Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
2.1. Mapping Transmission Electron Microscopy
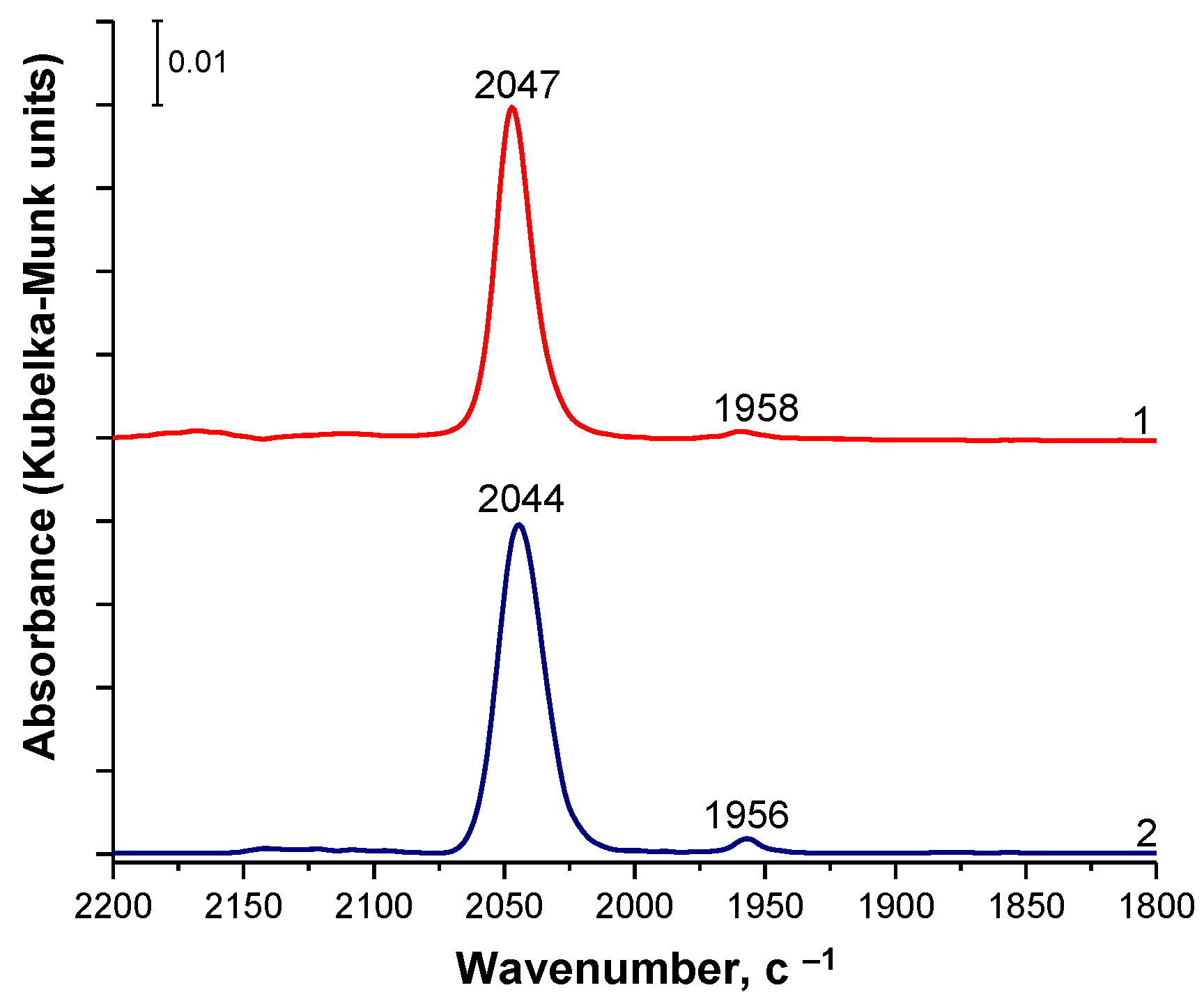
2.2. DRIFT Spectroscopy of Adsorbed CO (DRIFTS-CO)
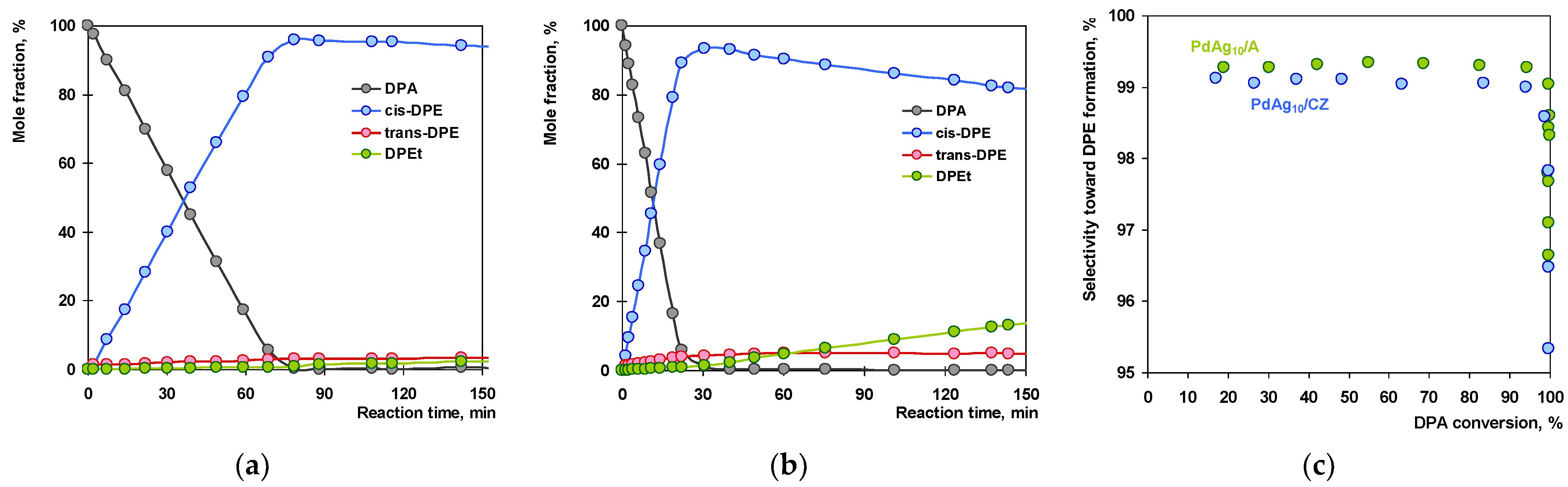
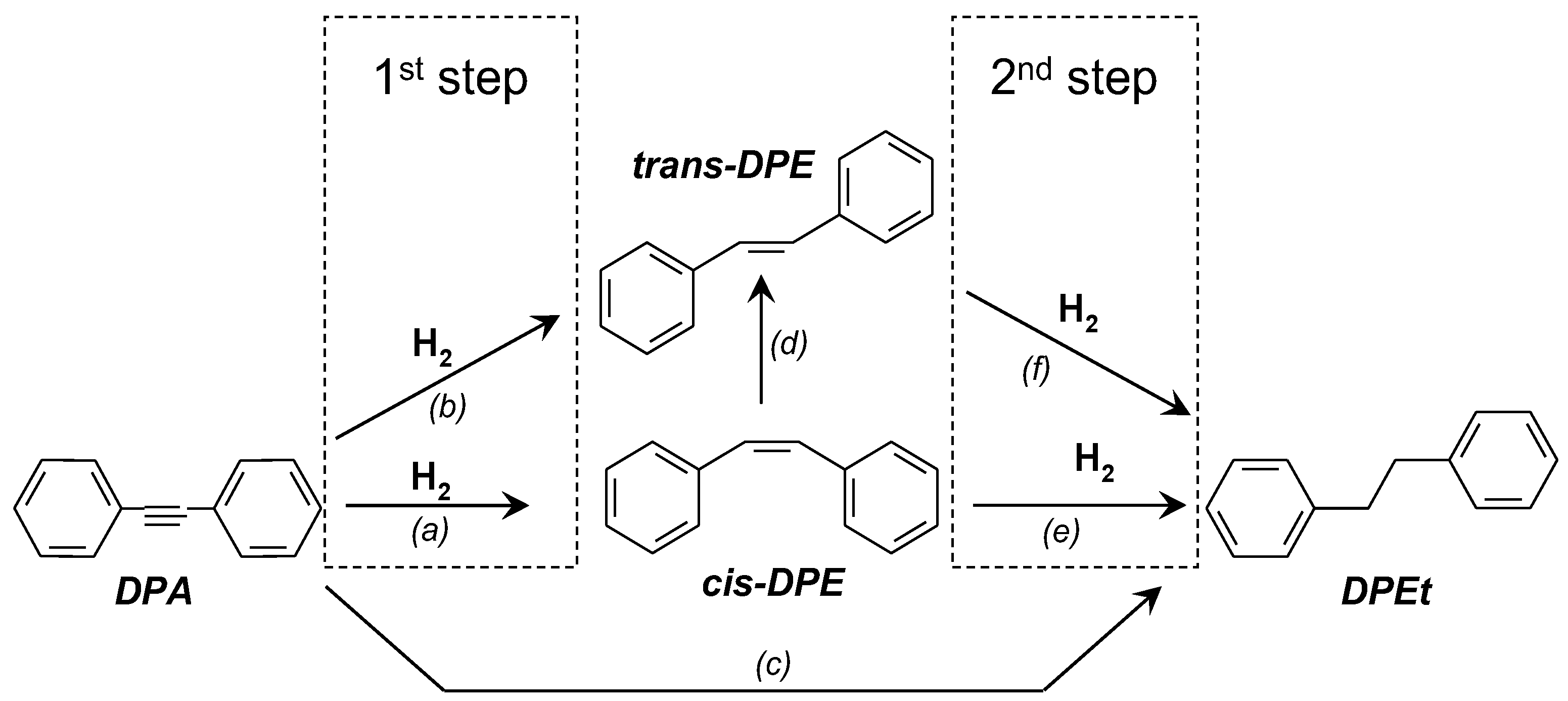
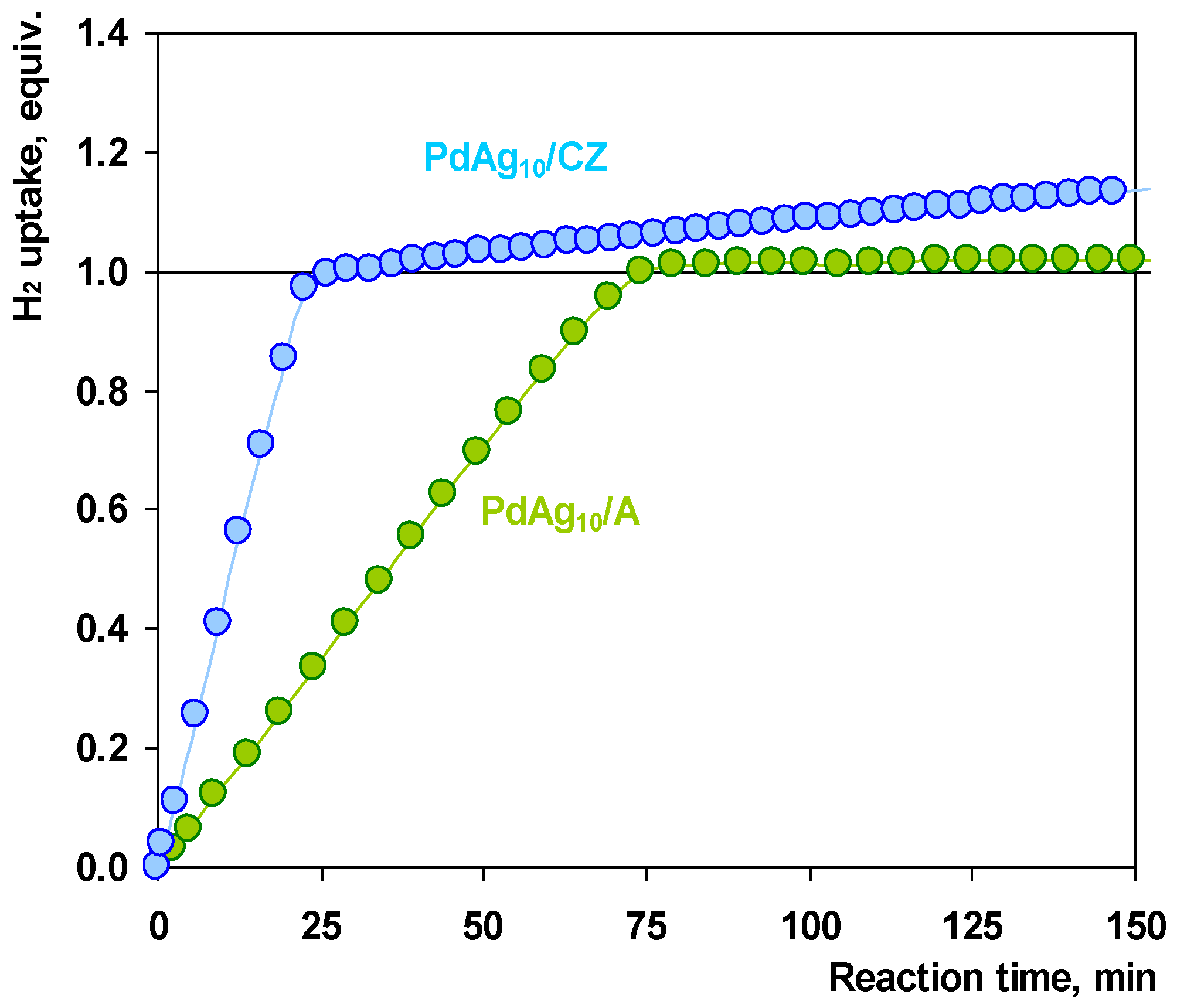
2.3. Catalytic Tests
2.4. Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. High-Resolution Transmission Electron Microscopy
3.4. DRIFT Spectroscopy of Adsorbed CO
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, K.; Pinchuk, A.O. Noble Metal Nanomaterials: Synthetic Routes, Fundamental Properties, and Promising Applications. Solid State Phys. 2015, 66, 131–211. [Google Scholar]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chemistry 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Patil, K.N.; Manikanta, P.; Srinivasappa, P.M.; Jadhav, A.H.; Nagaraja, B.M. State-of-the-art and perspectives in transition metal-based heterogeneous catalysis for selective hydrogenation of cinnamaldehyde. J. Environ. Chem. Eng. 2023, 11, 109168. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. A critical review of research progress for metal alloy materials in hydrogen evolution and oxygen evolution reaction. Environ. Sci. Pollut. Res. 2022, 30, 11302–11320. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, Y.; Chen, W.-J.; Wu, Q.; Pan, X.; Chen, K.; Weng, B. Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation. ACS Omega 2022, 7, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Glyzdova, D.V.; Smirnova, N.S.; Shlyapin, D.A.; Tsyrul’nikov, P.G. Gas-Phase and Liquid-Phase Hydrogenation of Acetylene in Lean and Enriched Mixtures over Supported Modified Palladium Catalysts. Russ. J. Gen. Chem. 2020, 90, 1120–1140. [Google Scholar] [CrossRef]
- Shittu, T.D.; Ayodele, O.B. Catalysis of semihydrogenation of acetylene to ethylene: Current trends, challenges, and outlook. Front. Chem. Sci. Eng. 2022, 16, 1031–1059. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Liang, C. Highly selective catalysts for the hydrogenation of alkynols: A review. Chin. J. Catal. 2021, 42, 2105–2121. [Google Scholar] [CrossRef]
