Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites
Abstract
1. Introduction
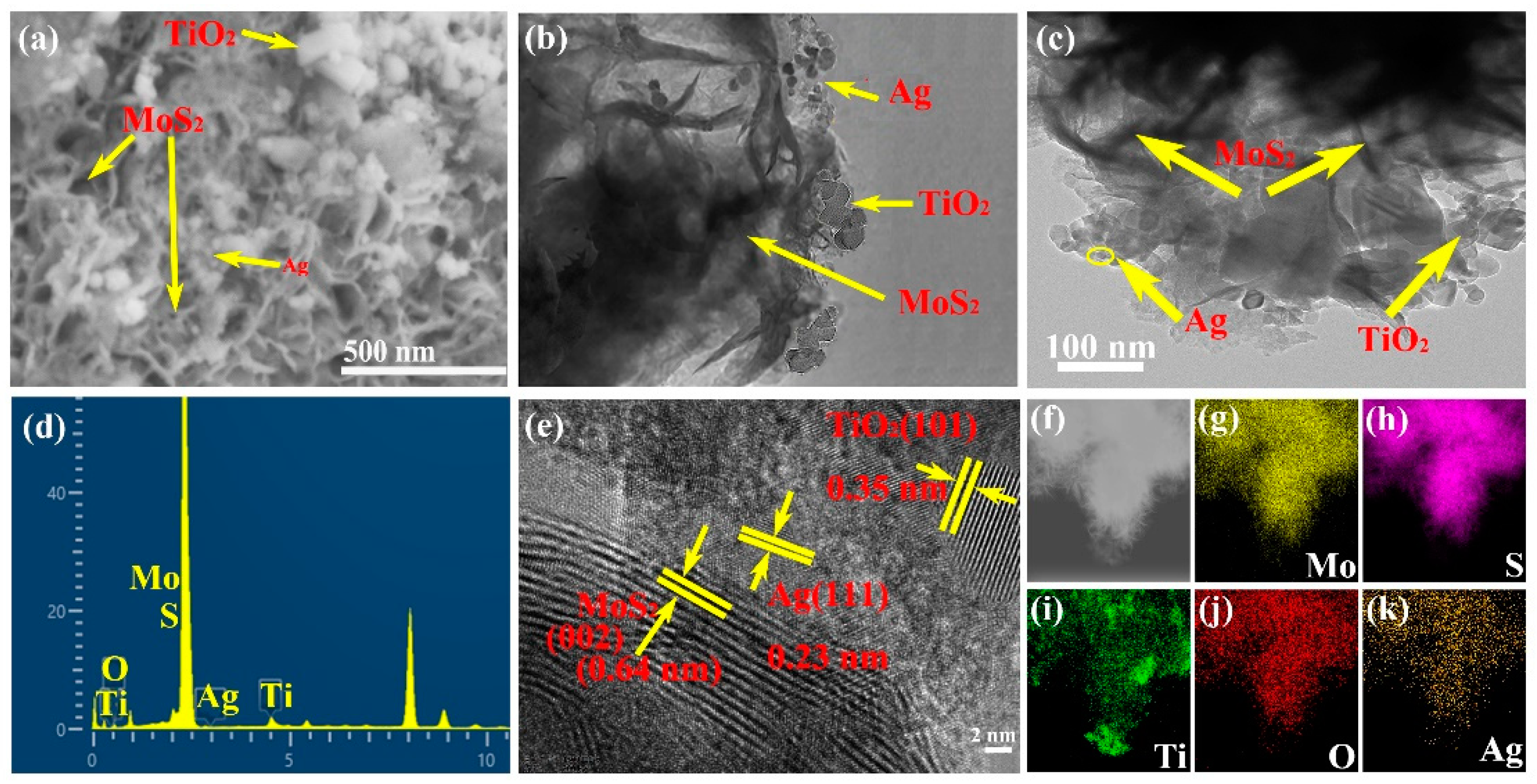
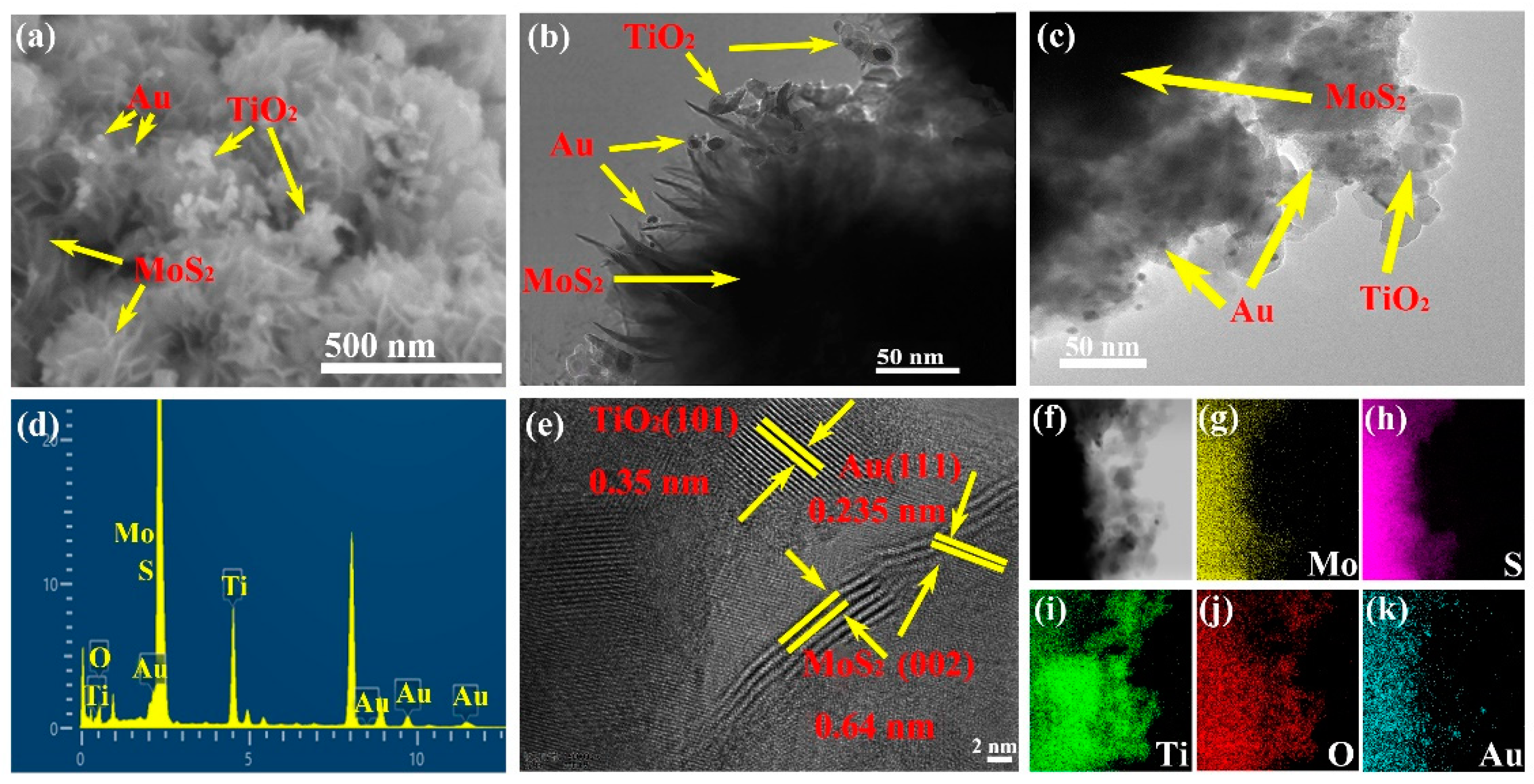
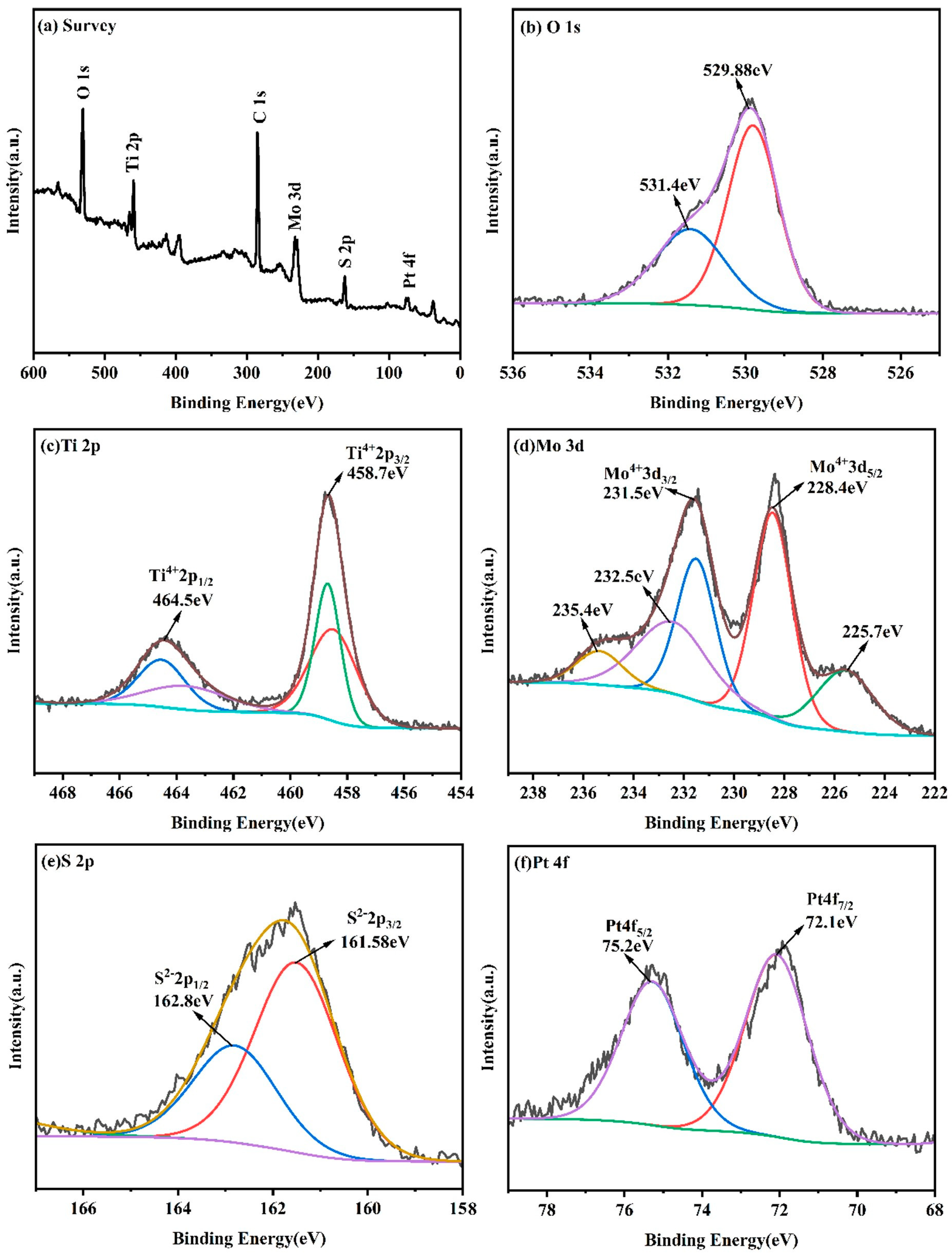
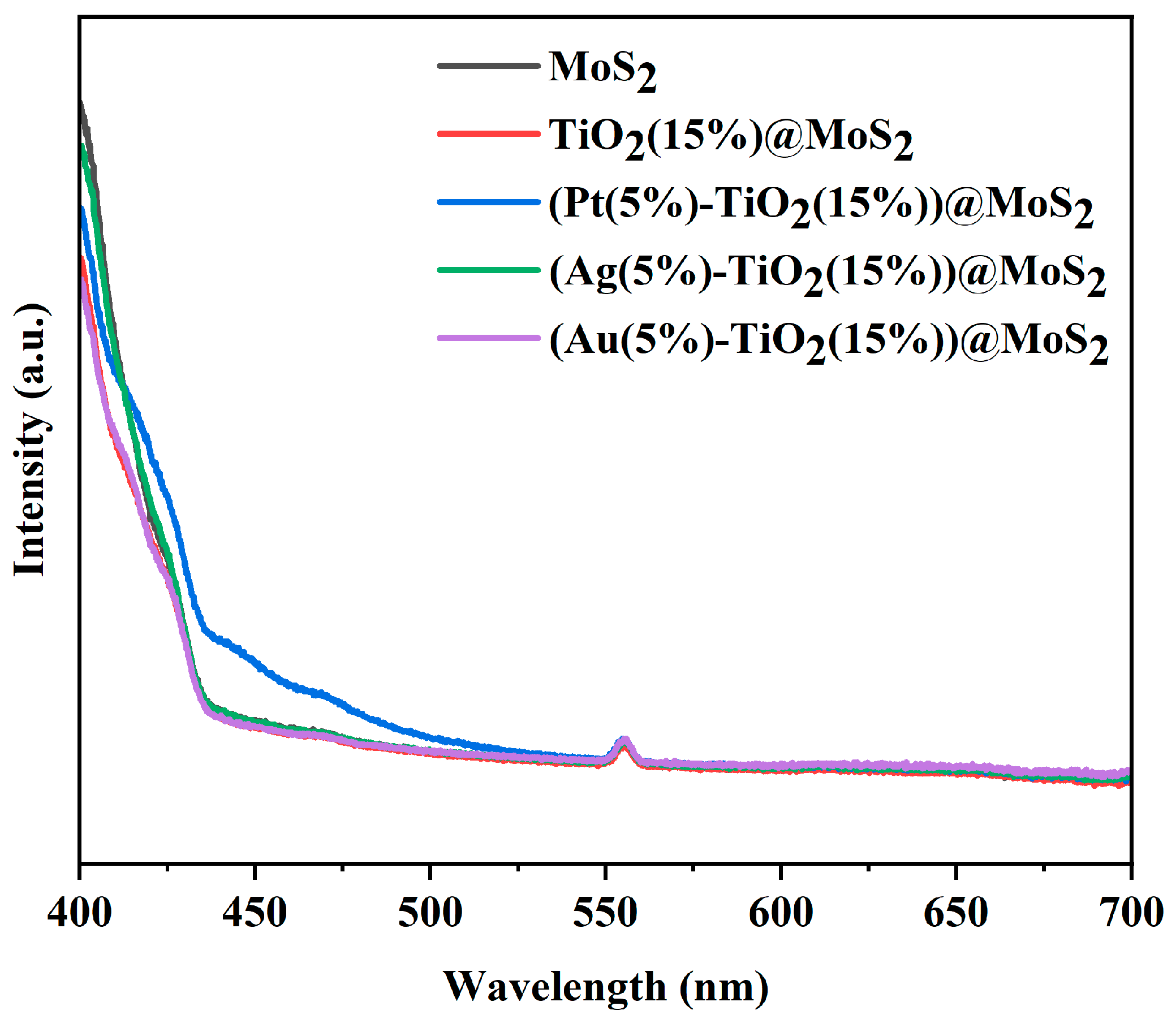
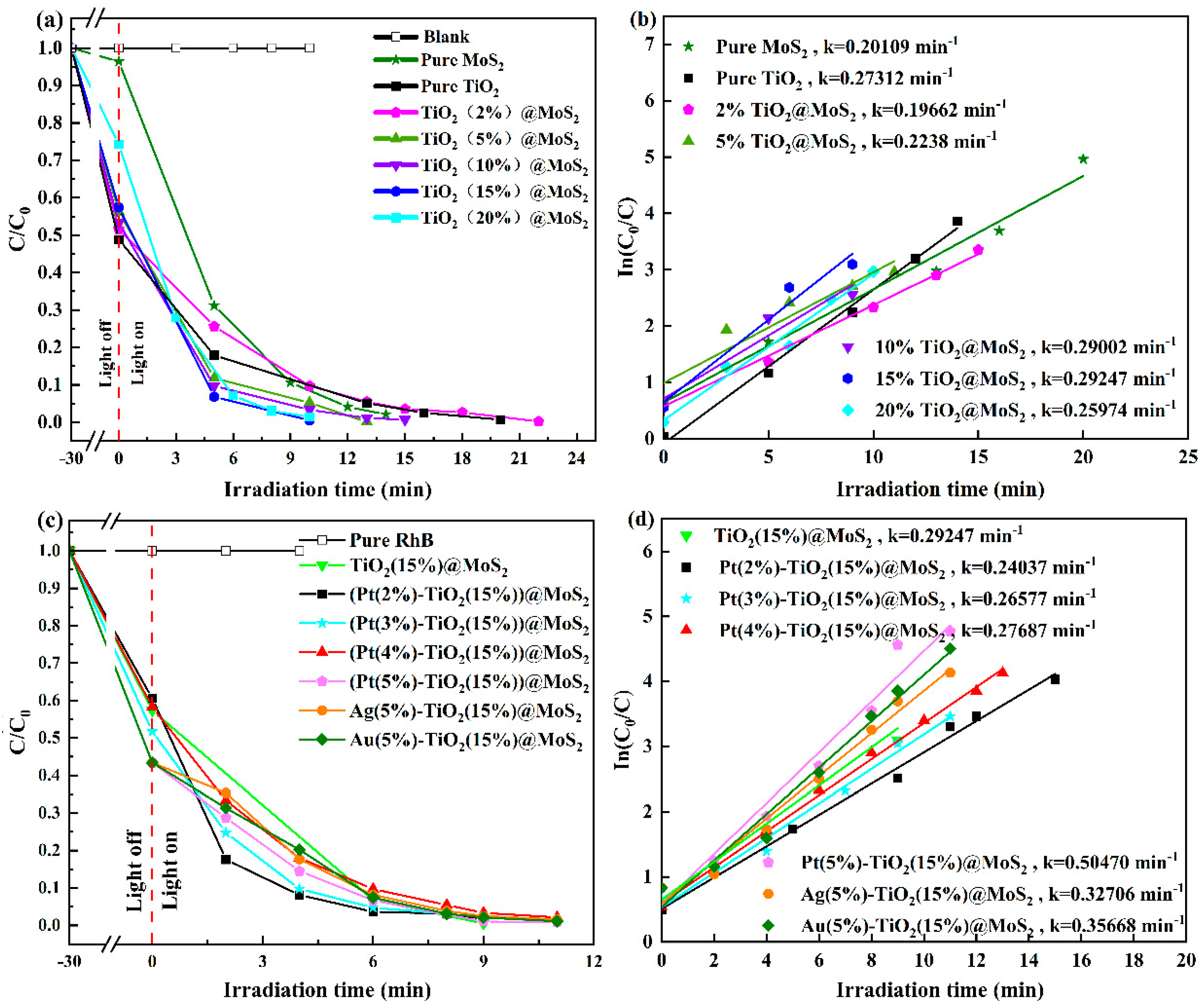
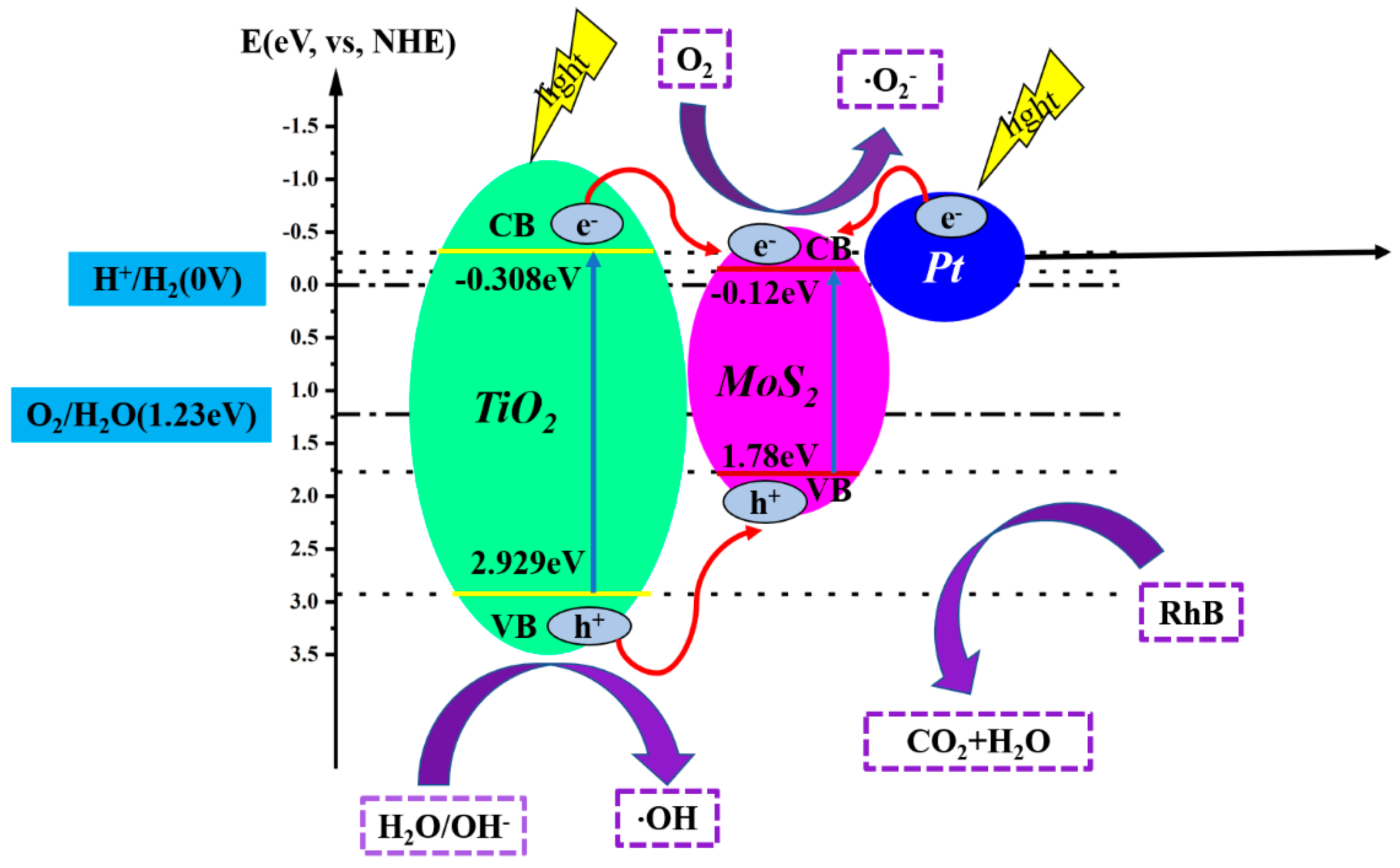
2. Results and Discussion
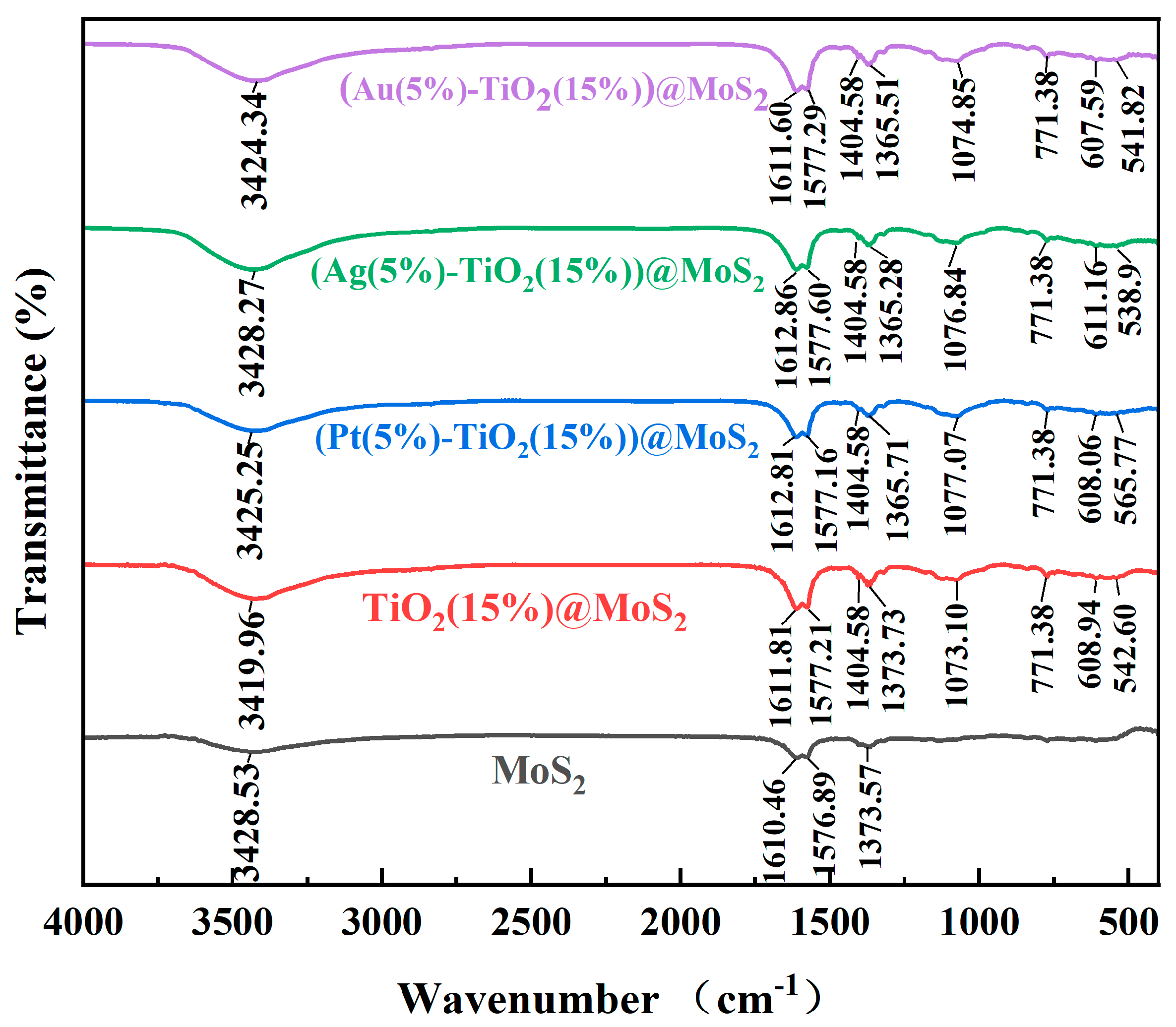
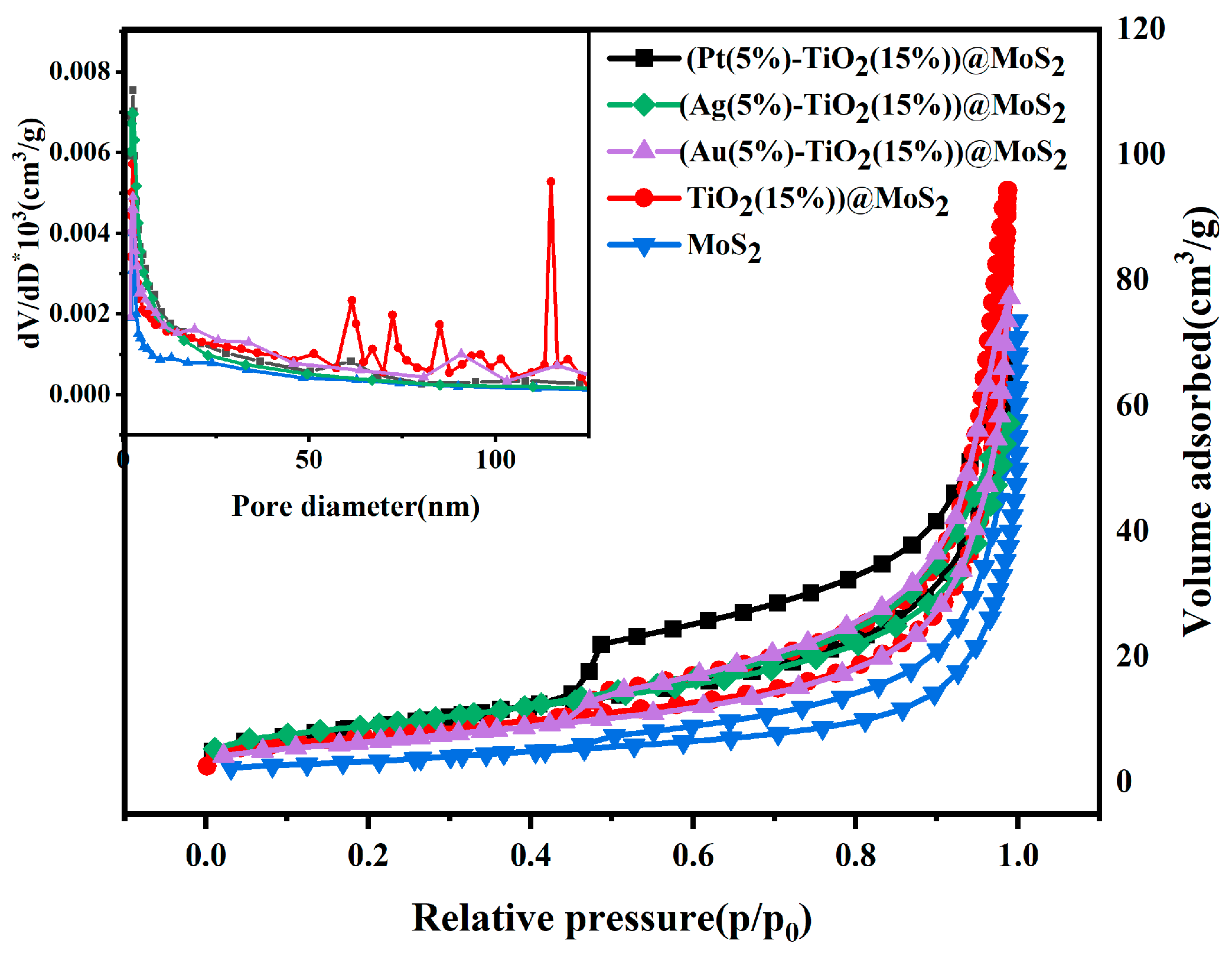
Characterization of the Synthesized Materials
3. Experiments
3.1. Materials
3.2. Preparation of a Series of MoS2, TiO2@MoS2, and (Pt-TiO2)@MoS2 Nanocomposites
3.2.1. Preparation of MoS2 Matrix Materials
3.2.2. Preparation of TiO2@MoS2 Nanocomposites
3.2.3. Preparation of (Pt-TiO2)@MoS2, (Ag-TiO2)@MoS2, and (Au-TiO2)@MoS2 Nanocomposites
3.3. Characterization
3.4. Photocatalytic Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core-shell structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, R.; Zhang, Q. Few-layer MoS2: A promising layered semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Vélez, N.; Zanella, R. Comparative study of transition metal (Mn, Fe or Co) catalysts supported on titania: Effect of Au nanoparticles addition towards CO oxidation and soot combustion reactions. Chem. Eng. J. 2020, 385, 123848. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Kumar, A. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrog. Energy 2019, 45, 2729–2744. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Shi, Q.; Yang, J.; Xie, M.; Han, W. Toward photocatalytic hydrogen generation over BiVO4 by controlling particle size. Chin. Chem. Lett. 2021, 32, 2419–2422. [Google Scholar] [CrossRef]
- Suarez-Escobar, A.F.; Conde-Rivera, L.R.; Lopez-Suarez, F.E.; Illán-Gómez, M.J.; Gonzalez-Hernandez, K.S.; Chalapud-Morales, J.S. Heterogeneous Photocatalytic Degradation of Ibuprofen over TiO2–Ag Supported on Activated Carbon from Waste Tire Rubber. Top. Catal. 2021, 64, 51–64. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Nada, E.A.; El-Maghrabi, H.H.; Raynaud, P.; Ali, H.R.; Abd El-Wahab, S.; Sabry, D.Y.; Moustafa, Y.M.; Nada, A.A. Enhanced Photocatalytic Activity of WS2/TiO2 Nanofibers for Degradation of Phenol under Visible Light Irradiation. Inorganics 2022, 10, 54. [Google Scholar]
- Nwankwo, U.; Bucher, R.; Ekwealor, A.B.C.; Maaza, M.; Ezema, F.I. Synthesis and characterizations of rutile-TiO2 nanoparticles derived from chitin for potential photocatalytic applications. Vacuum 2018, 161, 49–54. [Google Scholar] [CrossRef]
