Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity
Abstract
1. Introduction
2. Results and Discussion
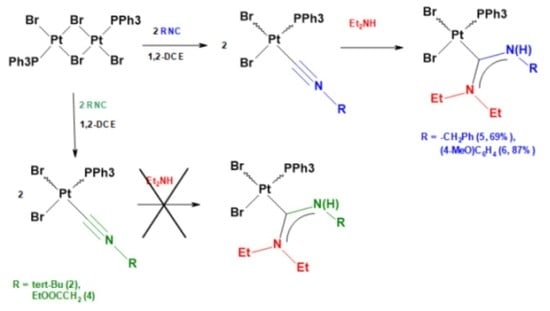
2.1. Synthesis of Isocyanide Complexes [PtBr2(PPh3)(CNR)]
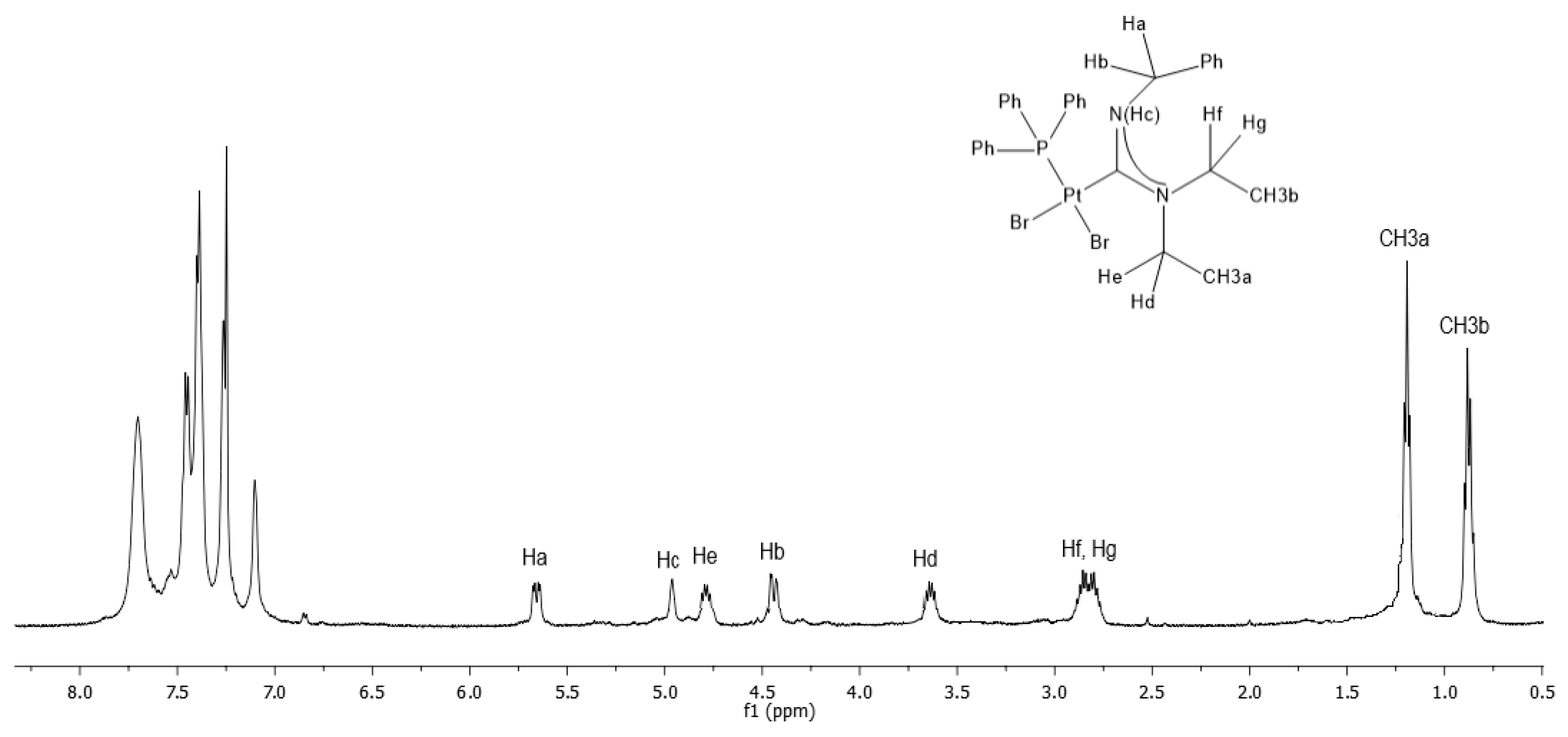
2.2. Synthesis of Carbene Complexes [PtBr2(PPh3)(Et2N(H)CNR)]
2.3. Stability of Complexes in DMSO
3. Conclusions
4. Materials and Methods
4.1. General Procedure for the Synthesis of [PtBr2(PPh3)(CNR)]
4.2. General Procedure for the Synthesis of [PtBr2(PPh3)(Et2N(H)CNR)]
5. Single-Crystal X-ray Diffraction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyeraci, M.; Agnarelli, L.; Labella, L.; Marchetti, F.; Di Paolo, M.L.; Samaritani, S.; Dalla Via, L. Trans-Dichloro(Triphenylarsino)(N,N-Dialkylamino)Platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance. Molecules 2022, 27, 644. [Google Scholar] [CrossRef] [PubMed]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New Platinum(II) Complexes Affecting Different Biomolecular Targets in Resistant Ovarian Carcinoma Cells. Chemmedchem 2021, 16, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Scalcon, V.; Hyeraci, M.; Folda, A.; Labella, L.; Samaritani, S.; Dalla Via, L.; Rigobello, M.P. Insights into the Redox Activity of Platinum(II) Complexes Bearing a Mitochondriotropic Ligand in Cisplatin-Resistant Ovarian Cancer Cell Lines. Free Rad. Biol. Med. 2020, 159, S60–S61. [Google Scholar] [CrossRef]
- Hyeraci, M.; Colalillo, M.; Labella, L.; Marchetti, F.; Samaritani, S.; Scalcon, V.; Rigobello, M.P.; Dalla Via, L. Platinum(II) Complexes Bearing Triphenylphosphine and Chelating Oximes: Antiproliferative Effect and Biological Profile in Resistant Cells. Chemmedchem 2020, 15, 1464–1472. [Google Scholar] [CrossRef]
- Bondi, R.; Biver, T.; Dalla Via, L.; Guarra, F.; Hyeraci, M.; Sissi, C.; Labella, L.; Marchetti, F.; Samaritani, S. DNA Interaction of a Fluorescent, Cytotoxic Pyridinimino Platinum(II) Complex. J. Inorg. Biochem. 2020, 202, 7. [Google Scholar] [CrossRef] [PubMed]
- Belli Dell’ Amico, D.; Colalillo, M.; Dalla Via, L.; Dell’ Acqua, M.; García-Argáez, A.N.; Hyeraci, M.; Labella, L.; Marchetti, F.; Samaritani, S. Synthesis and Reactivity of Cytotoxic Platinum(II) Complexes of Bidentate Oximes: A Step towards the Functionalization of Bioactive Complexes. Eur. J. Inorg. Chem. 2018, 2018, 1589–1594. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef]
