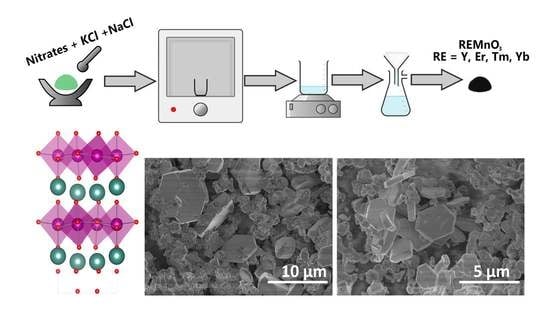
Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb)
Abstract
1. Introduction
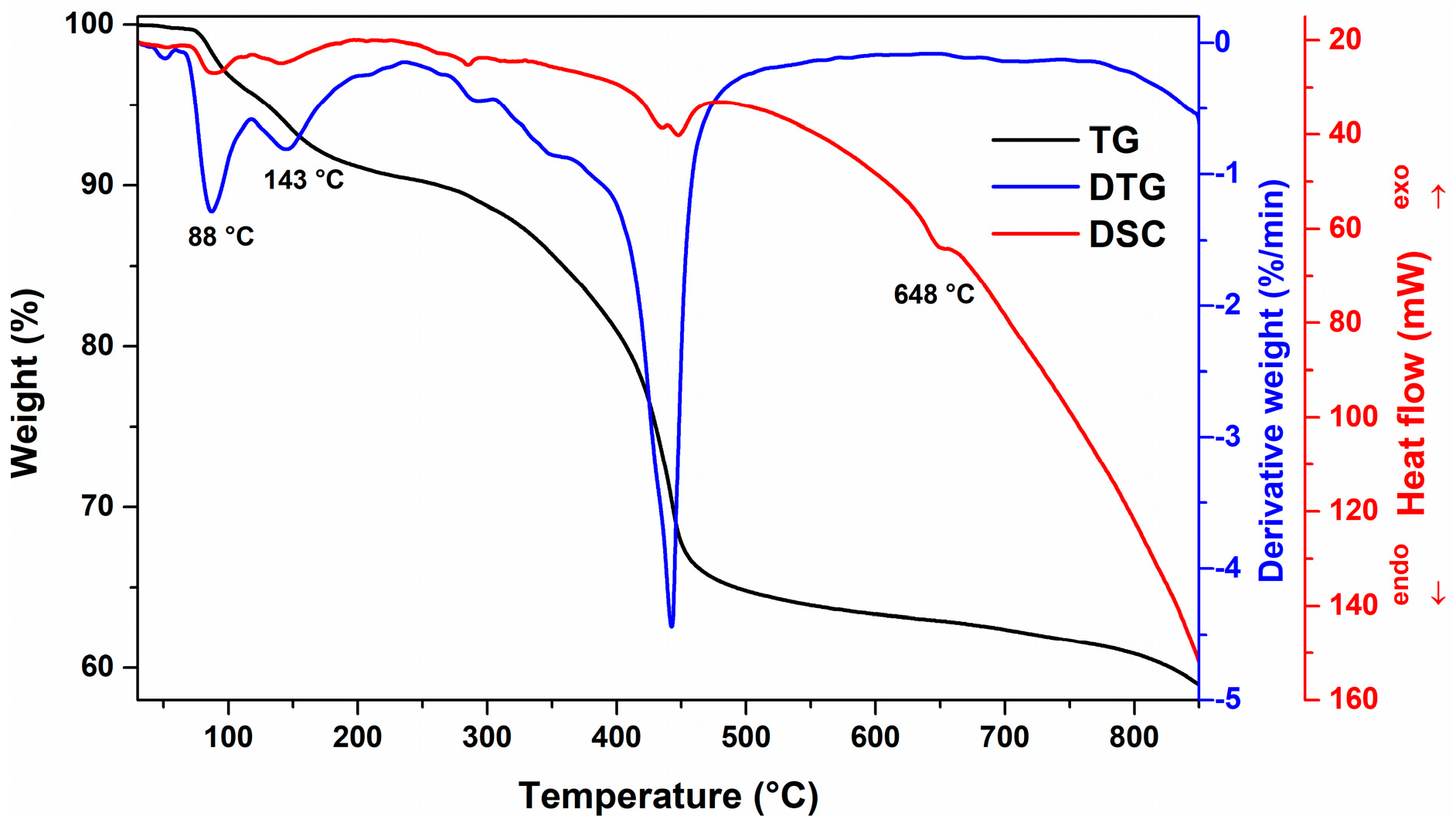
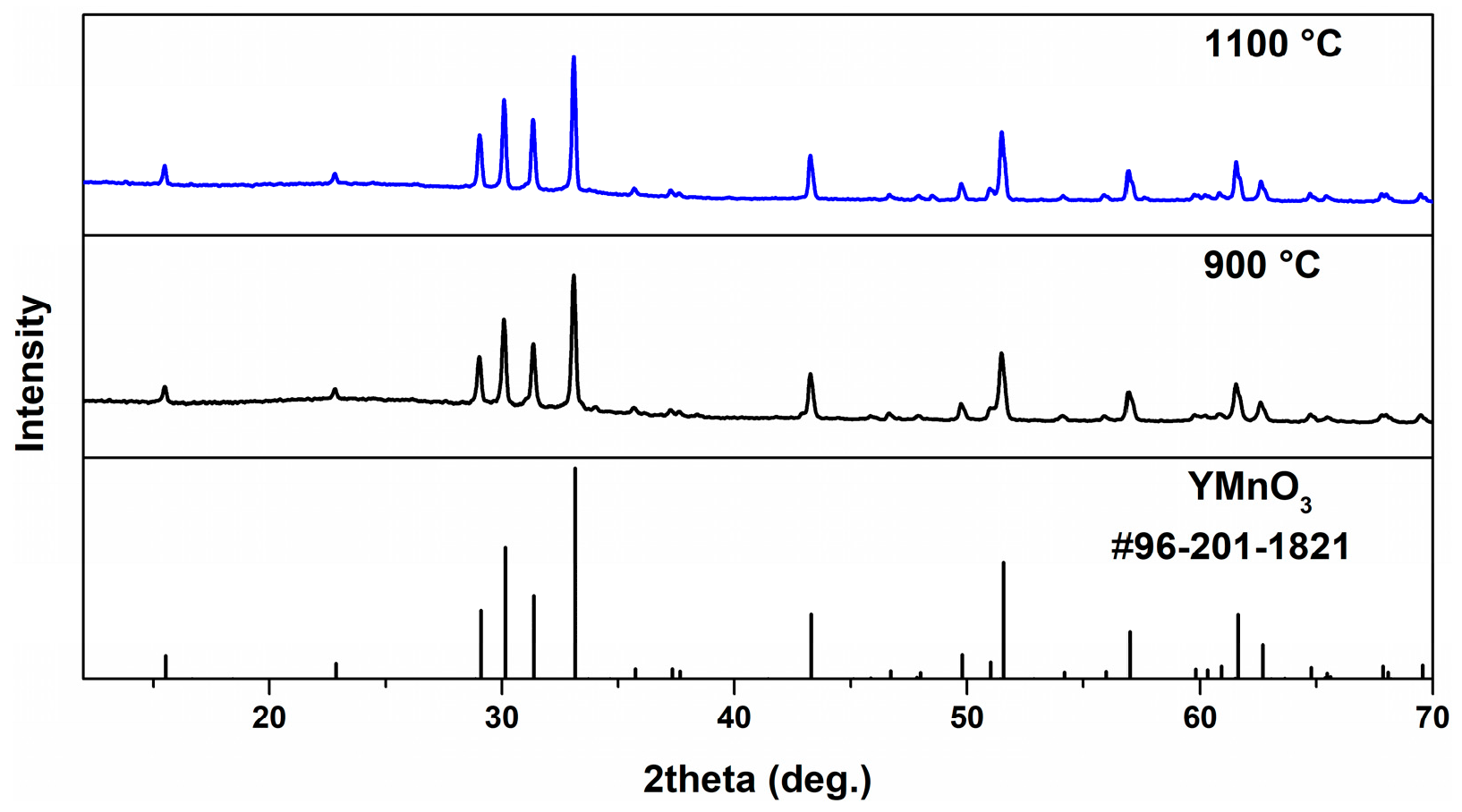
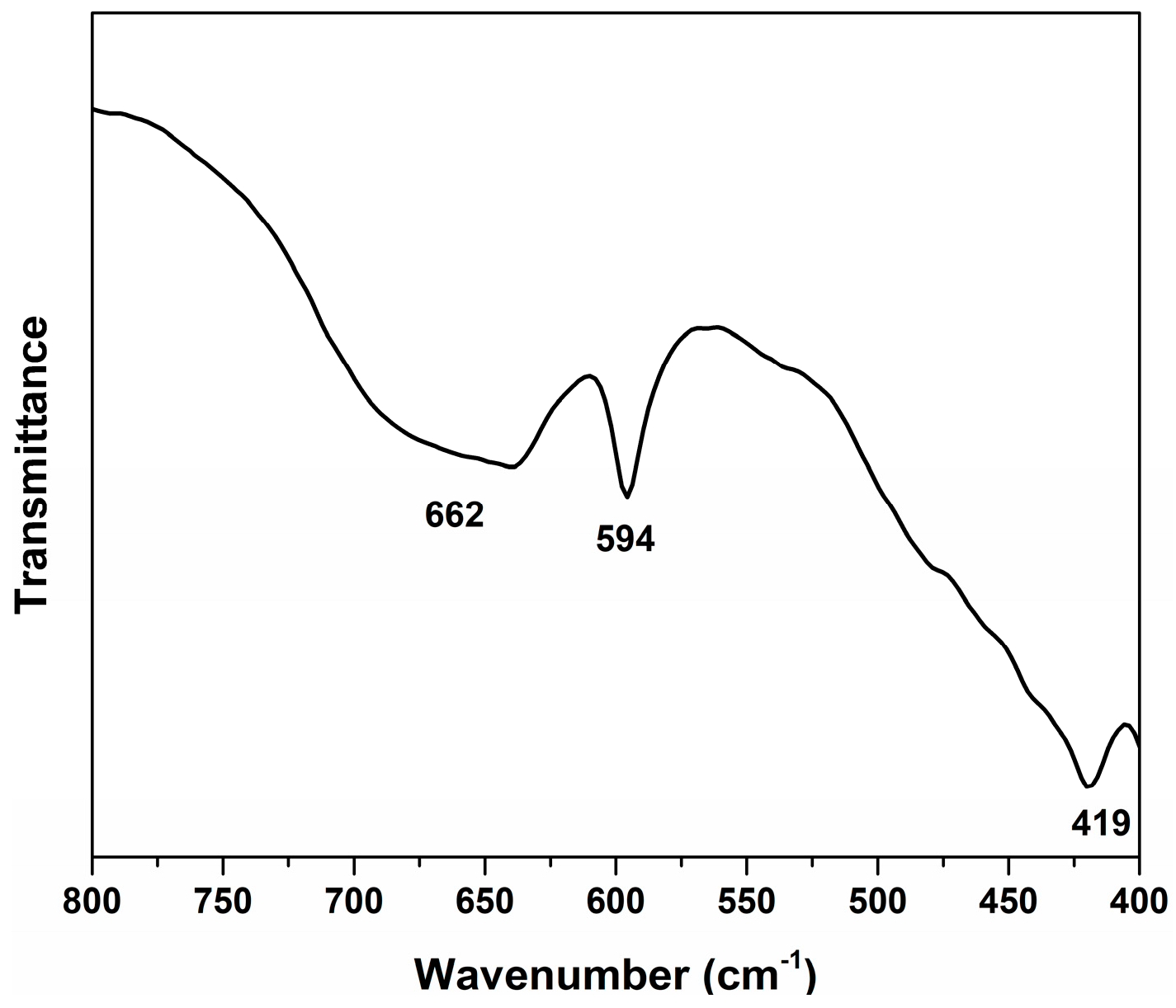
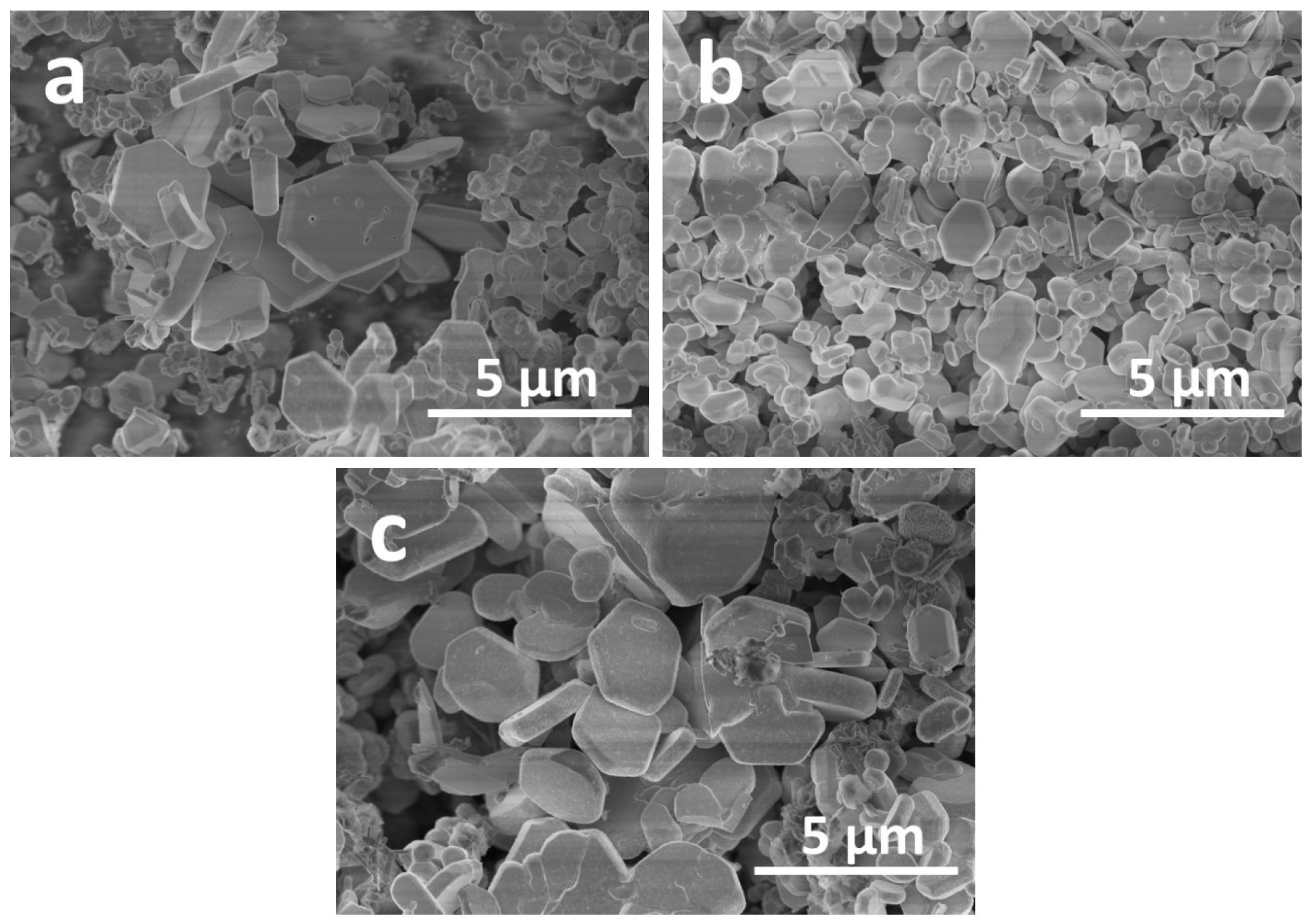
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lilienblum, M.; Lottermoser, T.; Manz, S.; Selbach, S.M.; Cano, A.; Fiebig, M. Ferroelectricity in the Multiferroic Hexagonal Manganites. Nat. Phys. 2015, 11, 1070–1073. [Google Scholar] [CrossRef]
- Das, H.; Wysocki, A.L.; Geng, Y.; Wu, W.; Fennie, C.J. Bulk Magnetoelectricity in the Hexagonal Manganites and Ferrites. Nat. Commun. 2014, 5, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B. Hexagonal Manganites—(RMnO3): Class (I) Multiferroics with Strong Coupling of Magnetism and Ferroelectricity. Int. Sch. Res. Not. 2013, 2013, 497073. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Duan, W. Hexagonal Rare-Earth Manganites and Ferrites: A Review of Improper Ferroelectricity, Magnetoelectric Coupling, and Unusual Domain Walls. Phys. Chem. Chem. Phys. 2020, 22, 14415–14432. [Google Scholar] [CrossRef] [PubMed]
- Manipatruni, S.; Nikonov, D.E.; Lin, C.-C.; Gosavi, T.A.; Liu, H.; Prasad, B.; Huang, Y.-L.; Bonturim, E.; Ramesh, R.; Young, I.A. Scalable Energy-Efficient Magnetoelectric Spin–Orbit Logic. Nature 2019, 565, 35–42. [Google Scholar] [CrossRef]
