In Vitro Biological Activity of α-Diimine Rhenium Dicarbonyl Complexes and Their Reactivity with Different Functional Groups
Abstract
1. Introduction
2. Results and Discussion
Biological Activity of Rhenium Dicarbonyl Complexes
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instruments and Analysis
3.3. In Vitro Antiproliferative Activity Assay
3.4. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirri, D.; Bartoli, F.; Pratesi, A.; Baglini, E.; Barresi, E.; Marzo, T. Strategies for the Improvement of Metal-Based Chemotherapeutic Treatments. Biomedicines 2021, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.; Zhang, Q.; Zhang, P. Chirality in metal-based anticancer agents. Dalton Trans. 2018, 47, 4017–4026. [Google Scholar] [CrossRef]
- Nasiri Sovari, S.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Schrage, B.R.; Frisinger, B.R.; Schmidtke Sobeck, S.J.; Ziegler, C.J. Lipophilic Re(CO)3pyca complexes for Mid-IR imaging applications. Dalton Trans. 2021, 50, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Raszeja, L.J.; Siegmund, D.; Cordes, A.L.; Güldenhaupt, J.; Gerwert, K.; Hahn, S.; Metzler-Nolte, N. Asymmetric rhenium tricarbonyl complexes show superior luminescence properties in live cell imaging. Chem. Commun. 2017, 53, 905–908. [Google Scholar] [CrossRef]
- Herrick, R.S.; Wrona, I.; McMicken, N.; Jones, G.; Ziegler, C.J.; Shaw, J. Preparation and characterization of rhenium(I) compounds with amino ester derivatized diimine ligands. Investigations of luminescence. Crystal structures of Re(CO)3Cl(pyca-β-Ala-OEt) and Re(CO)3Cl(pyca-l-Asp(OMe)-OMe). J. Organomet. Chem. 2004, 689, 4848–4855. [Google Scholar] [CrossRef]
- Saleh, N.; Srebro, M.; Reynaldo, T.; Vanthuyne, N.; Toupet, L.; Chang, V.Y.; Muller, G.; Williams, J.A.G.; Roussel, C.; Autschbach, J.; et al. enantio-Enriched CPL-active helicene–bipyridine–rhenium complexes. Chem. Commun. 2015, 51, 3754–3757. [Google Scholar] [CrossRef]
- Rattat, D.; Schubiger, P.A.; Berke, H.G.; Schmalle, H.; Alberto, R. Dicarbonyl-Nitrosyl-Complexes of Rhenium (Re) and Technetium (Tc), A Potentially New Class of Compounds for the Direct Radiolabeling of Biomolecules. Cancer Biother. Radiopharm. 2001, 16, 339–343. [Google Scholar] [CrossRef]
- Burzlaff, N.; Schenk, W.A. Chiral Rhenium Complexes of Functionalized Thioaldehydes. Eur. J. Inorg. Chem. 1999, 1999, 1435–1443. [Google Scholar] [CrossRef]
- Faller, J.W.; Lavoie, A.R. Diastereoselective Synthesis and Electronic Asymmetry of Chiral Nonracemic Rhenium(V) Oxo Complexes Containing the Hydrotris(1-pyrazolyl)borate Ligand. Organometallics 2000, 19, 3957–3962. [Google Scholar] [CrossRef]
- De Montigny, F.; Guy, L.; Pilet, G.; Vanthuyne, N.; Roussel, C.; Lombardi, R.; Freedman, T.B.; Nafie, L.A.; Crassous, J. Subtle chirality in oxo- and sulfidorhenium(v) complexes. Chem. Commun. 2009, 32, 4841–4843. [Google Scholar] [CrossRef] [PubMed]
- Procopio, E.Q.; Dova, D.; Cauteruccio, S.; Forni, A.; Licandro, E.; Panigati, M. Dirhenium Coordination Complex Endowed with an Intrinsically Chiral Helical-Shaped Diphosphine Oxide. ACS Omega 2018, 3, 11649–11654. [Google Scholar] [CrossRef] [PubMed]