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M.N. Recent Advances in Structural Modifications of Photo-Catalysts for Organic Pollutants Degradation—A Comprehensive Review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Hu, G.; Yang, C.; Chen, Y.; Liu, Q.; Ren, S.; Li, J. Optimization of TiO2/ZSM-5 Photocatalysts: Energy Band Engineering by Solid State Diffusion Method with Calcination. J. Environ. Chem. Eng. 2021, 9, 105563. [Google Scholar] [CrossRef]
- Xia, C.; Nguyen, T.H.C.; Nguyen, X.C.; Kim, S.Y.; Nguyen, D.L.T.; Raizada, P.; Singh, P.; Nguyen, V.-H.; Nguyen, C.C.; Hoang, V.C.; et al. Emerging Cocatalysts in TiO2-Based Photocatalysts for Light-Driven Catalytic Hydrogen Evolution: Progress and Perspectives. Fuel 2022, 307, 121745. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction. Adv. Opt. Mater. 2018, 6, 1800911. [Google Scholar] [CrossRef]
- Hu, X.; Lu, S.; Tian, J.; Wei, N.; Song, X.; Wang, X.; Cui, H. The selective deposition of MoS2 nanosheets onto (101) facets of TiO2 nanosheets with exposed (001) facets and their enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2019, 241, 329–337. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, H.; Miao, H.; Zhu, H.; Liu, X.; Lin, H.; Shi, G. Fabrication of a three-dimensional bionic Si/TiO2/MoS2 photoelectrode for efficient solar water splitting. ACS Appl. Energy Mater. 2020, 4, 730–736. [Google Scholar] [CrossRef]