- Ferson, N.D.; Uhl, A.M.; Andrew, J.S. Piezoelectric and Magnetoelectric Scaffolds for Tissue Regeneration and Biomedicine: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2020, 68, 229–241. [Google Scholar] [CrossRef]
- Arya, G.; Yogiraj, J.; Negi, N.S.; Shah, J.; Kotnala, R.K. Observation of Enhanced Multiferroic, Magnetoelectric and Photocatalytic Properties in Sm-Co Codoped BiFeO3 Nanoparticles. J. Alloys Compd. 2017, 723, 983–994. [Google Scholar] [CrossRef]
- Mansour, S.F.; Imam, N.G.; Goda, S.; Abdo, M.A. Constructive Coupling between BiFeO3 and CoFe2O4; Promising Magnetic and Dielectric Properties. J. Mater. Res. Technol. 2020, 9, 1434–1446. [Google Scholar] [CrossRef]
- Lee, K.; Hajra, S.; Sahu, M.; Kim, H.J. Colossal Dielectric Response, Multiferroic Properties, and Gas Sensing Characteristics of the Rare Earth Orthoferrite LaFeO3 Ceramics. J. Alloys Compd. 2021, 882, 160634. [Google Scholar] [CrossRef]
- Huang, Z.J.; Cao, Y.; Sun, Y.Y.; Xue, Y.Y.; Chu, C.W. Coupling between the Ferroelectric and Antiferromagnetic Orders in YMnO3. Phys. Rev. B 1997, 56, 2623. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, G.; Gu, L.; Yao, Y.; Jin, C.; Wang, Y.; Duan, X.; Yu, R. Direct Observation of Multiferroic Vortex Domains in YMnO3. Sci. Rep. 2013, 3, 2741. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.M.; Lilienblum, M.; Delaney, K.T.; Kumagai, Y.; Fiebig, M.; Spaldin, N.A. Scaling Behavior and beyond Equilibrium in the Hexagonal Manganites. Phys. Rev. X 2012, 2, 041022. [Google Scholar] [CrossRef]
- van Aken, B.B.; Palstra, T.T.M.; Filippetti, A.; Spaldin, N.A. The Origin of Ferroelectricity in Magnetoelectric YMnO3. Nat. Mater. 2004, 3, 164–170. [Google Scholar] [CrossRef]
- Wang, S.F.; Yang, H.; Xian, T.; Liu, X.Q. Size-Controlled Synthesis and Photocatalytic Properties of YMnO3 Nanoparticles. Catal. Commun. 2011, 12, 625–628. [Google Scholar] [CrossRef]
- Han, A.; Zhao, M.; Ye, M.; Liao, J.; Zhang, Z.; Li, N. Crystal Structure and Optical Properties of YMnO3 Compound with High Near-Infrared Reflectance. Sol. Energy 2013, 91, 32–36. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas Sensing Properties of YMnO3 Based Materials for the Detection of NOx and CO. Sens. Actuators B Chem. 2017, 244, 1054–1070. [Google Scholar] [CrossRef]
- Tian, M.; Liu, X.; Gong, A.; Zhang, S.; Wang, G.; Han, P.; Li, Y.; Lou, X.; Hao, X. Efficient Ultraviolet–Visible-near Infrared Self-Powered Photodetector Based on Hexagonal YMnO3-Based Ferroelectric Thin Film by Multiscale Polarity Structure Optimization. Chem. Eng. J. 2023, 452, 139040. [Google Scholar] [CrossRef]