- Farasat, A.; Labella, L.; Marchetti, F.; Samaritani, S. Addition of Aliphatic, Secondary Amines to Coordinated Isonitriles. N-Acyclic Carbene (NAC) Platinum(II) Complexes from Trans-[Pt(μ-Cl)Cl(PPh3)]2. Inorg. Chim. Acta 2022, 540, 121062. [Google Scholar] [CrossRef]
- Belli, D.; Labella, L.; Marchetti, F.; Samaritani, S. A Convenient Route to Dinuclear Chloro-Bridged Platinum(II) Derivatives via Nitrile Complexes. Dalton Trans. 2012, 41, 1389–1396. [Google Scholar] [CrossRef]
- Marzo, T.; Bartoli, G.; Gabbiani, C.; Pescitelli, G.; Severi, M.; Pillozzi, S.; Michelucci, E.; Fiorini, B.; Arcangeli, A.; Quiroga, A.G.; et al. Cisplatin and Its Dibromido Analogue: A Comparison of Chemical and Biological Profiles. BioMetals 2016, 29, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marzo, T.; Cirri, D.; Gabbiani, C.; Coletti, C.; Marrone, A.; Paciotti, R.; Messori, L.; Re, N. Reactions of Cisplatin and Cis-[PtI2(NH3)2] with Molecular Models of Relevant Protein Sidechains: A Comparative Analysis. J. Inorg. Biochem. 2020, 209, 111096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, Z.; Jiang, G.; Huang, S.; Bian, M.; Lu, Y.; Liu, W. An Overview of Anticancer Platinum N-Heterocyclic Carbene Complexes. Coord. Chem. 2021, 449, 214217. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S. N-Heterocyclic Carbene Platinum Complexes: A Big Step Forward for Effective Antitumor Compounds. Eur. J. Inorg. Chem. 2020, 2020, 10–20. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New Insights in Au-NHCs Complexes as Anticancer Agents. Europ. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Pessotto, I.; Cavarzerani, E.; Canzonieri, V.; Orian, L.; Demitri, N.; Schmidt, C.; Casini, A.; Bortolamiol, E.; Visentin, F.; et al. Indenyl and Allyl Palladate Complexes Bearing N-Heterocyclic Carbene Ligands: An Easily Accessible Class of New Anticancer Drug Candidates. Eur. J. Inorg. Chem. 2022, 2022, e202200103. [Google Scholar] [CrossRef]
- Marichev, K.O.; Patil, S.A.; Patil, S.A.; Heras Martinez, H.M.; Bugarin, A. N-Heterocyclic Carbene Metal Complexes as Therapeutic Agents: A Patent Review. Expert Opin. Ther. Pat. 2022, 32, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marzo, T.; Coletti, C.; La Mendola, D.; Storchi, L.; Re, N.; Marrone, A. Reactivity of Antitumor Coinage Metal-Based N-Heterocyclic Carbene Complexes with Cysteine and Selenocysteine Protein Sites. J. Inorg. Biochem. 2021, 223, 111533. [Google Scholar] [CrossRef]
