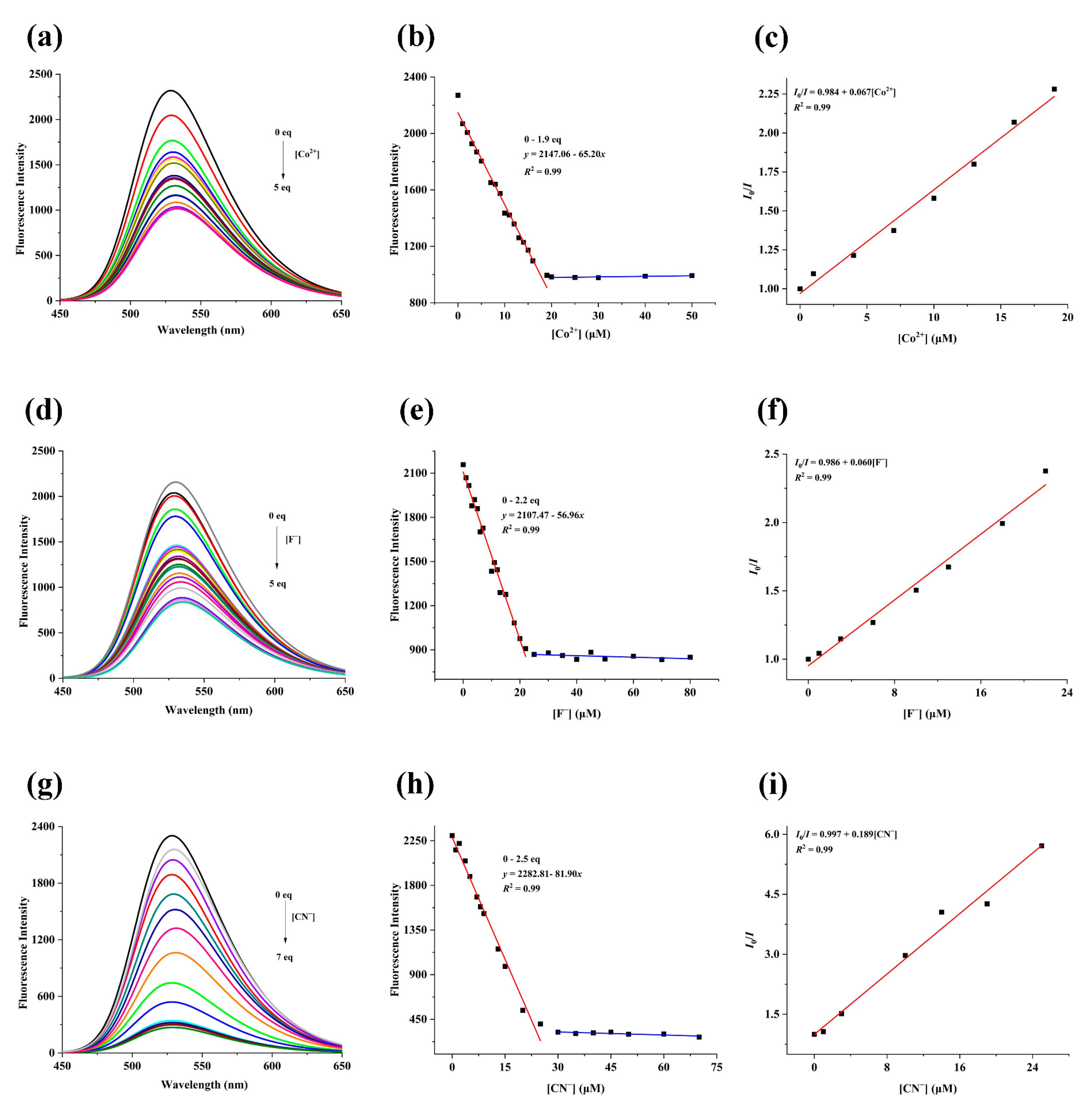
2.2. The Spectral Properties of TMN for the Detection of Co2+, F−, and CN−
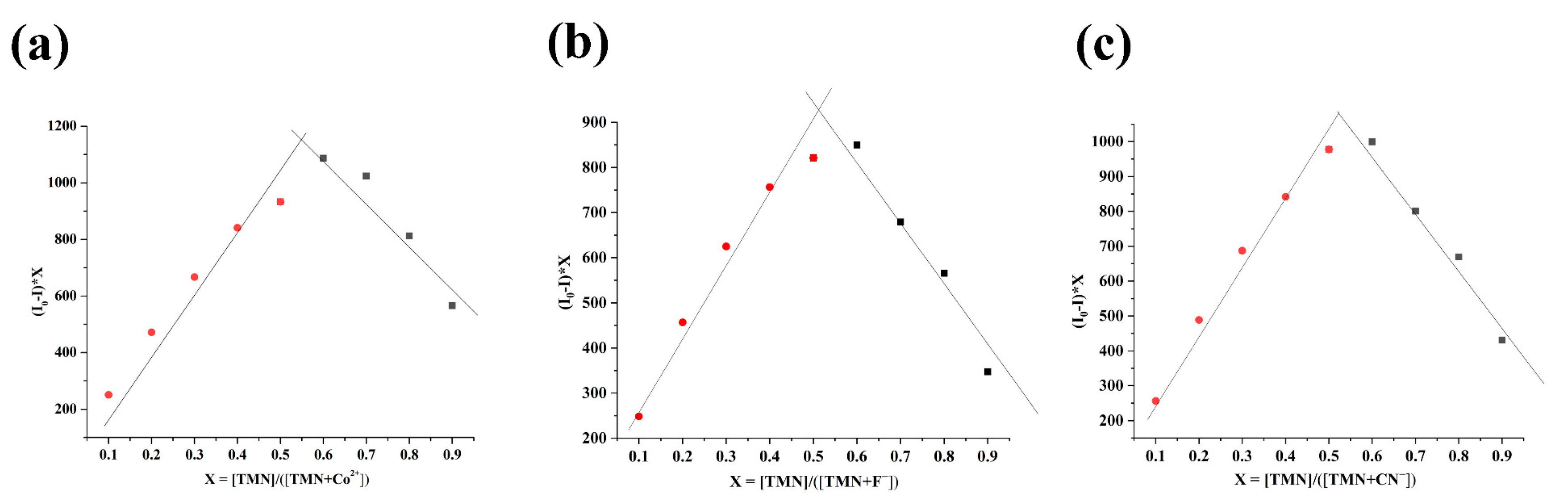
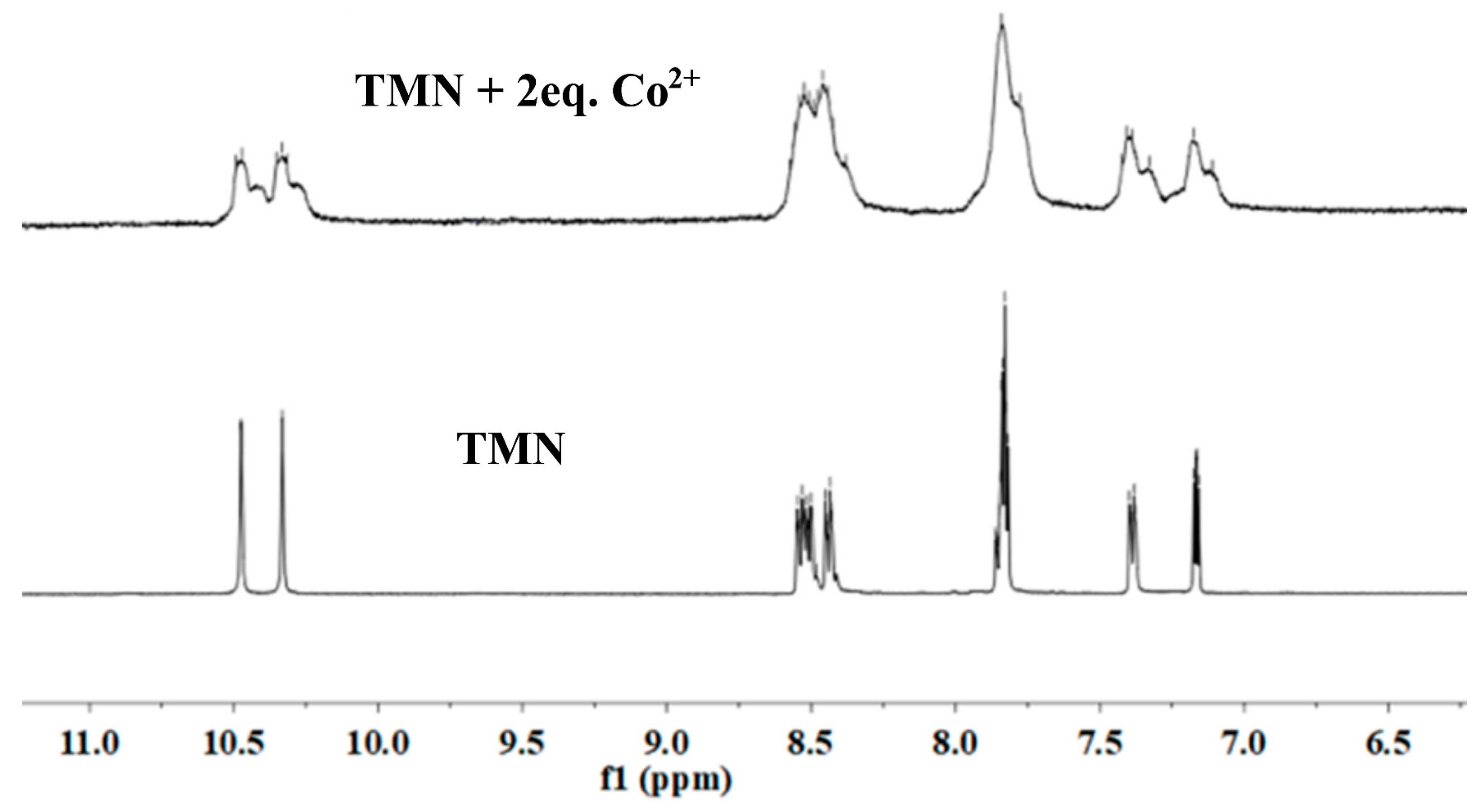
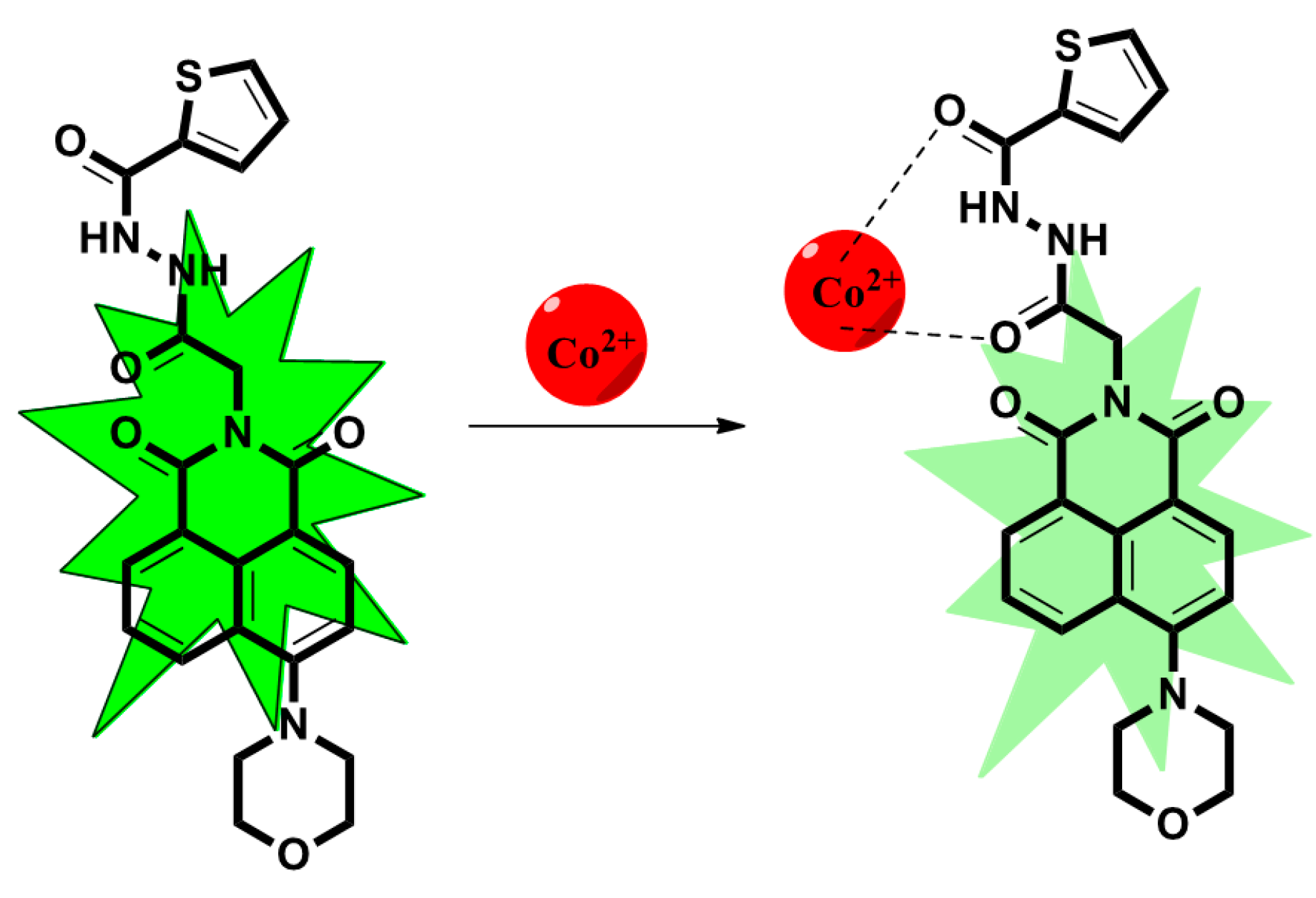
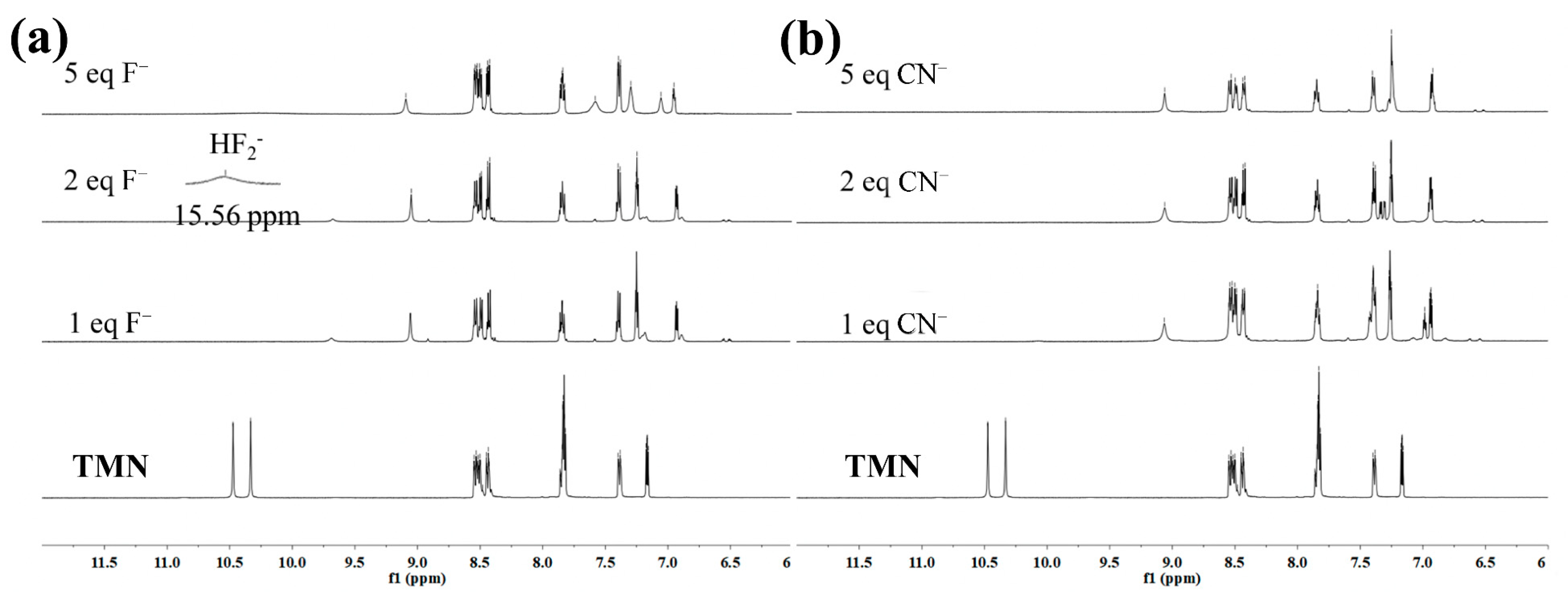
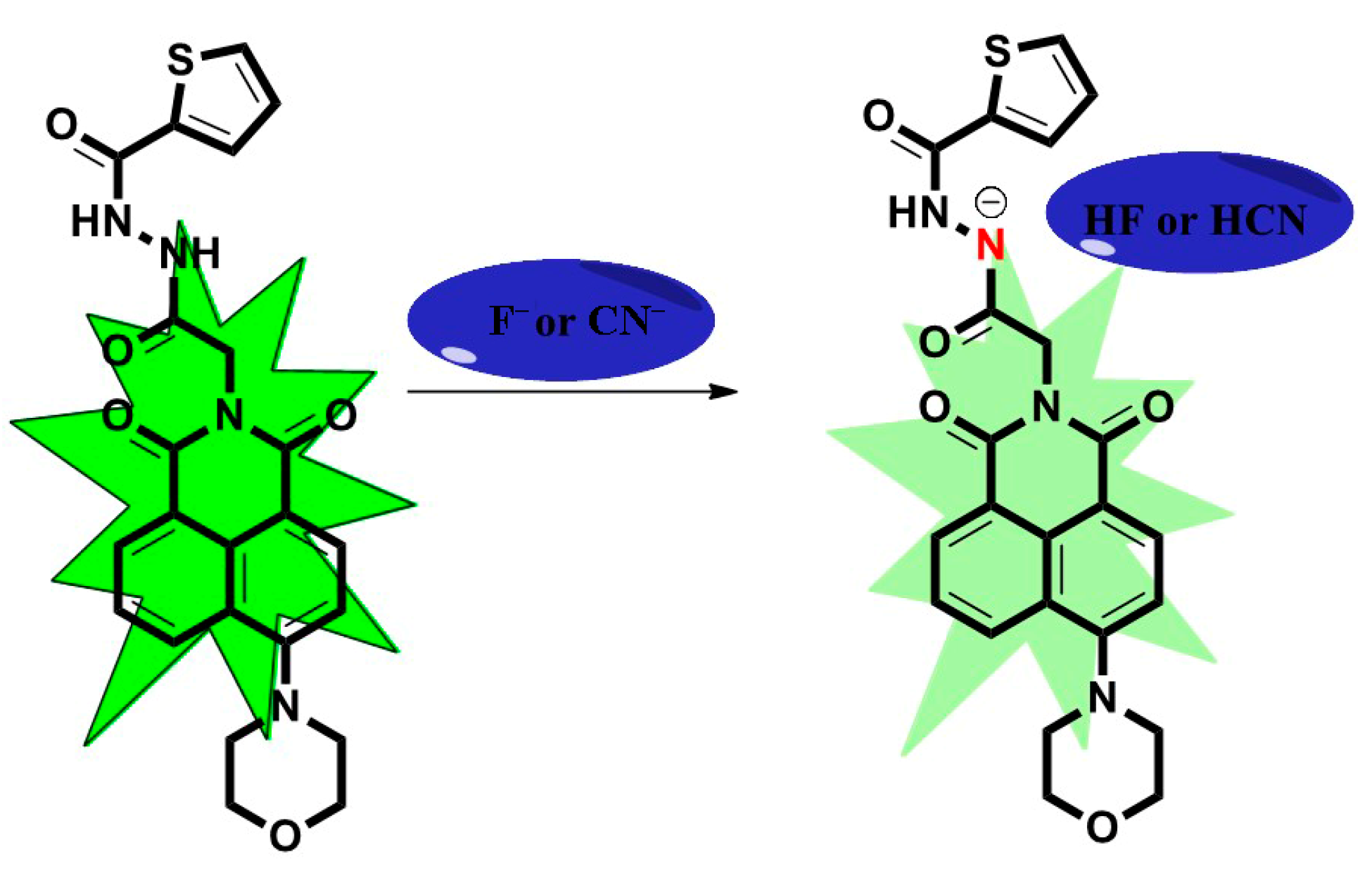
2.3. The Mechanism of TMN for the Detection of Co2+, F−, and CN−
2.4. Detecting Co2+, F−, and CN− in Real Samples
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Synthesis of N-(2-thiophenhydrazide)acetyl-4-morpholine-1,8-naphthalimide (TMN)
3.3. General Preparation for the Spectral Experiments
3.4. Detecting Co2+, F−, and CN− in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Lee, J.J.; Bok, K.H.; Kim, S.Y.; Kim, C. Highly selective and sensitive colorimetric chemosensor for detection of Co2+ in a near-perfect aqueous solution. RSC Adv. 2016, 6, 28081–28088. [Google Scholar] [CrossRef]
- Liu, K.; Guo, P.; Liu, L.; Shi, X. Fluorescence enhancement of a novel pyrazine coupled rhodamine derivative for the paramagnetic Co2+ detection. Sens. Actuators B Chem. 2017, 250, 667–672. [Google Scholar] [CrossRef]
- Santander, P.J.; Kajiwara, Y.; Williams, H.J.; Scott, A.I. Structural characterization of novel cobalt corrinoids synthesized by enzymes of the vitamin B12 anaerobic pathway. Bioorg. Med. Chem. 2006, 14, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shan, Q.; Chen, H.; Zhang, G. A salicylal-derived Schiff base as Co (II) selective fluorescent probe. Sens. Actuators B Chem. 2017, 239, 203–210. [Google Scholar] [CrossRef]
- Zelder, F.; Zhou, K.; Sonnay, M. Peptide B12: Emerging trends at the interface of inorganic chemistry, chemical biology and medicine. Dalton Trans. 2013, 42, 854–862. [Google Scholar] [CrossRef]
- Seldén, A.I.; Norberg, C.; Karlson-Stiber, C.; Hellström-Lindberg, E. Cobalt release from glazed earthenware: Observations in a case of lead poisoning. Environ. Toxicol. Pharmacol. 2007, 23, 129–131. [Google Scholar] [CrossRef]
