Preparation of Glass-Ceramics in the R2O-Bi2O3-B2O3-SiO2 System Applied in Automobile Glass Enamel
Abstract
1. Introduction
2. Experimental Procedure
2.1. Preparation of Automotive Glass Enamel
2.2. Performance Testing and Characterization of Materials
3. Results and Discussion
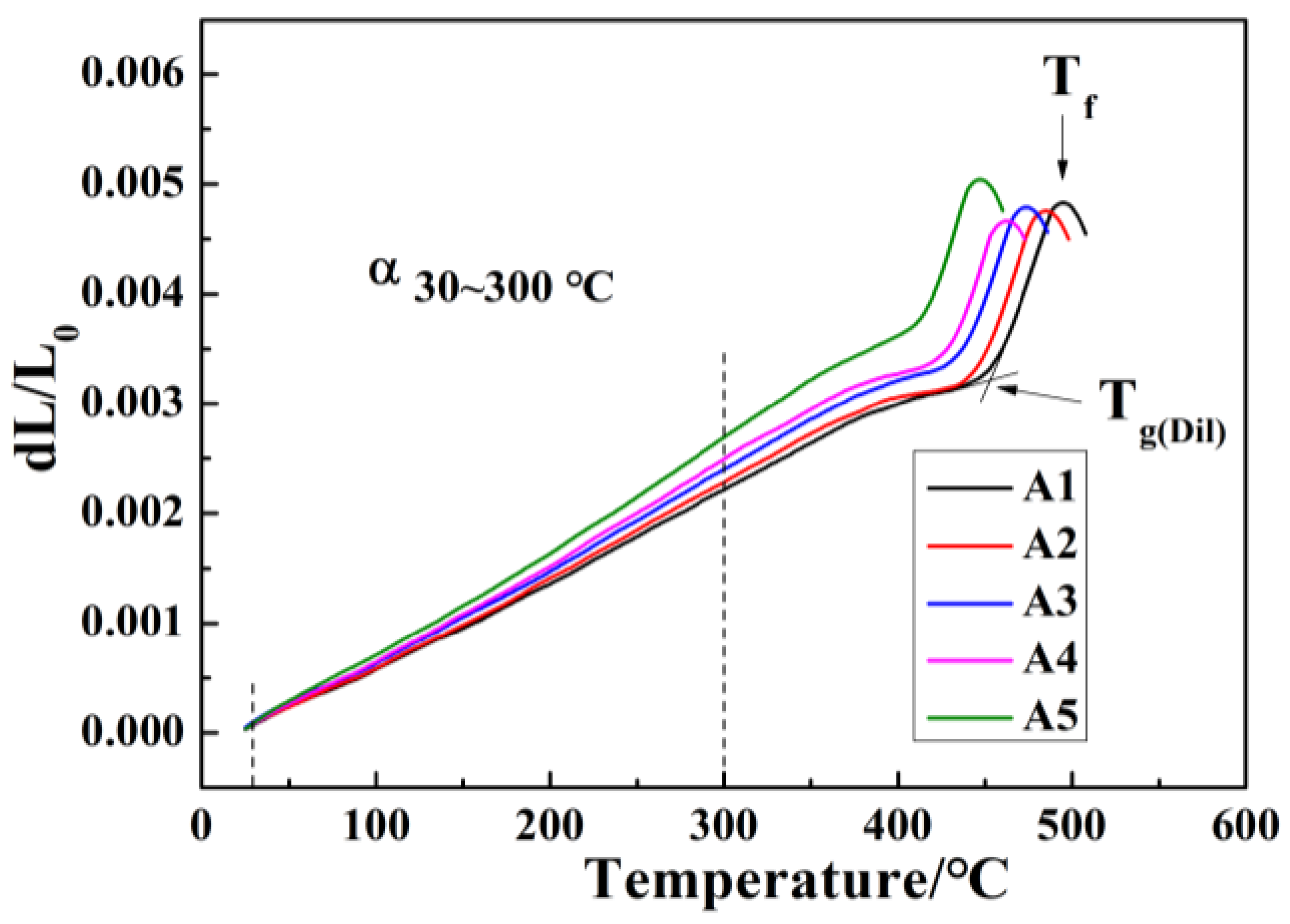
3.1. Glass Forming Ability and Thermal Properties
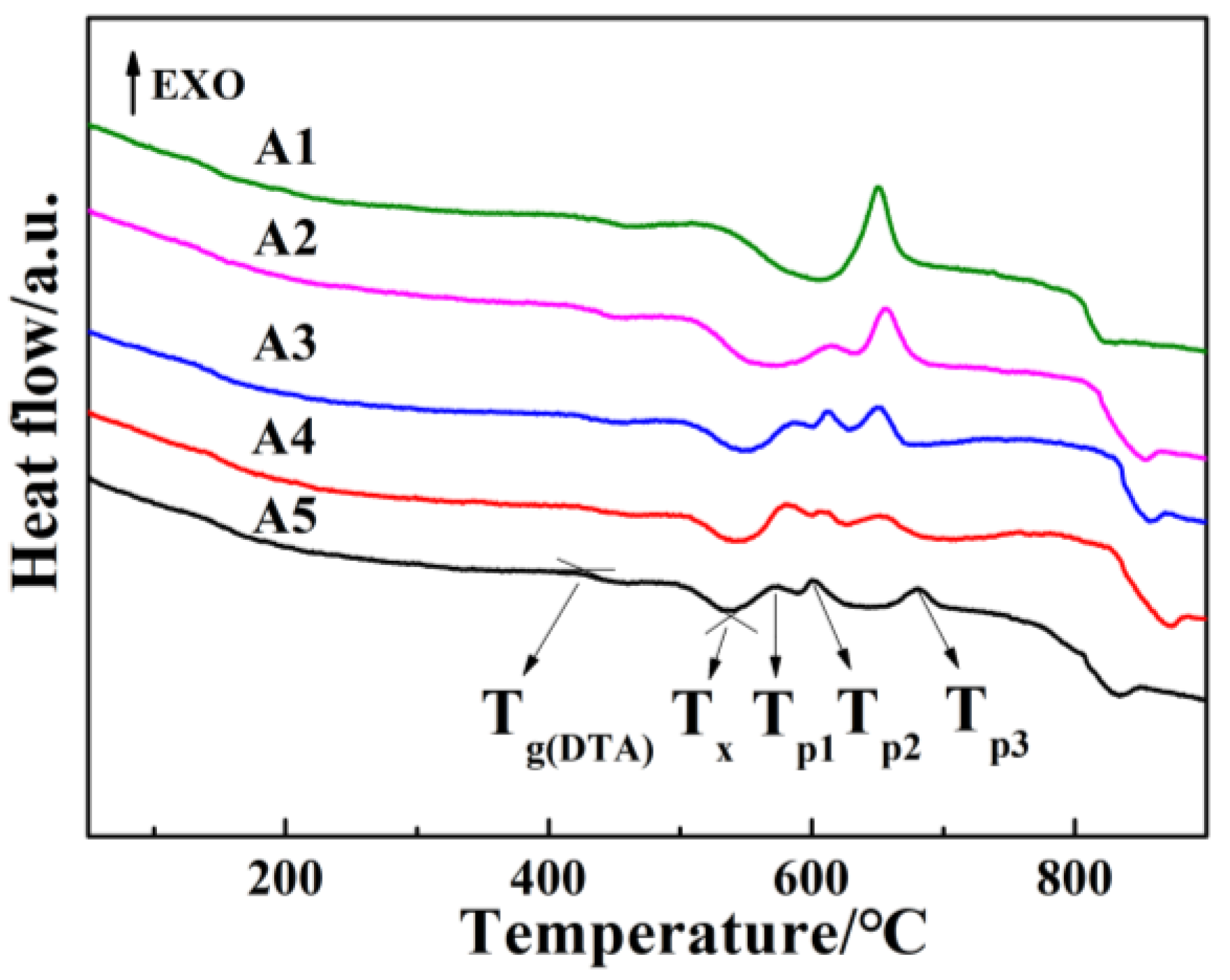