- Guarra, F.; Pratesi, A.; Gabbiani, C.; Biver, T. A Focus on the Biological Targets for Coinage Metal-NHCs as Potential Anticancer Complexes. J. Inorg. Biochem. 2021, 217, 111355. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Chapter Three—Anti-Cancer Gold, Platinum and Iridium Compounds with Porphyrin and/or N-Heterocyclic Carbene Ligand(s). In Advances in Inorganic Chemistry; Sadler, P.J., van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 87–119. ISBN 0898-8838. [Google Scholar]
- APatil, S.; PHoagland, A.; APatil, S.; Bugarin, A. N-Heterocyclic Carbene-Metal Complexes as Bio-Organometallic Antimicrobial and Anticancer Drugs, an Update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar] [CrossRef]
- Hu, D.; Yang, C.; Lok, C.-N.; Xing, F.; Lee, P.-Y.; Fung, Y.M.E.; Jiang, H.; Che, C.-M. An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. Angew. Chem. Int. Ed. 2019, 58, 10914–10918. [Google Scholar] [CrossRef] [PubMed]
- Harlepp, S.; Chardon, E.; Bouché, M.; Dahm, G.; Maaloum, M.; Bellemin-Laponnaz, S. N-Heterocyclic Carbene-Platinum Complexes Featuring an Anthracenyl Moiety: Anti-Cancer Activity and DNA Interaction. Int. J. Mol. Sci. 2019, 20, 4198. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Imbimbo, P.; Petruk, G.; Ferraro, G.; Pinto, V.; Tuzi, A.; Monti, D.M.; Merlino, A.; et al. A Highly Efficient and Selective Antitumor Agent Based on a Glucoconjugated Carbene Platinum(Ii) Complex. Dalton Trans. 2019, 48, 7794–7800. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer Metal-N-Heterocyclic Carbene Complexes of Gold, Platinum and Palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gust, R. Update on Metal N-Heterocyclic Carbene Complexes as Potential Anti-Tumor Metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Dahm, G.; Bailly, C.; Karmazin, L.; Bellemin-Laponnaz, S. Synthesis, Structural Characterization and In Vitro Anti-Cancer Activity of Functionalized N-Heterocyclic Carbene Platinum and Palladium Complexes. J. Organomet. Chem. 2015, 794, 115–124. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-Heterocyclic Carbene Complexes as Potential Antitumor Metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Cisnetti, F.; Gautier, A. Metal/N-Heterocyclic Carbene Complexes: Opportunities for the Development of Anticancer Metallodrugs. Angew. Chem. Int. Ed. 2013, 52, 11976–11978. [Google Scholar] [CrossRef]
