Abstract
Membrane permeability of copper complexes with potential anti-inflammatory activity were measured using an artificial membrane in a modified Franz cell. Using CuCl2 as the control, all the ligands tested enhanced the diffusion of copper, with enhancement factors ranging from 2 to 7. Octanol/water partition coefficients (log Ko/w) were measured and correlated with the permeability coefficients (Kp). In addition, chemical speciation was used to determine the predominant complex in solution at physiological pH. No correlation was found between the measured permeability coefficients and either molecular weight (MW) or log Ko/w.
1. Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory disease, which mainly affects the articulate joints and in severe cases can lead to joint destruction [1]. In the early stages of the disease, serum copper levels are significantly elevated [2,3]. Whether this is causative or a response to the inflammation is unknown, but serum copper levels are a potential biomarker of disease activity in RA patients [4]. Sorenson [5] and Jackson et al. [6,7] have shown that the inflammation associated with RA can be reduced by the subcutaneous injection of Cu(II) complexes. Walker et al. observed that topical applications of an ethanolic Cu(II) salicylate-containing preparation (Alcusal) produces anti-inflammatory effects in human volunteers. A gel-based version is available in Australia [8]. Puranik et al. showed that the copper complexes of non-steroidal anti-inflammatory drugs inhibit acute inflammation in vivo [9].
Dermal absorption is the preferred route of administration for long-term drug therapy because it is slow, tolerable and less painful compared to injection or oral administration. This leads to improved patient compliance. For oral administration, there is the added complication that the stomach pH may affect the drug. The efficacy of the dermal absorption route, however, depends on the ability of the drug to pass through the skin. Hostynek and Maibach have reviewed the interaction between copper and the skin [10]. Preliminary dermal absorption studies have been performed on some of the copper complexes using Cu-67 and BALB/c mice [7,11,12,13]. However, these studies are expensive, time consuming and require ethical approval. For the rapid screening of different copper complexes, an efficient model of dermal absorption is needed. Since Flynn [14] proposed the use of physicochemical properties to predict skin permeation, several experimental and theoretical models have been proposed [15,16,17,18]. Much of this work have been based on immobilized artificial membranes [19]. Meanwhile, the skin is composed of three main layers with the outer layer—the epidermis—being the main barrier to dermal absorption. The difficulty is to find an artificial mimic of this layer. Cerasome 9005 is a mixture of lipids similar to those found in the stratum corneum. Krulikowska et al. [20] found a 95% correlation between the penetration coefficients of porcine skin and Cerasome 9005. For this reason, Cerasome has been used in this study.
2. Results and Discussion
The ligands used in this study were chosen because they have been investigated as potential anti-inflammatory copper(II) drugs [7,11,12,13,21]. Figure 1 shows the structure of some of the ligands. Figure 2 shows the results for the diffusion of several copper complexes, over 24 h, through an immobilized Cerasome membrane. For each complex, there was a slow induction period (8 h) before a steady state flux was obtained. From the slope of the curves, the steady state flux and the permeability coefficients, Kp, were calculated (Table 1). The use of Kp is now encouraged [22] although it has been criticized [23].
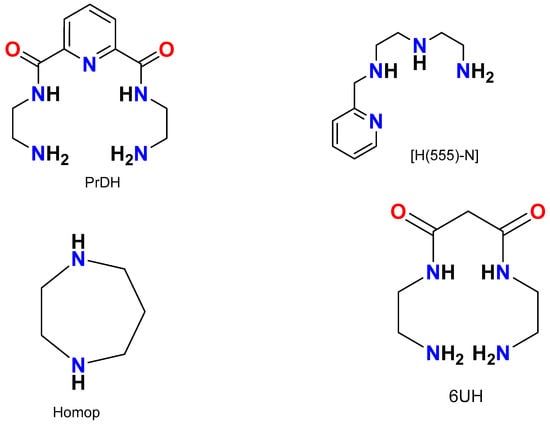
Figure 1.
