Structural and Antimicrobial Investigation of Some New Nanoparticles Mixed Ligands Metal Complexes of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate in Presence of 1,10-Phenanthroline
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis and Molar Conductance
2.2. FT-IR Spectra
2.3. UV-vis. Spectra and Magnetic Moment
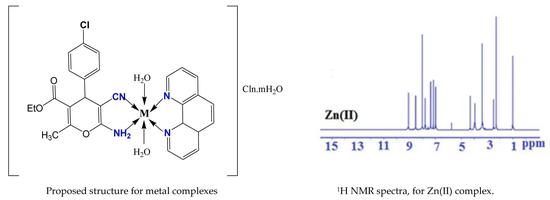
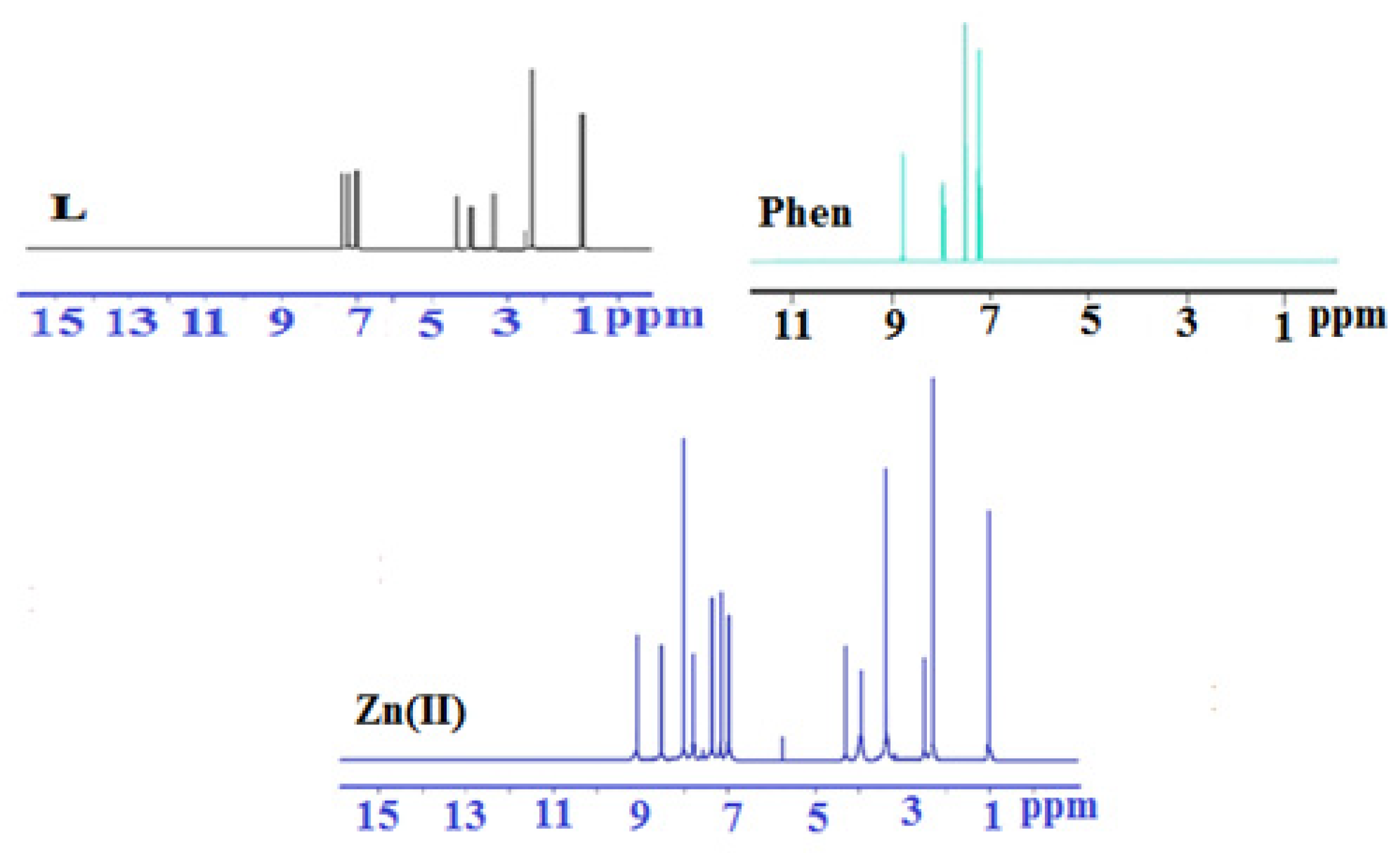
2.4. 1H NMR and 13C NMR Spectra
2.5. Thermal Analyses
2.6. The Kinetic Data
2.7. XRD
2.8. Antimicrobial Efficiency
3. Experimental
3.1. Materials
3.2. Physical Techniques
3.3. Synthesis of L
3.4. Syntheses of Metal Complexes
3.5. Antimicrobial and Minimal Inhibitory Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boger, D.L.; Weinreb, S.M. Hetero Diels-Alder Methodology in Organic Synthesis; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar] [CrossRef]
- Tietze, L.F.; Kettschau, G. Hetero Diels-Alder reactions in organic chemistry. Top. Curr. Chem. 1997, 12, 189. [Google Scholar] [CrossRef]
- Grivsky, E.M.; Lee, S.; Sigel, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem. 1980, 23, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Broom, A.D.; Shim, J.L.; Anderson, G.L. Pyrido[2,3-d]pyrimidines. IV. Synthetic studies leading to various oxopyrido[2,3-d]pyrimidines. J. Org. Chem. 1976, 41, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Heers, C.; Ravens, U. Positive inotropic activity of 5-amino-6-cyano-1,3-dimethyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. Part 6: Compounds with positive inotropic activity. Die Pharm. 1993, 48, 537–541. [Google Scholar]
- Asadian, M.; Davoodnia, A.; Beyramabadi, S.A. Efficient Synthesis of New Pyrimido[5′,4′:5,6]pyrano[2,3-d]pyrimidine-2,4,6(1H,3H)-triones via the Tandem Intramolecular Pinner–Dimroth Rearrangement, and Their Antibacterial Activity. Russ. J. Gen. Chem. 2018, 88, 2658–2663. [Google Scholar] [CrossRef]
- Poola, S.; Shaik, M.S.; Sudileti, M.; Yakkate, S.; Nalluri, V.; Chippada, A.; Cirandur, S.R. Nano CuO–Ag-catalyzed synthesis of some novel pyrano[2,3-d] pyrimidine derivatives and evaluation of their bioactivity. J. Chin. Chem. Soc. 2020, 67, 805–820. [Google Scholar] [CrossRef]
- Amininia, A.; Pourshamsian, K.; Sadeghi, B. Nano-ZnO Impregnated on Starch—A Highly Efficient Heterogeneous Bio-Based Catalyst for One-Pot Synthesis of Pyranopyrimidinone and Xanthene Derivatives as Potential Antibacterial Agents. Russ. J. Org. Chem. 2020, 56, 1279–1288. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Ertan, M.; Kelicen, P.; Demirdamar, R. Synthesis and anti-inflammatory activities of some thiazolo[3,2-a]pyrimidine derivatives. Il Farm. 1999, 54, 588–593. [Google Scholar] [CrossRef]
- Agarwal, A.; Srivastava, K.; Puri, S.; Chauhan, P.M. Synthesis of 2,4,6-trisubstituted pyrimidines as antimalarial agents. Bioorganic Med. Chem. 2005, 13, 4645–4650. [Google Scholar] [CrossRef]
- Cerecetto, H.; Di Maio, R.; González, M.; Risso, M.; Sagrera, G.; Seoane, G.; Denicola, A.; Peluffo, G.; Quijano, C.; Stoppani, A.O.; et al. Synthesis and antitrypanosomal evaluation of E-isomers of 5-nitro-2-furaldehyde and 5-nitrothiophene-2-carboxaldehyde semicarbazone derivatives. Structure–activity relationships. Eur. J. Med. Chem. 2000, 35, 343–350. [Google Scholar] [CrossRef]