- Deng, X.; Wang, J.; Guan, N.; Li, L. Catalysts and mechanisms for the selective heterogeneous hydrogenation of carbon-carbon triple bonds. Cell Rep. Phys. Sci. 2022, 3, 101017. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Li, Y. The electronic structure and geometric structure of nanoclusters as catalytic active sites. Nanotechnol. Rev. 2013, 2, 515–528. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloy Catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lu, J.; Wang, M.; Wang, Y.; Wang, F. Single Atom Alloy Preparation and Applications in Heterogeneous Catalysis. Chin. J. Chem. 2019, 37, 977–988. [Google Scholar] [CrossRef]
- Mao, J.; Yin, J.; Pei, J.; Wang, D.; Li, Y. Single atom alloy: An emerging atomic site material for catalytic applications. Nano Today 2020, 34, 100917. [Google Scholar] [CrossRef]
- Ciriminna, R.; Ghahremani, M.; Karimi, B.; Pagliaro, M.; Luque, R. Single-atom catalysis: A practically viable technology? Curr. Opin. Green Sustain. Chem. 2020, 25, 100358. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Wu, C.; Sui, X.; Wang, Q.; He, J. Effects of Ag Atomic Segregation from Different Planes on the Size-Dependent Melting of Ag–Pd Clusters. J. Cluster Sci. 2016, 27, 55–62. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Cherepanova, S.V.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Semihydrogenation of 2-methyl-3-butyn-2-ol on Pd-Zn nanoalloys: Effect of composition and heterogenization. Appl. Catal. A General 2018, 549, 245–253. [Google Scholar] [CrossRef]
- Concepción, P.; García, S.; Hernández-Garrido, J.C.; Calvino, J.J.; Corma, A. A promoting effect of dilution of Pd sites due to gold surface segregation under reaction conditions on supported Pd–Au catalysts for the selective hydrogenation of 1,5-cyclooctadiene. Catal. Today 2016, 259, 213–221. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kirichenko, O.A.; Kozlova, L.M.; Kapustin, G.I.; Mishin, I.V.; Strelkova, A.A.; Kustov, L.M. Liquid-phase hydrogenation of phenylacetylene to styrene on silica-supported Pd–Fe nanoparticles. Mendeleev Commun. 2016, 26, 228–230. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Liu, J.; Murphy, C.J.; Liriano, M.L.; Wasio, N.A.; Lucci, F.R.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective Formic Acid Dehydrogenation on Pt-Cu Single-Atom Alloys. ACS Catal. 2016, 7, 413–420. [Google Scholar] [CrossRef]