- Slate, A.J.; Shalamanova, L.; Akhidime, I.D.; Whitehead, K.A. Rhenium and yttrium ions as antimicrobial agents against multidrug resistant Klebsiella pneumoniae and Acinetobacter baumannii biofilms. Lett. Appl. Microbiol. 2019, 69, 168–174. [Google Scholar] [CrossRef]
- Kama, D.V.; Frei, A.; Brink, A.; Braband, H.; Alberto, R.; Roodt, A. New approach for the synthesis of water soluble fac-[MI(CO)3]+ bis(diarylphosphino)alkylamine complexes (M = 99Tc, Re). Dalton Trans. 2021, 50, 17506–17514. [Google Scholar] [CrossRef]
- Frei, A.; Amado, M.; Cooper, M.A.; Blaskovich, M.A.T. Light-Activated Rhenium Complexes with Dual Mode of Action against Bacteria. Chem. Eur. J. 2020, 26, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Vazquez-Hernandez, M.; Prochnow, P.; Bandow, J.E.; Metzler-Nolte, N. A CuAAC Click Approach for the Introduction of Bidentate Metal Complexes to a Sulfanilamide-Derived Antibiotic Fragment. Inorg. Chem. 2019, 58, 9404–9413. [Google Scholar] [CrossRef]
- Pagoni, C.-C.; Xylouri, V.-S.; Kaiafas, G.C.; Lazou, M.; Bompola, G.; Tsoukas, E.; Papadopoulou, L.C.; Psomas, G.; Papagiannopoulou, D. Organometallic rhenium tricarbonyl–enrofloxacin and –levofloxacin complexes: Synthesis, albumin-binding, DNA-interaction and cell viability studies. J. Biol. Inorg. Chem. 2019, 24, 609–619. [Google Scholar] [CrossRef]
- Wenzel, M.; Patra, M.; Senges, C.H.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N.; et al. Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: A structurally new class of antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef]
- Siegmund, D.; Lorenz, N.; Gothe, Y.; Spies, C.; Geissler, B.; Prochnow, P.; Nuernberger, P.; Bandow, J.E.; Metzler-Nolte, N. Benzannulated Re(i)–NHC complexes: Synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 2017, 46, 15269–15279. [Google Scholar] [CrossRef]
- Cooper, S.M.; Siakalli, C.; White, A.J.P.; Frei, A.; Miller, P.W.; Long, N.J. Synthesis and anti-microbial activity of a new series of bis(diphosphine) rhenium(v) dioxo complexes. Dalton Trans. 2022, 51, 12791–12795. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.S.; Marques, J.; Mesterházy, E.; Straetener, J.; Arts, M.; Pissarro, T.; Reginold, J.; Berscheid, A.; Bornikoel, J.; Kluj, R.M.; et al. Synergetic Antimicrobial Activity and Mechanism of Clotrimazole-Linked CO-Releasing Molecules. ACS Bio Med Chem Au 2022, 2, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Schindler, K.; Zobi, F. Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives. Molecules 2022, 27, 539. [Google Scholar] [CrossRef]
- Huang, Z.; Wilson, J.J. Therapeutic and Diagnostic Applications of Multimetallic Rhenium(I) Tricarbonyl Complexes. Eur. J. Inorg. Chem. 2021, 2021, 1312–1324. [Google Scholar] [CrossRef]
- Mkhatshwa, M.; Moremi, J.M.; Makgopa, K.; Manicum, A.-L.E. Nanoparticles Functionalised with Re(I) Tricarbonyl Complexes for Cancer Theranostics. Int. J. Mol. Sci. 2021, 22, 6546. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.S.; Mai, C.-W.; Zulkefeli, M.; Madheswaran, T.; Kiew, L.V.; Delsuc, N.; Low, M.L. Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules 2020, 25, 4176. [Google Scholar] [CrossRef]