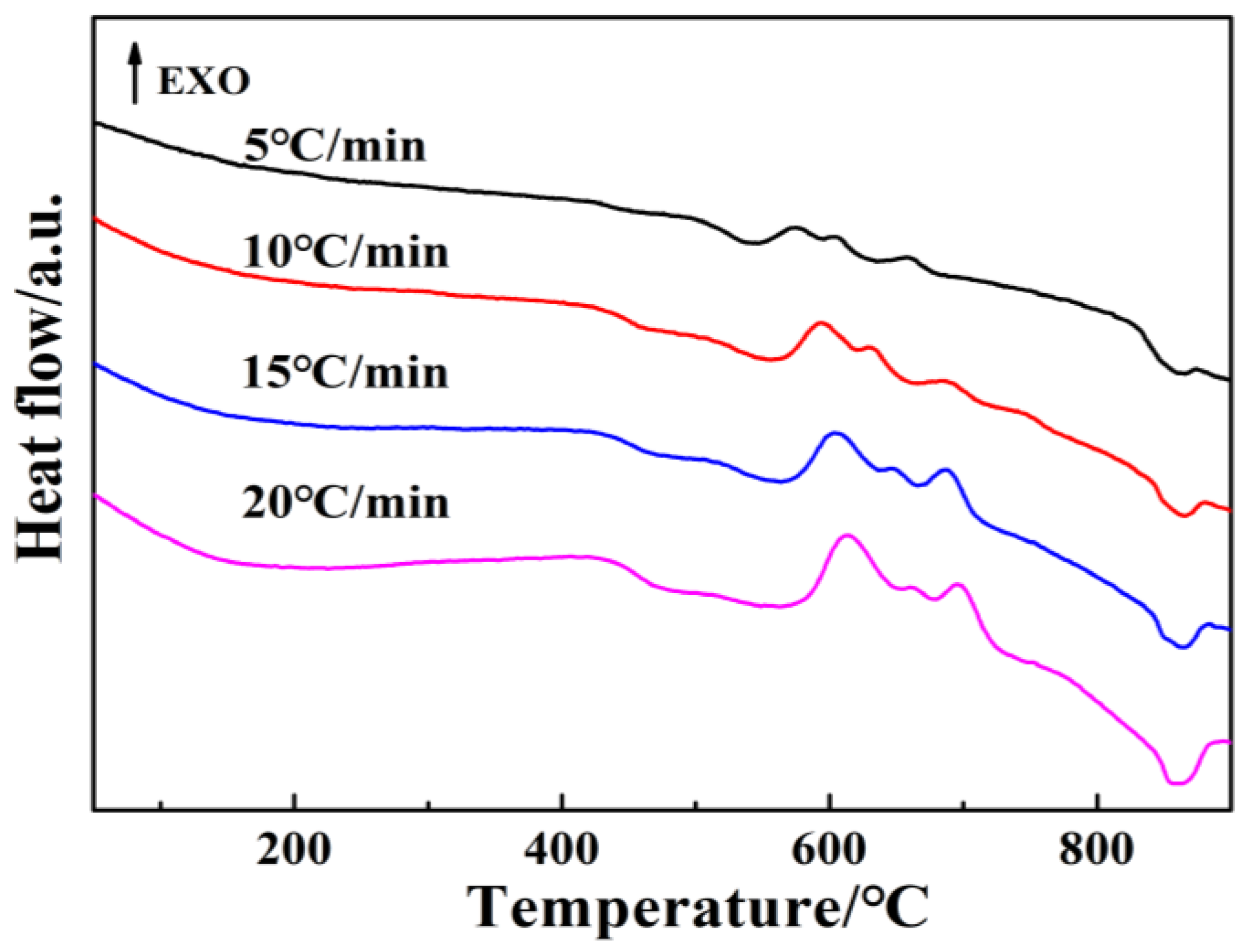
3.2. Crystallization Kinetics Analysis of Base Glass
3.3. Preparation and Properties of Glass-Ceramics
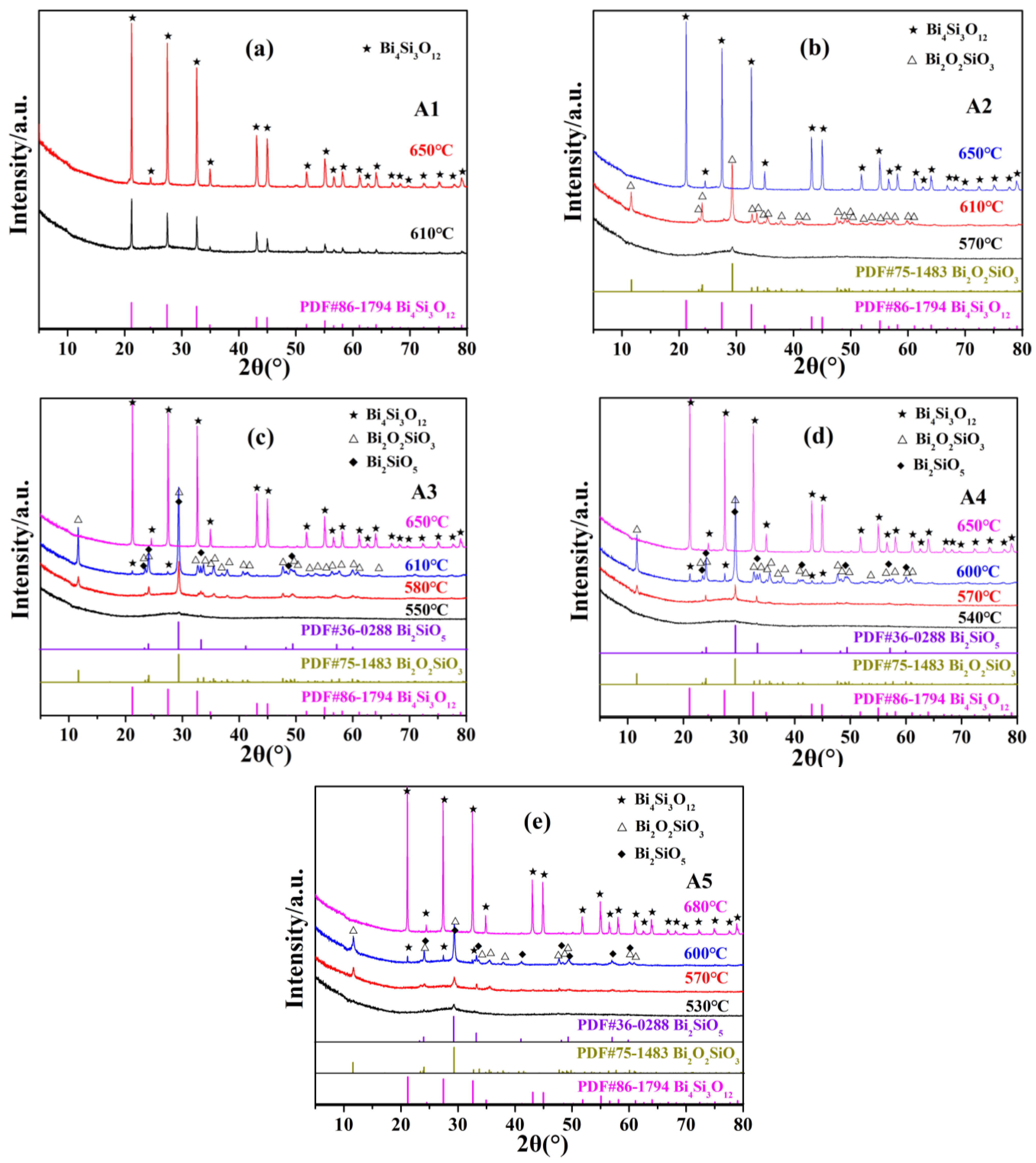
3.3.1. Phase Identification of Heat-Treated Base Glasses
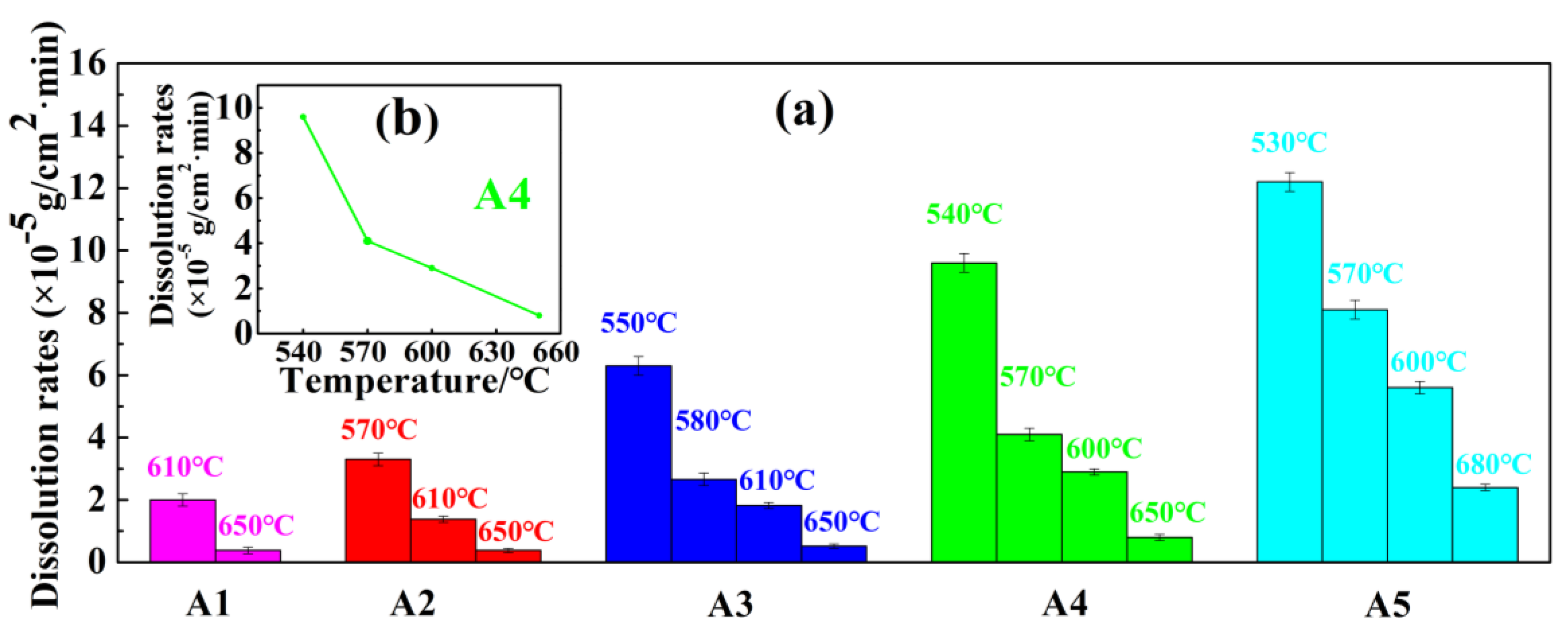
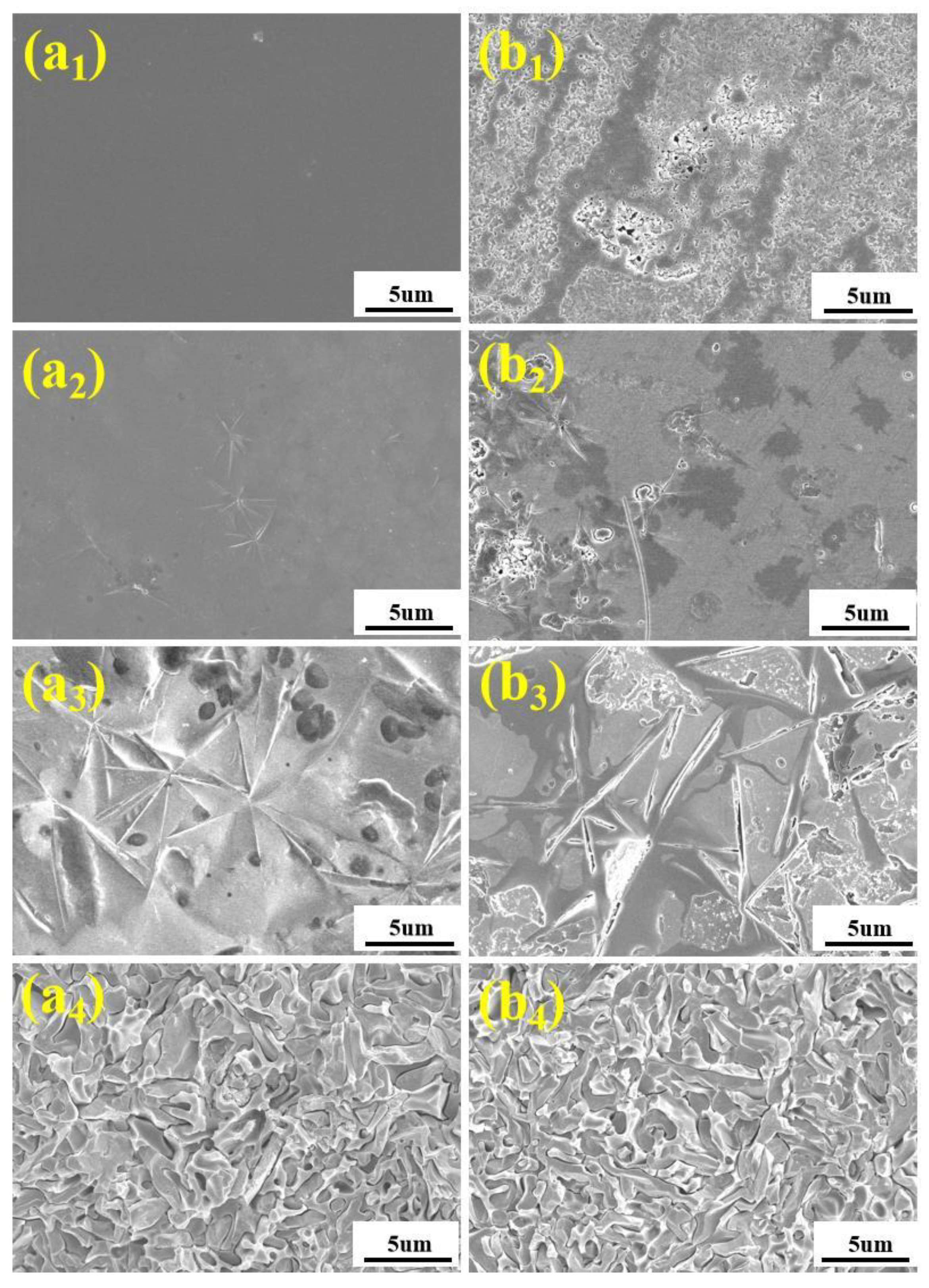
3.3.2. Microstructure and Properties of Heat-Treated Base Glasses
3.4. Preparation and Properties of Glass-Ceramic Enamel