- Wadati, H.; Okamoto, J.; Garganourakis, M.; Scagnoli, V.; Staub, U.; Yamasaki, Y.; Nakao, H.; Murakami, Y.; Mochizuki, M.; Nakamura, M. Origin of the Large Polarization in Multiferroic YMnO3 Thin Films Revealed by Soft-and Hard-X-Ray Diffraction. Phys. Rev. Lett. 2012, 108, 047203. [Google Scholar] [CrossRef]
- Ahmad, T.; Lone, I.H.; Ubaidullah, M. Structural Characterization and Multiferroic Properties of Hexagonal Nano-Sized YMnO3 Developed by a Low Temperature Precursor Route. RSC Adv. 2015, 5, 58065–58071. [Google Scholar] [CrossRef]
- Turut, A.; Coșkun, M.; Coșkun, F.M.; Polat, O.; Durmuș, Z.; Çağlar, M.; Efeoğlu, H. The Current-Voltage Characteristics of the Ferroelectric p-YMnO3 Thin Film/Bulk p-Si Heterojunction over a Broad Measurement Temperature Range. J. Alloys Compd. 2019, 782, 566–575. [Google Scholar] [CrossRef]
- Ren, P.; Fan, H.; Wang, X. Bulk Conduction and Nonlinear Behaviour in Multiferroic YMnO3. Appl. Phys. Lett. 2013, 103, 152905. [Google Scholar] [CrossRef]
- DiSalvo, F.J. Solid-State Chemistry: A Rediscovered Chemical Frontier. Science 1990, 247, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Song, S.; Ravi, M.; Liu, R.; Ji, S. Enhanced Multiferroic Properties of YMnO3 Ceramics Fabricated by Spark Plasma Sintering along with Low-Temperature