- Lucci, F.R.; Liu, J.; Marcinkowski, M.D.; Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit. Nat. Commun. 2015, 6, 8550. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Chen, S.; Fauth, C.; Häring, T.; Bansmann, J. Fundamental Aspects of Ceria Supported Au Catalysts Probed by In Situ/Operando Spectroscopy and TAP Reactor Studies. ChemPhysChem 2021, 22, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzakc, V.; Jürgen Behm, R. CO Oxidation over Supported Gold Catalysts—“Inert” and “Active” Support Materials and Their Role for the Oxygen Supply during Reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Razmgar, K.; Altarawneh, M.; Oluwoye, I.; Senanayake, G. Ceria-Based Catalysts for Selective Hydrogenation Reactions: A Critical Review. Catal. Surv. Asia 2021, 25, 27–47. [Google Scholar] [CrossRef]
- Anand, S.; Pinheiro, D.; Sunaja Devi, K.R. Recent Advances in Hydrogenation Reactions Using Bimetallic Nanocatalysts: A Review. Asian J. Org. Chem. 2021, 10, 3068. [Google Scholar] [CrossRef]
- Nelson, N.C.; Manzano, J.S.; Sadow, A.D.; Overbury, S.H.; Slowing, I.I. Selective Hydrogenation of Phenol Catalyzed by Palladium on High-Surface-Area Ceria at Room Temperature and Ambient Pressure. ACS Catal. 2015, 5, 2051–2061. [Google Scholar] [CrossRef]
- Velu, S.; Kapoor, M.P.; Inagaki, S.; Suzuki, K. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2. Appl. Catal. A General 2003, 245, 317–331. [Google Scholar] [CrossRef]
- Teng, M.; Luo, L.; Yang, X. Synthesis of mesoporous Ce1−xZrxO2 (x = 0.2−0.5) and catalytic properties of CuO based catalysts. Microporous Mesoporous Mater. 2009, 119, 158–164. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Zhang, J.; Wang, J.; Guo, M.; Chen, S.; Li, C.; Hu, C.; Xie, Y. One step hydrothermal synthesis of CeO2–ZrO2 nanocomposites and investigation of the morphological evolution. RSC Adv. 2015, 5, 89976–89984. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Intramolecular selective hydrogenation of cinnamaldehyde over CeO2–ZrO2-supported Pt catalysts. J. Catal. 2012, 285, 31–40. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, Y.; Fan, G.; Yang, L.; Li, F. Structure-dependent selective hydrogenation of cinnamaldehyde over high-surface-area CeO2–ZrO2 composites supported Pt nanoparticles. Chem. Eng. J. 2017, 322, 234–245. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Chemoselective Hydrogenation of Cinnamaldehyde over Pd/CeO2–ZrO2 Catalysts. Catal. Lett. 2010, 140, 55–64. [Google Scholar] [CrossRef]
- Vikanova, K.V.; Redina, E.A.; Kapustin, G.I.; Davshan, N.A.; Kustov, L.M. Hydrogenation of Acetophenone at Room Temperature and Atmospheric Pressure over Pt-Containing Catalysts Supported on Reducible Oxides. Russ. J. Phys. Chem. A 2019, 93, 231–235. [Google Scholar] [CrossRef]
- Redina, E.A.; Vikanova, K.V.; Kapustin, G.I.; Mishin, I.V.; Tkachenko, O.P.; Kustov, L.M. Selective Room-Temperature Hydrogenation of Carbonyl Compounds under Atmospheric Pressure over Platinum Nanoparticles Supported on Ceria-Zirconia Mixed Oxide. Eur. J. Org. Chem. 2019, 2019, 4159–4170. [Google Scholar] [CrossRef]
- Redina, E.A.; Vikanova, K.V. Highly Efficient Pt-Catalyst Supported on Mesoporous Ceria-Zirconia Oxide for Hydrogenation of Nitroaromatic Compounds to Anilines. Russ. J. Phys. Chem. A 2018, 92, 2374–2378. [Google Scholar] [CrossRef]
- Vikanova, K.V.; Redina, E.A. Influence of the Specific Surface Area of CeO2–ZrO2 Supports on the Activity of Pt-Containing Catalysts in the Cinnamaldehyde Hydrogenation Reaction. Russ. J. Phys. Chem. A 2018, 92, 2355–2358. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Mashkovsky, I.S.; Baeva, G.N.; Bragina, G.O.; Smirnova, N.S.; Markov, P.V.; Bukhtiyarov, A.V.; Wärnå, J.; Stakheev, A.Y.; Murzin, D.Y. Liquid-Phase Hydrogenation of 1-Phenyl-1-propyne on the Pd1Ag3/Al2O3 Single-Atom Alloy Catalyst: Kinetic Modeling and the Reaction Mechanism. Nanomaterials 2021, 11, 3286. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Bragina, G.O.; Baeva, G.N.; Mashkovsky, I.S.; Stakheev, A.Y. Alumina-Supported Palladium–Silver Bimetallic Catalysts with Single-Atom Pd1 Sites in the Liquid-Phase Hydrogenationof Substituted Alkynes. Kinet. Catal. 