4. Conclusions
- (1)
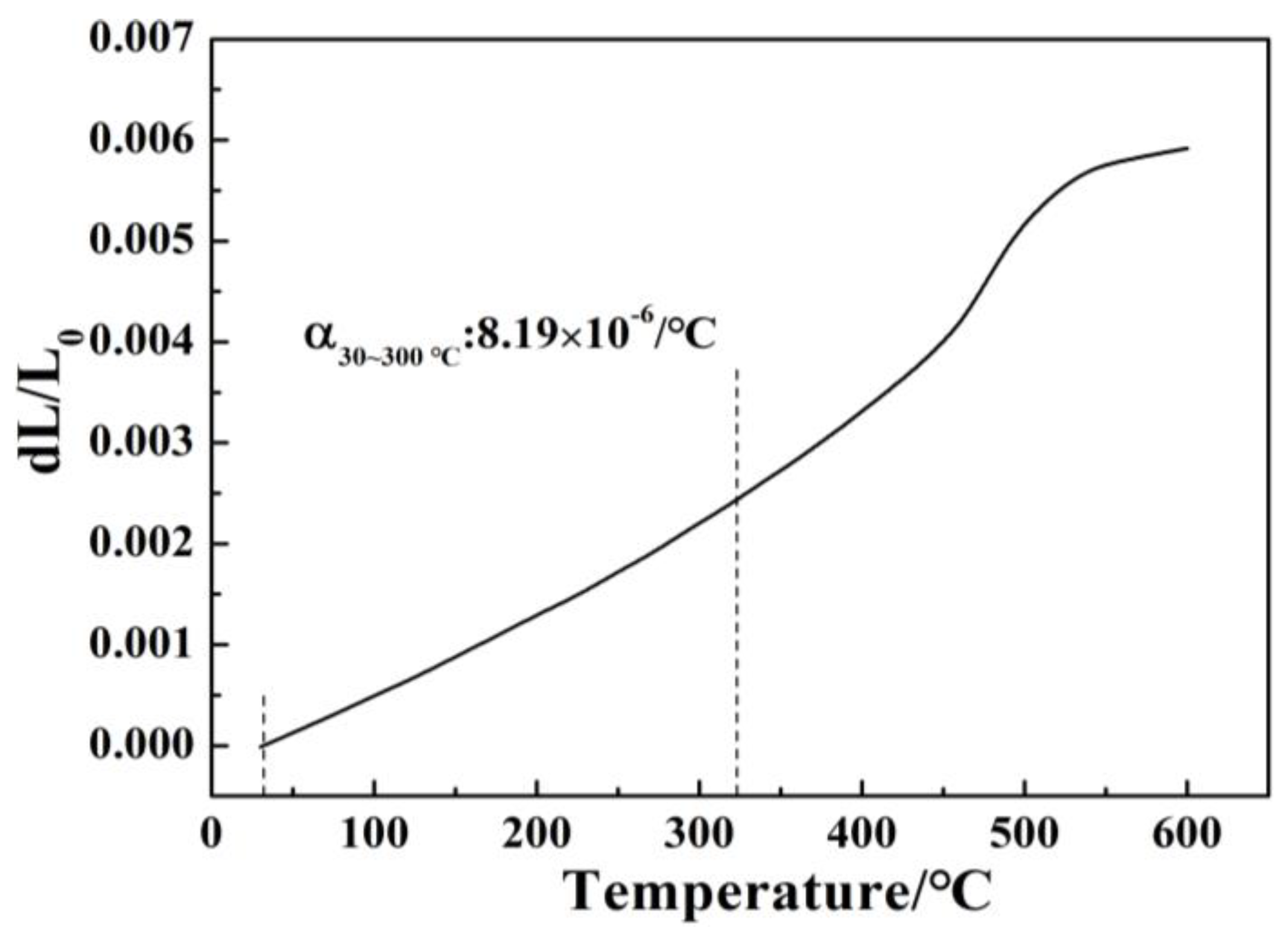
- For glasses in the 15R2O-xBi2O3-10B2O3-(75-x)SiO2 system, their Tg, Tf, and S values decrease regularly, their crystallization ability is improved, their crystallization temperature range is widened, and the number of crystallization exothermic peaks varies from one to two or even three with increasing Bi2O3/SiO2 ratio.
- (2)
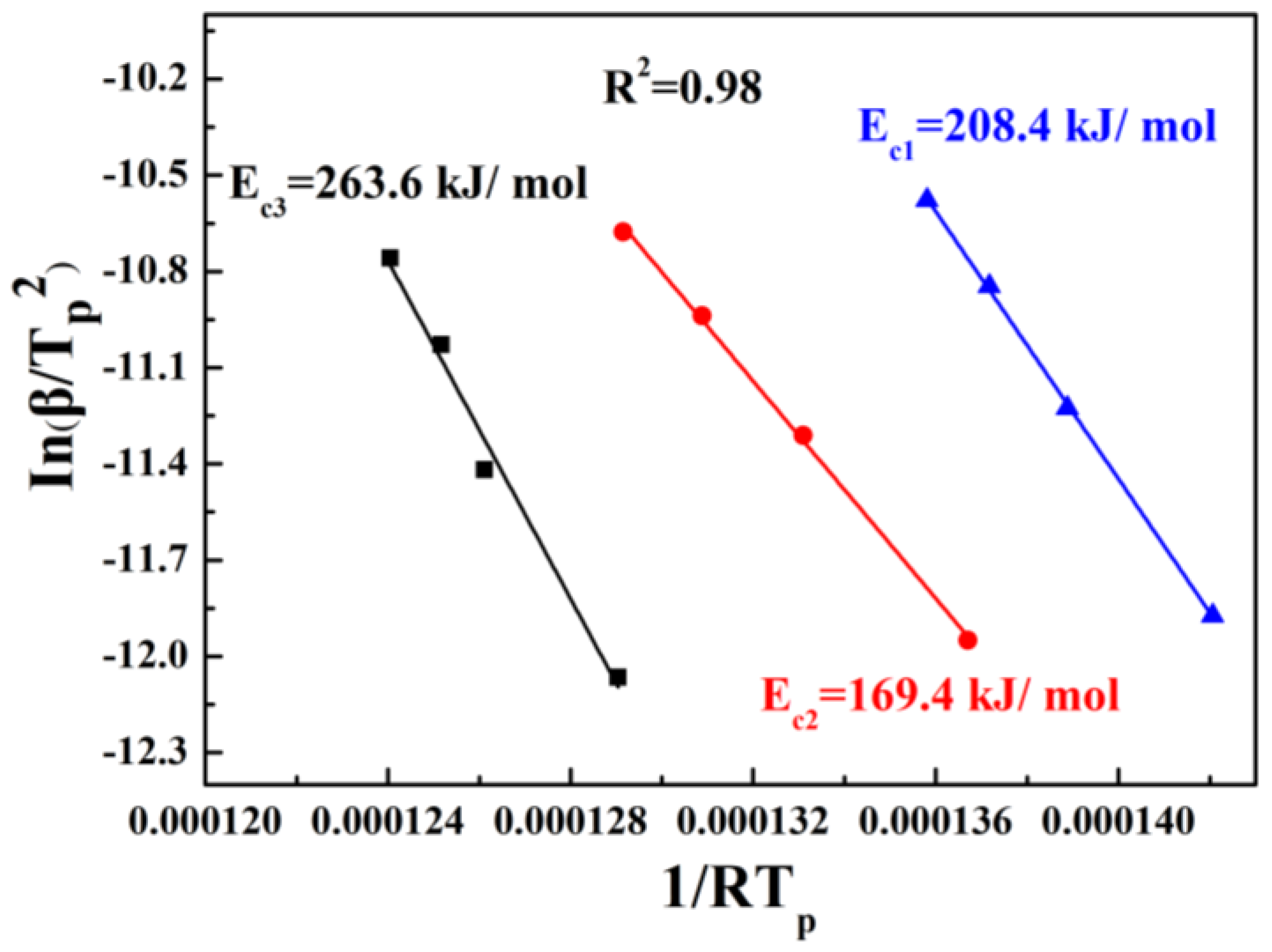
- The three crystallization exothermic peaks (Tp1, Tp2, and Tp3) in the DTA curve of A4 base glass correspond to the crystallization of Bi2SiO5, Bi2O2SiO3, and Bi4Si3O12 crystals, respectively. The crystallization activation energies of the three types of crystalline phases are 208.4 kJ/mol (Ec1), 169.4 kJ/mol (Ec2), and 263.6 kJ/mol (Ec3), respectively. The crystallization of A4 base glass is bulk crystallization.