- Bruno, O.; Brullo, C.; Schenone, S.; Bondavalli, F.; Ranise, A.; Tognolini, M.; Impicciatore, M.; Ballabeni, V.; Barocelli, E. Synthesis, antiplatelet and antithrombotic activities of new 2-substituted benzopyrano[4,3-d]pyrimidin-4-cycloamines and 4-amino/cycloamino-benzopyrano[4,3-d]pyrimidin-5-ones. Bioorganic Med. Chem. 2006, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chabchoub, F.; Messaâd, M.; Ben Mansour, H.; Chekir-Ghedira, L.; Salem, M. Synthesis and antigenotoxic activity of some naphtho[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Eur. J. Med. Chem. 2007, 42, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Feng, D.; Qu, Y.; Zhang, X.; Wang, J.; Loiseau, P.; Andrei, G.; Snoeck, R.; De Clercq, E. Practical and efficient synthesis of pyrano[3,2-c]pyridone, pyrano[4,3-b]pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents. Bioorganic Med. Chem. Lett. 2010, 20, 809–813. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.A.; Said, S.A. Direct Synthesis of Multi-functional Pyrimidine, Pyrazine, and Pyridine Scaffolds via Inter- and Intramolecular Annulations of 3-Amino-thieno[2,3- b ]pyridine-2-carboxylate. J. Heterocycl. Chem. 2019, 56, 1030–1037. [Google Scholar] [CrossRef]
- Assy, M.G.; El-Sayed, H.A.; Ouf, N.H.; Hamza, A.; Morsy, H.A. Cycloaddition of Aroyl Isothiocyanate: A Novel Synthesis of Triazine, Oxazine, Pyrimidine, and Pyridine Derivatives. J. Heterocycl. Chem. 2019, 56, 2954–2959. [Google Scholar] [CrossRef]
- El-Sayed, H.A. Design and synthesis of some tricyclic pyrimidines and triazines via cycloaddition and intermolecular cyclization of cyclic amidine. J. Iran. Chem. Soc. 2017, 14, 2239–2246. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Morsy, H.A. A facile synthesis of highly fluorescent pyrido[2,3-d]pyrimidines and 1,8-naphthyridines via oxazine transformation and enaminic addition reactions. J. Iran. Chem. Soc. 2019, 16, 723–732. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Hamid, A.M.A.; Assy, M.G.; Faraj, T.S. Intermolecular cyclization of cinnamoyl isothiocyanate: A new synthetic entry for pyrimidine, triazine, and triazole candidates. Synth. Commun. 2018, 48, 786–794. [Google Scholar] [CrossRef]
- Shijay, G.; Cheng, H.T.; Chi, T.; Ching-Fa, Y. Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tedrahedron 2008, 64, 9143–9149. [Google Scholar] [CrossRef]
- Oskooie, H.A.; Heravi, M.M.; Karimi, N.; Zadeh, M.E. Caro’s Acid–Silica Gel: An Efficient and Versatile Catalyst for the One-Pot Synthesis of Tetrahydrobenzo[b]pyran Derivatives. Synth. Commun. 2011, 41, 436–440. [Google Scholar] [CrossRef]
- Hafez, E.A.A.; Elnagdi, M.H.; Elagamey, A.G.A.; El-Taweel, F.M.A.A. Nitriles in Heterocyclic Synthesis: Novel Synthesis of Benzo[c]coumarin and of Benzo[c]pyrano[3,2-c]quinoline Derivatives. Heterocycles 1987, 26, 903–907. [Google Scholar] [CrossRef]
- Ellis, G.P. The Chemistry of Heterocyclic Compounds: Chromenes, Chromanes and Chromones; Weissberger, A., Taylor, E.C., Eds.; John Wiley: New York, NY, USA, 1977; pp. 11–139. [Google Scholar]
- Gao, Y.; Yang, W.; Du, D.M. Efficient organocatalytic asymmetric synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives. Tetrahedron Asymm. 2012, 23, 339–344. [Google Scholar] [CrossRef]
- Reynolds, G.; Drexhage, K. New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar] [CrossRef]
- Zollinger, H. Color chemistry. In Syntheses, Properties, and Applications of Organic Dyes and Pigments/Heinrich Zollinger, 3rd ed.; Verlag Helvetica Chimica Acta; Zurikh and Wiley-VCH: Cambridge, UK, 2003. [Google Scholar]
- Bissell, E.R.; Mitchell, A.R.; Smith, R.E. Synthesis and chemistry of 7-amino-4-(trifluoromethyl)coumarin and its amino acid and peptide derivatives. J. Org. Chem. 1980, 45, 2283–2287. [Google Scholar] [CrossRef]
- Khaksar, S.; Rouhollahpour, A.; Talesh, S.M. A facile and efficient synthesis of 2-amino-3-cyano-4H-chromenes and tetrahydrobenzo[b]pyrans using 2,2,2-trifluoroethanol as a metal-free and reusable medium. J. Fluor. Chem. 2012, 141, 11–15. [Google Scholar] [CrossRef]