Schematic diagram of some of the ligands used in this study: N,N′-di(aminoethylene)-2,6-pyridine-dicarbonylamine (PrDH), N-[2-(2-aminoethylamino)ethyl]picolinamide (H(555)-N), homopiperazine (homop) and N,N′-bis[aminoethyl]propanediamide (6UH).
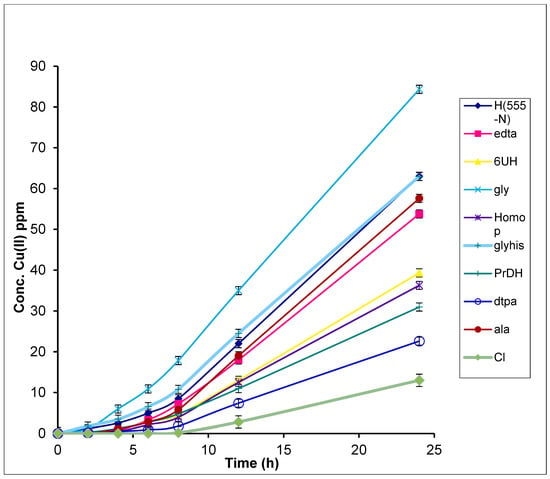
Figure 2.
Concentration vs. time of Cu(II) in receiver phase after passing through a Cerasome 9005 membrane at 25 °C. The error bars represent the standard deviation in the concentration.

Table 1.
Diffusion and distribution coefficients of different copper complex species present in solution at pH 7.0. Copper concentration was 20 mM.
The increased uptake of Cu(II) in the presence of amino acids is in agreement with previous results obtained with Ehrlich ascites tumor, brain, liver and kidney cells [24,25,26]. The Kp of Cu(II) complexed to alanine (6.31 ± 0.01) × 10−6 cm/s and glycine (5.79 ± 0.04) × 10−6 cm/s are comparable to values found by Mazurowsky for the same complexes (alanine (1.90 ± 0.16) × 10−6 cm/s; glycine (1.62 ± 0.06) × 10−6 cm/s) [24,25,26]. Mazurowsky used liposomes as a model membrane, with potassium phosphate buffered at pH 7.4 as the acceptor phase.
CuCl2, was included in the results as a control. The copper CuCl2 experiments were performed at pH 4.23 since, at pH 7 and the concentrations used, Cu(OH)2 would precipitate. Our results show that the ligands used were able to keep Cu(II) in solution at physiological pH and increase the rate of diffusion of copper through the membrane. From Equation (1), these resulted in an enhancement factor (EF) which can be calculated to provide the relative effect of the ligand upon Cu(II) diffusion through the membrane. The EF ranged from 2 for dtpa to 6.8 for H(555N) (Table 1).
2.1. Partition Coefficient
Table 1 lists the partition coefficients (log Ko/w) of the different Cu(II) complexes, measured at room temperature and a physiological pH of 7.00. Cu-gly and Cu-H(555-N) have the highest lipophilicity to the other complexes studied, although none of the complexes are very lipophilic. Zvimba has suggested that, for reasonably absorption, a log Ko/w of at least 0.6 is needed [27]. The negative values of log Ko/w found in our study indicate that these complexes are largely hydrophilic.
2.2. Data Analysis
One aim of this study was to derive a simple relationship between permeability coefficient and another more easily measured physical parameter of copper complexes, such as Ko/w or molecular weight (MW). Hence, it is necessary to know the MW of the complexes. For organic molecules, this is relatively easy, but for labile inorganic complexes such as copper, the speciation and, hence, MW changes with pH. In this study, we have used measured equilibrium constants [7,8,9,10,11,12,13,21] to calculate the speciation of the different complexes in solution. This is illustrated in Figure 3, which shows the concentration of the copper species as a function of pH. At pH 7, copper is 100% complexed to dtpa (Figure 3a), but with PrDH, the copper is distributed between two main complexes of different stoichiometry, [Cu(PrDH)(H2O)3] (12%) and [Cu(PrDH)(H2O)3H−1] (60%) (Figure 3b). An added complication is that the number of coordinated water molecules is not specified by the equilibrium constant. However, in this study, we have assumed that the copper is 6-coordinate with any vacant sites occupied by water molecules. The resultant stoichiometries and MWs are given in Table 1.