Solid-State Reaction. Materials 2017, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Mao, Y. Recent Developments on Molten Salt Synthesis of Inorganic Nanomaterials: A Review. J. Phys. Chem. C 2021, 125, 6508–6533. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A Review on Molten Salt Synthesis of Metal Oxide Nanomaterials: Status, Opportunity, and Challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Wang, Z.; Liu, H.; Li, Y.; Wang, X.; Wei, H.; Cheng, G.J. Controllable Near-Infrared Reflectivity and Infrared Emissivity with Substitutional Iron-Doped Orthorhombic YMnO3 Coatings. Sol. Energy 2020, 206, 778–786. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Wang, Z.; Wang, X.; Liu, H.; Cheng, G.J. Molten Salt Synthesis of YMnO3 Powder with High Near-Infrared Reflectivity. Mater. Lett. 2018, 229, 171–173. [Google Scholar] [CrossRef]
- McBean, C.L.; Lewis, C.S.; Tiano, A.L.; Simonson, J.W.; Han, M.-G.; Gannon, W.J.; Yue, S.; Patete, J.M.; Corrao, A.A.; Santulli, A.C. A Generalizable Multigram Synthesis and Mechanistic Investigation of YMnO3 Nanoplates. Ind. Eng. Chem. Res. 2017, 56, 5573–5585. [Google Scholar] [CrossRef]
