Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling
Abstract
1. Introduction
2. Carbon Dioxide and Global Warming

3. Nitrous and Sulfur Oxides and Acid Rain
4. Chlorofluorocarbons and the Ozone Layer
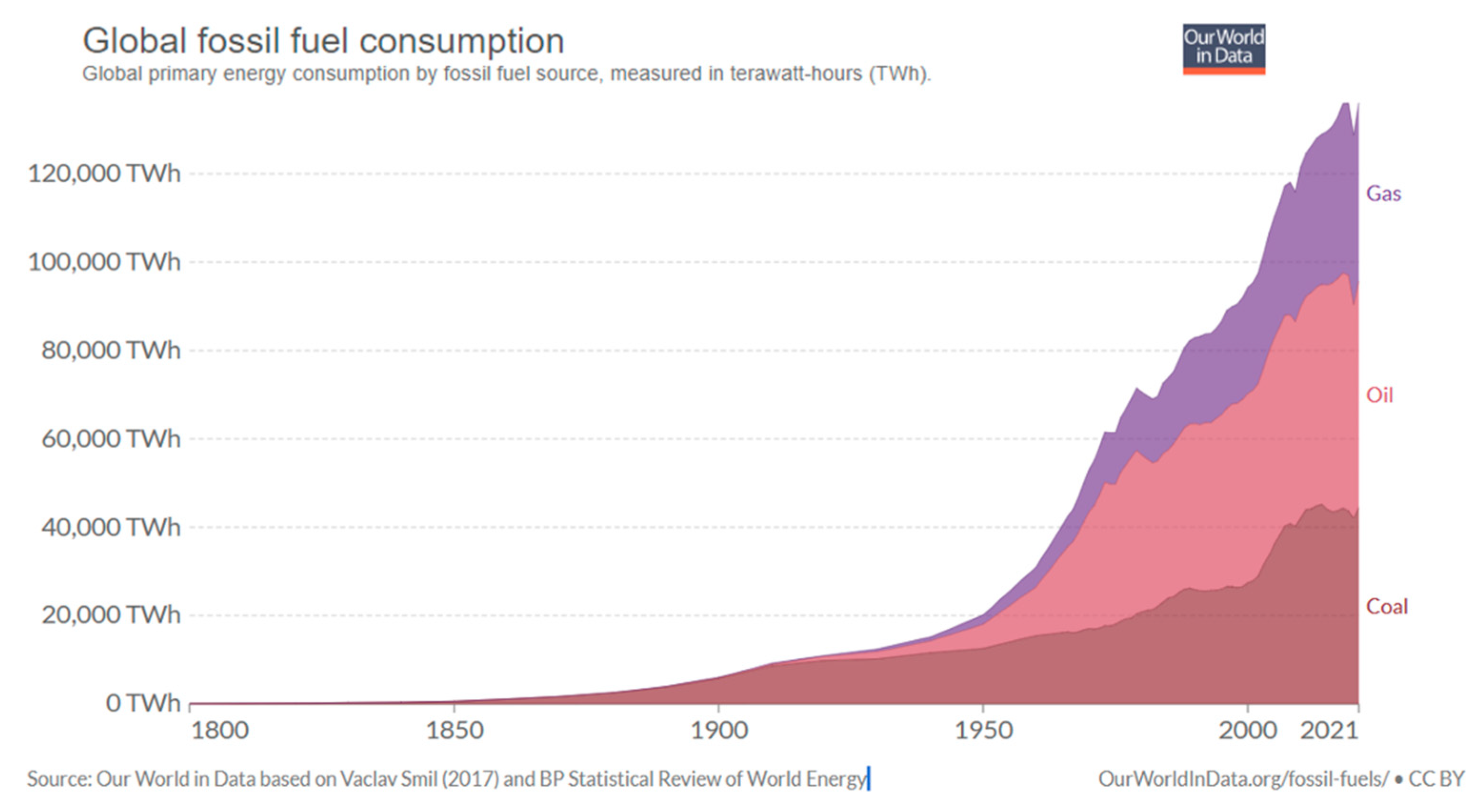
5. Energy Dependence
6. Catalysts
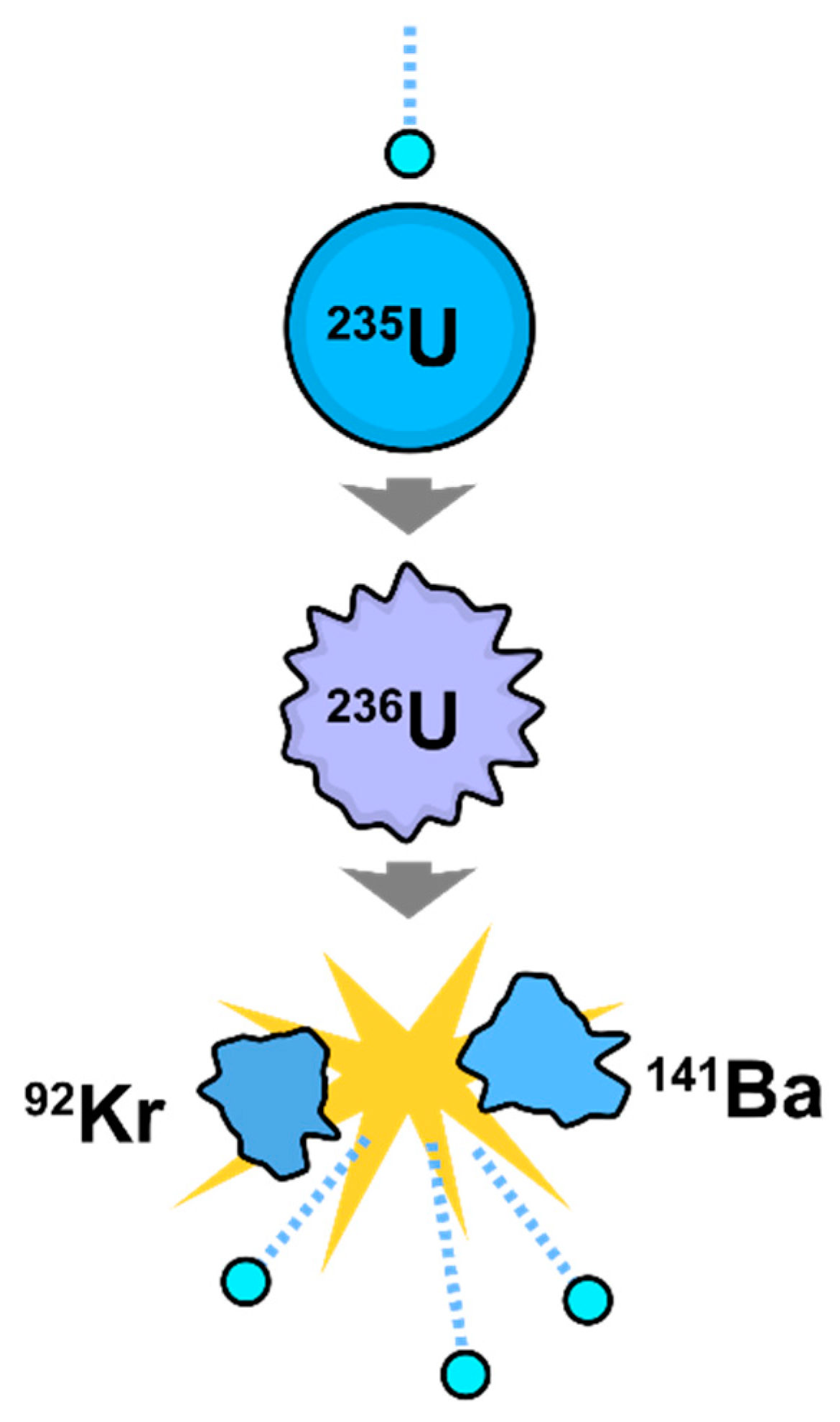
7. Hydrogen Production