2020, 61, 869–878. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Bragina, G.O.; Baeva, G.N.; Mashkovsky, I.S.; Smirnova, N.S.; Gerasimov, E.Y.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. Highly Active Bimetallic Single-Atom Alloy PdAg Catalysts on Cerium-Containing Supports in the Hydrogenation of Alkynes to Alkenes. Kinet. Catal. 2022, 63, 756–764. [Google Scholar] [CrossRef]
- Meunier, F.C. Relevance of IR Spectroscopy of Adsorbed CO for the Characterization of Heterogeneous Catalysts Containing Isolated Atoms. J. Phys. Chem. C 2021, 125, 21810–21823. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Zhang, J.; Huang, Y.; Liu, B. In Situ/Operando Techniques for Characterization of Single-Atom Catalysts. ACS Catal. 2019, 9, 2521–2531. [Google Scholar] [CrossRef]
- Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T.V.; Jentoft, F.C.; Knop-Gericke, A.; Grin, Y.; Schlögl, R. A new approach to well-defined, stable and site-isolated catalysts. Sci. Technol. Adv. Mater. 2007, 8, 420–427. [Google Scholar] [CrossRef]
- Pei, G.H.; Liu, X.Y.; Wang, A.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.; Yang, X.; Wang, X.; Tai, Z.; et al. Ag Alloyed Pd Single-Atom Catalysts for Efficient Selective Hydrogenation of Acetylene to Ethylene in Excess Ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Bragina, G.O.; Baeva, G.N.; Smirnova, N.S.; Kazakov, A.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. Formation of Isolated Single-Atom Pd1 Sites on the Surface of Pd–Ag/Al2O3 Bimetallic Catalysts. Kinet. Catal. 2020, 61, 758–767. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Bragina, G.O.; Baeva, G.N.; Smirnova, N.S.; Kazakov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Liquid-Phase Hydrogenation of Internal and Terminal Alkynes on Pd–Ag/Al2O3 Catalyst. Kinet. Catal. 2019, 60, 642–649. [Google Scholar] [CrossRef]