- Collery, P.; Desmaele, D.; Vijaykumar, V. Design of Rhenium Compounds in Targeted Anticancer Therapeutics. Curr. Pharm. Des. 2019, 25, 3306–3322. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Lee, L.C.; Leung, K.K.; Lo, K.K. Recent development of luminescent rhenium(I) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents. Dalton Trans. 2017, 46, 16357–16380. [Google Scholar] [CrossRef]
- Leonidova, A.; Gasser, G. Underestimated Potential of Organometallic Rhenium Complexes as Anticancer Agents. ACS Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Vaibhavi, N.; Kar, B.; Das, U.; Paira, P. Target-specific mononuclear and binuclear rhenium(i) tricarbonyl complexes as upcoming anticancer drugs. RSC Adv. 2022, 12, 20264–20295. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Kalaj, M.; Gembicky, M.; Cohen, S.M. ReI Tricarbonyl Complexes as Coordinate Covalent Inhibitors for the SARS-CoV-2 Main Cysteine Protease. Angew. Chem. Int. Ed. 2021, 60, 10716–10723. [Google Scholar] [CrossRef] [PubMed]
- Valentová, J.; Lintnerová, L. Chirality in Anticancer Agents. In Current Topics in Chirality; Takashiro, A., Ed.; IntechOpen: Rijeka, Croatia, 2021; Volume Chapter 8. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Williams, D.S.; Bregman, H.; Pagano, N.; Meggers, E. Organometallic Compounds with Biological Activity: A Very Selective and Highly Potent Cellular Inhibitor for Glycogen Synthase Kinase 3. Chembiochem 2006, 7, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, I.P.; Colbon, P.J.J. The Growing Importance of Chirality in 3D Chemical Space Exploration and Modern Drug Discovery Approaches for Hit-ID. ACS Med. Chem. Lett. 2021, 12, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Ortiz, G.A.; Hernández-Correa, R.; Morales-Moreno, M.D.; Toscano, R.A.; Ramirez-Apan, M.T.; Hernandez-Garcia, A.; Amézquita-Valencia, M.; Araiza-Olivera, D. Diastereomeric Separation of Chiral fac-Tricarbonyl(iminopyridine) Rhenium(I) Complexes and Their Cytotoxicity Studies: Approach toward an Action Mechanism against Glioblastoma. J. Med. Chem. 2022, 65, 9281–9294. [Google Scholar] [CrossRef]
- Rossier, J.; Hauser, D.; Kottelat, E.; Rothen-Rutishauser, B.; Zobi, F. Organometallic cobalamin anticancer derivatives for targeted prodrug delivery via transcobalamin-mediated uptake. Dalton Trans. 2017, 46, 2159–2164. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in Vitro Anticancer Activity of Rhenium(I) Tricarbonyl Complexes Bearing Water-Soluble Phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef]
- Muñoz-Osses, M.; Siegmund, D.; Gómez, A.; Godoy, F.; Fierro, A.; Llanos, L.; Aravena, D.; Metzler-Nolte, N. Influence of the substituent on the phosphine ligand in novel rhenium(i) aldehydes. Synthesis, computational studies and first insights into the antiproliferative activity. Dalton Trans. 2018, 47, 13861–13869. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Marker, S.C.; Wilson, J.J.; Visser, H.G. Aquation and Anation Kinetics of Rhenium(I) Dicarbonyl Complexes: Relation to Cell Toxicity and Bioavailability. Inorg. Chem. 2020, 59, 15888–15897. [Google Scholar] [CrossRef]