- Skander, M.; Retailleau, P.; Bourrié, B.; Schio, L.; Mailliet, P.; Marinetti, A. N-Heterocyclic Carbene-Amine Pt(II) Complexes, a New Chemical Space for the Development of Platinum-Based Anticancer Drugs. J. Med. Chem. 2010, 53, 2146–2154. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Balova, I.A. Alternative Transformations of N-Heterocyclic Carbene Complexes of the Group 11 Metals in Transmetalation Reactions (A Review). Russ. J. Gen. Chem. 2021, 91, 2194–2248. [Google Scholar] [CrossRef]
- Baczewska, P.; Śniady, K.; Kośnik, W.; Michalak, M. Acenaphthene-Based N-Heterocyclic Carbene Metal Complexes: Synthesis and Application in Catalysis. Catalysts 2021, 11, 972. [Google Scholar] [CrossRef]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-Heterocyclic Carbenes: Emerging Powerful Catalysts. Trends Chem. 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Voloshkin, V.A.; Tzouras, N.V.; Nolan, S.P. Recent Advances in the Synthesis and Derivatization of N-Heterocyclic Carbene Metal Complexes. Dalton Trans. 2021, 50, 12058–12068. [Google Scholar] [CrossRef] [PubMed]
- Bołt, M.; Mermela, A.; Żak, P. Influence of Bis-NHC Ligand on Platinum-Catalyzed Hydrosilylation of Internal Alkynes. Eur. J. Inorg. Chem. 2023, 26, e202200622. [Google Scholar] [CrossRef]
- Strausser, S.L.; Jenkins, D.M. Synthesis of D10 N-Heterocyclic Carbene Complexes with a Perimidine Scaffold. Organometallics 2021, 40, 1706–1712. [Google Scholar] [CrossRef]
- Koy, M.; Bellotti, P.; Das, M.; Glorius, F. N-Heterocyclic Carbenes as Tunable Ligands for Catalytic Metal Surfaces. Nat. Catal. 2021, 4, 352–363. [Google Scholar] [CrossRef]
- Pertschi, R.; Hatey, D.; Pale, P.; de Frémont, P.; Blanc, A. Synthesis, Characterization, and Catalytic Activity of Chiral NHC Platinum(II) Pyridine Dihalide Complexes. Organometallics 2020, 39, 804–812. [Google Scholar] [CrossRef]
- Maliszewski, B.P.; Tzouras, N.V.; Guillet, S.G.; Saab, M.; Beliš, M.; Van Hecke, K.; Nahra, F.; Nolan, S.P. A General Protocol for the Synthesis of Pt-NHC (NHC = N-Heterocyclic Carbene) Hydrosilylation Catalysts. Dalton Trans. 2020, 49, 14673–14679. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC Heterocycle Complexes in Catalysis and Biological Applications: Systematic Review. Mater. Today Proc. 2020, 31, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Afanasenko, A.M.; Chulkova, T.G.; Boyarskaya, I.A.; Islamova, R.M.; Legin, A.A.; Keppler, B.K.; Selivanov, S.I.; Vereshchagin, A.N.; Elinson, M.N.; Haukka, M. C,N-Chelated Diaminocarbene Platinum(II) Complexes Derived from 3,4-Diaryl-1H-Pyrrol-2,5-Diimines and Cis-Dichlorobis(Isonitrile)Platinum(II): Synthesis, Cytotoxicity, and Catalytic Activity in Hydrosilylation Reactions. J. Organomet. Chem. 2020, 923, 121435. [Google Scholar] [CrossRef]
- Correa, A.; Nolan, S.P.; Cavallo, L. N-Heterocyclic Carbene Complexes of Au, Pd, and Pt as Effective Catalysts in Organic Synthesis. In Computational Mechanisms of Au and Pt Catalyzed Reactions; Soriano, E., Marco-Contelles, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 131–155. ISBN 978-3-642-21083-9. [Google Scholar]
- Hashmi, A.S.K.; Lothschütz, C.; Böhling, C.; Hengst, T.; Hubbert, C.; Rominger, F. Carbenes Made Easy: Formation of Unsymmetrically Substituted N-Heterocyclic Carbene Complexes of Palladium(II), Platinum(II) and Gold(I) from Coordinated Isonitriles and Their Catalytic Activity. Adv. Synth. Catal. 2010, 352, 3001–3012. [Google Scholar] [CrossRef]
- Brissy, D.; Skander, M.; Retailleau, P.; Frison, G.; Marinetti, A. Platinum(II) Complexes Featuring Chiral Diphosphines and N-Heterocyclic Carbene Ligands: Synthesis and Evaluation as Cycloisomerization Catalysts. Organometallics 2009, 28, 140–151. [Google Scholar] [CrossRef]
- Fioco, D.; Belli Dell’Amico, D.; Labella, L.; Marchetti, F.; Samaritani, S. A Sound Sequence to Triphenylphosphino Dibromoplatinum(II) Complexes—Solvothermal Preparation of Trans-[PtBr(µ-Br)(PPh3)]2. Eur. J. Inorg. Chem. 2019, 2019, 3970–3974. [Google Scholar] [CrossRef]
- Priqueler, J.R.L.; Butler, I.S.; Rochon, F.D. An Overview of 195Pt Nuclear Magnetic Resonance Spectroscopy. Appl. Spectrosc. Rev. 2006, 41, 185–226. [Google Scholar] [CrossRef]
- Pregosin, P.S. Platinum-195 Nuclear Magnetic Resonance. Coord. Chem. Rev. 1982, 44, 247–291. [Google Scholar] [CrossRef]
- Bouché, M.; Vincent, B.; Achard, T.; Bellemin-Laponnaz, S. N-Heterocyclic Carbene Platinum(IV) as Metallodrug Candidates: Synthesis and 195Pt NMR Chemical Shift Trend. Molecules 2020, 25, 3148. [Google Scholar] [CrossRef]
- Sarju, J.; Arbour, J.; Sayer, J.; Rohrmoser, B.; Scherer, W.; Wagner, G. Synthesis and Characterisation of Mixed Ligand Pt(Ii) and Pt(Iv) Oxadiazoline Complexes. Dalton Trans. 2008, 5302–5312. [Google Scholar] [CrossRef]