- Jian, G.; Xu, Y.; Lai, L.-C.; Wang, C.; Zachariah, M.R. Mn3O4 Hollow Spheres for Lithium-Ion Batteries with High Rate and Capacity. J. Mater. Chem. A Mater. 2014, 2, 4627–4632. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl System. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Gizowska, M.; Piątek, M.; Perkowski, K.; Konopka, G.; Witosławska, I. Fabrication of Nanoyttria by Method of Solution Combustion Synthesis. Nanomaterials 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Mishra, A. Structural and Electrical Properties of YMnO3 Manganites: Influence of Cr Ion Doping. J. Solid State Chem. 2021, 301, 122364. [Google Scholar] [CrossRef]
- Karoblis, D.; Zarkov, A.; Garskaite, E.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Beganskiene, A.; Kareiva, A. Study of Gadolinium Substitution Effects in Hexagonal Yttrium Manganite YMnO3. Sci. Rep. 2021, 11, 2875. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Rao, C.N.R. Infrared and Electronic Spectra of Rare Earth Perovskites. Ortho- Chromites, -Manganites and -Ferrites. Appl. Spectrosc. 1970, 24, 436–444. [Google Scholar] [CrossRef]
- Vishnuvardhan, T.K.; Kulkarni, V.R.; Basavaraja, C.; Raghavendra, S.C. Synthesis, Characterization and a.c. Conductivity of Polypyrrole/Y2O3 Composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Cherian, E.; Rajan, A.; Baskar, G. Synthesis of Manganese Dioxide Nanoparticles Using Co-Precipitation Method and Its Antimicrobial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 17–22. [Google Scholar]