- Keane, M.J.; Hornsby-Myers, J.L.; Stephens, J.W.; Harrison, J.C.; Myers, J.R.; Wallace, W.E. Characterization of hard metal dusts from sintering and detonation coating processes and comparative hydroxyl radical production. Chem. Res. Toxicol. 2002, 15, 1010–1016. [Google Scholar] [CrossRef]
- Shree, G.J.; Murugesan, S.; Siva, A. A highly sensitive and selective Schiff-base probe as a colorimetric sensor for Co2+ and a fluorimetric sensor for F− and its utility in bio-imaging, molecular logic gate and real sample analysis. Spectrochim. Acta Part A 2020, 226, 117613. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J.Y. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef]
- Kim, H.N.; Guo, Z.Q.; Zhu, W.H.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, L.X.; Jiang, C.Y.; Li, P.; Liu, Y.; Fu, Y.; Ye, F. A naked-eye visible colorimetric and fluorescent chemosensor for rapid detection of fluoride anions: Implication for toxic fluorine-containing pesticides detection. J. Mol. Liq. 2020, 302, 112549. [Google Scholar] [CrossRef]
- Zhang, S.W.; Swager, T.M. Fluorescent detection of chemical warfare agents: Functional group specific ratiometric chemosensors. J. Am. Chem. Soc. 2003, 125, 3420–3421. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.; Nairb, M.; Kumar, D. Analytical methods for determination and sensing of fluoride in biotic and abiotic sources: A review. Anal. Methods 2016, 8, 5338–5352. [Google Scholar] [CrossRef]
- Sun, G.; Chen, W.; Liu, Y.; Jin, X.; Zhang, Z.; Su, J. A novel colorimetric and fluorometric probe for the detection of CN− with high selectivity in aqueous media. Dyes Pigm. 2020, 176, 108224. [Google Scholar] [CrossRef]
- Lin, W.C.; Fang, S.K.; Hu, J.W.; Tsai, H.Y.; Chen, K.Y. Ratiometric fluorescent/colorimetric cyanide-selective sensor based on excited-state intramolecular charge transfer-excited-state intramolecular proton transfer switching. Anal. Chem. 2014, 86, 4648–4652. [Google Scholar] [CrossRef]
- Ma, K.H.; Lippner, D.S.; Basi, K.A.; Deleon, S.M.; Cappuccio, W.R.; Rhoomes, M.O.; Hildenberger, D.M.; Hoard-Fruchey, H.M.; Rockwood, G.A. Cyanide poisoning compromises gene pathways modulating cardiac injury in vivo. Chem. Res. Toxicol. 2021, 34, 1530–1541. [Google Scholar] [CrossRef]