- Delasoie, J.; Schiel, P.; Vojnovic, S.; Nikodinovic-Runic, J.; Zobi, F. Photoactivatable Surface-Functionalized Diatom Microalgae for Colorectal Cancer Targeted Delivery and Enhanced Cytotoxicity of Anticancer Complexes. Pharmaceutics 2020, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Sovari, S.N.; Radakovic, N.; Roch, P.; Crochet, A.; Pavic, A.; Zobi, F. Combatting AMR: A molecular approach to the discovery of potent and non-toxic rhenium complexes active against C. albicans-MRSA co-infection. Eur. J. Med. Chem. 2021, 226, 113858. [Google Scholar] [CrossRef] [PubMed]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Rossier, J.; Derszniak, K.; Dybas, J.; Oetterli, R.M.; Kottelat, E.; Chlopicki, S.; Zelder, F.; Zobi, F. Modified biovectors for the tuneable activation of anti-platelet carbon monoxide release. Chem. Commun. 2017, 53, 6840–6843. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Beltrami, R.; Kottelat, E.; Blacque, O.; Bogdanova, A.Y.; Zobi, F. N-Nitrosamine-{cis-Re[CO]2}2+ cobalamin conjugates as mixed CO/NO-releasing molecules. Dalton Trans. 2016, 45, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.B.; Zobi, F.; Piantadosi, C.A. Heme Oxygenase-1/Carbon Monoxide System and Embryonic Stem Cell Differentiation and Maturation into Cardiomyocytes. Antioxid. Redox Signal. 2016, 24, 345–360. [Google Scholar] [CrossRef]
- Schindler, K.; Crochet, A.; Zobi, F. Aerobically stable and substitutionally labile α-diimine rhenium dicarbonyl complexes. RSC Adv. 2021, 11, 7511–7520. [Google Scholar] [CrossRef]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Biancalana, L.; Batchelor, L.K.; Dyson, P.J.; Zacchini, S.; Schoch, S.; Pampaloni, G.; Marchetti, F. α-Diimine homologues of cisplatin: Synthesis, speciation in DMSO/water and cytotoxicity. New J. Chem. 2018, 42, 17453–17463. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.A.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. [Google Scholar]
- Amoroso, A.J.; Coogan, M.P.; Dunne, J.E.; Fernández-Moreira, V.; Hess, J.B.; Hayes, A.J.; Lloyd, D.; Millet, C.; Pope, S.J.A.; Williams, C. Rhenium fac tricarbonyl bisimine complexes: Biologically useful fluorochromes for cell imaging applications. Chem. Commun. 2007, 29, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Louie, M.-W.; Ho-Chuen Lam, M.; Kam-Wing Lo, K. Luminescent Polypyridinerhenium(I) Bis-Biotin Complexes as Crosslinkers for Avidin. Eur. J. Inorg. Chem. 2009, 2009, 4265–4273. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Adams, L.A.; Barlow, N.; Ferrari, S.; Graham, B.; Gasser, G. Enhanced Cytotoxicity through Conjugation of a “Clickable” Luminescent Re(I) Complex to a Cell-Penetrating Lipopeptide. ACS Med. Chem. Lett. 2014, 5, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.R.; Tan, C.P.; Chen, M.H.; Hao, L.; Ji, L.N.; Mao, Z.W. Mono- and Dinuclear Phosphorescent Rhenium(I) Complexes: Impact of Subcellular Localization on Anticancer Mechanisms. Chem. Eur. J. 2016, 22, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Clede, S.; Lambert, F.; Saint-Fort, R.; Plamont, M.A.; Bertrand, H.; Vessieres, A.; Policar, C. Influence of the Side-Chain Length on the Cellular Uptake and the Cytotoxicity of Rhenium Triscarbonyl Derivatives: A Bimodal Infrared and Luminescence Quantitative Study. Chem. Eur. J. 2014, 20, 8714–8722. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Sun, T.; Lin, W.; Guan, X.; Zheng, M.; Xie, Z.; Jing, X. BODIPY Fluorescent Chemosensor for Cu2+ Detection and Its Applications in Living Cells: Fast Response and High Sensitivity. J. Fluoresc. 2014, 24, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Potocny, A.M.; Teesdale, J.J.; Marangoz, A.; Yap, G.P.A.; Rosenthal, J. Spectroscopic and 1O2 Sensitization Characteristics of a Series of Isomeric Re(bpy)(CO)3Cl Complexes Bearing Pendant BODIPY Chromophores. Inorg. Chem. 2019, 58, 5042–5050. [Google Scholar] [CrossRef]