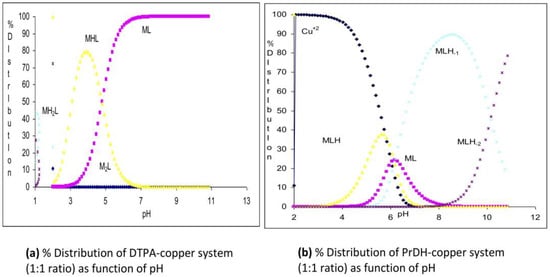
Figure 3.
Species distribution curves for (a) Cu(II)/dtpa and (b) Cu(II)/PrDH. The stoichiometry of the complex is denoted by MHL, where M = Cu(II), L = dtpa or PrDH and H = H+.
Linear regression analysis of log Kp against log Ko/w presented an R2 of 0.49, while log Kp versus MW presented an R2 of 0.35. Consequently, for these compounds, there is no correlation between log Kp and either log Ko/w or MW.
Potts and Guy [17] have proposed a quantitative structure–permeability relationship model (Equation (2), which depends upon both the size of the drug (MW) and Ko/w.
where, h is the membrane thickness; D0 is the diffusivity of a hypothetical molecule of zero molecular weight; f is a constant which accounts for the difference between the membrane lipids and octanol; β’ converts molecular volume to molecular weight.
Log Kp = log (D0/h) + f log Ko/w − β′ MW
Multiple linear regression of log Kp, log Ko/w and MW presented an R2 of 0.53. Thus, these two parameters on their own are poor descriptors of log Kp and indicates that only 53% of log Kp is explained by log Ko/w and MW. This is not surprising as the different copper complexes have different charges, which would affect their diffusivity. Even though the correlation was poor, values were obtained for β’ (0.01), log (D0/h) (−17.4) and f (−2.7). Flynn [14,22] found that the values of β’, Log (D0/h) and f for 90 drugs were 0.0061, −2.72 and 0.71, respectively. The diffusivity of these charged complexes would be expected to be much lower, as was found, but that f should be similar as the charge of the complex should have the same effect on partitioning for both octanol and the membrane. Thus, for labile metal complexes, factors other than size and partition coefficient are important.
3. Materials and Methods
CuCl2.2H2O, glycine (gly), ethylenediaminetetraacetic acid (edta), alanine (ala), diethylenetriaminepentaacetic acid (dtpa) and homopiperazine (homop) were obtained commercially. Ligands, N,N′-di(aminoethylene)-2,6-pyridine-dicarbonylamine (PrDH), N-[2-(2-aminoethylamino)ethyl]picolinamide (H(555)-N) and N,N′-bis[aminoethyl]propanediamide (6UH) (Figure 1) were synthesized in our laboratory [7,12,21]. Cerasome (product name: Cerasome 9005) was kindly donated by Lipoid GMBH (Ludwigshafen, Germany). Cerasome 9005 is composed of hydrogenated lecithin, cholesterol, ceramides (NP and NS) and fatty acids (palmitic acid and oleic acid) in distilled water with ~10% ethanol as a preservative. The concentration of total lipids is 6.60 g/100 g. The particle size and pH value of Cerasome, offered by Lipoid GMBH, were 48.1 nm and 7.3, respectively. Cerasome was stored between 15 °C and 25 °C, as recommended in the product information sheet. A comparison of the structure of Cerasome 9005 and the stratum corneum is detailed in Figure 1 of [28].