- Jackson, R.; Oda, R.P.; Bhandari, R.K.; Mahon, S.B.; Brenner, M.; Rockwood, G.A.; Logue, B.A. Development of a fluorescence-based sensor for rapid diagnos- is of cyanide exposure. Anal. Chem. 2014, 86, 1845–1852. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.Y.; Si, S.H.; Zhu, D.R.; Fung, Y.S. Piezoelectric quartz crystal (PQC) with photochemically deposited nano-sized Ag particles for determining cyanide at trace levels in water. Sens. Actuators B Chem. 2005, 108, 925–932. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Liu, C.G.; Fu, Y.; Ye, F. A built-in self-calibrating luminescence sensor based on RhB@Zr-MOF for detection of cations, nitro explosives and pesticides. RSC Adv. 2020, 10, 19149–19156. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yue, M.; Yang, L.; Sun, F.; Xu, G.; Fu, Y.; Ye, F. A Switch-On fluorescent probe for detection of mesotrione based on the straightforward cleavage of carbon-nitrogen double bond of Schiff base. Chem. Eng. J. 2022, 430, 132758. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, G.M.; Zhang, Y.; Tang, L.; Chen, J.; Cheng, M.; Zhang, L.H.; He, L.; Guo, Y.; He, X.X.; et al. Highly sensitive electrochemical sensor using a MWCNTs/GNPs-modified electrode for lead (II) detection based on Pb2+-induced G-rich DNA conformation. Analyst 2014, 139, 5014–5020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Wang, Y.; Cai, C.; Lin, H. Bright-yellow-emissive N-doped carbon dots: Preparation, cellular imaging, and bifunctional sensing. ACS Appl. Mater. Interfaces 2015, 7, 23231–23238. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Y.; Xie, J.; Li, P.; Lu, Q.; Liu, M.; Yin, P.; Li, H.; Zhang, Y.; Yao, S. Facile preparation of MnO2 quantum dots with enhanced fluorescence via microenvironment engineering with the assistance of some reductive biomolecules. ACS Appl. Mater. Interf. 2020, 12, 15919–15927. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Li, P.; Liu, Y.L.; Liang, X.M.; Fu, Y.; Ye, F. A dual-mode colorimetric/fluorescent probe based on perylene: Response to acidic pH values. J. Taiwan Inst. Chem. Eng. 2021, 129, 97–103. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, D.; Wu, D.; Chen, Z.; Zhang, J.; Chen, X.; Liu, S.; Yin, J. A colorimetric and ratiometric fluorescent probe for mercury (II) in lysosome. Sens. Actuators B Chem. 2016, 224, 907–914. [Google Scholar] [CrossRef]
- Ye, Z.; Xiong, C.; Pan, J.; Su, D.; Zeng, L. Highly photostable, lysosometargeted BODIPYs with green to near-infrared emission for lysosome imaging in living cells. Dyes Pigm. 2018, 155, 30–35. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, R.; Liang, Q.; Su, X.; Deng, L.; Yang, L.; Ma, L.J. A sensitive pH fluorescent probe based on triethylenetetramine bearing double dansyl groups in aqueous solutions and its application in cells. Spectrochim. Acta A 2020, 229, 117881. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Ji, X.X.; Liu, C.G.; Fu, Y.; Ye, F. A novel luminescent sensor based on Tb@UiO-66 for highly detecting Sm3+ and teflubenzuron. J. Taiwan Inst. Chem. Eng. 2021, 126, 173–181. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, W.; Xu, Y.; Zhou, T.; Xia, M.; Hao, Q. Determination of trace uric acid in serum using porous graphitic carbon nitride (g-C3N4) as a fluorescent probe. Microchim. Acta 2018, 185, 39. [Google Scholar] [CrossRef]
- Xu, S.; Ni, Y. NH2-MIL-53(Al) nanocrystals: A fluorescent probe for the fast detection of aromatic nitro-compounds and ions in aqueous systems. Analyst 2019, 144, 1687. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.C.; Trogler, W.C. Efficient blue-emitting silafluorene–fluorene-conjugated copolymers: Selective turn-off/turn-on detection of explosives. J. Mater. Chem. 2008, 18, 3143–3156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Lu, Q.; Wang, Y.; Fu, W.; Tan, Q.; Luo, W. Water-soluble MoS2 quantum dots are a viable fluorescent probe for hypochlorite. Microchim. Acta 2018, 185, 233. [Google Scholar] [CrossRef] [PubMed]