- Teesdale, J.J.; Pistner, A.J.; Yap, G.P.A.; Ma, Y.-Z.; Lutterman, D.A.; Rosenthal, J. Reduction of CO2 using a rhenium bipyridine complex containing ancillary BODIPY moieties. Catal. Today 2014, 225, 149–157. [Google Scholar] [CrossRef]
- Andrade, G.A.; Pistner, A.J.; Yap, G.P.A.; Lutterman, D.A.; Rosenthal, J. Photocatalytic Conversion of CO2 to CO Using Rhenium Bipyridine Platforms Containing Ancillary Phenyl or BODIPY Moieties. ACS Catal. 2013, 3, 1685–1692. [Google Scholar] [CrossRef]
- Schindler, K.; Cortat, Y.; Nedyalkova, M.; Crochet, A.; Lattuada, M.; Pavic, A.; Zobi, F. Antimicrobial Activity of Rhenium Di- and Tricarbonyl Diimine Complexes: Insights on Membrane-Bound S. aureus Protein Binding. Pharmaceuticals 2022, 15, 1107. [Google Scholar] [CrossRef]
- Kitanovic, I.; Can, S.Z.; Alborzinia, H.; Kitanovic, A.; Pierroz, V.; Leonidova, A.; Pinto, A.; Spingler, B.; Ferrari, S.; Molteni, R.; et al. A Deadly Organometallic Luminescent Probe: Anticancer Activity of a ReI Bisquinoline Complex. Chem. Eur. J. 2014, 20, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In Vitro Anticancer Activity and in Vivo Biodistribution of Rhenium(I) Tricarbonyl Aqua Complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef] [PubMed]
- König, M.; Siegmund, D.; Raszeja, L.J.; Prokop, A.; Metzler-Nolte, N. Resistance-breaking profiling and gene expression analysis on an organometallic ReI–phenanthridine complex reveal parallel activation of two apoptotic pathways. Med. Chem. Commun. 2018, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Konkankit, C.C.; King, A.P.; Knopf, K.M.; Southard, T.L.; Wilson, J.J. In Vivo Anticancer Activity of a Rhenium(I) Tricarbonyl Complex. ACS Med. Chem. Lett. 2019, 10, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Liang, J.H.; Zhang, H.; Wang, Z.H.; Wan, Q.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Mitochondria-Accumulating Rhenium(I) Tricarbonyl Complexes Induce Cell Death via Irreversible Oxidative Stress and Glutathione Metabolism Disturbance. ACS Appl. Mater. Interfaces 2019, 11, 13123–13133. [Google Scholar] [CrossRef]
- Munoz-Osses, M.; Godoy, F.; Fierro, A.; Gomez, A.; Metzler-Nolte, N. New organometallic imines of rhenium(I) as potential ligands of GSK-3 beta: Synthesis, characterization and biological studies. Dalton Trans. 2018, 47, 1233–1242. [Google Scholar] [CrossRef]
- Kaplanis, M.; Stamatakis, G.; Papakonstantinou, V.D.; Paravatou-Petsotas, M.; Demopoulos, C.A.; Mitsopoulou, C.A. Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory, and anti-coagulant effects towards platelet activating factor. J. Inorg. Biochem. 2014, 135, 1–9. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Rajendran, T.; Senthil Murugan, K.; Sathish Kumar, M.; Sivasubramanian, V.K.; Ganesan, M.; Mahesh, A.; Thirunalasundari, T.; Rajagopal, S. Interaction of rhenium(I) complex carrying long alkyl chain with Calf Thymus DNA: Cytotoxic and cell imaging studies. Inorg. Chim. Acta 2015, 434, 51–59. [Google Scholar] [CrossRef]