10 mM or 5 mM solutions of the copper complexes were prepared from CuCl2.2H2O and the different ligands in MilliQ-water. The pH of the solutions was adjusted to 7.00 using concentrated NaOH or HCl. Different metal/ligand ratios were used contingent upon the ligand so as to avoid the formation of precipitate. In order to avoid precipitation of copper hydroxide, a pH of 4.23 was used for the CuCl2 control. This was the highest pH that could be used and still avoid precipitation.
The artificial membrane was made using filter paper (Macherey-Nagel) of 3.2 cm2 diameter and thickness 0.12 cm. The filter paper was submerged in the Cerasome 9005 lipid solution for a few minutes at 25 °C and then weighed. The amount of lipid absorbed was determined by mass difference and was typically 0.0131g with variability ≈ 7%.
Figure 4 shows a modified Franz cell consisting of two 50 cm3 cylinders connected through a sintered glass membrane. This arrangement had the advantage that samples could be removed for analysis without disturbing the hydrostatic pressure. Each solution could be stirred and the whole apparatus was placed in a temperature-controlled environment. The disadvantage of this apparatus, relative to the Franz cell, is that the donor and receiver phases could not be independently thermostated. Each experiment was repeated 3 times.
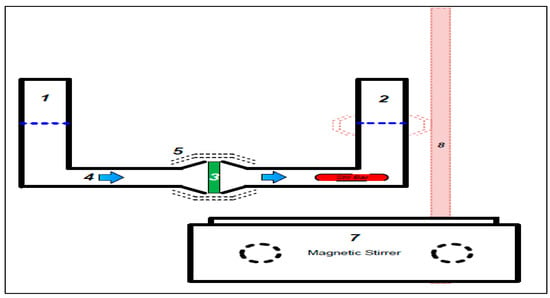
Figure 4.
Modified Franz cell apparatus used in this study, where: (1) donor phase filled with 20 mL of Cu(II) complex; (2) receiver phase filled with blank solution (distilled/deionized water; (3) the artificial membrane; (4) passive diffusion direction; (5) clamp; (6) stirrer bar; (7) magnetic stirrer; and (8) burette stands with clamp.
The shake flask method was used to measure partition coefficients where the organic phase was 1-octanol pre-saturated with water [29]. An amount of 40 mL of 1-octanol was mixed with 10 mL of the aqueous complex solution and shaken for 5 min. Afterward, 1 mL of the aqueous layer and 38 mL of the organic layer were removed using micropipettes. The copper in the organic layer was back-extracted using 5% v/v HNO3. The concentration of copper in the two layers was measured by atomic absorption spectroscopy (AAS) using a Varian AA-5 spectrometer. The instrument was calibrated in the 1–15 ppm range and sample concentrations were adjusted accordingly. The spectrometer settings used were as follows: 3 mA (copper lamp), 1.5 units/70 kPa (acetylene), 350 kPa (compress air), 324.7 nm (wavelength), 2 s (time), abs. exp. factor (1) and 0.03–10 μg/mL. The analytical standard deviation was found to be <1%.
Analysis of Data
Permeability coefficients (Kp) were determined from Fick’s first law of diffusion (Equation (3)) [22,23]:
where Ci is the initial permeant concentration in the donor solution and J is the mass passing through unit area of the membrane per unit time and is given by:
where J = flux in g/cm2s; S = cross-section of barrier in cm2; and dM/dt = rate of diffusion in g/s.
4. Conclusions
In this study, we have demonstrated that a simple artificial membrane, Cerasome, in a modified Franz cell, can be used to study the diffusion of copper complexes. In addition, we showed that simple ligands can be used to enhance the membrane permeability of copper. However, the partition coefficient and/or molecular weight cannot be used to predict tissue permeability.