- Mutuyimana, F.P.; Liu, J.; Nsanzamahoro, S.; Na, M.; Chen, H.; Chen, X. Yellow-emissive carbon dots as a fluorescent probe for chromium(VI). Microchim. Acta 2019, 186, 163. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, Y.; Ding, X.J.; Sun, Y.X.; Liu, H.B.; Xie, C.Z.; Qian, J.; Li, Q.Z.; Xu, J.Y. A highly selective colorimetric and fluorescent probe for quantitative detection of Cu2+/Co2+: The unique ON-OFF-ON fluorimetric detection strategy and applications in living cells/zebrafish. Spectrochim. Acta Part A 2020, 228, 117763. [Google Scholar] [CrossRef]
- Gao, L.L.; Li, S.P.; Wang, Y.; Wu, W.N.; Zhao, X.L.; Li, H.J.; Xu, Z.H. Quinoline-based hydrazone for colorimetric detection of Co2+ and fluorescence turn-on response of Zn2+. Spectrochim. Acta Part A 2020, 230, 118025. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Chang, L.F.; He, X.W.; Chen, L.X.; Zhang, Y.K. A fluorescent sensing for glycoproteins based on the FRET between quantum dots and Au nanoparticles. Sens. Actuators B Chem. 2017, 250, 17–23. [Google Scholar] [CrossRef]
- Song, Y.F.; Wu, W.N.; Zhao, X.L.; Wang, Y.; Fan, Y.C.; Dong, X.Y.; Xu, Z.H. A simple colorimetric and fluorometric probe for rapid detection of CN– with large emission shift. Spectrochim. Acta Part A 2022, 280, 121540. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.; Wu, X.; Li, Y.; Du, J.; Qi, S.; Yang, Q.; Xu, H.; Li, Y. A novel Near-Infrared fluorescent probe for Zn2+ and CN– double detection based on dicyanoisfluorone derivatives with highly sensitive and selective, and its application in Bioimaging. Spectrochim. Acta Part A 2022, 267, 120621. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, H.A. Spin crossover in cobalt(II) systems, topics. Curr. Chem. 2004, 234, 23–47. [Google Scholar]
- Shirazi, A.; Goff, H.M. Carbon-13 and proton NMR spectroscopy of four- and five-coordinate cobalt(II) porphyrins: Analysis of NMR isotropic shifts. Inorg. Chem. 1982, 21, 3420–3425. [Google Scholar] [CrossRef]