- Coşkun, M.; Polat, Ö.; Coşkun, F.M.; Durmuş, Z.; Çağlar, M.; Turut, A. Effect of Os Doping on Electrical Properties of YMnO3 Multiferroic Perovskite-Oxide Compounds. Mater. Sci. Semicond Process 2019, 91, 281–289. [Google Scholar] [CrossRef]
- Wan, F.; Li, L.; Bai, X.; Wang, Y.; Gao, L.; Li, J.; Cao, C. Structure and Dielectric Relaxation Behaviors of Co-Doped YMnO3 Multiferroic Ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 17361–17371. [Google Scholar] [CrossRef]
- Banerjee, D.; Nesbitt, H.W. XPS study of reductive dissolution of birnessite by oxalate: Rates and mechanistic aspects of dissolution and redox processes. Geochim. Et Cosmochim. Acta 1999, 51, 3025–3038. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Joshi, Z.; Dhruv, D.; Rathod, K.N.; Boricha, H.; Gadani, K.; Pandya, D.D.; Joshi, A.D.; Solanki, P.S.; Shah, N.A. Low Field Magnetoelectric Studies on Sol–Gel Grown Nanostructured YMnO3 Manganites. Prog. Solid State Chem. 2018, 49, 23–36. [Google Scholar] [CrossRef]
- Chanu, L.P.; Phanjoubam, S. Study on the Structural and Electrical Properties of YMnO3 Co-Substituted with Transition Metal Ions at Mn-Site and Their Conduction Mechanism. J. Mater. Sci. Mater. Electron. 2022, 33, 6107–6120. [Google Scholar] [CrossRef]
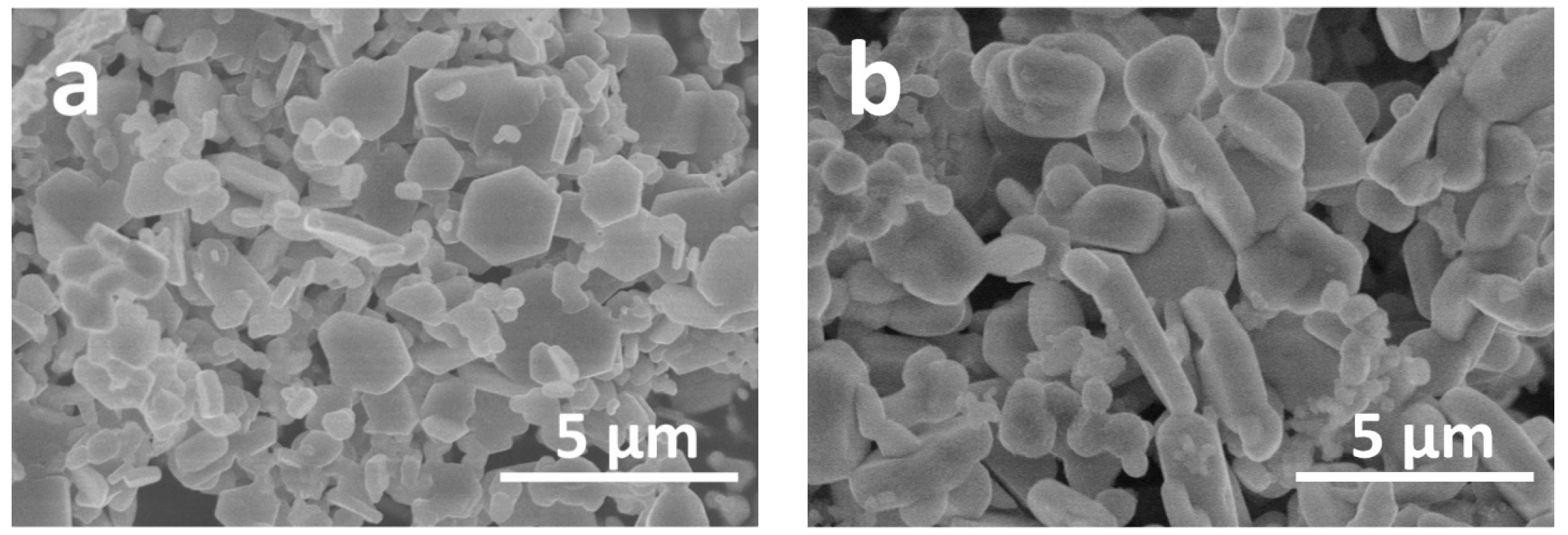