- (3)
- When the crystallization temperature is in the range of 530–650 °C, the metastable Bi2SiO5 and Bi2O2SiO3 crystalline phases are mainly precipitated. Above 650 °C, only Bi4Si3O12 crystalline phases are precipitated, and Bi4Si3O12 crystals are intermingled with each other. The crystallinity of the base glass increases significantly with the increase in heat treatment temperature. The CTE of the glass-ceramic is significantly lower than that of the corresponding base glass. DRAcid values of the heat-treated base glasses are in the range of 10−5~10−6 g·cm−2·min−1. In comparison with base glass, the DRAcid values of base glass heated at temperatures higher than 650 °C decrease by an order of magnitude. It proves that increasing the heat treatment temperature is beneficial for improving the acid resistance of base glass. Enamel was prepared by mixing A4-4 glass-ceramic powder with copper-chromium black and varnish, then printed on automotive glass substrates, and then sintered. The indicators of our product meet the requirements of the current mainstream automobile manufacturers, and it has much higher acid resistance than commercial products.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Pan, H.Z.; Zheng, W.H. Assessment of the incentives on electric vehicle promotion in China. Transport. Res. A Pol. 2017, 101, 177–189. [Google Scholar] [CrossRef]
- Gong, H.M.; Wang, M.; Wang, H.W. New energy vehicles in China: Policies, demonstration, and progress. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 207–228. [Google Scholar] [CrossRef]
- Li, Y.S.; Kong, X.X.; Zhang, M. Industrial upgrading in global production networks: The case of the Chinese automotive industry. Asia Pac. Bus. Rev. 2016, 22, 21–37. [Google Scholar] [CrossRef]
- Liu, B.C.; Song, C.Y.; Wang, Q.S.; Zhang, X.M.; Chen, J.L.; Lund, H.; Kaiser, M.J. Research on regional differences of China’s new energy vehicles promotion policies: A perspective of sales volume forecasting. Energy 2022, 248, 123541. [Google Scholar] [CrossRef]
- Rossi, S.; Russo, F.; Calovi, M. Durability of vitreous enamel coatings and their resistance to abrasion, chemicals, and corrosion: A review. J. Coat. Technol. Res. 2021, 18, 39–52. [Google Scholar] [CrossRef]
- Malnieks, K.; Mezinskis, G.; Pavlovska, I.; Bidermanis, L.; Pludons, A. Black enamel for concentrated solar-power receivers. Ceram. Int. 2014, 40, 13321–13327. [Google Scholar] [CrossRef]
- Ren, B.Y.; Luo, D.S.; Dai, Y.X.; Tong, Z. Preparation and properties of anti-adhering and acid-resistant tempered glass ink applied to automotive. J. South China Univ. Technol. 2013, 41, 112–116+132. [Google Scholar]
- Mahdi, S. Organic/inorganic coatings cured by ultraviolet light and moisture for automotive glass. SAE Int. J. Mater. Manuf. 2012, 5, 499–502. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y. Preparation of highly dispersible glass frit powders and its application in ink-jet printing ink. J. Eur. Ceram. Soc. 2020, 40, 3489–3493. [Google Scholar] [CrossRef]
- Jiao, J.X.; Yang, M.; Li, J.W.; Xiong, D.H.; Li, H. A novel high reflective glass-ceramic ink with Bi2Ti2O7 nanocrystals used for the photovoltaic glass backplane. J. Eur. Ceram. Soc. 2023, 43, 3630–3636. [Google Scholar] [CrossRef]
- Kulikova, O.V.; Shchegoleva, N.E.; Lebedeva, Y.E.; Vaganova, M.L.; Chainikova, A.S. Insulating Enamel Based on Lead-Borate Glass. Glass Ceram. 2022, 78, 436–441. [Google Scholar] [CrossRef]
- Schalm, O.; Van der Linden, V.; Frederickx, P.; Luyten, S.; Van der Snickt, G.; Caen, J.; Schryvers, D.; Janssens, K.; Cornelis, E.; Van Dyck, D.; et al. Enamels in stained glass windows: Preparation, chemical composition, microstructure and causes of deterioration. Spectrochim. Acta B 2009, 64, 812–820. [Google Scholar] [CrossRef]
- Yin, S.H.; Wang, H.; Wang, S.F.; Zhang, J.; Zhu, Y.Z. Effect of B2O3 on the radiation shielding performance of telluride lead glass system. Crystals 2022, 12, 178. [Google Scholar] [CrossRef]
- Butenkov, D.A.; Runina, K.I.; Petrova, O.B. Synthesis and properties of Nd-doped chlorofluorosilicate lead glasses. Glass Ceram. 2021, 78, 135–139. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Tijani, S.A. The use of lead-free transparent 50BaO-(50-x) borosilicate-xBi2O3 glass system as radiation shields in nuclear medicine. J. Alloys Compd. 2019, 803, 625–630. [Google Scholar] [CrossRef]
- Tijani, S.A.; Al-Hadeethi, Y. The influence of TeO2 and Bi2O3 on the shielding ability of lead-free transparent bismuth tellurite glass at low gamma energy range. Ceram. Int. 2019, 45, 23572–23577. [Google Scholar] [CrossRef]