- Liang, H.; Bai, J.; Xu, T.; Li, C. Controllable growth of foxtail-like MoS2 on one-dimensional carbon nanofibers with enhanced photocatalytic activity. Vacuum 2020, 172, 109059. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Peng, F.; Lin, Y.; Yang, S.; Zhang, S.; Wang, H.; Cao, Y.; Yu, H. 2H-and 1T-mixed phase few-layer MoS2 as a superior to Pt cocatalyst coated on TiO2 nanorod arrays for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 241, 236–245. [Google Scholar] [CrossRef]
- Lathe, A.; Ansari, A.; Badhe, R.; Palve, A.M.; Garje, S.S. Single-Step Production of a TiO2@MoS2 Heterostructure and Its Applications as a Supercapacitor Electrode and Photocatalyst for Reduction of Cr(VI) to Cr(III). ACS Omega 2021, 6, 13008–13014. [Google Scholar] [CrossRef]
- Nkele, A.C.; Chime, U.K.; Asogwa, L.; Nwanya, A.C.; Nwankwo, U.; Ukoba, K.; Jen, T.; Maaza, M.; Ezema, F.I. A study on titanium dioxide nanoparticles synthesized from titanium isopropoxide under SILAR-induced gel method: Transition from anatase to rutile structure. Inorg. Chem. Commun. 2020, 112, 107705. [Google Scholar] [CrossRef]