Author Contributions
Conceptualization, G.E.J.; Methodology, G.E.J.; Writing—original draft, E.U.-T.; Writing—review and editing, G.E.J. and A.N.H. The project was conceptualized by G.E.J., executed by E.U.-T. and the data was interpreted by all of the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of South Africa, grant number 93450 and 85466 and the University of Cape Town Research Committee. E.U. thanks the Eric Abraham Foundation for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors wish to thank Lipoid GmbH Company (Germany) for supplying Cerasome 9005.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
Kp = permeability coefficient defined according to Fick’s first law of diffusion; dtpa = diethylenetriamine; edta = ethylenediamine; gly = glycine; MW = molecular weight.
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Strecker, D.; Mierzecki, A.; Radomska, K. Copper levels in patients with rheumatoid arthritis. Ann. Agric. Environ. Med. 2013, 20, 312–316. [Google Scholar]
- Wang, H.; Zhang, R.; Shen, J.; Jin, Y.; Chang, C.; Hong, M.; Guo, S.; He, D. Circulating Level of Blood Iron and Copper Associated with Inflammation and Disease Activity of Rheumatoid Arthritis. Biol. Trace Elem. Res. 2023, 201, 90–97. [Google Scholar] [CrossRef]
- Chakraborty, M.; Chutia, H.; Changkakati, R. Serum Copper as a Marker of Disease Activity in Rheumatoid Arthritis. J. Clin. Diag. Res. 2015, 9, BC09-11. [Google Scholar] [CrossRef]
- Sorenson, J.R. Copper chelates as possible active forms of the anti-arthritic agents. J. Med. Chem. 1976, 19, 135–148. [Google Scholar] [CrossRef]
- Jackson, G.E.; May, P.M.; Williams, D.R. Metal-ligand complexes involved in rheumatoid arthritis. I. Justification for Copper Administration. J. Inorg. Nucl. Chem. 1978, 40, 1227–1234. [Google Scholar] [CrossRef]
- Zvimba, J.N.; Jackson, G.E. Copper chelating anti-inflammatory agents: N1-(2-aminoethyl)-N2-(pyridin-2-ylmethyl)ethane-1,2-diamine and N-(2-(2-aminoethylamino)ethyl)picolinamide: An in vitro and in vivo study. J. Inorg. Biochem. 2007, 101, 148–158. [Google Scholar] [CrossRef]
- Walker, W.R.; Beveridge, S.J.; Whitehouse, M.W. Anti-inflammatory activity of a dermally applied copper salicylate preparation (Alcusal). Agents Actions 1980, 10, 38–47. [Google Scholar] [CrossRef]
- Puranik, R.; Bao, S.; Bonin, A.M.; Kaur, R.; Weder, J.E.; Casbolt, L.; Hambley, T.W.; Lay, P.A.; Barter, P.J.; Rye, K.A. A novel class of copper(II)- and zinc(II)-bound non-steroidal anti-inflammatory drugs that inhibits acute inflammation in vivo. Cell Biosci. 2016, 6, 9. [Google Scholar] [CrossRef]
- Hostynek, J.J.; Maibach, H.I. Copper and the Skin; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zvimba, J.N.; Jackson, G.E. Solution equilibria of copper(II) complexation with N,N′-(2,2′-azanediylbis(ethane-2,1-diyl))dipicolinamide: A bio-distribution and dermal absorption study. J. Inorg. Biochem. 2007, 101, 1120–1128. [Google Scholar] [CrossRef]
- Odisitse, S.; Jackson, G.E. In vitro and in vivo studies of the dermally absorbed Cu(II) complexes of N5O2 donor ligands—Potential anti-inflammatory drugs. Inorg. Chim. Acta 2009, 362, 125–135. [Google Scholar] [CrossRef]
- Odisitse, S.; Jackson, G.E. In vitro and in vivo studies of N,N′-bis[2(2-pyridyl)-methyl]pyridine-2,6-dicarboxamide-copper(II) and rheumatoid arthritis. Polyhedron 2008, 27, 453–464. [Google Scholar] [CrossRef]
- Dermal Exposure Assessment: Principles and Applications. January 1992 United States Environmental Protection Agency, EPA/600/8-91/011B. Interim Report. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=12188 (accessed on 17 February 2021).