- Saha, M.; Pal, A.K. Palladium (0) Nanoparticles: A Novel and Reusable Catalyst for the Synthesis of Various Pyran Derivatives. Adv. Nanoparticles 2012, 1, 61–70. [Google Scholar] [CrossRef]
- Abouzid, K.A.; Al-Ansary, G.H.; El-Naggar, A.M. Eco-friendly synthesis of novel cyanopyridine derivatives and their anticancer and PIM-1 kinase inhibitory activities. Eur. J. Med. Chem. 2017, 134, 357–365. [Google Scholar] [CrossRef]
- Sadeek, S.; El-Hamid, S.A. Preparation, characterization and cytotoxicity studies of some transition metal complexes with ofloxacin and 1,10-phenanthroline mixed ligand. J. Mol. Struct. 2016, 1122, 175–185. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Hamid, S.M.A. Synthesis, spectroscopic, thermal analysis and in vitro biological properties of some new metal complexes with gemifloxacin and 1,10-phenanthroline. J. Therm. Anal. Calorim. 2015, 124, 547–562. [Google Scholar] [CrossRef]
- Katsarou, M.E.; Efthimiadou, E.K.; Psomas, G.; Karaliota, A.; Vourloumis, D. Novel Copper(II) Complex of N-Propyl-norfloxacin and 1,10-Phenanthroline with Enhanced Antileukemic and DNA Nuclease Activities. J. Med. Chem. 2008, 51, 470–478. [Google Scholar] [CrossRef]
- Chen, M.-Z.; Zhou, C.-Q.; Lin, W.-E.; Chen, J.-X.; Chen, W.-H.; Jiang, Z.-H. Synthesis, Crystal Structures and DNA-Cleaving Activities of [Cemp]2[MCl4] (Cemp=N-Carbethoxymethyl-1,10-phenanthrolinium, M = CuII, ZnII, CoII, NiII and MnII). Chem. Pharm. Bull. 2013, 61, 714–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kharadi, G.J. Molar conductance, magnetic susceptibility, mass spectra, and thermal decomposition studies on Cu(II) compounds with substituted terpyridines and clioquinol drug. J. Therm. Anal. Calorim. 2014, 117, 333–341. [Google Scholar] [CrossRef]
- Bellér, G.; Lente, G.; Fábián, I. Central Role of Phenanthroline Mono-N-oxide in the Decomposition Reactions of Tris(1,10-phenanthroline)iron(II) and -iron(III) Complexes. Inorg. Chem. 2010, 49, 3968–3970. [Google Scholar] [CrossRef]
- Singh, C.P.; Singh, A.; Nibha; Daniliuc, C.G.; Kumar, B.; Singh, G. Preparation, crystal structure and thermal studies of cadmium perchlorate complex with 2,2′-bipyridine. J. Therm. Anal. Calorim. 2015, 121, 633–640. [Google Scholar] [CrossRef]
- El-Deen, I.; Shoair, A.; El-Bindary, M. Synthesis, characterization and biological properties of oxovanadium(IV) complexes. J. Mol. Struct. 2019, 1180, 420–437. [Google Scholar] [CrossRef]
- Sahyon, H.; El-Bindary, A.; Shoair, A.; Abdellatif, A. Synthesis and characterization of ruthenium(III) complex containing 2-aminomethyl benzimidazole, and its anticancer activity of in vitro and in vivo models. J. Mol. Liq. 2018, 255, 122–134. [Google Scholar] [CrossRef]
- Shoair, A.; El-Bindary, A.; El-Ghamaz, N.; Rezk, G. Synthesis, characterization, DNA binding and antitumor activities of Cu(II) complexes. J. Mol. Liq. 2018, 269, 619–638. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Hamid, S.M.A.; Mohamed, A.A.; Zordok, W.A.; El-Sayed, H.A. Spectroscopic characterization, thermogravimetry, density functional theory and biological studies of some mixed-ligand complexes of meloxicam and 2,2′-bipyridine with some transition metals. Appl. Organomet. Chem. 2019, 33, e4889. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological Investigations and Spectroscopic Studies of New Moxifloxacin/Glycine-Metal Complexes. Chem. Biodivers. 2019, 16, e1800633. [Google Scholar] [CrossRef]
- Abd El-Hamid, S.M.; Sadeek, S.A.; Zordok, W.A.; El-Shwiniy, W.H. Synthesis, spectroscopic studies, DFT calculations, cytotoxicity and antimicrobial activity of some metal complexes with ofloxacin and 2,2′-bipyridine. J. Mol. Struct. 2019, 1176, 422–433. [Google Scholar] [CrossRef]