- Still, B.M.; Kumar, P.G.A.; Aldrich-Wright, J.R.; Price, W.S. 195Pt NMR—Theory and Application. Chem. Soc. Rev. 2007, 36, 665–686. [Google Scholar] [CrossRef]
- Gudat, D.; Dogan, A.; Kaim, W.; Klein, A. Multinuclear NMR Study of Some Organoplatinum Complexes Containing Multifunctional Azines as Chelating Ligands. Magn. Reson. Chem. 2004, 42, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Bret, J.-M.; Castan, P.; Commenges, G.; Laurent, J.-P. NMR (195Pt and 13C) Contribution to the Study of Some Pt(II), Pt(IV) and Mixed-Valence Thioamido Complexes. Polyhedron 1983, 2, 901–905. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Brice, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-119-09306-0. [Google Scholar]
- Kotha, S.H.; Somnath, H. Ethyl Isocyanoacetate as a Useful Glycine Equivalent. Synlett 2010, 2010, 337–354. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin, and Other Platinum Complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.P.; Ortiz, D.; Höfer, D.; Menin, L.; Galanski, M.S.; Keppler, B.K.; Dyson, P.J. Oxaliplatin Reacts with DMSO Only in the Presence of Water. Dalton Trans. 2017, 46, 8929–8932. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Purkait, K.; Chatterjee, S.; Mukherjee, A. Anticancer Activity of a Cis-Dichloridoplatinum(II) Complex of a Chelating Nitrogen Mustard: Insight into Unusual Guanine Binding Mode and Low Deactivation by Glutathione. Dalton Trans. 2016, 45, 3599–3615. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C. Chapter 4—Purification of Organic Chemicals. In Purification of Laboratory Chemicals, 7th ed.; Armarego, W.L.F., Chai, C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 103–554. ISBN 978-0-12-382161-4. [Google Scholar]
- Armarego, W.L.F.; Chai, C. Chapter 5—Purification of Inorganic and Metal-Organic Chemicals. In Purification of Laboratory Chemicals, 7th ed.; Armarego, W.L.F., Chai, C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 555–661. ISBN 978-0-12-382161-4. [Google Scholar]


| Complex | R | % Yield | cis,trans % a |
|---|---|---|---|
| 1 | Bz | 93 | 59/41 |
| 2 | Tert-Bu | 98 | 85/15 |
| 3 | 4-(MeO)C6H4 | 75 | 76/24 |
| 4 | CH2COOEt | 83 | 100/0 |
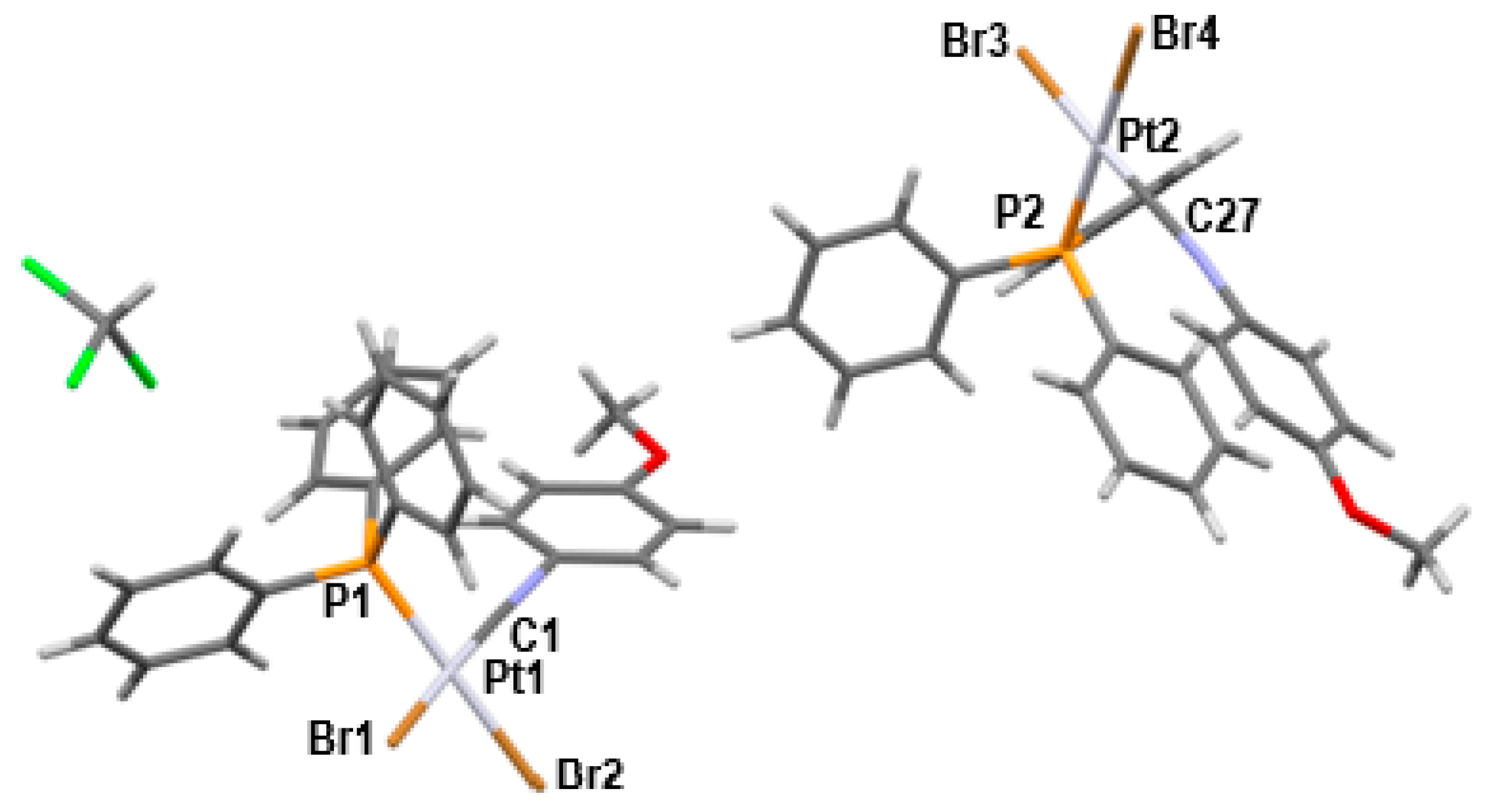
| Bond lengths (Å) | |||
| Pt1-C1 | 1.910 (5) | Pt1-P1 | 2.2563 (12) |
| Pt1-Br2 | 2.4317 (6) | Pt1-Br1 | 2.4886 (6) |
| Pt2-C27 | 1.897 (5) | Pt2-P2 | 2.2536 (10) |
| Pt2-Br3 | 2.4358 (5) | Pt2-Br4 | 2.4754 (5) |