- Halimah, M.K.; Azuraida, A.; Ishak, M.; Hasnimulyati, L. Influence of bismuth oxide on gamma radiation shielding properties of boro-tellurite glass. J. Non-Cryst. Solids 2019, 512, 140–147. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. BaO-Li2O-B2O3 glass systems: Potential utilization in gamma radiation protection. Prog. Nucl. Energy 2020, 129, 103511. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass wastes for the development of geopolymer composites–Durability, thermal and microstructural properties: A review. Constr. Build. Mater. 2019, 222, 673–687. [Google Scholar] [CrossRef]
- Stalin, S.; Gaikwad, D.K.; Al-Buriahi, M.S.; Srinivasu, C.; Ahmed, S.A.; Tekin, H.O.; Rahman, S. Influence of Bi2O3/WO3 substitution on the optical, mechanical, chemical durability and gamma ray shielding properties of lithium-borate glasses. Ceram. Int. 2021, 47, 5286–5299. [Google Scholar] [CrossRef]
- Kalio, A.; Leibinger, M.; Filipovic, A.; Krüger, K.; Glatthaar, M.; Wilde, J. Development of lead-free silver ink for front contact metallization. Sol. Energy Mater. Sol. Cells 2012, 106, 51–54. [Google Scholar] [CrossRef]
- Adeyemi, J.; Onwudiwe, D. Chemistry and some biological potential of bismuth and antimony dithiocarbamate complexes. Molecules 2020, 25, 305. [Google Scholar] [CrossRef] [PubMed]
- Maeder, T. Review of Bi2O3 based glasses for electronics and related applications. Int. Mater. Rev. 2013, 58, 3–40. [Google Scholar] [CrossRef]
- Hussein, E.M.A.; Madbouly, A.M.; El Alaily, N.A. Gamma ray interaction of optical, chemical, physical behavior of bismuth silicate glasses and their radiation shielding proficiency using Phy-X/PSD program. J. Non-Cryst. Solids 2021, 570, 121021. [Google Scholar] [CrossRef]
- Laourayed, M.; El Moudane, M.; Khachani, M.; Boudalia, M.; Guenbour, A.; Bellaouchou, A.; Tabyaoui, M. Effect of the Bi2O3 on the thermal, structural and chemical durability of some bismuth niobium phosphate glasses. Mater. Today 2019, 13, 974–981. [Google Scholar] [CrossRef]
- Emlemdi, H. Durable Functional Glass Enamel Coating for Automotive Applications. U.S. Patent 7528084, 5 May 2009. [Google Scholar]
- Singh, S.K.; Sakoske, G.E.; Klimas, D. Glass Enamel for Automotive Applications. U.S. Patent 20150013390, 15 January 2015. [Google Scholar]
- Li, S.; Zhang, J.; Li, M.M.; Liu, J.; Wang, X.Y.; Mei, X.H.; Zhang, Y. Study on preparation and properties of boron bismuth/fluorooxy glass and glass-ceramics. J. Non-Cryst. Solids 2021, 561, 120749. [Google Scholar] [CrossRef]
- El-Rehim, A.F.; Shaaban, K.S.; Zahran, H.Y.; Yahia, I.S.; Ali, A.M.; Halaka, M.M.A.; Makhlouf, S.A.; Wahab, E.A.A.; Shaaban, E.R. Structural and mechanical properties of Lithium bismuth borate glasses containing molybdenum (LBBM) together with their glass-ceramics. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1057–1065. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Folz, D.C.; Suchicital, C.T.; Clark, D. Estimate of the crystallization volume fraction in lithium disilicate glass-ceramics using Fourier transform infrared reflectance spectroscopy. J. Eur. Ceram. Soc. 2015, 35, 597–604. [Google Scholar] [CrossRef]
- Plekhovich, A.D.; Kut’in, A.M.; Rostokina, E.E.; Komshina, M.E.; Balueva, K.V.; Ignatova, K.F.; Shiryaev, V.S. Controlled crystallization of BaO–B2O3–Bi2O3 Glass in the temperature range of a supercooled melt in the presence of additional nucleation centers. J. Non-Cryst. Solids 2022, 588, 121629. [Google Scholar] [CrossRef]
- Yang, Y.B.; Wu, L.; Teng, Y.C.; Jiang, F.; Lei, J.; Chen, H.; Cheng, J.H. Effect of ZnO content on microstructure, crystallization behavior, and thermal properties of xZnO-30B2O3-(65-x) Bi2O3-5BaO glass. J. Non-Cryst. Solids 2019, 511, 29–35. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, M.; Zhang, C.Y.; Meng, F.C.; Lin, H.X. Effect of SrO Content on Structure, Thermal Properties and Chemical Stability of Bi2O3-B2O3-ZnO-SrO Low-melting Glass for Si-Al Alloy Package. J. Wuhan Univ. Technol. 2020, 35, 368–376. [Google Scholar] [CrossRef]
- Yang, J.K.; Liang, B.; Zhao, M.J.; Gao, Y.; Zhang, F.C.; Zhao, H.L. Reference of Temperature and Time during tempering process for non-stoichiometric FTO films. Sci. Rep. 2015, 5, 15001. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.A.; Madbouly, A.M.; Eldin, F.M.E.; ElAlaily, N.A. Evaluation of physical and radiation shielding properties of Bi2O3–B2O3 glass doped transition metals ions. Mater. Chem. Phys. 2021, 261, 124212. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, K.J.; Anand, V. Structural properties of Bi2O3–B2O3–SiO2–Na2O glasses for gamma ray shielding applications. Radiat. Phys. Chem. 2016, 120, 63–72. [Google Scholar] [CrossRef]