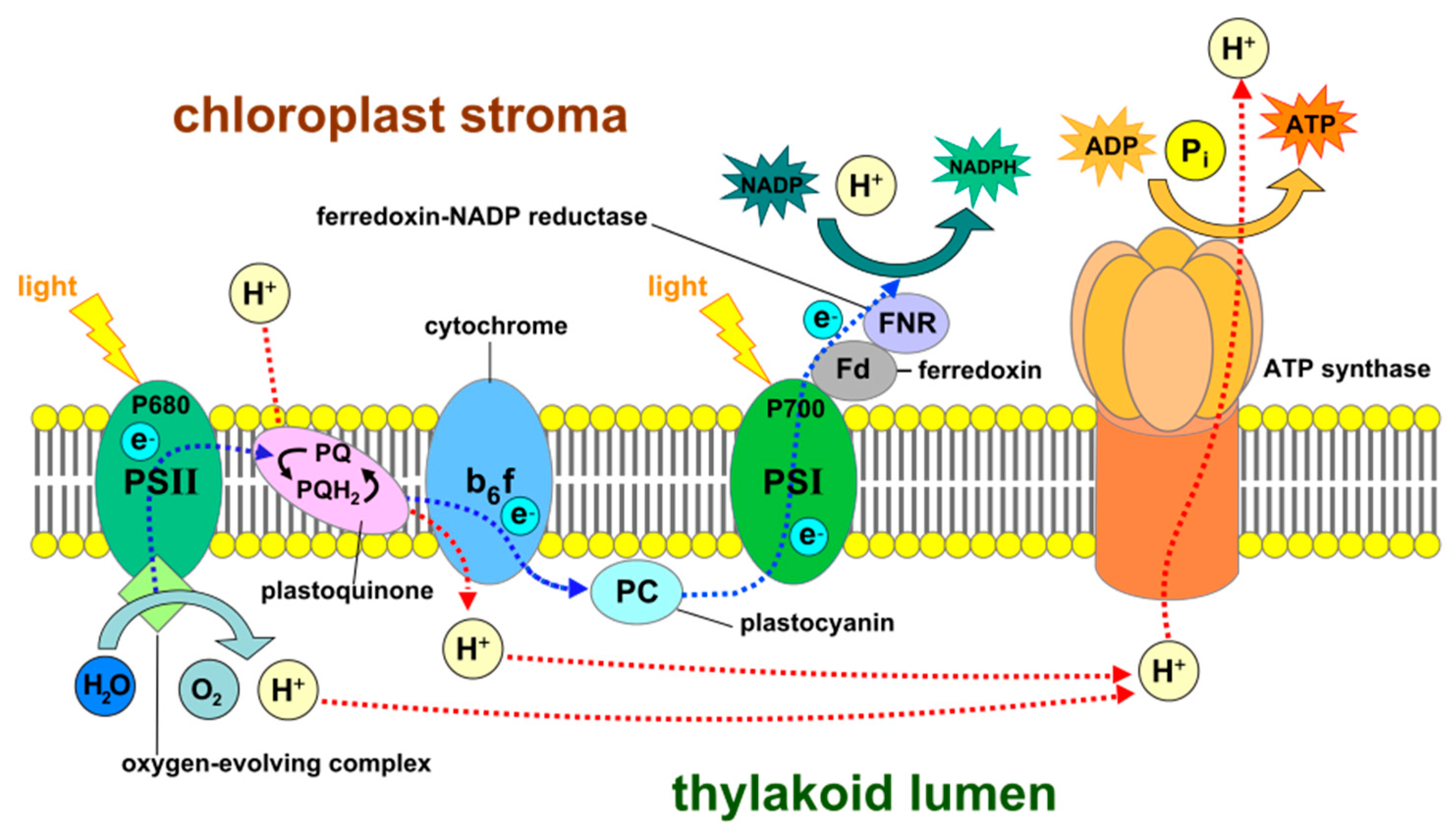
8. Water-Splitting
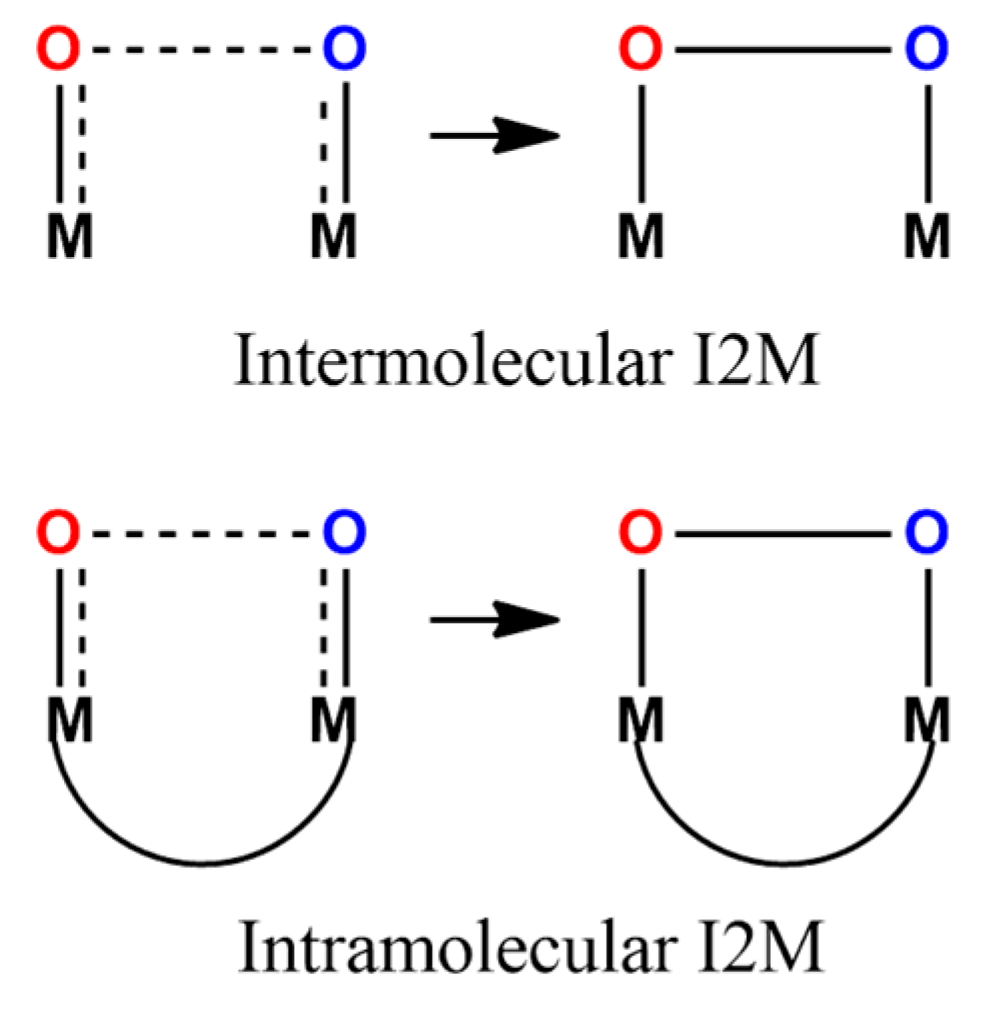
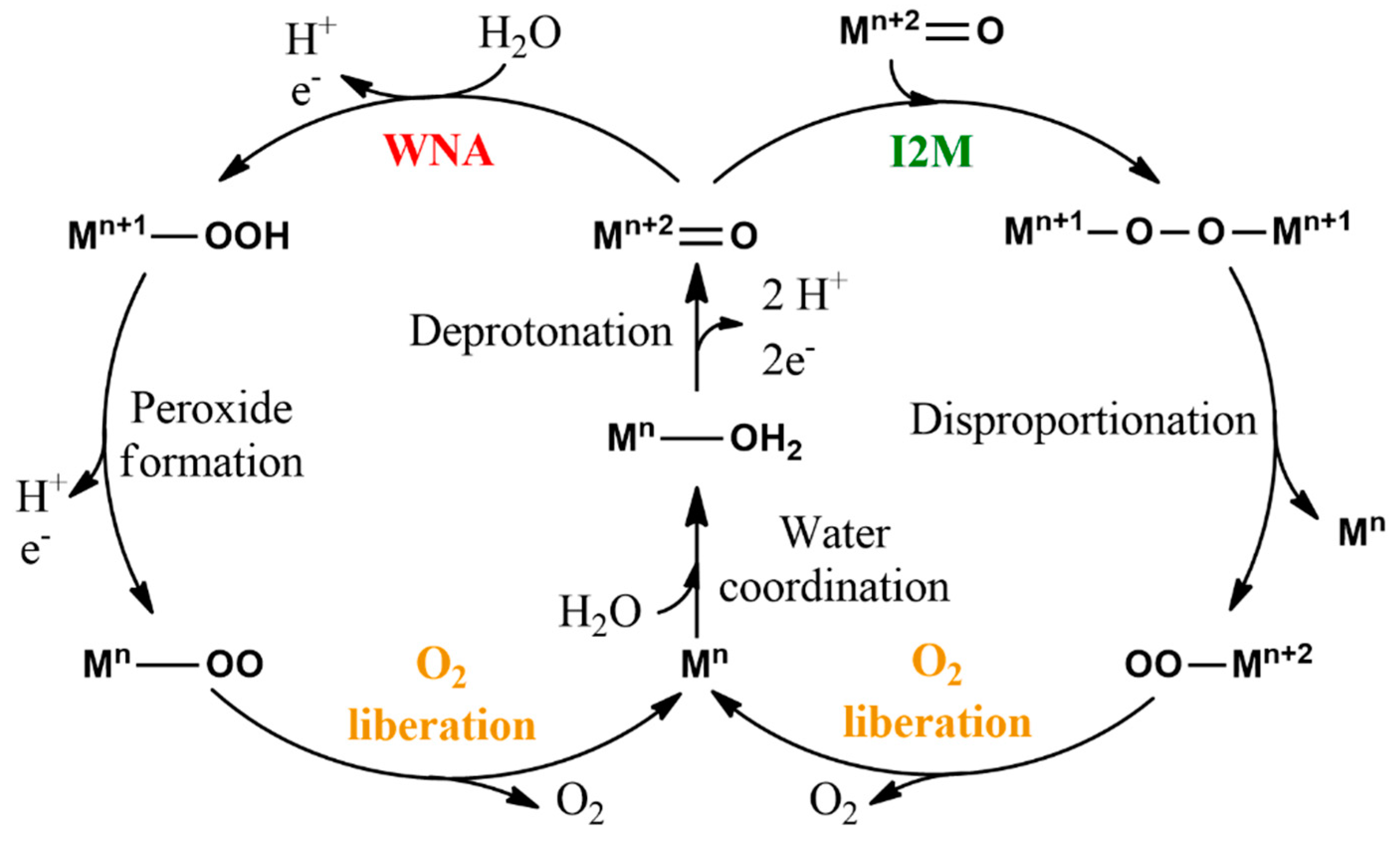
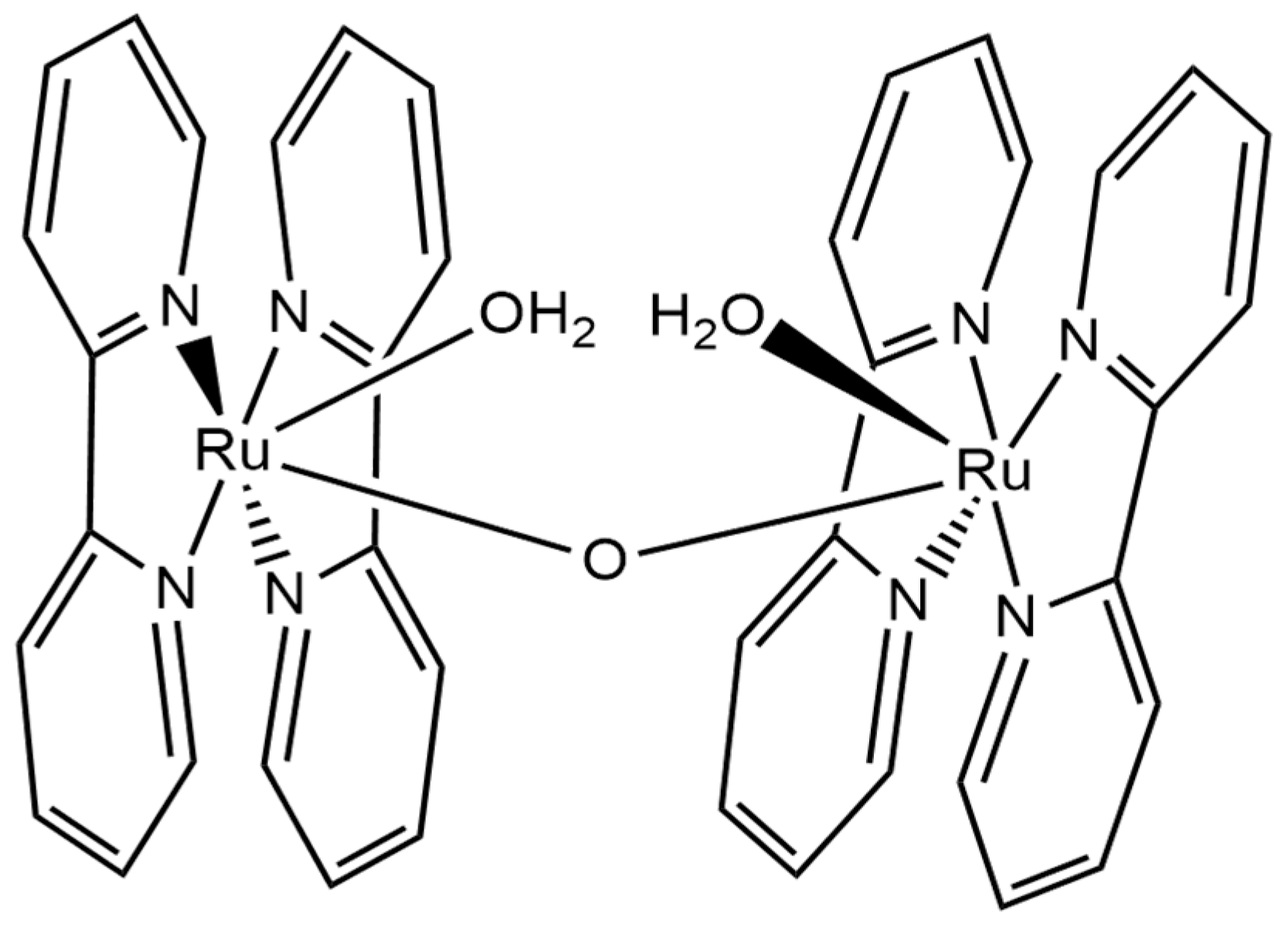
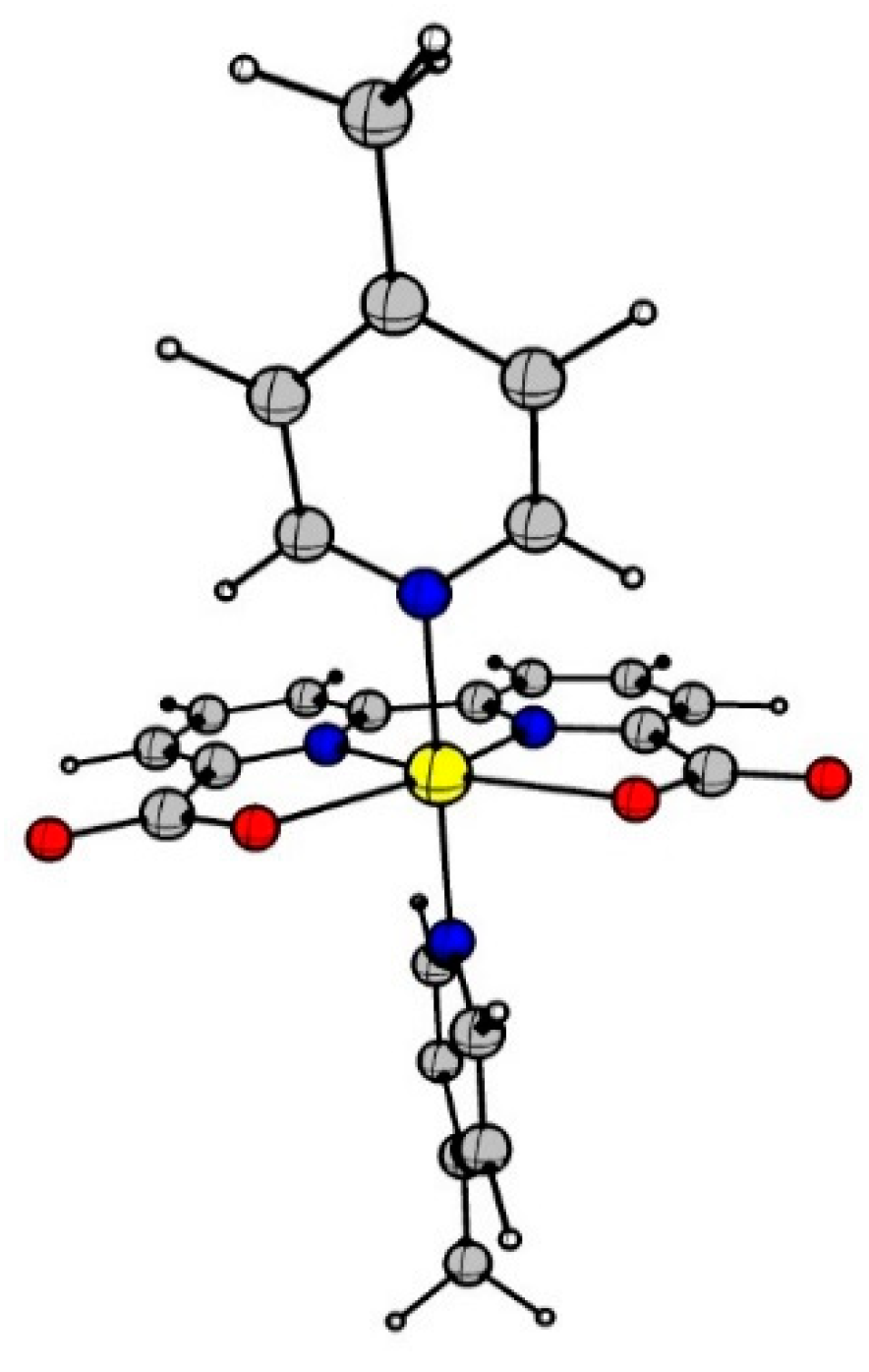
9. Water Oxidation Catalysis
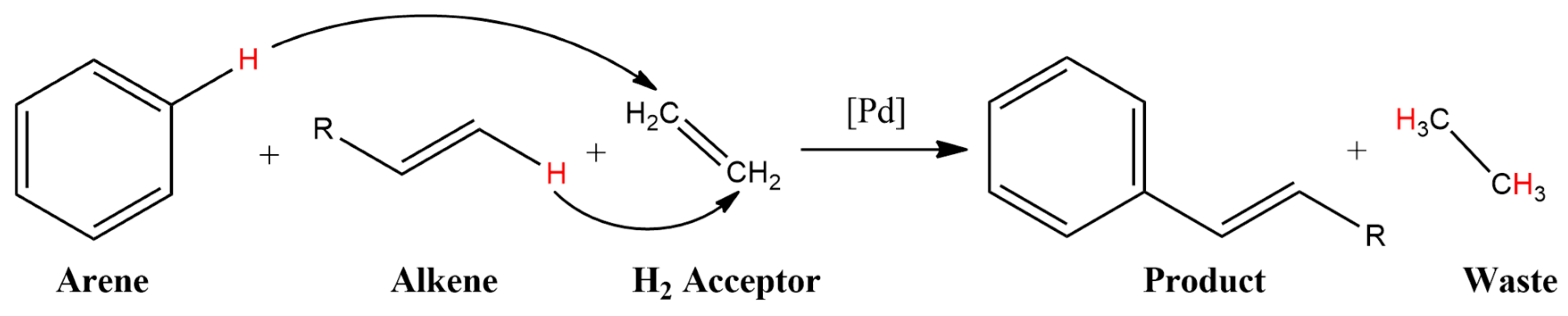
10. Acceptorless Dehydrogenative Coupling
