Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrogen Storage Properties of LiBH4-Doped MgH2
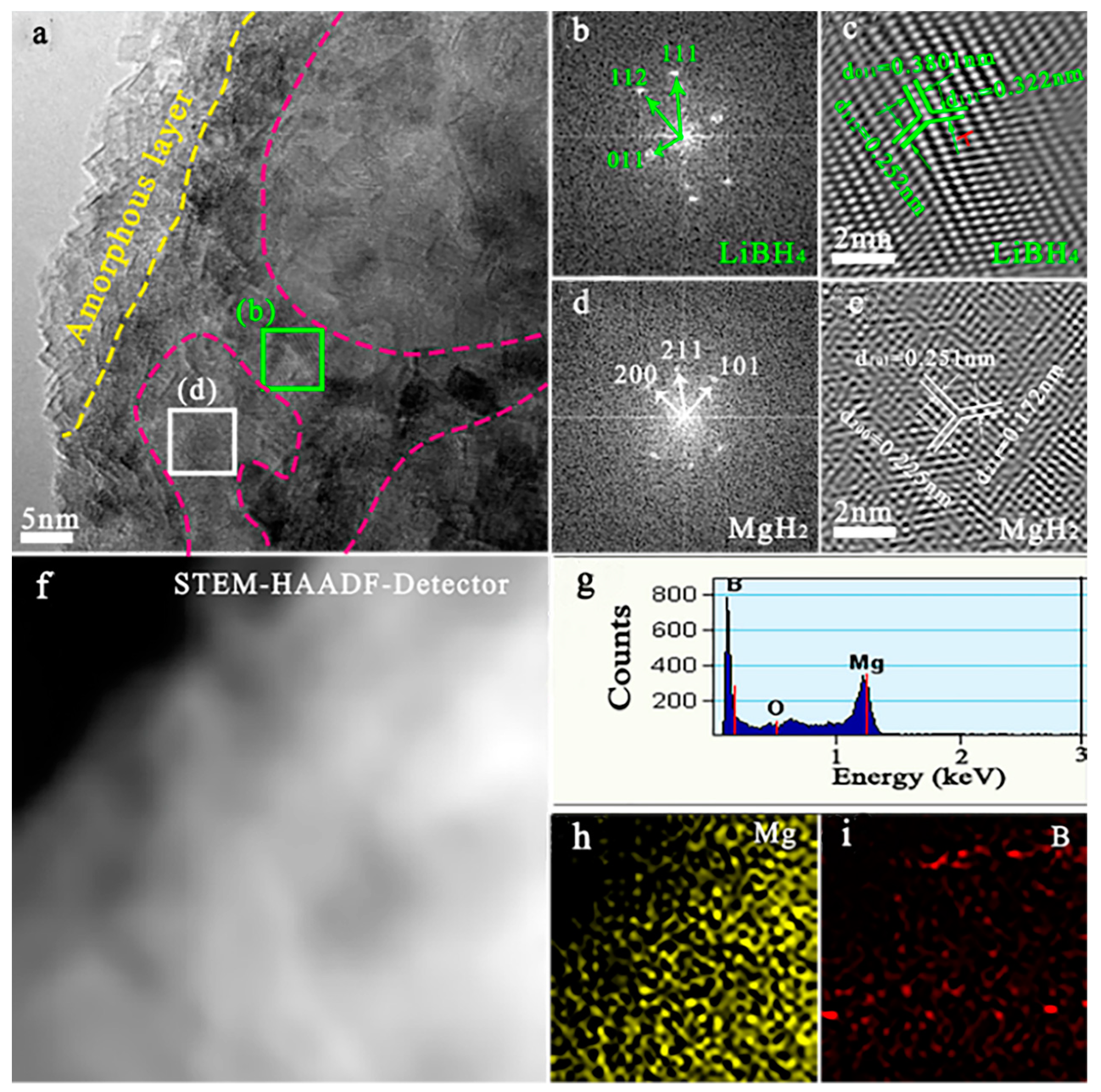
2.2. Structural Features of LiBH4-Doped MgH2
2.3. Electrochemical Analysis of LiBH4-Doped MgH2
3. Experimental Procedure
3.1. Material Preparation
3.2. Material Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fang, M.; Si, T.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Phase stability, structural transition, and hydrogen absorption-desorption features of the polymorphic La4MgNi19 compound. J. Phys. Chem. C 2010, 114, 11686–11692. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Fang, F.; Sun, D.L.; Zhang, Q. Hydrogen-induced magnesium–zirconium interfacial coupling: Enabling fast hydrogen sorption at lower temperatures. J. Mater. Chem. A 2017, 5, 5067–5076. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, C.; Fang, C.; Fang, F.; Sun, D.L.; Ouyang, L.; Zhu, M. Synthesis, Crystal Structure, and Thermal Decomposition of Strontium Amidoborane. J. Phys. Chem. C 2010, 114, 1709–1714. [Google Scholar] [CrossRef]
- Si, T.; Yin, F.; Zhang, X.; Zhang, Q.; Liu, D.; Li, Y. In-situ formation of medium-entropy alloy nanopump to boost hydrogen storage in Mg-based alloy. Scr. Mater. 2023, 222, 115052. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, D.; Wang, Q.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Superior hydrogen storage kinetics of Mg12YNi alloy with a long-period stacking ordered phase. Scr. Mater. 2011, 65, 233–236. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Fang, F.; Sun, D.; Li, X.; Guo, Z.; Yu, X. Oxygen-free Layer-by-Layer Assembly of Lithiated Composites on Graphene for Advanced Hydrogen Storage. Adv. Sci. 2017, 4, 1600257. [Google Scholar] [CrossRef]
- Li, Y.; Ding, X.; Zhang, Q. Self-Printing on Graphitic Nanosheets with Metal Borohydride Nanodots for Hydrogen Storage. Sci. Rep. 2016, 6, 31144. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Fang, M.; Liu, C.; Hu, Q.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. (Nd1.5Mg0.5)Ni7-based compounds: Structural and hydrogen storage properties. Inorg. Chem. 2012, 51, 2976–2983. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, H.; Wang, S.; Jiang, L.; Du, J.; Zhang, J.; Latroche, M.; Cuevas, F. Mechanochemistry and hydrogen storage properties of 2Li3N+Mg mixture. Rare. Met. 2022, 41, 4223–4229. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Lin, H.; Lu, Y.; Zhang, L.; Liu, H.; Edalati, K. Recent advances in metastable alloys for hydrogen storage: A review. Rare. Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, H.; Lu, X.; Song, M.; Wu, F.; Zheng, J.; Yuan, Z.; Zhang, L. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare. Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Yao, Q.; Lu, Z.; Zhang, N.; Xia, J.; Yang, K.; Wang, Y.; Zhang, K.; Liu, H.; et al. 2022 roadmap on hydrogen energy from production to utilizations. Rare. Met. 2022, 41, 3251–3267. [Google Scholar] [CrossRef]