- Dararutana, P.; Duchaneephet, J.; Pongkrapan, S.; Sirikulrat, N.; Wathanakul, P. Effect of Bi2O3 content on optical and radiation shielding properties of (Na2O, K2O)-SiO2–CaO-Al2O3–B2O3 glass system. Optoelectron. Adv. Mat. 2009, 3, 525–527. [Google Scholar]
- Xu, J.J.; Lin, P.P.; Chen, Q.Q.; Zhao, X.; He, P.; Lin, T.S.; Jiang, C.L.; Liu, Y.; Liu, H.Z.; Long, W.M. Evolutionary mechanism of YBO3 whisker and its effect on the properties of YIG ferrite joint brazed by Bi2O3–B2O3–SiO2–ZnO glass. Ceram. Int. 2021, 47, 973–983. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Maier, J. Basic properties of the F-type centers in halides, oxides and perovskites. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 3084–3089. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Kostyk, L.; Tsvetkova, O.; Popov, A.I. Shallow and deep trap levels in X-ray irradiated β-Ga2O3: Mg. Nucl. Instrum. Meth. B 2019, 441, 12–17. [Google Scholar] [CrossRef]
- Klym, H.; Ingram, A.; Shpotyuk, O.; Hotra, O.; Popov, A.I. Positron trapping defects in free-volume investigation of Ge–Ga–S–CsCl glasses. Radiat. Meas. 2016, 90, 117–121. [Google Scholar] [CrossRef]
- Li, X.Y.; Xiao, Z.H.; He, Y.T.; Wang, Y.Q.; Luo, J.Z.; Shi, J.J.; Huang, Q.; Kong, L.B. Crystallization behavior, structure and properties of glasses in SrO–Fe2O3–P2O5 system. J. Non-Cryst. Solids 2019, 523, 119588. [Google Scholar] [CrossRef]
- Qiang, L.Y.; Zhang, X.Z.; Ai, Y.Z.T.; Zhuan, Y.; Sheng, J.; Zhao, H.Y.; Ni, J.X.; Yang, K. Crystallization behavior and thermal stability mechanism of plasma sprayed Al2O3-GAP amorphous ceramic coatings. J. Eur. Ceram. Soc. 2022, 42, 2018–2121. [Google Scholar] [CrossRef]
- Jiang, H.R.; Hu, J.Y.; Neuber, N.; Bochtler, B.; Adam, B.; Riegler, S.S.; Frey, M.; Ruschel, L.; Lu, W.F.; Feng, A.H.; et al. Effect of sulfur on the glass-forming ability, phase transformation, and thermal stability of Cu-Zr-Al bulk metallic glass. Acta Mater. 2021, 212, 116923. [Google Scholar] [CrossRef]
- Mohammed, A.; Lakshminarayana, G.; Baki, S.O.; Bashar, K.A.; Kityk, I.V.; Mahdi, M.A. Optical and dielectric studies for Tb3+/Sm3+ co-doped borate glasses for solid-state lighting applications. Opt. Mater. 2018, 86, 387–393. [Google Scholar] [CrossRef]
- Çelikbilek Ersundu, M.; Ersundu, A.E. Structure and crystallization kinetics of lithium tellurite glasses. J. Non-Cryst. Solids 2016, 453, 150–157. [Google Scholar] [CrossRef]
- Wang, S.H.; Li, X.N.; Wang, C.; Bai, M.M.; Zhou, X.J.; Zhang, X.Z.; Wang, Y.Q. Anorthite-based transparent glass-ceramic glaze for ceramic tiles: Preparation and crystallization mechanism. J. Eur. Ceram. Soc. 2022, 42, 1132–1140. [Google Scholar] [CrossRef]
- Baranowska, A.; Leśniak, M.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Dorosz, D. Crystallization kinetics and structural properties of the 45S5 bioactive glass and glass-ceramic fiber doped with Eu3+. Materials 2020, 13, 1281. [Google Scholar] [CrossRef]
- Hivrekar, M.M.; Sable, D.B.; Solunke, M.B.; Jadhav, K.M. Network structure analysis of modifier CdO doped sodium borate glass using FTIR and Raman spectroscopy. J. Non-Cryst. Solids 2017, 474, 58–65. [Google Scholar] [CrossRef]
- Shi, X.Z.; Gu, Y.; Liu, T.Y.; Jiang, Z.H.; Li, R.; Zeng, F.H. Effect of different P2O5/SnF2 ratios on the structure and properties of phosphate glass. J. Non-Cryst. Solids 2022, 578, 121350. [Google Scholar] [CrossRef]
- Saritha, D.; Markandeya, Y.; Salagram, M.; Vithal, M.; Singh, A.K.; Bhikshamaiah, G. Effect of Bi2O3 on physical, optical and structural studies of ZnO-Bi2O3-B2O3 glasses. J. Non-Cryst. Solids 2008, 354, 5573–5579. [Google Scholar] [CrossRef]
- Rada, S.; Pascuta, P.; Bosca, M.; Pop, L.; Culea, E. Structural properties of the boro-bismuthate glasses containing gadolinium ions. Vib. Spectrosc. 2008, 48, 255–258. [Google Scholar] [CrossRef]
- Emami, S.; Martins, J.; Andrade, L.; Mendes, J.; Mendes, A. Low temperature hermetic laser-assisted glass frit encapsulation of soda-lime glass substrates. Opt. Lasers Eng. 2017, 96, 107–116. [Google Scholar] [CrossRef]
- Lei, W.C.; Luo, Z.W.; He, Y.; Zhang, P.; Liu, S.X.; Lu, A.X. ZrO2-doped transparent glass-ceramics embedding ZnO nano-crystalline with enhanced defect emission for potential yellow-light emitter applications. Ceram. Int. 2021, 47, 35073–35080. [Google Scholar] [CrossRef]