11. Reuse of Atmospheric Gases for Waste Management
12. Solar Cells and Batteries
13. Limitations and Perspectives
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Acceptorless Dehydrogenative Coupling |
| ATP | Adenosine TriPhosphate |
| bda | 2,2′-Bipyridine-6,6′-Dicarboxylic Acid |
| CFC | ChloroFluoroCarbons |
| DFT | Density Functional Theory |
| FNR | Ferredoxin NADP Reductase |
| I2M | Interaction between 2 Metal-oxo centers |
| NADP | Nicotinamide Adenine Dinucleotide Phosphate |
| NADPH | Hydrogenated NADP |
| NOAA | National Oceanic and Atmospheric Administration |
| PCET | Proton Coupled Electron Transfer |
| PNP | Phosphorous-Nitrogen-Phosphorous |
| PSII | Photosystem II |
| SMR | Steam Methane Reforming |
| TOF | TurnOver Frequency |
| TON | TurnOver Number |
| WNA | Water Nucleophilic Attack |
| WOC | Water Oxidation Catalysis |
References
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Luque Urrutia, J.A. Computational Studies Oriented towards the Development of a Greener Chemistry. Ph.D. Thesis, Universitat de Girona, Girona, Spain, 2021. [Google Scholar]
- Ruocco, C.; Martino, M. Catalysts for Sustainable Hydrogen Production: Preparation, Applications and Process Integration. Catalysts 2022, 12, 322. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Somepics. Available online: https://commons.wikimedia.org/w/index.php?curid=38088695 (accessed on 1 February 2023).