- Zobi, F.; Spingler, B.; Alberto, R. Guanine and plasmid DNA binding of mono- and trinuclear fac-[Re(CO)3]+ complexes with amino acid Ligands. Chembiochem 2005, 6, 1397–1405. [Google Scholar] [CrossRef]
- Caspar, J.V.; Sullivan, B.P.; Meyer, T.J. Synthetic Routes to Luminescent 2,2′-Bipyridyl Complexes of Rhenium—Preparation and Spectral and Redox Properties of Mono(Bipyridyl) Complexes of Rhenium(Iii) and Rhenium(I). Inorg. Chem. 1984, 23, 2104–2109. [Google Scholar] [CrossRef]
- Smithback, J.L.; Helms, J.B.; Schutte, E.; Woessner, S.M.; Sullivan, B.P. Preparative Routes to Luminescent Mixed-Ligand Rhenium(I) Dicarbonyl Complexes. Inorg. Chem. 2006, 45, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Skiba, J.; Kowalczyk, A.; Stączek, P.; Bernaś, T.; Trzybiński, D.; Woźniak, K.; Schatzschneider, U.; Czerwieniec, R.; Kowalski, K. Luminescent fac-[Re(CO)3(phen)] carboxylato complexes with non-steroidal anti-inflammatory drugs: Synthesis and mechanistic insights into the in vitro anticancer activity of fac-[Re(CO)3(phen)(aspirin)]. New J. Chem. 2019, 43, 573–583. [Google Scholar] [CrossRef]
- Ragone, F.; Saavedra, H.H.M.; Gara, P.M.D.; Ruiz, G.T.; Wolcan, E. Photosensitized Generation of Singlet Oxygen from Re(I) Complexes: A Photophysical Study Using LIOAS and Luminescence Techniques. J. Phys. Chem. A 2013, 117, 4428–4435. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, L.; Hevia, E.; Morales, D.; Pérez, J.; Riera, L.; Miguel, D. Reactivity of Molybdenum and Rhenium Hydroxo Complexes toward Organic Electrophiles: Reactions that Afford Carboxylato Products. Organometallics 2006, 25, 1717–1722. [Google Scholar] [CrossRef]
- Kisel, K.S.; Melnikov, A.S.; Grachova, E.V.; Hirva, P.; Tunik, S.P.; Koshevoy, I.O. Linking ReI and PtII Chromophores with Aminopyridines: A Simple Route to Achieve a Complicated Photophysical Behavior. Chem. Eur. J. 2017, 23, 11301–11311. [Google Scholar] [CrossRef] [PubMed]
- Hevia, E.; Pérez, J.; Riera, V.; Miguel, D. New Octahedral Rhenium(I) Tricarbonyl Amido Complexes. Organometallics 2002, 21, 1966–1974. [Google Scholar] [CrossRef]
- Cuesta, L.; Huertos, M.A.; Morales, D.; Pérez, J.; Riera, L.; Riera, V.; Miguel, D.; Menéndez-Velázquez, A.; García-Granda, S. Synthesis, Structure, and Reactivity of Mononuclear Re(I) Oximato Complexes. Inorg. Chem. 2007, 46, 2836–2845. [Google Scholar] [CrossRef]
- Coogan, M.P.; Platts, J.A. Blue rhenium tricarbonyl DPPZ complexes—Low energy charge-transfer absorption at tissue-penetrating wavelengths. Chem. Commun. 2016, 52, 12498–12501. [Google Scholar] [CrossRef]
- Capper, M.S.; Enriquez Garcia, A.; Macia, N.; Lai, B.; Lin, J.-B.; Nomura, M.; Alihosseinzadeh, A.; Ponnurangam, S.; Heyne, B.; Shemanko, C.S.; et al. Cytotoxicity, cellular localization and photophysical properties of Re(I) tricarbonyl complexes bound to cysteine and its derivatives. J. Biol. Inorg. Chem. 2020, 25, 759–776. [Google Scholar] [CrossRef]
- Cañadas, P.; Ziegler, S.; Fombona, S.; Hevia, E.; Miguel, D.; Pérez, J.; Riera, L. Molybdenum and rhenium carbonyl complexes containing thiolato ligands. J. Organomet. Chem. 2019, 896, 113–119. [Google Scholar] [CrossRef]