- El-Hamid, S.M.A.; Sadeek, S.A.; Mohammed, S.F.; Ahmed, F.M.; El-Gedamy, M.S. N2O2-chelate metal complexes with Schiff base ligand: Synthesis, characterisation and contribution as a promising antiviral agent against human cytomegalovirus. Appl. Organomet. Chem. 2023, 37, e6958. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Co-ord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- El-Shwiniy, W.; Sadeek, S. Synthesis and characterization of new 2-cyano-2-(p-tolyl-hydrazono)-thioacetamide metal complexes and a study on their antimicrobial activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zordok, W.; Sadeek, S. Synthesis, spectroscopic characterization, biological studies and DFT calculations on some transition metal complexes of NO donor ligand. J. Mol. Struct. 2018, 1158, 205–220. [Google Scholar] [CrossRef]
- Mayr, A.; Mao, L.F. Cyanoisocyanoarene Metal Complexes as Building Blocks for Coordination Polymers: Structural Nonrigidity of a Metal−Nitrile Linkage1. Inorg. Chem. 1998, 37, 5776–5780. [Google Scholar] [CrossRef]
- Cordiner, R.L.; Jove´, D.A.; Roberts, R.L.; Farmer, J.D.; Puschmann, H.; Corcoran, D.; Goeta, A.E.; Howard, J.A.K.; Low, P.J. Syntheses and molecular structures of group 8 benzonitrile complexes. J. Organomet. Chem. 2005, 690, 4908–4919. [Google Scholar] [CrossRef]
- Li, Y.; Chai, Y.; Yuan, R.; Liang, W. Synthesis and application of a new copper(II) complex containing oflx and leof. Russ. J. Inorg. Chem. 2008, 53, 704–706. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; p. 230. [Google Scholar]
- Mohamed, A.A.; Nassr, A.A.; Sadeek, S.A.; Elshafie, H.S. Synthesis and Spectral, Thermal and Antimicrobial Investigation of Mixed Ligand Metal Complexes of N-Salicylidene Aniline and 1,10-Phenanthroline. Compounds 2023, 3, 298–309. [Google Scholar] [CrossRef]
- Kamal, H.M.; El-Sayed, H.A.; Sadeek, S.A.; Zordok, W.A.-A.; El-Attar, M.S. Spectroscopic characterization, DFT modeling and antimicrobial studies of some novel nanoparticles mixed ligand complexes of NS bidentate ligand in presence of 2,2′-bipyridine. J. Mol. Liq. 2023, 376, 121404. [Google Scholar] [CrossRef]
- Almond, M.J.; Redman, H.; Rice, D.A. Growth of thin layers of metal sulfides by chemical vapour deposition using dual source and single source precursors: Routes to Cr2S3, α-MnS and FeS. J. Mater. Chem. 2000, 10, 2842–2846. [Google Scholar] [CrossRef]
- Yousef, T.; Abu El-Reash, G.; Rakha, T.; El-Ayaan, U. First row transition metal complexes of (E)-2-(2-(2-hydroxybenzylidene) hydrazinyl)-2-oxo-N-phenylacetamide complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Lach, J.; Loose, C.; Hahn, T.; Kersting, B.; Kortus, J. Binuclear nickel complexes with an edge sharing bis(square-pyramidal) N3Ni(μ-S2)NiN3core: Synthesis, characterization, crystal structure and magnetic properties. Dalton Trans. 2013, 42, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Sathyadevi, P.; Krishnamoorthy, P.; Jayanthi, E.; Butorac, R.R.; Cowley, A.H.; Dharmaraj, N. Studies on the effect of metal ions of hydrazone complexes on interaction with nucleic acids, bovine serum albumin and antioxidant properties. Inorganica Chim. Acta 2012, 384, 83–96. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biochemical Characterization of New Gemifloxacin Schiff Base (GMFX-o-phdn) Metal Complexes and Evaluation of Their Antimicrobial Activity against Some Phyto- or Human Pathogens. Int. J. Mol. Sci. 2022, 23, 2110. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Rahmouni, N.T.; Bensiradj, N.E.H.; Megatli, S.A.; Djebbar, S.; Baitich, O.B. New mixed amino acids complexes of iron(III) and zinc(II) with isonitrosoacetophenone: Synthesis, spectral characterization, DFT study and anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 235–248. [Google Scholar] [CrossRef]