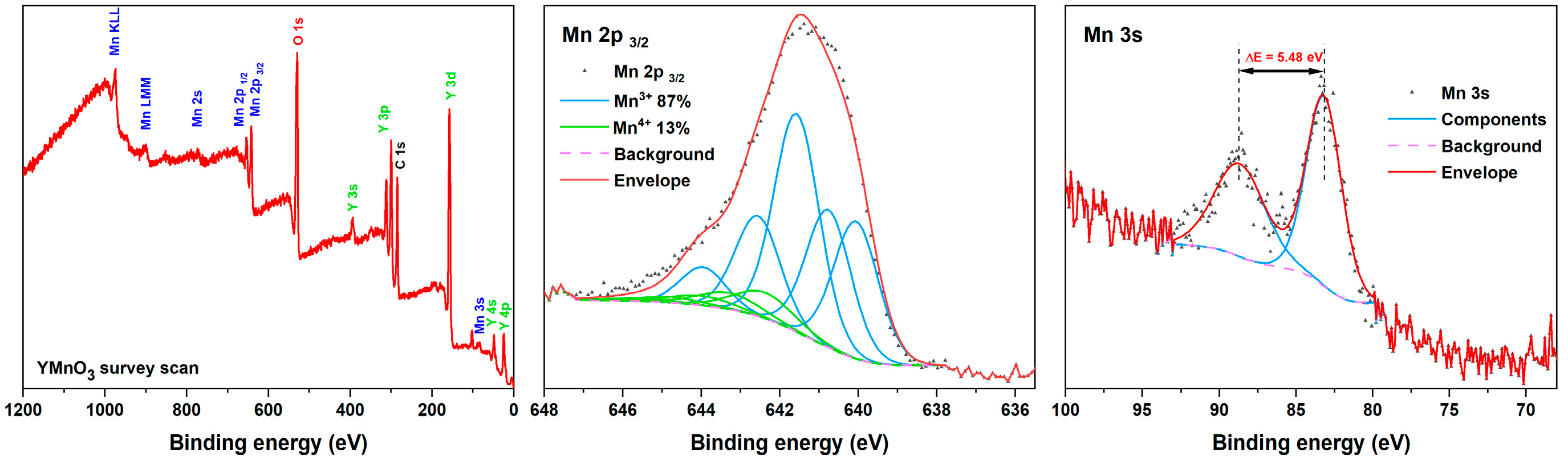
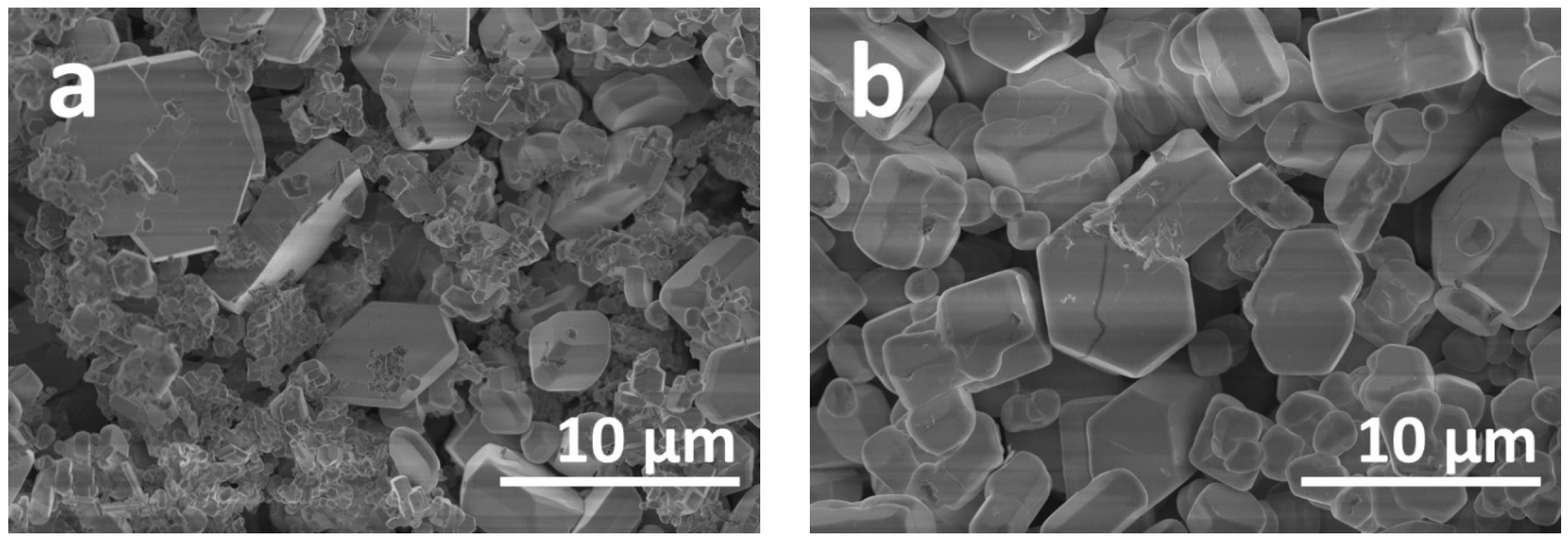
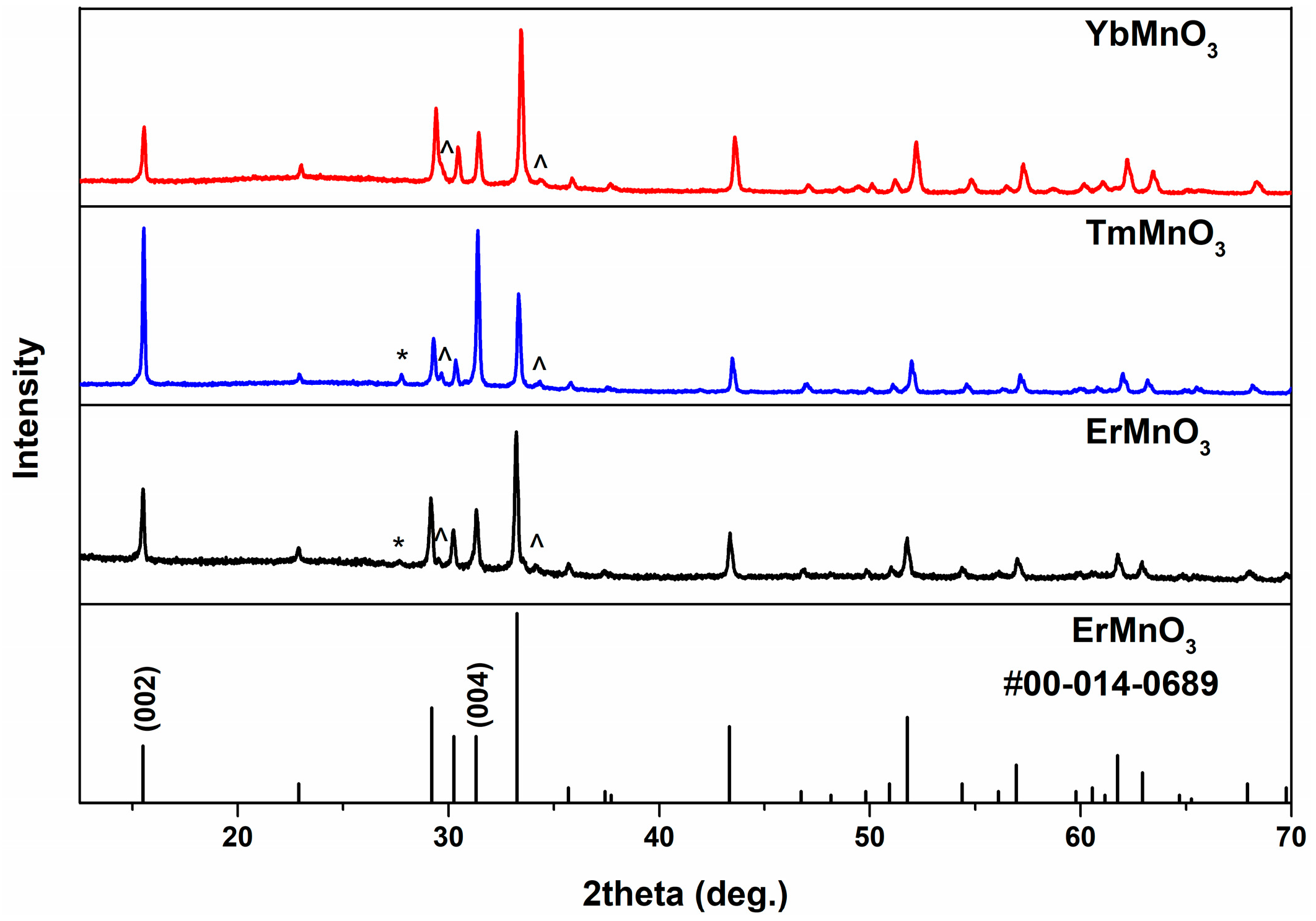
| Synthesis Conditions | a, Å | b, Å | c, Å | V, Å3 | |
|---|---|---|---|---|---|
| Temperature, °C | Molar Ratio between Precursors and Chlorides | ||||
| 900 | 1:2 | 6.1490 (6) | 6.1490 (6) | 11.4065 (7) | 373.51 (0) |
| 1100 | 1:2 | 6.1359 (4) | 6.1359 (4) | 11.3902 (5) | 371.38 (6) |
| 1000 | 1:10 | 6.1432 (5) | 6.1432 (5) | 11.4039 (0) | 372.71 (8) |
| 1100 | 1:10 | 6.1376 (1) | 6.1376 (1) | 11.3907 (3) | 371.60 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoblis, D.; Zarkov, A.; Murauskas, T.; Kareiva, A. Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics 2023, 11, 178. https://doi.org/10.3390/inorganics11050178
Karoblis D, Zarkov A, Murauskas T, Kareiva A. Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics. 2023; 11(5):178. https://doi.org/10.3390/inorganics11050178
Chicago/Turabian StyleKaroblis, Dovydas, Aleksej Zarkov, Tomas Murauskas, and Aivaras Kareiva. 2023. "Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb)" Inorganics 11, no. 5: 178. https://doi.org/10.3390/inorganics11050178
APA StyleKaroblis, D., Zarkov, A., Murauskas, T., & Kareiva, A. (2023). Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics, 11(5), 178. https://doi.org/10.3390/inorganics11050178