- Primet, M.; Mathieu, M.V.; Sachtler, W.M.H. Infrared Spectra of Carbon Monoxide Adsorbed on Silica-Supported PdAg Alloys. J. Catal. 1976, 44, 324–327. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Lopez-Sanchez, J.A.; Jackson, S.D.; Klapotke, T.M.; Baumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Baeva, G.N.; Stakheev, A.Y. Hydroisomerization of cis-Stilbene into trans-Stilbene on Supported Heterogeneous Metal Catalysts (Rh, Pd, Pt, Ru, Ir/α-Al2O3). Kinet. Catal. 2017, 58, 771–779. [Google Scholar] [CrossRef]
- Markov, P.V.; Smirnova, N.S.; Baeva, G.N.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Intermetallic PdxIny/Al2O3 catalysts with isolated single-atom Pd sites for one-pot hydrogenation of diphenylacetylene into trans-stilbene. Mendeleev Commun. 2020, 30, 468–471. [Google Scholar] [CrossRef]
- Reddy, B.M.; Rao, K.N.; Reddy, G.K. Controlled Hydrogenation of Acetophenone Over Pt/CeO2–MOx (M = Si, Ti, Al, and Zr) Catalysts. Catal. Lett. 2009, 131, 328–336. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luettich, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Effect of the support composition on the vapor-phase hydrogenation of crotonaldehyde over Pt/CexZr1−xO2 catalysts. J. Catal. 2006, 241, 45–55. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Mashkovsky, I.S.; Bragina, G.O.; Baeva, G.N.; Markov, P.V.; Smirnova, N.S.; Wärnå, J.; Stakheev, A.Y.; Murzin, D.Y. Kinetics of liquid-phase diphenylacetylene hydrogenation on “single-atom alloy” Pd-Ag catalyst: Experimental study and kinetic analysis. Mol. Catal. 2021, 506, 111550. [Google Scholar] [CrossRef]
- Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloys as a Reductionist Approach to the Rational Design of Heterogeneous Catalysts. Acc. Chem. Res. 2019, 52, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Uhlman, M.B.; Montemore, M.M.; Trimpalis, A.; Giannakakis, G.; Shan, J.; Cao, S.; Hannagan, R.T.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Integrated Catalysis-Surface Science-Theory Approach to Understand Selectivity in the Hydrogenation of 1-Hexyne to 1-Hexene on PdAu Single-Atom Alloy Catalysts. ACS Catal. 2019, 9, 8757–8765. [Google Scholar] [CrossRef]