- Lu, C.; Ma, Y.; Li, F.; Zhu, H.; Zeng, X.; Ding, W.; Deng, T.; Wu, J.; Zou, J. Visualization of fast “hydrogen pump” in core–shell nanostructured Mg@Pt through hydrogen-stabilized Mg3PtC. J. Mater. Chem. A 2019, 7, 14629–14637. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, P.; Guo, X.; Zhou, C.; Fang, Z. The effects of crystalline defects on hydrogen absorption kinetics of catalyzed MgH2 at ambient conditions. J. Alloys Compd. 2022, 927, 167090. [Google Scholar] [CrossRef]
- Ouyang, L.; Cao, Z.; Wang, H.; Liu, J.; Sun, D.; Zhang, Q. Dual-tuning effect of In on the thermodynamic and kinetic properties of Mg2Ni dehydrogenation. Int. J. Hydrogen Energy 2013, 38, 8881–8887. [Google Scholar] [CrossRef]
- Conceição, M.; Brum, M.; Santos, D. The effect of V, VCl3 and VC catalysts on the MgH2 hydrogen sorption properties. J. Alloys Compd. 2014, 586, S101–S104. [Google Scholar] [CrossRef]
- Rousselot, S.; Guay, D.; Roue, L. Synthesis of fcc Mg–Ti–H alloys by high energy ball milling: Structure and electrochemical hydrogen storage properties. J. Power Sources 2010, 195, 4370–4373. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Ma, T.; Li, K.; Ye, F.; Wang, X.; Jiao, L.; Yuan, H.; Wang, Y. Facile synthesis of small MgH2 nanoparticles confined in different carbon materials for hydrogen storage. J. Alloys Compd. 2020, 825, 153953. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, S.; Dixit, V.; Shukla, V.; Shahi, R.; Shaz, M.; Srivastava, O.N. Catalytic effect of carbon nanostructures on the hydrogen storage properties of MgH2–NaAlH4 composite. Int. J. Hydrogen Energy 2014, 39, 14240–14246. [Google Scholar] [CrossRef]
- Inoishi, A.; Sato, H.; Chen, Y.; Saito, H.; Sakamoto, R.; Sakaebe, H.; Okada, S. High capacity all-solid-state lithium battery enabled by in situ formation of an ionic conduction path by lithiation of MgH2. RSC Adv. 2022, 12, 10749–10754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Fang, F.; Sun, D.; Zhang, Q.; Wei, S.; Cao, F.; Sun, H.; Ouyang, L.; Zhu, M. Formation of Mg2Ni with enhanced kinetics: Using MgH2 instead of Mg as a starting material. J. Solid State Chem. 2012, 192, 210–214. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Zhang, D.; Fan, Y.; Liu, B. Striking enhanced effect of PrF3 particles on Ti3C2 MXene for hydrogen storage properties of MgH2. J. Alloys Compd. 2022, 914, 165291. [Google Scholar] [CrossRef]
- Kammerer, J.; Duan, X.; Neubrech, F.; Schroder, R.; Liu, N.; Pfannmoller, M. Stabilizing γ-MgH2 at Nanotwins in Mechanically Constrained Nanoparticles. Adv. Mater. 2021, 33, 2008259. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Qiu, N.; Zhang, Y.; Zhao, G.; Xu, L.; Lan, Z.; Guo, J. Cycling hydrogen desorption properties and microstructures of MgH2–AlH3–NbF5 hydrogen storage materials. Rare. Mat. 2021, 40, 1003–1007. [Google Scholar] [CrossRef]