- Ray, C.S.; Yang, Q.Z.; Huang, W.H.; Day, D.E. Surface and internal crystallization in glasses as determined by differential thermal analysis. J. Am. Ceram. Soc. 1996, 79, 3155–3160. [Google Scholar] [CrossRef]
- Todea, M.; Turcu, R.V.F.; Vasilescu, M.; Trandafir, D.L.; Simon, S. Structural characterization of heavy metal SiO2-Bi2O3 glasses and glass–ceramics. J. Non-Cryst. Solids 2016, 432, 271–276. [Google Scholar] [CrossRef]
- Guo, H.W.; Wang, X.F.; Gao, D.N. Non-isothermal crystallization kinetics and phase transformation of Bi2O3-SiO2 glass-ceramics. Sci. Sinter. 2011, 43, 353–362. [Google Scholar] [CrossRef]
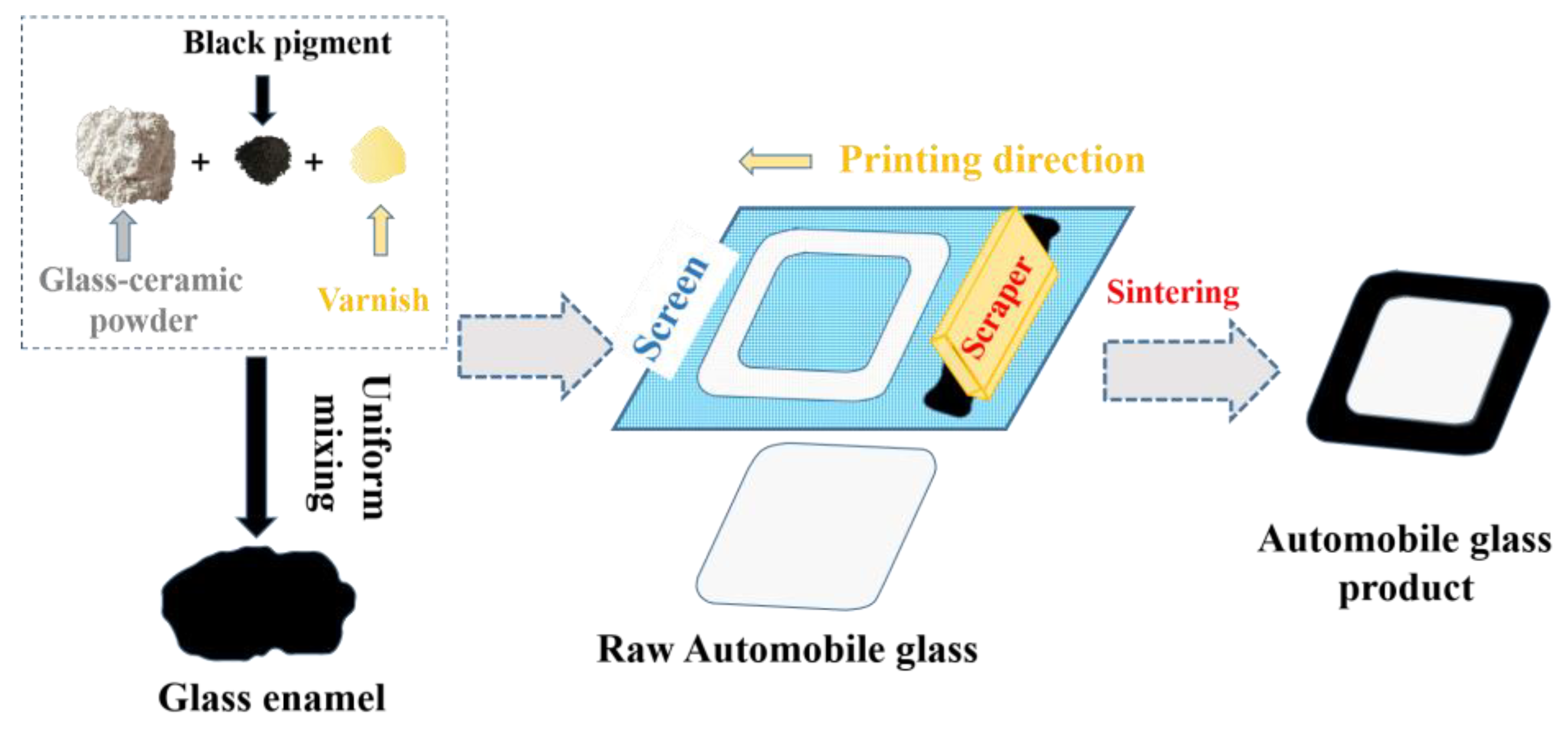
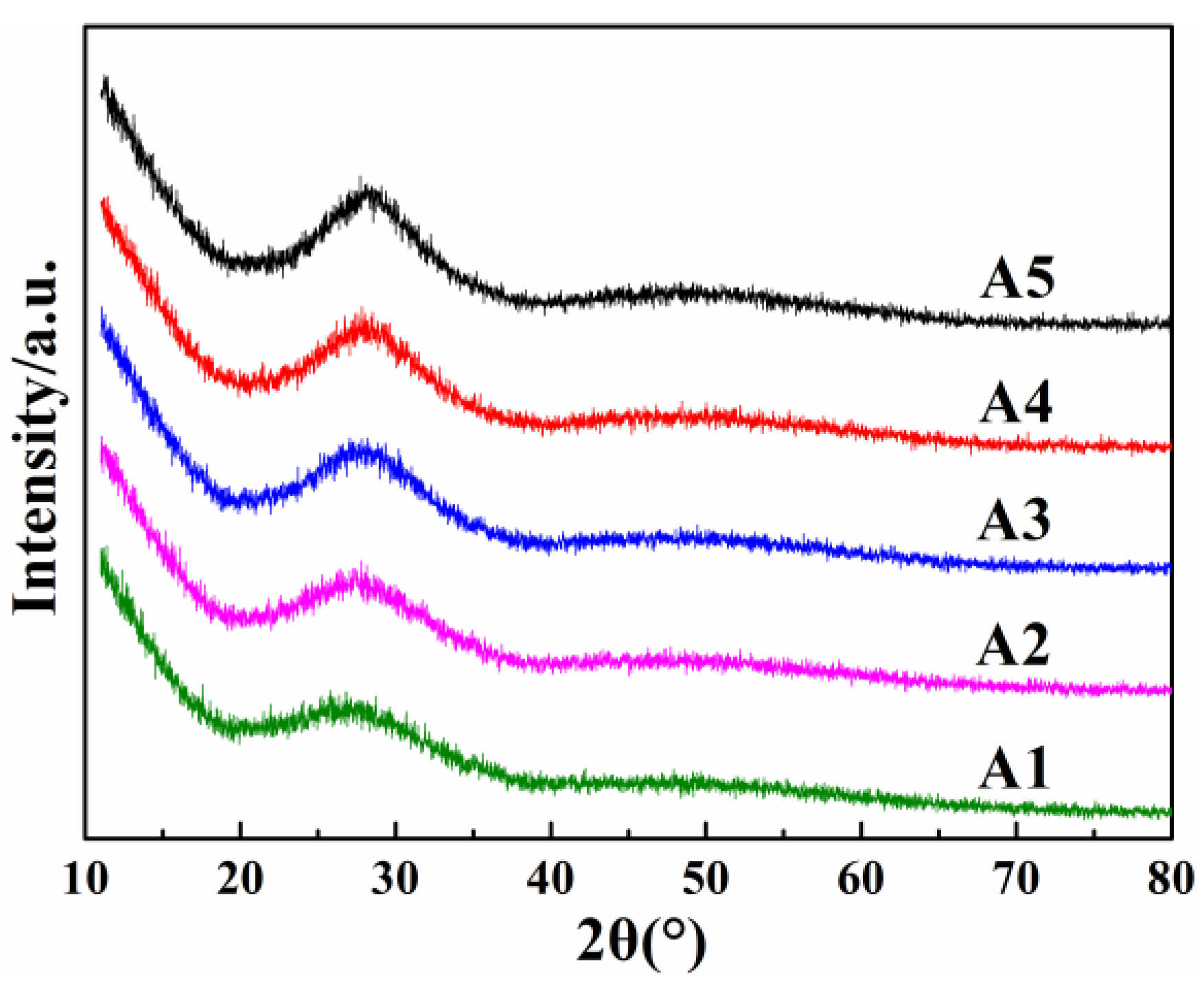


| Sample Codes | R2O (mol%) | Bi2O3 (mol%) | B2O3 (mol%) | SiO2 (mol%) |
|---|---|---|---|---|
| A1 | 15 | 10 | 10 | 65 |
| A2 | 15 | 15 | 10 | 60 |
| A3 | 15 | 20 | 10 | 55 |
| A4 | 15 | 25 | 10 | 50 |
| A5 | 15 | 30 | 10 | 45 |
| Sample Codes | α30~300 °C (±0.2 × 10−6/°C) | Tg(Dil) (±5 °C) | Tf (±5 °C) |
|---|---|---|---|
| A1 | 7.93 | 449 | 497 |
| A2 | 8.32 | 441 | 484 |
| A3 | 8.77 | 435 | 473 |
| A4 | 9.05 | 430 | 462 |
| A5 | 9.89 | 419 | 446 |
| Sample Codes | Tg(DTA) (±5 °C) | Tx (±5 °C) | Tp1 (±5 °C) | Tp2 (±5 °C) | Tp3 (±5 °C) | S (±5 °C) |
|---|---|---|---|---|---|---|
| A1 | 442 | 617 | 653 | / | / | 175 |
| A2 | 437 | 578 | 615 | 654 | / | 141 |
| A3 | 427 | 554 | 585 | 611 | 651 | 127 |
| A4 | 426 | 545 | 574 | 606 | 659 | 119 |
| A5 | 421 | 539 | 573 | 602 | 681 | 118 |
| Heating Rates (°C/min) | Tp1 (±5 °C) | Tp2 (±5 °C) | Tp3 (±5 °C) |
|---|---|---|---|
| 5 | 574 | 606 | 659 |
| 10 | 593 | 630 | 681 |
| 15 | 604 | 646 | 688 |
| 20 | 613 | 658 | 696 |
| Ec and n values | |||
| Ec (kJ/mol) | Ec1 = 208.4 | Ec2 = 169.4 | Ec3 = 263.6 |
| n | 2.06 | 2.58 | 2.23 |
| Base Glass Codes | Heat Treatment Schedules | Heated Base Glass Codes | Crystalline Phases | DRAcid (×10−5 g·cm−2·min−1) |
|---|---|---|---|---|
| A1 | 610 °C/2 h | A1-1 | Bi4Si3O12 | 2.01 |
| 650 °C/2 h | A1-2 | Bi4Si3O12 | 0.38 | |
| A2 | 570 °C/2 h | A2-1 | No crystals | 3.32 |
| 610 °C/2 h | A2-2 | Bi2O2SiO3 | 1.38 | |
| 650 °C/2 h | A2-3 | Bi4Si3O12 | 0.38 | |
| A3 | 550 °C/2 h | A3-1 | No crystals | 6.30 2.66 1.82 0.52 |
| 580 °C/2 h | A3-2 | Bi2O2SiO3 (major phase); Bi2SiO5 (minor phase) | 2.66 | |
| 610 °C/2 h | A3-3 | Bi2O2SiO3 (major phase); Bi2SiO5 (secondary phase); Bi4Si3O12 (minor phase) | 1.82 | |
| 650 °C/2 h | A3-4 | Bi4Si3O12 | 0.52 | |
| A4 | 540 °C/2 h | A4-1 | No crystals | 9.63 |
| 570 °C/2 h | A4-2 | Bi2O2SiO3 (major phase); Bi2SiO5 (minor phase) | 4.14 | |
| 600 °C/2 h | A4-3 | Bi2O2SiO3 (major phase); Bi2SiO5 (secondary phase); Bi4Si3O12 (minor phase) | 2.95 | |
| 650 °C/2 h | A4-4 | Bi4Si3O12 | 0.83 | |
| A5 | 530 °C/2 h | A5-1 | No crystals | 12.24 |
| 570 °C/2 h | A5-2 | Bi2O2SiO3 (major phase); Bi2SiO5 (minor phase) | 8.14 | |
| 600 °C/2 h | A5-3 | Bi2O2SiO3 (major phase); Bi2SiO5 (secondary phase); Bi4Si3O12 (minor phase) | 5.61 | |
| 680 °C/2 h | A5-4 | Bi4Si3O12 | 2.45 |
| Properties | Our Products | Similar Product in Market |
|---|---|---|
| DRAcid (g·cm−2·min−1) | 2.9 × 10−6 | 6.4 × 10−5 |
| Adhesive force, (ISO grade) | 0 | 0 |
| Chroma (L, a, and b) | L: 6.39, a: −0.08, b: −0.08 | L: 6.17, a: −0.10, b: −0.09 |
| Optical density, (OD) | 3.71 | 3.55 |
| Glossiness, G (Gs) | 6.5 | 7.8 |
| Roughness, Ra, Rz (μm) | Ra: 0.624 Rz: 4.393 | Ra: 0.611 Rz: 4.256 |
| Surface tension, σ (mN/m) | 44 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Wang, W.; Liu, K.; Liu, L.; Dong, W.; Bao, Q.; Xu, H.; Zhou, J. Preparation of Glass-Ceramics in the R2O-Bi2O3-B2O3-SiO2 System Applied in Automobile Glass Enamel. Inorganics 2023, 11, 166. https://doi.org/10.3390/inorganics11040166
Zhao T, Wang W, Liu K, Liu L, Dong W, Bao Q, Xu H, Zhou J. Preparation of Glass-Ceramics in the R2O-Bi2O3-B2O3-SiO2 System Applied in Automobile Glass Enamel. Inorganics. 2023; 11(4):166. https://doi.org/10.3390/inorganics11040166
Chicago/Turabian StyleZhao, Tiangui, Wei Wang, Kun Liu, Li Liu, Weixia Dong, Qifu Bao, Heliang Xu, and Jianer Zhou. 2023. "Preparation of Glass-Ceramics in the R2O-Bi2O3-B2O3-SiO2 System Applied in Automobile Glass Enamel" Inorganics 11, no. 4: 166. https://doi.org/10.3390/inorganics11040166
APA StyleZhao, T., Wang, W., Liu, K., Liu, L., Dong, W., Bao, Q., Xu, H., & Zhou, J. (2023). Preparation of Glass-Ceramics in the R2O-Bi2O3-B2O3-SiO2 System Applied in Automobile Glass Enamel. Inorganics, 11(4), 166. https://doi.org/10.3390/inorganics11040166