- Chandrabose, G.; Dey, A.; Gaur, S.S.; Pitchaimuthu, S.; Jagadeesan, H.; Braithwaite, N.S.J.; Selvaraj, V.; Kumar, V.; Krishnamurthy, S. Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 2021, 279, 130467. [Google Scholar] [CrossRef] [PubMed]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S. TiO2 nanosheets with exposed {001} facets for photocatalytic applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics 2022, 10, 29. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.-Q.; Huang, Y.-W.; Yu, Z.-T.; Zhong, J.-S.; Chen, T.-T.; Tu, W.-G.; Guan, Z.-J.; Cao, D.-P.; Zou, Z.-G. MoS2 Nanosheet-Modified CuInS2 Photocatalyst for Visible-Light-Driven Hydrogen Production from Water. ChemSusChem 2016, 9, 1003–1009. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Machín, A.; Soto-Vázquez, L.; Colón-Cruz, C.; Valentín-Cruz, C.A.; Claudio-Serrano, G.J.; Fontánez, K.; Resto, E.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics 2021, 6, 4. [Google Scholar] [CrossRef]
- Limón-Rocha, I.; Marizcal-Barba, A.; Guzmán-González, C.A.; Anaya-Esparza, L.M.; Ghotekar, S.; González-Vargas, O.A.; Pérez-Larios, A. Co, Cu, Fe, and Ni Deposited over TiO2 and Their Photocatalytic Activity in the Degradation of 2,4-Dichlorophenol and 2,4-Dichlorophenoxyacetic Acid. Inorganics 2022, 10, 157. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Phan, D.T.; Chung, G.S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 207, 362–369. [Google Scholar] [CrossRef]
- Al Jitan, S.; Li, Y.; Bahamon, D.; Žerjav, G.; Tatiparthi, V.S.; Aubry, C.; Sinnokrot, M.; Matouk, Z.; Rajput, N.; Gutierrez, M.; et al. Unprecedented Photocatalytic Conversion of Gaseous and Liquid CO2 on Graphene-Impregnated Pt/Cu-TiO2. SSRN J. 2022, 11, 109485. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Li, Q.; Guan, Z.; Zhang, M.; Yang, J. The Photothermal Effect Enhance Visible Light-Driven Hydrogen Evolution Using Urchin-like Hollow RuO2/TiO2/Pt/C Nanomaterial. J. Alloy. Compd. 2022, 890, 161722. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simoňcǐc, B.; Tomšǐc, B. Recent Advances in TiO2 -Functionalized Textile Surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Cao, T.M.; Balayeva, N.O.; Pham, V.V. Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance. Catalysts 2021, 11, 857. [Google Scholar] [CrossRef]
- Li, S.; Pu, T.; Wang, J.; Fang, X.; Liu, Y.; Kang, S.; Cui, L. Efficient visible-light-driven hydrogen evolution over ternary MoS2/Pt-TiO2 photocatalysts with low overpotential. Int. J. Hydrog. Energy 2018, 43, 16534–16542. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Rodríguez-Pérez, M.; Ruiz-Gómez, M.Á.; Rodríguez-Gattorno, G.; Villanueva-Cab, J. Synergistic Correlation in the Colloidal Properties of TiO2 Nanoparticles and Its Impact on the Photocatalytic Activity. Inorganics 2022, 10, 125. [Google Scholar] [CrossRef]
- Abdolvahabpour, N.L.; Michael, S.; Minoo, T. Photocatalytic Degradation of Phenol Using Photodeposited Pt Nanoparticles on Titania. J. Nanosci. Nanotechnol. 2020, 20, 1056–1065. [Google Scholar]
- Fan, G.; Huang, X.; Li, D.; Li, A. TiO-Graphene 3D Hydrogel Supported on Ni Foam for Photoelectrocatalysis Removal of Organic Contaminants. J. Nanosci. Nanotechnol. 2020, 20, 2645–2649. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xue, M.; Ye, X.; Lei, W.; Tang, H.; Li, C. Hydrothermal synthesis and characterization of 3D flower-like MoS2 microspheres. Mater. Lett. 2015, 148, 67–70. [Google Scholar] [CrossRef]
- Neri, G.; Leonardi, S.G.; Latino, M.; Donato, N.; Baek, S.; Conte, D.E.; Russo, P.A.; Pinna, N. Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens. Actuators B Chem. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- The International Center for Diffraction Data (ICDD) No. 00-004-0784; ICDD: Newtown Square, PA, USA, 2008.