- Si, T.; Zhang, X.; Feng, J.; Ding, X.; Li, Y. Enhancing hydrogen sorption in MgH2 by controlling particle size and contact of Ni catalysts. Rare. Met. 2021, 40, 995–1002. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.; Sohn, H.; Ronnebro, E. Hydrogen Storage Properties of Nanosized MgH2−0.1TiH2 Prepared by Ultrahigh-Energy−High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef]
- Cuan, J.; Zhou, Y.; Zhou, T.; Ling, S.; Rui, K.; Guo, Z.; Liu, H.; Yu, X. Borohydride-Scaffolded Li/Na/Mg Fast Ionic Conductors for Promising Solid-State Electrolytes. Adv. Mater. 2019, 31, 1803533. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Zhang, J.; Zhang, Y.; Xu, F.; Xing, Y.; Li, F.; Zhao, J.; Du, Y.; Hu, W.; et al. Hydrogen storage properties of destabilized MgH2–Li3AlH6 systemInt. J. Hydrog. Energy 2010, 35, 8122–8129. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Li, C.; Gao, M.; Pan, H. In situ formation of lithium fast-ion conductors and improved hydrogen desorption properties of the LiNH2–MgH2 system with the addition of lithium halides. J. Mater. Chem. A 2014, 2, 3155–3162. [Google Scholar] [CrossRef]
- Kato, S.; Borgschulte, A.; Bielmann, M.; Zuttel, A. Interface reactions and stability of a hydride composite (NaBH4 + MgH2). Phys. Chem. Chem. Phys. 2012, 14, 8360–8368. [Google Scholar] [CrossRef] [PubMed]
- Paik, B.; Li, H.; Wang, J.; Akiba, E. A Li–Mg–N–H composite as H2 storage material: A case study with Mg(NH2)2–4LiH–LiNH2. Chem. Commun. 2015, 51, 10018–10021. [Google Scholar] [CrossRef]
- Yan, Y.G.; Grinderslev, J.B.; Lee, Y.; Jorgensen, M.; Cho, Y.; Cerny, R.; Jensen, T. Ammonia-assisted fast Li-ion conductivity in a new hemiammine lithium borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 28, 3971–3974. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Liu, H.; Gao, S.; Yan, M. Improved hydrogen storage properties of LiBH4 confined with activated charcoal by ball milling. Rare Met. 2019, 38, 321–326. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.-K.; He, L.-Q.; Ding, X.-L.; Si, T.-Z.; Cui, P.; Li, H.-W.; Li, Y.-T. Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption. Inorganics 2023, 11, 216. https://doi.org/10.3390/inorganics11050216
Qin Z-K, He L-Q, Ding X-L, Si T-Z, Cui P, Li H-W, Li Y-T. Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption. Inorganics. 2023; 11(5):216. https://doi.org/10.3390/inorganics11050216
Chicago/Turabian StyleQin, Zhi-Kang, Li-Qing He, Xiao-Li Ding, Ting-Zhi Si, Ping Cui, Hai-Wen Li, and Yong-Tao Li. 2023. "Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption" Inorganics 11, no. 5: 216. https://doi.org/10.3390/inorganics11050216
APA StyleQin, Z.-K., He, L.-Q., Ding, X.-L., Si, T.-Z., Cui, P., Li, H.-W., & Li, Y.-T. (2023). Liquid Channels Built-In Solid Magnesium Hydrides for Boosting Hydrogen Sorption. Inorganics, 11(5), 216. https://doi.org/10.3390/inorganics11050216