- Długopolska, K.; Ruman, T.; Danilczuk, M.; Pogocki, D. Analysis of NMR shifts of high-spin cobalt(II) pyrazolylborate complexes. Appl. Magn. Reson. 2009, 35, 271–283. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N.; Khokhlova, A.Y.; Gashnikova, A.V.; Aleksandriiskii, V.V.; Sharnin, V.A. Complexation between nickel(II), cobalt(III) and hydrazones derived from pyridoxal 5′-phosphate and hydrazides of 2-,3-,4-pyridinecarboxylic acids in aqueous solution. J. Coord. Chem. 2018, 71, 3304–3314. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Gamov, G.A.; Khokhlova, A.Y.; Gashnikova, A.V.; Sharnin, V.A. Stability of Co(III), Ni(II), and Cu(II) complexes with 2-Furan- and 2-thiophenecarboxyhydrazones of pyridoxal 5-phosphate in neutral aqueous solutions. Russ. J. Inorg. Chem. 2020, 65, 119–125. [Google Scholar] [CrossRef]
- Baruah, S.; Aier, M.; Puzari, A. Fluorescent probe sensor based on (R)-(-)-4-phenyl-2-oxazolidone for effective detection of divalent cations. Luminescence 2020, 35, 1206–1216. [Google Scholar] [CrossRef]
- Niu, L.; Liu, J.; Gao, S.; Gao, J.; Zhou, Y.; Liu, S.; Ma, C.; Zhao, Y. Fluoride ions detection in aqueous media by unprecedented ring opening of fluorescein dye: A novel multimodal sensor for fluoride ions and its utilization in live cell imaging. Spectrochim. Acta Part A 2023, 287, 122001. [Google Scholar] [CrossRef]
- Malkondu, S.; Altinkaya, N.; Erdemir, S.; Kocak, A. A reaction-based approach for fluorescence sensing of fluoride through cyclization of an O-acyl pyrene amidoxime derivative. Sensor. Actuat. B-Chem. 2018, 276, 296–303. [Google Scholar] [CrossRef]
- Cho, J.; Kim, I.; Moon, J.H.; Singh, H.; Jung, H.S.; Kim, J.S.; Lee, J.Y.; Kim, S. Triazolium-promoted highly selective fluorescence “turn-on” detection of fluoride ions. Dyes Pigm. 2016, 132, 248–254. [Google Scholar] [CrossRef]
- Shenderovich, I.G.; Limbach, H.H.; Smirnov, S.N.; Tolstoy, P.M.; Denisov, G.S.; Golubev, N.S. H/D isotope effects on the low-temperature NMR parameters and hydrogen bond geometries of (FH)2F− and (FH)3F− dissolved in CDF3/CDF2Cl. Phys. Chem. Chem. Phys. 2002, 4, 5488–5497. [Google Scholar] [CrossRef]