- Moore, J.W.; Pearson, R.G. Kinetic and Mechanism; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Ilhan, S.; Temel, H.; Yilmaz, I.; Şekerci, M. Synthesis and characterization of new macrocyclic Schiff base derived from 2,6-diaminopyridine and 1,7-bis(2-formylphenyl)-1,4,7-trioxaheptane and its Cu(II), Ni(II), Pb(II), Co(III) and La(III) complexes. Polyhedron 2007, 26, 2795–2802. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Ibrahim, H.H.; Al-Tamimi, A.A. Antioxidant, antimicrobial, and theoretical studies of the thiosemicarbazone derivative Schiff base 2-(2-imino-1-methylimidazolidin-4-ylidene)hydrazinecarbothioamide (IMHC). Org. Med. Chem. Lett. 2012, 2, 4. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.; Hussain, I.; Gul, S.; Iqbal, M.; Rahman, I.U.; Khuda, F. Spectral, XRD, SEM and biological properties of new mononuclear Schiff base transition metal complexes. Inorg. Chem. Commun. 2013, 35, 104–109. [Google Scholar] [CrossRef]
- El-Bindary, A.; El-Sonbati, A.; Diab, M.; Morgan, S. Geometrical structure, potentiometric and thermodynamic studies of rhodanine azodye and its metal complexes. J. Mol. Liq. 2014, 201, 36–42. [Google Scholar] [CrossRef]
- Abu-Eittah, R.H.; Zordok, W.A. A molecular orbital treatment of piroxicam and its M2+-complexes: The change of the drug configuration in a time of bond formation. J. Mol. Struct. THEOCHEM 2010, 951, 14–20. [Google Scholar] [CrossRef]
- Defazio, S.; Cini, R. Synthesis, X-ray structure and molecular modelling analysis of cobalt(ii), nickel(ii), zinc(ii) and cadmium(ii) complexes of the widely used anti-inflammatory drug meloxicam. J. Chem. Soc. Dalton Trans. 2002, 9, 1888–1897. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Q.; Fan, H.; Chen, J.; Sun, Z.; Li, T.; Liu, X.; Zhang, G.; Xu, D.; Liu, W. Preparation, crystal structure, spectrographic characterization, thermal and third order nonlinear optical properties of benzyltriethylamine bis(2-thioxo-1,3-dithiole-4,5-dithiolato)nickel(III). J. Cryst. Growth 2010, 312, 2206–2214. [Google Scholar] [CrossRef]
- Altürk, S.; Avcı, D.; Başoğlu, A.; Tamer, Ö.; Atalay, Y.; Dege, N. Copper(II) complex with 6-methylpyridine-2-carboxyclic acid: Experimental and computational study on the XRD, FT-IR and UV–Vis spectra, refractive index, band gap and NLO parameters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 220–230. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shindia, A.A.; Zeid, A.A.A.; Yassin, A.M.; Sitohy, M.Z.; Sitohy, B. Aspergillus nidulans thermostable arginine deiminase-Dextran conjugates with enhanced molecular stability, proteolytic resistance, pharmacokinetic properties and anticancer activity. Enzym. Microb. Technol. 2019, 131, 109432. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Abdel-Azeim, S.; Ibrahim, H.M.; Yassin, M.A.; Abdel-Ghany, S.E.; Esener, S.; Ali, G.S. Biochemical stability and molecular dynamic characterization of Aspergillus fumigatus cystathionine γ-lyase in response to various reaction effectors. Enzym. Microb. Technol. 2015, 81, 31–46. [Google Scholar] [CrossRef]
- Zhao, P.; Shangguan, R.; Wang, H.; Qing, Y.; Jian, F. Synthesis, characterization, crystal structure and ab initio studies on 5-ethoxycarbonly-6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 61–67. [Google Scholar] [CrossRef]