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Shen, T.; Xiang, Q. Roles of MoS2 and Graphene as Cocatalysts in the Enhanced Visible-Light Photocatalytic H2 Production Activity of Multiarmed CdS Nanorods. ChemCatChem 2015, 7, 943–951. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, J.; Yan, K.; Wang, H.; Wang, Z.; Yang, S. Space-Confined Growth of MoS2 Nanosheets within Graphite: The Layered Hybrid of MoS2 and Graphene as an Active Catalyst for Hydrogen Evolution Reaction. Chem. Mater. A Publ. Am. Chem. Soc. 2014, 26, 2344–2353. [Google Scholar] [CrossRef]
- Drmosh, Q.; Yamani, Z. Yamani. Hydrogen sensing properties of sputtered ZnO films decorated with Pt nanoparticles. Ceram. Int. 2016, 42, 12378–12384. [Google Scholar] [CrossRef]
- Jung, W.Y.; Lee, G.D.; Park, S.S.; Lim, K.T.; Hong, S.-S. Photocatalytic decomposition of methylene blue over yttrium ion doped Ti-SBA-15 catalysts. Catal. Today 2011, 164, 395–398. [Google Scholar] [CrossRef]
- Deghiche, A.; Haddaoui, N.; Zerriouh, A.; Fenni, S.E.; Cavallo, D.; Erto, A.; Benguerba, Y. Effect of the stearic acid-modified TiO2 on PLA nanocomposites: Morphological and thermal properties at the microscopic scale. J. Environ. Chem. Eng. 2021, 9, 106541. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Zheng, Q.; Shao, Y.; Li, D. Amorphous MoSx on CdS nanorods for highly efficient photocatalytic hydrogen evolution. J. Solid State Chem. 2017, 246, 230–236. [Google Scholar] [CrossRef]
- Zheng, L.; Han, S.; Liu, H.; Yu, P.; Fang, X. Hierarchical MoS2 Nanosheet@TiO2 Nanotube Array Composites with Enhanced Photocatalytic and Photocurrent Performances. Small 2016, 12, 1527.e36. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Zheng, L.; Wan, C. Fabrication of TiO2/MoS2@zeolite photocatalyst and its photocatalytic activity for degradation of methyl orange under visible light. Appl. Surf. Sci. A J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2015, 358, 468–478. [Google Scholar] [CrossRef]
- Xu, H.; Tao, X.; Wang, D.-T.; Zheng, Y.-Z.; Chen, J.-F. Enhanced efficiency in dye-sensitized solar cells based on TiO2 nanocrystal/nanotube double-layered films. Electrochim. Acta 2009, 55, 2280–2285. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.H.; Kim, H.G.; Lee, J.S. Location and State of Pt in Platinized CdS/TiO2 Photocatalysts for Hydrogen Production from Water under Visible Light. J. Phys. Chem. C Nanomater. Interfaces 2008, 112, 17200–17205. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, H.; Li, Y.; Fu, L.; Gao, Y.; Song, W.; Shao, Z.; Yi, B. Simple synthesis of Pt/TiO2 nanotube arrays with high activity and stability. J. Electroanal. Chem. 2013, 701, 14–19. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic H2 evolution on MoS2-TiO2 catalysts synthesized via mechanochemistry. Phys. Chem. Chem. Phys. 2015, 17, 933–940. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, W.; Yu, X.; Zhang, H.; Li, Y.; Sun, X.; Wang, X.; Wang, H.; Wang, J.; Luo, J.; et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Chen, X.; Du, J.; Xiong, Y. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2015, 8, 175–183. [Google Scholar] [CrossRef]
- Wang, B.; Xia, Y.; Wang, G.; Zhou, Y.; Wang, H. Core shell MoS2/C nanospheres embedded in foam-like carbon sheets composite with an interconnected macroporous structure as stable and high-capacity anodes for sodium ion batteries. Chem. Eng. J. 2017, 309, 417–425. [Google Scholar] [CrossRef]
- Yan, X.; Bao, R.; Yu, S.; Li, Q.; Jing, Q. The roles of hydroxyl radicals, photo-generated holes and oxygen in the photocatalytic degradation of humic acid. Russ. J. Phys. Chem. 2012, 86, 1479–1485. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Yang, Z.; Wang, Q. Facile synthesis, characterization and gas sensing performance of ZnO nanoparticles-coated MoS2 nanosheets. J. Alloy. Compd. 2016, 662, 118–125. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Lei, X.; Guo, B.; Li, C.; Fua, H.; Zhu, Z. Synthesis of MoS2@CuO Heterogeneous Structure with Improved Photocatalysis Performance and H2O Adsorption Analysis. Dalton Trans. 2015, 44, 10438. [Google Scholar] [CrossRef]
- Hong, D.; Cao, G.; Qu, J.; Deng, Y.; Tang, J. Antibacterial activity of Cu2O and Ag co-modified rice grains-like ZnO nanocomposites. J. Mater. Sci. Technol. 2018, 34, 2359–2367. [Google Scholar] [CrossRef]
- Zhang, D.; Pang, M.; Wu, J.; Cao, Y. Experimental and density functional theory investigation of Pt-loaded titanium dioxide/molybdenum disulfide nanohybrid for SO2 gas sensing. New J. Chem. 2019, 43, 4900–4907. [Google Scholar] [CrossRef]
- Abniel, M.; Kenneth, F.; Diego, G.; Paola, S.; Carla, C.; Gerardo, C.J.; Loraine, S.; Edgard, R.; Florian, P.I.; Carmen, M.; et al. Hydrogen Production and Degradation of Ciprofloxacin by Ag@TiO2-MoS2 Photocatalysts. Catalysts 2022, 12, 267. [Google Scholar]
- Naldoni, A.; D’Arienzo, M.; Altomare, M.; Marelli, M.; Scotti, R.; Morazzoni, F.; Selli, E.; Dal Santo, V. Pt and Au/TiO2 photocatalysts for methanol reforming: Role of metal nanoparticles in tuning charge trapping properties and photoefficiency. Appl. Catal. B Environ. 2013, 130–131, 239–248. [Google Scholar] [CrossRef]




| Sample | (Pt(5 wt%)-TiO2(15 wt%))@MoS2 | (Ag(5 wt%)-TiO2(15 wt%))@MoS2 | (Au(5 wt%)-TiO2(15 wt%)) @MoS2 | TiO2(15 wt%))@MoS2 | MoS2 |
|---|---|---|---|---|---|
| SBET (m2 × g−1) | 32.85 | 32.72 | 23.85 | 26.78 | 12.62 |
| Average pore size (nm) | 12.61 | 10.83 | 20.05 | 21.78 | 19.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Hong, D.; Jin, X.; Liu, H.; Yang, X.; Nie, L.; Liu, Q.; Gao, Z.; Zhu, W.; Wang, Y.; et al. Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites. Inorganics 2023, 11, 258. https://doi.org/10.3390/inorganics11060258
Ju L, Hong D, Jin X, Liu H, Yang X, Nie L, Liu Q, Gao Z, Zhu W, Wang Y, et al. Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites. Inorganics. 2023; 11(6):258. https://doi.org/10.3390/inorganics11060258
Chicago/Turabian StyleJu, Liying, Dunhua Hong, Xing Jin, Hongxian Liu, Xiude Yang, Liying Nie, Qibin Liu, Zhixi Gao, Wei Zhu, Yi Wang, and et al. 2023. "Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites" Inorganics 11, no. 6: 258. https://doi.org/10.3390/inorganics11060258
APA StyleJu, L., Hong, D., Jin, X., Liu, H., Yang, X., Nie, L., Liu, Q., Gao, Z., Zhu, W., Wang, Y., & Yang, X. (2023). Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites. Inorganics, 11(6), 258. https://doi.org/10.3390/inorganics11060258