- Hayoz, P.; von Zelewsky, A. New versatile optically active bipyridines as building blocks for helicating and caging ligands. Tetrahedron Lett. 1992, 33, 5165–5168. [Google Scholar] [CrossRef]
- Abram, U.; Hübener, R.; Alberto, R.; Schibli, R. Darstellung und Strukturen von (Et4N)2[Re(CO)3(NCS)3] und (Et4N)[Re(CO)2Br4]. Z. Anorg. Allg. Chem. 1996, 622, 813–818. [Google Scholar] [CrossRef]
- De Clercq, E.; Field, H.J. Antiviral prodrugs—The development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 2006, 147, 1–11. [Google Scholar] [CrossRef]
- Collery, P.; Mohsen, A.; Kermagoret, A.; Corre, S.; Bastian, G.; Tomas, A.; Wei, M.; Santoni, F.; Guerra, N.; Desmaele, D.; et al. Antitumor activity of a rhenium (I)-diselenoether complex in experimental models of human breast cancer. Investig. New Drugs 2015, 33, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F.; Alberto, R. Redox-Induced Binding of [(tacn)ReBrII(CO)2]+ to Guanine, Oligonucleotides, and Peptides. Chem. Eur. J. 2010, 16, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F.; Blacque, O.; Sigel, R.K.O.; Alberto, R. Binding interaction of [Re(H2O)3(CO)3]+ with the DNA fragment d(CpGpG). Inorg. Chem. 2007, 46, 10458–10460. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.M.; Marzilli, L.G. fac-[Re(CO)3(H2O)3]+ Nucleoside Monophosphate Adducts Investigated in Aqueous Solution by Multinuclear NMR Spectroscopy. Inorg. Chem. 2007, 46, 4926–4936. [Google Scholar] [CrossRef]
- Oriskovich, T.A.; White, P.S.; Thorp, H.H. Luminescent Labels for Purine Nucleobases: Electronic Properties of Guanine Bound to Rhenium(I). Inorg. Chem. 1995, 34, 1629–1631. [Google Scholar] [CrossRef]
- Williams, J.D.; Kampmeier, F.; Badar, A.; Howland, K.; Cooper, M.S.; Mullen, G.E.D.; Blower, P.J. Optimal His-Tag Design for Efficient [99mTc(CO)3]+ and [188Re(CO)3]+ Labeling of Proteins for Molecular Imaging and Radionuclide Therapy by Analysis of Peptide Arrays. Bioconjugate Chem. 2021, 32, 1242–1254. [Google Scholar] [CrossRef]
- Pospíšil, P.; Sýkora, J.; Takematsu, K.; Hof, M.; Gray, H.B.; Vlček, A. Light-Induced Nanosecond Relaxation Dynamics of Rhenium-Labeled Pseudomonas aeruginosa Azurins. J. Phys. Chem. B 2020, 124, 788–797. [Google Scholar] [CrossRef]
- De Tommaso, G.; Celentano, V.; Malgieri, G.; Fattorusso, R.; Romanelli, A.; D’Andrea, L.D.; Iuliano, M.; Isernia, C. fac-[Re(H2O)3(CO)3]+ Complexed with Histidine and Imidazole in Aqueous Solution: Speciation, Affinity and Binding Features. ChemistrySelect 2016, 1, 3739–3744. [Google Scholar] [CrossRef]
- Simpson, E.J.; Hickey, J.L.; Breadner, D.; Luyt, L.G. Investigation of isomer formation upon coordination of bifunctional histidine analogues with 99mTc/Re(CO)3. Dalton Trans. 2012, 41, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Binkley, S.L.; Leeper, T.C.; Rowlett, R.S.; Herrick, R.S.; Ziegler, C.J. Re(CO)3(H2O)3+ binding to lysozyme: Structure and reactivity. Metallomics 2011, 3, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulou, D.; Tsoukalas, C.; Makris, G.; Raptopoulou, C.P.; Psycharis, V.; Leondiadis, L.; Gniazdowska, E.; Koźmiński, P.; Fuks, L.; Pelecanou, M.; et al. Histidine derivatives as tridentate chelators for the fac-[MI(CO)3] (Re, 99mTc, 188Re) core: Synthesis, structural characterization, radiochemistry and stability. Inorg. Chim. Acta 2011, 378, 333–337. [Google Scholar] [CrossRef]