- Mukherjee, S.; Paul, A.K. Pyrene based chemosensor for selective sensing of fluoride in aprotic and protic environment. J. Fluoresc. 2015, 25, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.M.; Dhahagani, K.; Rajesh, J.; Anitha, K.; Chakkaravarthi, G.; Kanakachalam, N.; Marappan, M.; Rajagopal, G. Synthesis, structural analysis and cytotoxic effect of copper (II)-thiosemicarbazone complexes having heterocyclic bases: A selective naked eye sensor for F− and CN−. Polyhedron 2015, 85, 830–840. [Google Scholar] [CrossRef]
- Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.; Kiatkamjornwong, S. Furan/thiophene-based fluorescent hydrazones as fluoride and cyanide sensors. J. Photochem. Photobiol. A Chem. 2019, 385, 112038. [Google Scholar] [CrossRef]
- Zuo, B.; Liu, L.; Feng, X.; Li, D.; Li, W.; Huang, M.; Deng, Q. A novel fluorescent sensor based on triphenylamine with AIE properties for the highly sensitive detection of CN-. Dyes Pigm. 2021, 193, 109534. [Google Scholar] [CrossRef]
- Dai, H.; Xu, H. Selective and sensitive fluorescent chemosensors for Cu2+ ion based upon bis-1,8-naphthalimide dyads. Chin. J. Chem. 2012, 30, 267–272. [Google Scholar] [CrossRef]
- Liang, S.; Yu, H.; Xiang, J.; Yang, W.; Chen, X.; Liu, Y.; Gao, C.; Yan, G. New naphthalimide modified polyethylenimine nanoparticles as fluorescent probe for DNA detection. Spectrochim. Acta. A. 2012, 97, 359–365. [Google Scholar] [CrossRef]


| Probes | Solution System | LOD | Liner Ranges | Applications | Ref. |
|---|---|---|---|---|---|
 | EtOH/H2O (Co2+) | 4.55 × 10−3 μM | 0–0.5 μM | Cell imaging | [37] |
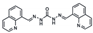 | MeOH/H2O (Co2+) | 0.21 μM | 0–12.5 μM | Cell imaging | [38] |
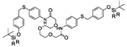 | DMF/PBS (F−) | 120 μM | 120 μM–1.5 mM | No statement | [39] |
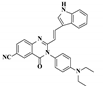 | DMSO (F−) | 1.096 μM | 0–1 mM | No statement | [40] |
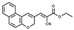 | DMSO/H2O (CN−) | 0.70 μM | 0–30 μM | Cell imaging | [41] |
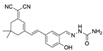 | DMSO/HEPES (CN−) | 0.79 μM | 0–50 μM | Live animal imaging | [42] |
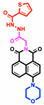 | MeCN | 0.15 μM (Co2+), 0.18 μM (F−), 0.12 μM (CN−) | 0–19 μM (Co2+), 0–22 μM (F−), 0–25 μM (CN−) | Real water samples detection | TMN |
| TMN | Add (μM) (Co2+) | Found (μM) (Co2+) | Recovery (%) (Co2+) | RSDb (%) (Co2+) |