- Gregg, J.S.; Andres, R.J.; Marland, G. China: Emissions pattern of the world leader in CO2 emissions from fossil fuel consumption and cement production. Geophys. Res. Lett. 2008, 35, L08806. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef] [PubMed]
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B-Environ. 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Husain, T. Extinguishing of Kuwaiti oil fires—Challenges, technology, and success. Atmos. Environ. 1994, 28, 2139–2147. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Ren, X.; He, C.; Liu, J.; Zhang, Q. Ultra-low-loaded Ni−Fe Dimer Anchored to Nitrogen/Oxygen Sites for Boosting Electroreduction of Carbon Dioxide. ChemSusChem 2021, 14, 4499–4506. [Google Scholar] [CrossRef]
- Jackson, R.B.; Friedlingstein, P.; Andrew, R.M.; Canadell, J.G.; Le Quéré, C.; Peters, G.P. Persistent fossil fuel growth threatens the Paris Agreement and planetary health. Environ. Res. Lett. 2019, 14, 121001. [Google Scholar] [CrossRef]
- Marland, G.; Boden, T.A.; Andres, R.J. Global, Regional, and National CO2 Emissions. In Trends: A Compendium of Data on Global Change; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 2003. [Google Scholar]
- Feng, C.; Zheng, C.-J.; Shan, M.-L. The clarification for the features, temporal variations, and potential factors of global carbon dioxide emissions. J. Clean. Prod. 2020, 255, 120250. [Google Scholar] [CrossRef]
- Yang, S.; Lei, L.; Zeng, Z.; He, Z.; Zhong, H. An Assessment of Anthropogenic CO2 Emissions by Satellite-Based Observations in China. Sensors 2019, 19, 1118. [Google Scholar] [CrossRef] [PubMed]
- Charlock, T.P. CO2-induced climatic change and spectral variations in the outgoing terrestrial infrared radiation. Tellus B 2017, 36, 139–148. [Google Scholar] [CrossRef]
- Thacker, I.; Sinatra, G. Visualizing the Greenhouse Effect: Restructuring Mental Models of Climate Change Through a Guided Online Simulation. Educ. Sci. 2019, 9, 14. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. Climate at a Glance: Global Mapping. Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 17 February 2023).
- Zanna, L.; Khatiwala, S.; Gregory, J.M.; Ison, J.; Heimbach, P. Global reconstruction of historical ocean heat storage and transport. Proc. Natl. Acad. Sci. USA 2019, 116, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Carrington, D. Global Warming of Oceans Equivalent to an Atomic Bomb per Second. Available online: https://www.theguardian.com/environment/2019/jan/07/global-warming-of-oceans-equivalent-to-an-atomic-bomb-per-second (accessed on 17 February 2023).
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-Term Climate Change Projections, Commitments and Irreversibility. In Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Cole, S.; Jacobs, P. NASA, NOAA Analyses Reveal 2019 Second Warmest Year on Record. Available online: https://www.giss.nasa.gov/research/news/20200115/ (accessed on 1 February 2023).
- Shultz, J.M.; Sands, D.E.; Kossin, J.P.; Galea, S. Double Environmental Injustice—Climate Change, Hurricane Dorian, and the Bahamas. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.; Ahlstrom, A.; Goelzer, H.; Lipscomb, W.; Nowicki, S. Rising Oceans Guaranteed: Arctic Land Ice Loss and Sea Level Rise. Curr. Clim. Chang. Rep. 2018, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Prather, M.J.; Hsu, J.; DeLuca, N.M.; Jackman, C.H.; Oman, L.D.; Douglass, A.R.; Fleming, E.L.; Strahan, S.E.; Steenrod, S.D.; Sovde, O.A.; et al. Measuring and modeling the lifetime of nitrous oxide including its variability. J. Geophys. Res. Atmos. 2015, 120, 5693–5705. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Methane and Nitrous Oxide Emissions from Natural Sources. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100717T.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000017%5CP100717T.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 1 February 2023).
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant. Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- CICA. Nitrogen Oxides (NOx), Why and How They Are Controlled; ITPID, Ed.; Environmental Protection Agency: Washington, DC, USA, 1999; p. 24.