| Bond angles (°) | |||
| P1-Pt1-Br2 | 89.77 (3) | P1-Pt1-Br1 | 179.32 (3) |
| C1-Pt1-P1 | 92.48 (16) | C1-Pt1-Br2 | 177.19 (17) |
| C1-Pt1-Br1 | 86.88 (16) | C1-N1-C2 | 179.4 (6) |
| C27-Pt2-Br4 | 88.43 (13) | C27-Pt2-Br3 | 177.91 (14) |
| C27-Pt2-P2 | 91.74 (13) | C27-N2-C28 | 172.5 (5) |
| P2-Pt2-Br4 | 179.69 (3) | P2-Pt2-Br3 | 88.86 (3) |
| Br3-Pt2-Br4 | 90.986 (19) | Br2-Pt1-Br1 | 90.86 (3) |
| Identification code | CP9 | |
| Empirical formula | C53H45Br4Cl3N2O2P2Pt2 | |
| Formula weight | 1620.02 g/mol | |
| Temperature | 293(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Triclinic | |
| Space group | P-1 | |
| Unit cell dimensions | a = 10.6145(3)Å | α = 103.8190(10)° |
| b = 14.8776(4) Å | β = 103.3390(10)° | |
| c = 18.7841(4)Å | γ = 90.3250(10)° | |
| Volume | 2796.98(13) Å3 | |
| Z | 2 | |
| Density (calculated) | 1.924 g/cm3 | |
| Absorption coefficient | 8.93 mm−1 | |
| F(000) | 1540 | |
| Theta range for data collection | 1.98 to 28.27° | |
| Index ranges | −14 ≤ h ≤ 14, −19 ≤ k ≤ 19, −25 ≤ l ≤ 24 | |
| Reflections collected | 100,548 | |
| Independent reflections | 13,634 [R(int) = 0.0502] | |
| Max. and min. transmission | 0.4980 and 0.1400 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 13,634/0/615 | |
| Goodness-of-fit on F2 | 1.099 | |
| Final R indices | 12,364 data; I > 2σ(I) | R1 = 0.0367, wR2 = 0.1041 |
| all data | R1 = 0.0406, wR2 = 0.1114 | |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.0675P)2 + 3.3100P] | |
| Largest diff. peak and hole | 1.183 and −2.282 eÅ−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farasat, A.; Nerli, F.; Labella, L.; Taddei, M.; Samaritani, S. Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity. Inorganics 2023, 11, 137. https://doi.org/10.3390/inorganics11040137
Farasat A, Nerli F, Labella L, Taddei M, Samaritani S. Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity. Inorganics. 2023; 11(4):137. https://doi.org/10.3390/inorganics11040137
Chicago/Turabian StyleFarasat, Anna, Francesca Nerli, Luca Labella, Marco Taddei, and Simona Samaritani. 2023. "Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity" Inorganics 11, no. 4: 137. https://doi.org/10.3390/inorganics11040137
APA StyleFarasat, A., Nerli, F., Labella, L., Taddei, M., & Samaritani, S. (2023). Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity. Inorganics, 11(4), 137. https://doi.org/10.3390/inorganics11040137