|---|---|---|---|---|
| 3.00 | 3.27 ± 0.04 | 109.00 ± 1.33 | 1.22 | |
| Songhua River | 6.00 | 5.94 ± 0.02 | 99.00 ± 0.33 | 0.34 |
| 9.00 | 9.19 ± 0.05 | 102.11 ± 0.56 | 0.54 | |
| 3.00 | 3.30 ± 0.06 | 110.00 ± 2.00 | 3.30 | |
| Tap water | 6.00 | 6.31 ± 0.03 | 105.17 ± 0.50 | 0.48 |
| 9.00 | 9.38 ± 0.06 | 104.22 ± 0.67 | 0.52 | |
| TMN | Add (μM) (F−) | Found (μM) (F−) | Recovery (%) (F−) | RSDb (%) (F−) |
| 3.00 | 3.14 ± 0.04 | 104.67 ± 1.33 | 1.92 | |
| Songhua River | 6.00 | 6.28 ± 0.05 | 104.67 ± 0.83 | 3.67 |
| 9.00 | 9.16 ± 0.03 | 101.78 ± 0.33 | 0.79 | |
| 3.00 | 3.32 ± 0.04 | 110.67 ± 1.33 | 3.48 | |
| Tap water | 6.00 | 6.30 ± 0.03 | 105.17 ± 0.50 | 0.72 |
| 9.00 | 9.29 ± 0.05 | 104.22 ± 0.56 | 1.08 | |
| TMN | Add (μM) (CN−) | Found (μM) (CN−) | Recovery (%) (CN−) | RSDb (%) (CN−) |
| 3.00 | 3.18 ± 0.03 | 106.00 ± 1.00 | 0.63 | |
| Songhua River | 6.00 | 6.25 ± 0.03 | 104.17 ± 0.50 | 0.48 |
| 9.00 | 8.87 ± 0.05 | 98.56 ± 0.56 | 0.56 | |
| 3.00 | 3.25 ± 0.03 | 108.33 ± 1.00 | 1.12 | |
| Tap water | 6.00 | 6.33 ± 0.04 | 105.50 ± 0.67 | 0.47 |
| 9.00 | 9.42 ± 0.06 | 104.67 ± 0.67 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ji, X.-X.; Xu, M.-Y.; Liu, Y.-L.; Yang, L. A Multifunctional Fluorescent Probe Based on 1,8-Naphthalimide for the Detection of Co2+, F−, and CN−. Inorganics 2023, 11, 265. https://doi.org/10.3390/inorganics11070265
Li P, Ji X-X, Xu M-Y, Liu Y-L, Yang L. A Multifunctional Fluorescent Probe Based on 1,8-Naphthalimide for the Detection of Co2+, F−, and CN−. Inorganics. 2023; 11(7):265. https://doi.org/10.3390/inorganics11070265
Chicago/Turabian StyleLi, Ping, Xian-Xian Ji, Ming-Yao Xu, Yu-Long Liu, and Liu Yang. 2023. "A Multifunctional Fluorescent Probe Based on 1,8-Naphthalimide for the Detection of Co2+, F−, and CN−" Inorganics 11, no. 7: 265. https://doi.org/10.3390/inorganics11070265
APA StyleLi, P., Ji, X.-X., Xu, M.-Y., Liu, Y.-L., & Yang, L. (2023). A Multifunctional Fluorescent Probe Based on 1,8-Naphthalimide for the Detection of Co2+, F−, and CN−. Inorganics, 11(7), 265. https://doi.org/10.3390/inorganics11070265