- Chen, B.; Dingerdissen, U.; Krauter, J.G.E.; Lansink Rotgerink, H.G.J.; Möbus, K.; Ostgard, D.J.; Panster, P.; Riermeier, T.H.; Seebald, S.; Tacke, T.; et al. New developments in hydrogenation catalysis particularly in synthesis of fine and intermediate chemicals. Appl. Catal. A General 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Tkachenko, O.P.; Mashkovsky, I.S.; Yakushev, I.A.; Kozitsyna, N.Y.; Vargaftik, M.N.; Stakheev, A.Y. Pd–Cu Catalysts from Acetate Complexes in Liquid Phase Diphenylacetylene Hydrogenation. Kinet. Catal. 2015, 56, 591–597. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Bragina, G.O.; Wärnå, J.; Gerasimov, E.Y.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Murzin, D.Y. Particle size effect in liquid-phase hydrogenation of phenylacetylene over Pd catalysts: Experimental data and theoretical analysis. Chem. Eng. J. 2019, 35.8, 520–530. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Bragina, G.O.; Wärnå, J.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Murzin, D.Y. Experimental and theoretical analysis of particle size effect in liquid-phase hydrogenation of diphenylacetylene. Chem. Eng. J. 2021, 404, 126409. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Kustov, L.M. Effects of the support on the morphology and electronic properties of supported metal clusters: Modern concepts and progress in 1990s. Appl. Catal. A 1999, 188, 3–35. [Google Scholar] [CrossRef]
- Yudanov, I.V.; Sahnoun, R.; Neyman, K.M.; Rösch, N.; Hoffmann, J.; Schauermann, S.; Johánek, V.; Unterhalt, H.; Rupprechter, G.; Libuda, J.; et al. CO Adsorption on Pd Nanoparticles: Density Functional and Vibrational Spectroscopy Studies. J. Phys. Chem. B 2003, 107, 255–264. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Zanaveskin, L.N.; Smirnov, V.V.; Aver’yanov, V.A.; Zanaveskin, K.L. Catalytic hydrogenation of alkyne and alkadiene impurities in alkenes. Practical and theoretical aspects. Russ. Chem. Rev. 2009, 78, 231–247. [Google Scholar] [CrossRef]
- Alifanti, M.; Baps, B.; Blangenois, N.; Naud, J.; Grange, P.; Delmon, B. Characterization of CeO2–ZrO2 Mixed Oxides. Comparison of the Citrate and Sol-Gel Preparation Methods. Chem. Mater. 2003, 15, 395–403. [Google Scholar] [CrossRef]
- Liang, Q.; Wu, X.; Wu, X.; Weng, D. Role of Surface Area in Oxygen Storage Capacity of Ceria–Zirconia as Soot Combustion Catalyst. Catal. Lett. 2007, 119, 265–270. [Google Scholar] [CrossRef]