- Andruh, M.; Melanson, R.; Stager, C.V.; Rochon, F.D. [Cr(bipy)(C2O4)2]−—A versatile building block for the design of heteropolymetallic systems 2. Syntheses and crystal structures of CuCr2(bipy)2(μ-C2O4)4(C2O4)4(H2O)2·112H2O and (AgCr(bipy)(μ-C2O4)2(H2O)2)2 and magnetic properties of the copper(II) derivative. Inorganica Chim. Acta 1996, 251, 309–317. [Google Scholar] [CrossRef]
- Anderson, R.A.; Cheng, N.; A. Bryden, N.; Polansky, M.M.; Cheng, N.; Chi, J.; Feng, J. Elevated Intakes of Supplemental Chromium Improve Glucose and Insulin Variables in Individuals with Type 2 Diabetes. Diabetes 1997, 46, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.; Denney, R.C.; Jeffery, G.H.; Mendham, J. Vogel’s Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis, 4th ed.; Longman Group: London, UK, 1978. [Google Scholar]
- Lewis, J.; Wilkins, R.G. Modern Coordination Chemistry; Interscience: New York, NY, USA, 1960; pp. 403–406. [Google Scholar]
- El-Sayed, H.A.; Said, S.A.; El-Hamid, A.A.; Mohamed, A.S.A.; Amr, A.E.; Morsy, H.A. Functionalization of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate: Facile Synthesis of a New Series of Pyrano[2,3-d]pyrimidine Derivatives. Russ. J. Gen. Chem. 2021, 91, 1403–1408. [Google Scholar] [CrossRef]
- Lalhruaizela; Marak, B.N.; Sran, B.S.; Singh, V.P. Multicomponent Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking of 4H-Pyrans. Chemistryselect 2021, 6, 11249–11260. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C.L. Identification of Hemolysin BL-Producing Bacillus cereus Isolates by a Discontinuous Hemolytic Pattern in Blood Agar. Appl. Environ. Microb. 1994, 60, 1646–1651. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An Attempt of Biocontrol the Tomato-Wilt Disease Caused by Verticillium dahliae Using Burkholderia gladioli pv. agaricicola and Its Bioactive Secondary Metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.; Camele, I.; Bufo, S.A. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. Boll. Soc. Ital. Biol. Sper. 2017, 90, 96–103. [Google Scholar] [CrossRef]
- Casey, J.; O’Cleirigh, C.; Walsh, P.; O’Shea, D. Development of a robust microtiter plate-based assay method for assessment of bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef]
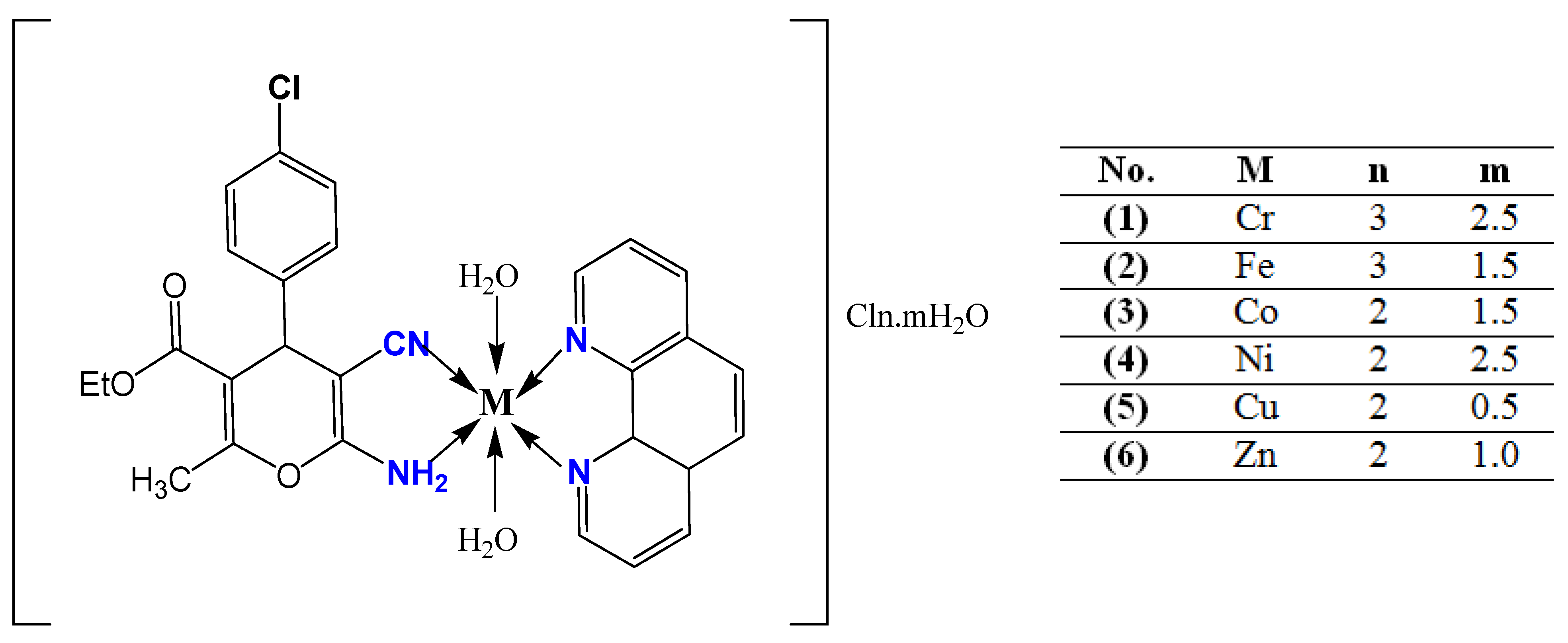

| Compounds M.Wt. (M.F.) and Cord. F. | Color Yield (%) | M.P. (°C) | Calc. (Found) (%) | Λ Ω cm2 mol−1 | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | M | Cl | ||||
| L 318.50 (C16H18ClN2O3) | Yellow 88.00 | 165 | 60.28 (60.01) | 4.71 (4.57) | 8.79 (8.68) | - | 11.14 (10.99) | 6.28 |
| Phen 198.20 (C12H10N2O) | White - | 100 | 72.61 (72.35) | 5.00 (4.89) | 14.05 (13.98) | - | - | 5.00 |
| (1) 737.99 (CrC28H32N4O7.5Cl4) [Cr(L)(Phen)(H2O)2] Cl3.2.5H2O | Faint Green 85.43 | 200 | 45.53 (45.22) | 4.34 (4.22) | 7.59 (7.37) | 7.04 (6.87) | 19.24 (19.01) | 198.65 |
| (2) 723.80 (FeC28H30N4O6.5Cl4) [Fe(L)(Phen)(H2O)2]Cl3.1.5H2O | Dark Red 74.17 | 95 | 46.42 (46.32) | 4.14 (4.05) | 7.73 (7.52) | 7.70 (7.46) | 19.61 (19.23) | 191.23 |
| (3) 691.50 (CoC28H30N4O6.5Cl3) [Co(L)(Phen)(H2O)2]Cl2.1.5H2O | Faint Green 78.33 | 210 | 48.59 (48.19) | 4.34 (4.24) | 8.10 (7.99) | 8.52 (8.22) | 15.40 (15.13) | 142.35 |
| (4) 709.20 (NiC28H32N4O7.5Cl3) [Ni(L)(Phen)(H2O)2]Cl2.2.5H2O | Pale Green 78.96 | 280 Decomposed | 47.38 (47.19) | 4.51 (4.43) | 7.90 (7.67) | 8.28 (8.11) | 15.02 (14.87) | 137.58 |
| (5) 678.00 (CuC28H28N4O5.5Cl3) [Cu(L)(Phen)(H2O)2]Cl2.0.5H2O | Green 76.69 | 180 | 49.56 (49.44) | 4.13 (3.99) | 8.26 (8.02) | 9.37 (9.13) | 15.71 (15.44) | 125.94 |
| (6) 688.90 (ZnC28H29N4O6Cl3) [Zn(L)(Phen)(H2O)2]Cl2.H2O | Buff 80.09 | 105 | 48.77 (48.34) | 4.21 (4.04) | 8.13 (8.01) | 9.51 (9.29) | 15.46 (15.25) | 128.32 |