- Herrick, R.S.; Ziegler, C.J.; Gambella, A. Reactions of [Re(CO)3]+ with Histidylhistidine and Modified Histidines. Eur. J. Inorg. Chem. 2010, 2010, 3905–3908. [Google Scholar] [CrossRef]
- Zobi, F.; Kromer, L.; Spingler, B.; Alberto, R. Synthesis and Reactivity of the 17 e− Complex [ReIIBr4(CO)2]2−: A Convenient Entry into Rhenium(II) Chemistry. Inorg. Chem. 2009, 48, 8965–8970. [Google Scholar] [CrossRef]
- Kolp, B.; Abeln, D.; Stoeckli-Evans, H.; von Zelewsky, A. Platinum(II) Compounds with Enantiomerically Pure Bis(pinene)-Fused Bipyridine Ligands − Diimine-Dichloro Complexes and Their Substitution Reactions. Eur. J. Inorg. Chem. 2001, 2001, 1207–1220. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]

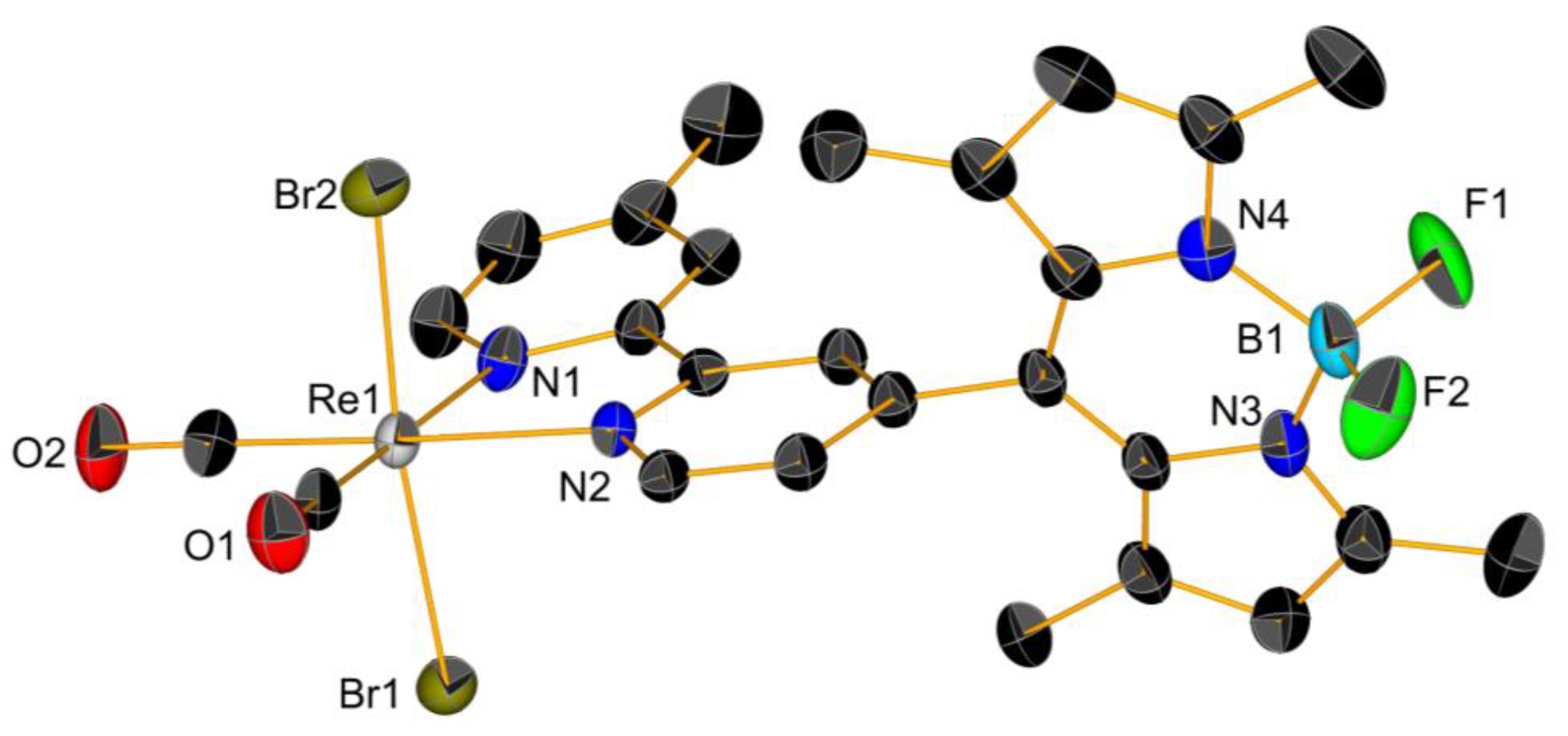

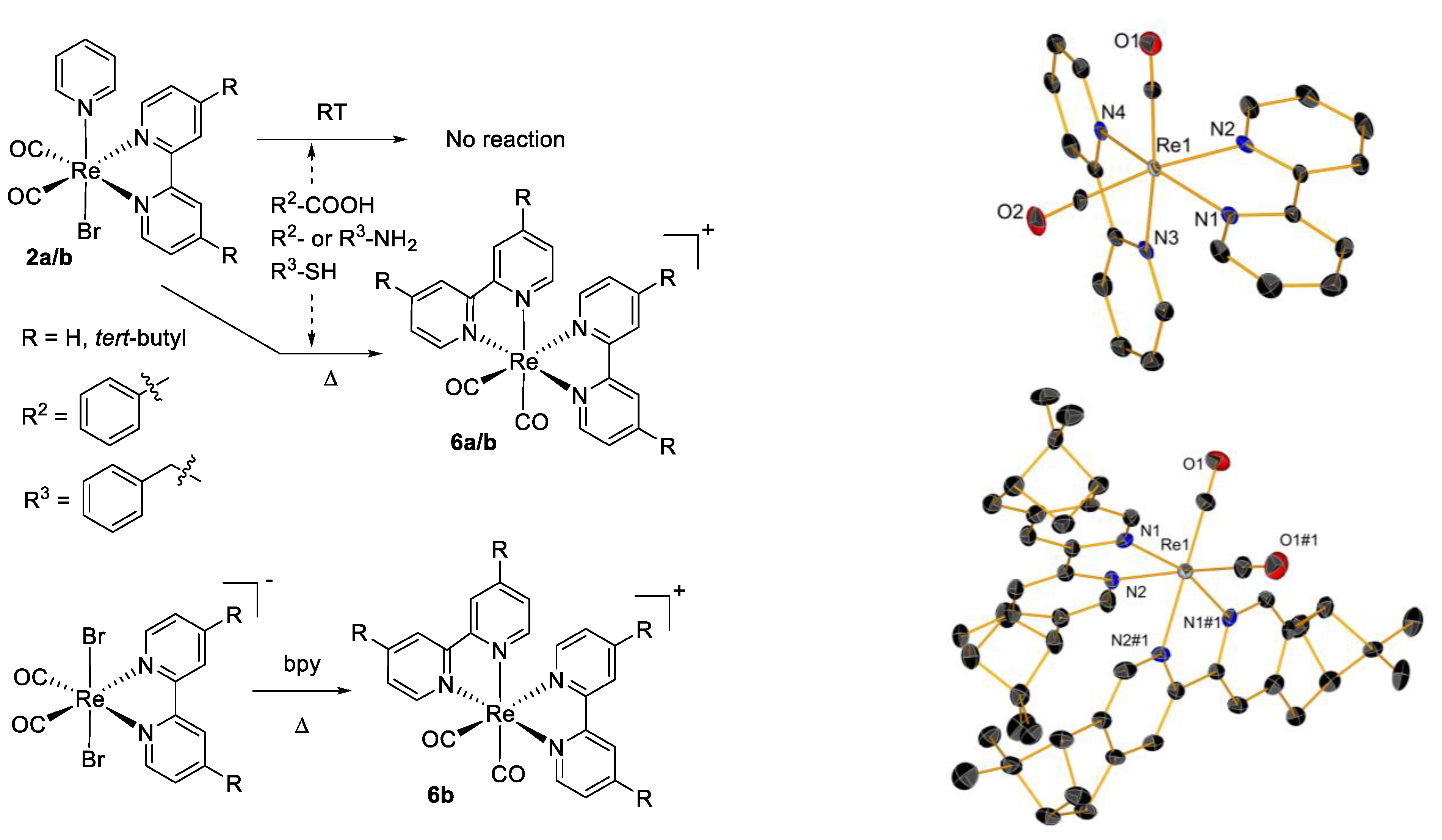
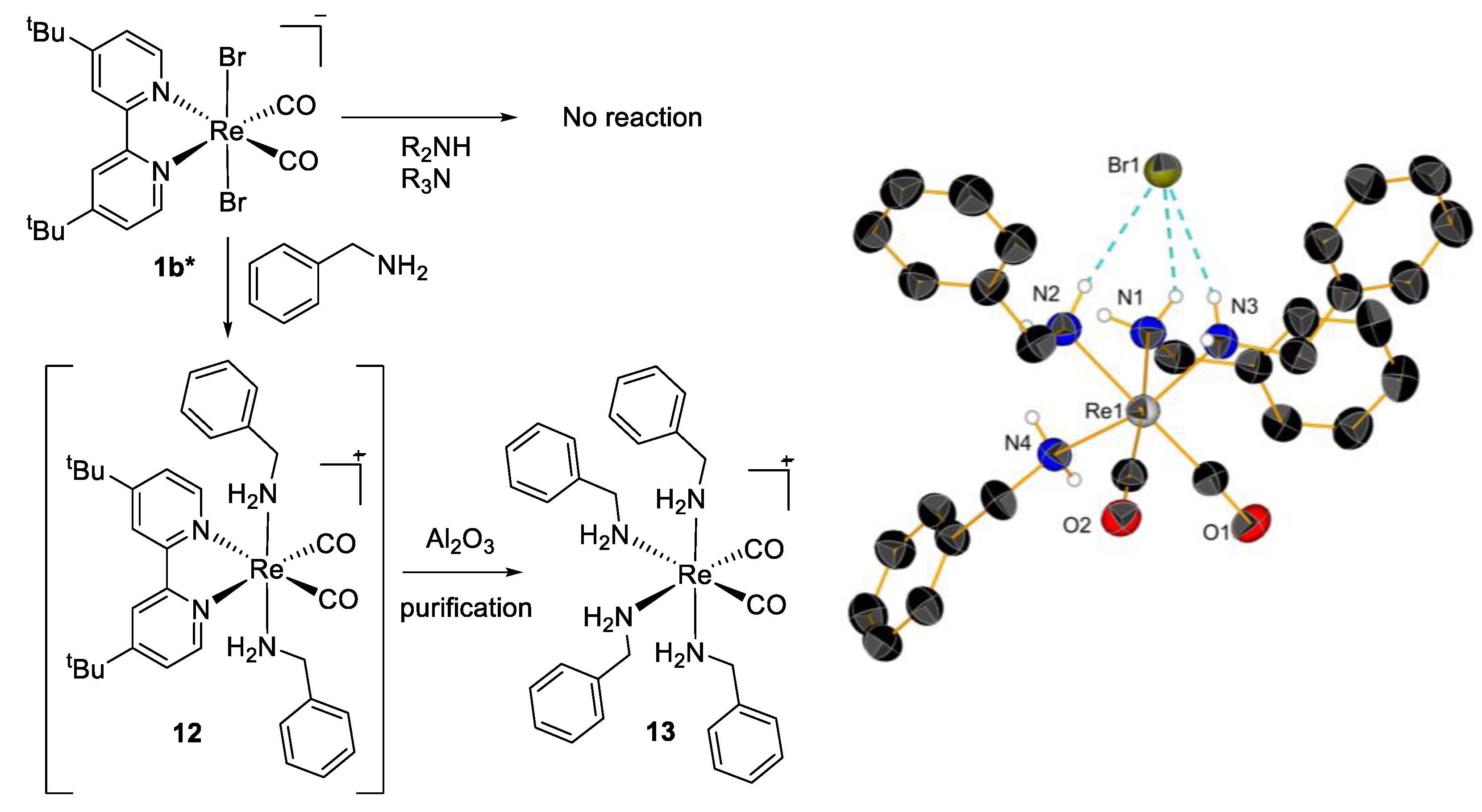

| Compounds | A549 | MCF-7 | HCT116 | HEK293 | Reference a |
|---|---|---|---|---|---|
| 1a | >10 | >10 | >10 | >10 | [61] |
| 1b | >10 | 2.3 | >10 | 3.0 | [48] |
| 1c | >10 | 3.5 | 6.2 | 1.5 | [61] |
| 2a | >10 | >10 | >10 | >10 | [61] |
| 2b | >10 | >10 | >10 | >10 | [48] |
| 3a | >10 | >10 | >10 | >10 | [61] |
| 3b | >10 | >10 | >10 | >10 | [48] |
| 4a | >10 | >10 | >10 | >10 | [61] |
| 4b | >10 | >10 | >10 | >10 | [48] |
| 5a | >10 | >10 | >10 | >10 | [61] |
| 5b | >10 | >10 | >10 | >10 | [48] |
| 6a | >10 | >10 | >10 | >10 | |
| 6b | 3.3 | 4.1 | 1.5 | 0.5 | |
| 7a-1 | >10 | >10 | >10 | 1.2 | |
| 7a-2 | >10 | >10 | 4.5 | 1.5 | |
| 7b-1 | >10 | >10 | >10 | 9.8 | |
| 7b-2 | >10 | >10 | >10 | 3.9 | |
| 7c-1 | 4.4 | 7.6 | 3.6 | 1.0 | |
| 7c-2 | 4.4 | 9.6 | 4.0 | 1.1 | |
| 8a | >10 | >10 | >10 | >10 | [44] |
| 8b | 9.7 | 7.1 | 6.3 | 4.9 | [44] |
| 8c | >10 | 8.2 | 6.9 | >10 | [61] |
| 9a | >10 | >10 | >10 | >10 | [43] |
| 9b | >10 | >10 | >10 | 8.8 | [43] |
| 10b | >10 | >10 | >10 | >10 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, K.; Horner, J.; Demirci, G.; Cortat, Y.; Crochet, A.; Mamula Steiner, O.; Zobi, F. In Vitro Biological Activity of α-Diimine Rhenium Dicarbonyl Complexes and Their Reactivity with Different Functional Groups. Inorganics 2023, 11, 139. https://doi.org/10.3390/inorganics11040139
Schindler K, Horner J, Demirci G, Cortat Y, Crochet A, Mamula Steiner O, Zobi F. In Vitro Biological Activity of α-Diimine Rhenium Dicarbonyl Complexes and Their Reactivity with Different Functional Groups. Inorganics. 2023; 11(4):139. https://doi.org/10.3390/inorganics11040139
Chicago/Turabian StyleSchindler, Kevin, Justine Horner, Gozde Demirci, Youri Cortat, Aurélien Crochet, Olimpia Mamula Steiner, and Fabio Zobi. 2023. "In Vitro Biological Activity of α-Diimine Rhenium Dicarbonyl Complexes and Their Reactivity with Different Functional Groups" Inorganics 11, no. 4: 139. https://doi.org/10.3390/inorganics11040139
APA StyleSchindler, K., Horner, J., Demirci, G., Cortat, Y., Crochet, A., Mamula Steiner, O., & Zobi, F. (2023). In Vitro Biological Activity of α-Diimine Rhenium Dicarbonyl Complexes and Their Reactivity with Different Functional Groups. Inorganics, 11(4), 139. https://doi.org/10.3390/inorganics11040139