- Spee, M.P.R.; Boersma, J.; Meijer, M.D.; Slagt, M.Q.; van Koten, G.; Geus, J.W. Selective Liquid-Phase Semihydrogenation of Functionalized Acetylenes and Propargylic Alcohols with Silica-Supported Bimetallic Palladium-Copper Catalysts. J. Org. Chem. 2001, 66, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.A.; Chaudhari, R.V. Three-Phase Catalytic Reactors; Gordon and Breach: Philadelphia, PA, USA, 1983; p. 32. [Google Scholar]
- Weisz, P.B.; Prater, C.D. Interpretation of Measurements in Experimental Catalysis. Adv. Catal. 1954, 6, 143–196. [Google Scholar]

| Catalyst | Calculation on the GC Data | Calculation on the Kinetic Data | ||||||
|---|---|---|---|---|---|---|---|---|
| r1 | r2 | r1/r2 | r1 | r2 | r1/r2 | A1 | A2 | |
| mmol DPA (DPEt) min−1 gcat−1 | mmol H2 min−1 gcat−1 | s−1 | ||||||
| PdAg10/CZ | 4.21 | 0.10 | 37.0 | 4.24 | 0.11 | 39.0 | 1.505 | 0.039 |
| PdAg10/A | 1.36 | 0.014 | 95.1 | 1.37 | 0.014 | 97.7 | 0.485 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markov, P.V.; Bragina, G.O.; Smirnova, N.S.; Baeva, G.N.; Mashkovsky, I.S.; Gerasimov, E.Y.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation. Inorganics 2023, 11, 150. https://doi.org/10.3390/inorganics11040150
Markov PV, Bragina GO, Smirnova NS, Baeva GN, Mashkovsky IS, Gerasimov EY, Bukhtiyarov AV, Zubavichus YV, Stakheev AY. Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation. Inorganics. 2023; 11(4):150. https://doi.org/10.3390/inorganics11040150
Chicago/Turabian StyleMarkov, Pavel V., Galina O. Bragina, Nadezhda S. Smirnova, Galina N. Baeva, Igor S. Mashkovsky, Evgeny Y. Gerasimov, Andrey V. Bukhtiyarov, Yan. V. Zubavichus, and Alexander Y. Stakheev. 2023. "Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation" Inorganics 11, no. 4: 150. https://doi.org/10.3390/inorganics11040150
APA StyleMarkov, P. V., Bragina, G. O., Smirnova, N. S., Baeva, G. N., Mashkovsky, I. S., Gerasimov, E. Y., Bukhtiyarov, A. V., Zubavichus, Y. V., & Stakheev, A. Y. (2023). Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation. Inorganics, 11(4), 150. https://doi.org/10.3390/inorganics11040150