| Compound | λmax (nm) | ν (cm−1) | Peak Assignment | ε (M−1cm−1) | 10Dq | CFSE | μeff (B.M) | |
|---|---|---|---|---|---|---|---|---|
| cm−1 | kJ/mol | |||||||
| L | 284 | 35,211 | π→π * | 12 | - | - | - | - |
| 343 | 29,154 | n→π * | 104 | |||||
| Phen | 275 | 36,364 | π→π * | 27 | - | - | - | - |
| 355 | 28,169 | n→π * | 235 | |||||
| (1) | 255, 305 | 39,215, 32,786 | π→π * | 42, 30 | ||||
| 354 | 28,248 | n→π * | 15 | |||||
| 475, 490 | 21,052, 20,408 | LMCT | 358, 270 | |||||
| 525 | 19,047 | d-d transition | 125 | 19,047 | 227.8 | −273.36 | 3.32 | |
| (2) | 250, 280 | 40,000, 35,714 | π→π * | 45, 100 | ||||
| 345 | 28,985 | n→π * | 55 | |||||
| 485, 570 | 20,618, 17,543 | LMCT | 600, 1341 | |||||
| 722 | 13,850 | d-d transition | 24 | 13,850 | 165.6 | 0.00 | 5.90 | |
| (3) | 290 | 34,482 | π→π* | 90 | ||||
| 350 | 28,571 | n→π* | 53 | |||||
| 465, 490 | 21,505, 20,408 | LMCT | 47, 285 | |||||
| 620 | 16,129 | d-d transition | 478 | 16,129 | 192.9 | −154.3 + 2p | 4.22 | |
| (4) | 250, 290 | 40,000, 34,482 | π→π * | 38, 40 | ||||
| 320 | 31,250 | n→π * | 20 | |||||
| 460, 490 | 21,739, 20,408 | LMCT | 442, 457 | |||||
| 650 | 15,384 | d-d transition | 43 | 15,384 | 184.0 | 220.8 + 3p | 3.05 | |
| (5) | 255 | 39,215 | π→π * | 34 | ||||
| 300, 330 | 33,333, 30,303 | n→π * | 25, 18 | |||||
| 460, 490 | 21,739, 20,408 | LMCT | 442, 445 | |||||
| 615 | 16,260 | d-d transition | 56 | 16,260 | 194.5 | 116.7 + 4p | 1.76 | |
| (6) | 255 | 39,215 | π→π * | 53 | ||||
| 310, 350 | 32,258, 28,571 | n→π * | 60, 30 | |||||
| 420, 470, 490 | 23,809, 21,276, 20,408 | LMCT | 66, 388, 299 | - | - | - | - | |
| Compounds | Decomposition Range (K) | Ts (K) | Method | Parameter | R a | SD b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | A (s−1) | ΔS* (kJ/mol·K) | ΔH* (kJ/mol) | ΔG* (kJ/mol) | ||||||
| L | 436–540 | 512 | CR HM | 185.56 157.18 | 5.08 × 1018 7.81 × 1015 | 0.0746 0.0208 | 181.30 152.92 | 143.07 142.27 | 0.981 0.970 | 0.034 0.036 |
| Phen | 394–572 | 551 | CR HM | 117.83 146.78 | 2.03 × 109 7.97 × 1011 | −0.071 −0.022 | 113.250 142.210 | 152.84 154.42 | 0.996 0.998 | 0.120 0.076 |
| (1) | 313–683 | 529 | CR HM | 35.11 47.13 | 5.72 × 102 2.11 × 1010 | −0.2309 −0.0860 | 30.71 42.73 | 152.87 88.24 | 0.948 0.951 | 0.105 0.097 |
| (2) | 310–690 | 656 | CR HM | 27.96 53.91 | 17.00 2.94 × 103 | −0.2619 −0.2190 | 22.51 48.46 | 194.32 192.17 | 0.909 0.883 | 0.102 0.198 |
| (3) | 292–617 | 198 | CR HM | 44.16 8.53 | 6.03 × 104 7.18 × 1010 | −0.1840 −0.0336 | 42.51 6.89 | 78.94 13.56 | 0.976 0.968 | 0.043 0.057 |
| (4) | 313–646 | 457 | CR HM | 30.38 40.49 | 1.89 × 102 9.87 × 103 | −0.2389 −0.2060 | 26.59 36.69 | 135.76 130.84 | 0.955 0.948 | 0.051 0.094 |
| (5) | 292–650 | 455 | CR HM | 48.70 38.55 | 2.33 × 104 5.95 × 103 | −0.1988 −0.2101 | 44.91 34.77 | 135.39 130.40 | 0.842 0.758 | 0.265 0.407 |
| (6) | 332–602 | 437 | CR HM | 42.50 35.10 | 4.88 × 103 3.45 × 103 | −0.2115 −0.2143 | 38.86 31.47 | 131.29 125.15 | 0.947 0.979 | 0.036 0.092 |
| Compounds | 2θ (°) | d (A°) | FWHM | Cs (nm) | D × 10−4 (nm−2) | ε × 10−2 (rad) |
|---|---|---|---|---|---|---|
| L | 17.99 | 4.95 | 0.1771 | 45.41 | 4.84 | 27.97 |
| Phen | 19.87 | 4.47 | 0.2170 | 38.93 | 7.24 | 30.97 |
| (1) | 21.90 | 4.07 | 0.0984 | 82.22 | 1.47 | 12.71 |
| (2) | 22.33 | 4.00 | 0.2558 | 31.65 | 9.98 | 32.40 |
| (3) | 28.54 | 3.14 | 0.1574 | 52.07 | 3.68 | 15.47 |
| (4) | 24.34 | 3.67 | 0.1378 | 58.97 | 2.87 | 15.97 |
| (5) | 27.66 | 3.24 | 0.2168 | 37.78 | 7.00 | 22.02 |
| (6) | 31.66 | 2.84 | 0.1771 | 46.62 | 4.60 | 15.62 |
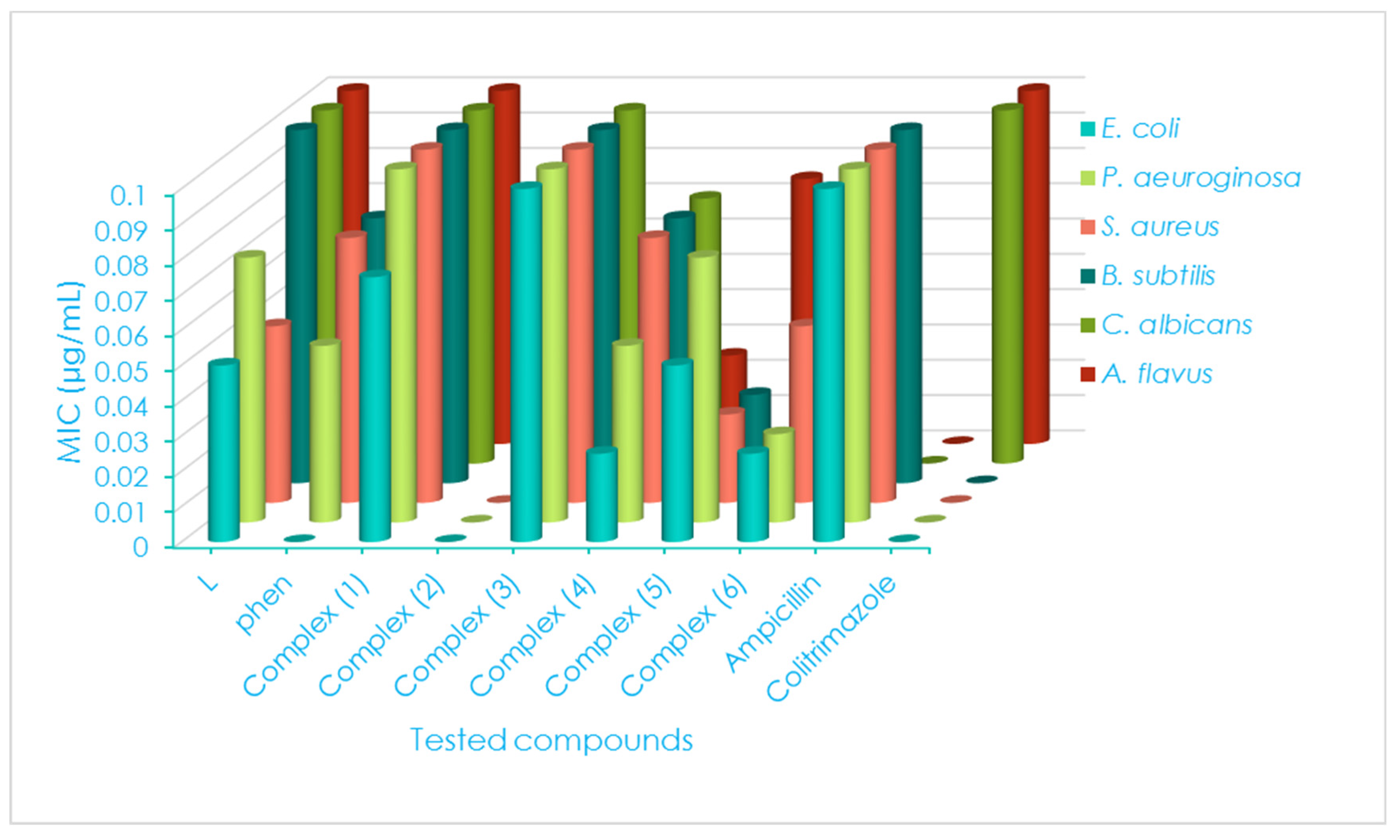
| Compounds | G(+ve) and G(−ve) Bacterial Strains | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | ||||||||||