- Zhang, K.; Chen, M.; Scriba, G.K.E.; Abraham, M.H.; Fahr, A.; Liu, X. Human Skin Permeation of Neutral Species and Ionic Species: Extended Linear Free-Energy Relationship Analyses. J. Pharm. Sci. 2012, 101, 2034–2044. [Google Scholar] [CrossRef]
- Geinoz, S.; Guy, R.H.; Testa, B.; Carrupt, P.A. Quantitative structure–permeation relationships (QSPeRs) to predict skin permeation: A critical evaluation. Pharm. Res. 2004, 21, 83–92. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting Skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Komatsu, T.; Sumi, M.; Numajiri, S.; Miyambo, M.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. In vitro permeation of several drugs through the human nail plate: Relationship between physicochemical properties and nail permeability of drugs. Eur. J. Pharm. Sci. 2004, 21, 471–477. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, M.; Scriba, K.E.G.; Abraham, M.H.; Fahr, A.; Liu, X. Linear Free Energy Relationship Analysis of Retention Factors in Cerasome Electrokinetic Chromatography Intended for Predicting Drug Skin Permeation. J. Pharm. Sci. 2011, 100, 3105–3113. [Google Scholar] [CrossRef]
- Krulikowska, M.; Arct, J.; Lucova, M.; Cetner, B.; Majewski, S. Artificial membranes as models in penetration Investigations. Skin Res. Technol. 2013, 19, 139–145. [Google Scholar] [CrossRef]
- Jackson, G.E.; Linder, P.W.; Voye, A. A potentiometric and spectroscopic study of copper(ii) diamidodiamino complexes. J. Chem. Soc. Dalton Trans. 1996, 24, 4605–4612. [Google Scholar] [CrossRef]
- Bartzatt, R. Determination of dermal permeability coefficient (Kp) by utilizing multiple descriptors in artificial neural network analysis and multiple regression analysis. J. Sci. Res. Rep. 2014, 3, 2884–2899. [Google Scholar] [CrossRef]
- Korinth, G.; Schaller, K.H.; Drexler, H. Is the permeability coefficient Kp a reliable tool in percutaneous absorption studies? Arch. Toxicol. 2005, 79, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Mazurowska, L.; Nowak-Buciak, K.; Mojski, M. ESI-MS method for in vitro investigation of skin penetration by copper-amino acid complexes: From an emulsion through a model membrane. Anal. Bioanal. Chem. 2007, 388, 1157–1163. [Google Scholar] [CrossRef]
- Mazurowska, L.; Mojski, M. Biological activities of selected peptides: Skin penetration ability of copper complexes with peptide. J. Cosmet. Sci. 2008, 59, 59–69. [Google Scholar] [PubMed]
- Mazurowska, L.; Mojski, M. ESI-MS study of the mechanism of glycyl-l-histidyl-l-lysine-Cu(II) complex transport through model membrane of stratum corneum. Talanta 2007, 72, 650–654. [Google Scholar] [CrossRef]
- Zvimba, J.N.; Jackson, G.E. Thermodynamic and spectroscopic study of the interaction of Cu(II), Ni(II), Zn(II) and Ca(II) ions with 2-amino-N-(2-oxo-2-(2-(pyridin-2-yl)ethyl amino)ethyl)acetamide, a pseudo-mimic of human serum albumin. Polyhedron 2007, 26, 2395–2404. [Google Scholar] [CrossRef]
- Wajda, R. Cerasomes—Liposomes with membranes formed from stratum corneum lipids. Euro Cosmet. 2001, 4, 1–2. [Google Scholar]
- Leo, A.; Hansch, A.C.; Elkins, D. Partition Coefficient and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).