| D.iz a (mm) | AI b (%) | MIC c (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | ||
| L | 9 ± 0.22 | 37.5 | 0.050 | 13 ± 0.05 | 56.5 | 0.100 | 8 ± 0.01 | 32.0 | 0.050 | 10 ± 0.33 | 43.5 | 0.075 | |
| Phen | 7NS ± 0.55 | 29.2 | 0.075 | 9NS ± 0.11 | 39.1 | 0.075 | ND | ---- | ---- | 4NS ± 0.11 | 17.4 | 0.050 | |
| (1) | 11+1 ± 0.08 | 45.8 | 0.100 | 15+1 ± 0.88 | 65.2 | 0.100 | 10NS ± 0.11 | 40.0 | 0.075 | 13+1 ± 0.05 | 56.5 | 0.100 | |
| (2) | ND | ---- | ---- | 3NS ± 0.60 | 13.0 | 0.025 | ND | ---- | ---- | ND | ---- | ---- | |
| (3) | 14+2 ± 0.06 | 58.3 | 0.100 | 17+2 ± 0.01 | 73.9 | 0.100 | 11+1 ± 0.09 | 44.0 | 0.100 | 16+2 ± 0.60 | 69.6 | 0.100 | |
| (4) | 9NS ± 0.44 | 37.5 | 0.075 | 11NS ± 0.03 | 47.8 | 0.075 | 8NS ± 0.77 | 32.0 | 0.025 | 9NS ± 0.09 | 39.1 | 0.050 | |
| (5) | 5NS ± 0.33 | 20.8 | 0.025 | 8NS ± 0.22 | 34.8 | 0.025 | 6NS ± 0.22 | 24.0 | 0.050 | 9NS ± 0.33 | 39.1 | 0.075 | |
| (6) | 4NS ± 0.07 | 16.7 | 0.050 | 6NS ± 0.09 | 26.1 | 0.050 | 3NS ± 0.01 | 12.0 | 0.025 | 5NS ± 0.77 | 21.7 | 0.025 | |
| Standards | Ampicillin | 24 ± 0.02 | 100 | 0.100 | 23 ± 0.07 | 100 | 0.100 | 23 ± 0.22 | 100 | 0.100 | 23 ± 0.22 | 100 | 0.100 |
| Clotrimazole | ND | ---- | ---- | ND | ---- | ---- | ND | ---- | ---- | ND | ---- | ---- | |
| Compounds | Fungal Strains | ||||||||||||
| C. albicans | A. flavus | ||||||||||||
| D.iz (mm) | AI (%) | MIC (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | ||||||||
| L | 12 ± 0.14 | 44.4 | 0.100 | 14 ± 0.16 | 56.0 | 0.100 | |||||||
| Phen | 10NS ± 0.11 | 37.0 | 0.075 | 12NS ± 0.11 | 48.0 | 0.050 | |||||||
| (1) | 16+1 ± 0.22 | 59.2 | 0.100 | 18+1 ± 0.09 | 72.0 | 0.100 | |||||||
| (2) | ND | ---- | ---- | ND | ---- | ---- | |||||||
| (3) | 15+1 ± 0.55 | 55.5 | 0.100 | 16+1 ± 0.8 | 64.0 | 0.050 | |||||||
| (4) | 7+1 ± 0.09 | 25.9 | 0.075 | 9NS ± 0.02 | 36.0 | 0.025 | |||||||
| (5) | ND | ---- | ---- | 7NS ± 0.11 | 28.0 | 0.075 | |||||||
| (6) | 5+1 ± 0.22 | 18.5 | 0.050 | 9NS ± 0.70 | 36.0 | 0.050 | |||||||
| Standards | Ampicillin | ND | ---- | ---- | ---- | ---- | ---- | ||||||
| Clotrimazole | 27 ± 0.11 | 100 | 0.100 | 25 ± 0.02 | 100 | 0.100 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Attar, M.S.; Sadeek, S.A.; El-Sayed, H.A.; Kamal, H.M.; Elshafie, H.S. Structural and Antimicrobial Investigation of Some New Nanoparticles Mixed Ligands Metal Complexes of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate in Presence of 1,10-Phenanthroline. Inorganics 2023, 11, 220. https://doi.org/10.3390/inorganics11050220
El-Attar MS, Sadeek SA, El-Sayed HA, Kamal HM, Elshafie HS. Structural and Antimicrobial Investigation of Some New Nanoparticles Mixed Ligands Metal Complexes of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate in Presence of 1,10-Phenanthroline. Inorganics. 2023; 11(5):220. https://doi.org/10.3390/inorganics11050220
Chicago/Turabian StyleEl-Attar, Mohamed S., Sadeek A. Sadeek, Hassan A. El-Sayed, Heba M. Kamal, and Hazem S. Elshafie. 2023. "Structural and Antimicrobial Investigation of Some New Nanoparticles Mixed Ligands Metal Complexes of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate in Presence of 1,10-Phenanthroline" Inorganics 11, no. 5: 220. https://doi.org/10.3390/inorganics11050220
APA StyleEl-Attar, M. S., Sadeek, S. A., El-Sayed, H. A., Kamal, H. M., & Elshafie, H. S. (2023). Structural and Antimicrobial Investigation of Some New Nanoparticles Mixed Ligands Metal Complexes of Ethyl 6-Amino-4-(4-chlorophenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate in Presence of 1,10-Phenanthroline. Inorganics, 11(5), 220. https://doi.org/10.3390/inorganics11050220