- Lee, C.; Martin, R.V.; van Donkelaar, A.; Lee, H.; Dickerson, R.R.; Hains, J.C.; Krotkov, N.; Richter, A.; Vinnikov, K.; Schwab, J.J. SO2 emissions and lifetimes: Estimates from inverse modeling using in situ and global, space-based (SCIAMACHY and OMI) observations. J. Geophys. Res. 2011, 116, D06304. [Google Scholar]
- Mehmood, A.; Alhasani, H.; Alamoodi, N.; AlWahedi, Y.F.; Ibrahim, S.; Raj, A.C. An evaluation of kinetic models for the simulation of Claus reaction furnaces in sulfur recovery units under different feed conditions. J. Nat. Gas Sci. Eng. 2020, 74, 103106. [Google Scholar] [CrossRef]
- Marsh, D.W.; Ulrichson, D.L. Rate and diffusional study of the reaction of calcium oxide with sulfur dioxide. Chem. Eng. Sci. 1985, 40, 423–433. [Google Scholar] [CrossRef]
- Brantley, S.L.; Borgiatt, A.; Rowe, G.; Fernandez, J.F.; Reynolds, J.R. Poás volcano crater lake acts as a condenser for acid metal-rich brine. Nature 1987, 330, 470–472. [Google Scholar] [CrossRef]
- Nachbar-Hapai, M.; Siegel, B.Z.; Russell, C.; Siegel, S.M.; Siy, M.L.; Priestley, D. Acid rain in the kilauea Volcano area (Hawaii). Arch. Environ. Con. Tox. 1989, 18, 65–73. [Google Scholar] [CrossRef]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, O.; Rodhe, H.; Cowling, E. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar] [PubMed]
- Busenberg, E.; Plummer, L.N. Use of chlorofluorocarbons (CCl3F and CCl2F2) as hydrologic tracers and age-dating tools: The alluvium and terrace system of central Oklahoma. Water Resour. Res. 1992, 28, 2257–2283. [Google Scholar] [CrossRef]
- Buis, A. The Atmosphere: Tracking the Ongoing Recovery of Earth’s Ozone Hole. Available online: https://climate.nasa.gov/news/2916/the-atmosphere-tracking-the-ongoing-recovery-of-earths-ozone-hole/ (accessed on 1 February 2023).
- Soni, P. The Year That Was. Available online: https://secondsguru.com/top-environmental-news-2019/ (accessed on 1 February 2023).
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy; BP Website: London, UK, 2019; p. 64. [Google Scholar]
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; Praeger: Westport, CT, USA, 2016. [Google Scholar]
- Ritchie, H.; Roser, M. Fossil Fuels. 2017. Available online: https://ourworldindata.org/fossil-fuels (accessed on 1 February 2023).
- Nagatani, K.; Kiribayashi, S.; Okada, Y.; Otake, K.; Yoshida, K.; Tadokoro, S.; Nishimura, T.; Yoshida, T.; Koyanagi, E.; Fukushima, M.; et al. Emergency response to the nuclear accident at the Fukushima Daiichi Nuclear Power Plants using mobile rescue robots. J. Field Robot. 2013, 30, 44–63. [Google Scholar] [CrossRef]
- Cardis, E.; Hatch, M. The Chernobyl accident—An epidemiological perspective. Clin. Oncol. (R. Coll. Radiol.) 2011, 23, 251–260. [Google Scholar] [CrossRef]
- Lee, H.K.H.; Telford, A.M.; Röhr, J.A.; Wyatt, M.F.; Rice, B.; Wu, J.; de Castro Maciel, A.; Tuladhar, S.M.; Speller, E.; McGettrick, J.; et al. The role of fullerenes in the environmental stability of polymer:fullerene solar cells. Energy Environ. Sci. 2018, 11, 417–428. [Google Scholar] [CrossRef]
- Zhang, F.; Inganäs, O.; Zhou, Y.; Vandewal, K. Development of polymer–fullerene solar cells. Nat. Sci. Rev. 2016, 3, 222–239. [Google Scholar] [CrossRef]
- Thompson, B.C.; Frechet, J.M. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Javaid, R. Catalytic hydrogen production, storage and application. Catalysts 2021, 11, 836. [Google Scholar] [CrossRef]
- Alternative Fuels Data Center. Fuel Properties Comparison. Available online: https://afdc.energy.gov/fuels/fuel_comparison_chart.pdf (accessed on 1 February 2023).
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Takeno, K.; Okabayashi, K.; Kouchi, A.; Nonaka, T.; Hashiguchi, K.; Chitose, K. Dispersion and explosion field tests for 40 MPa pressurized hydrogen. Int. J. Hydrogen Energy 2007, 32, 2144–2153. [Google Scholar] [CrossRef]
- Zuttel, A. Hydrogen storage methods. Sci. Nat. 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Rödl, A.; Wulf, C.; Kaltschmitt, M. Assessment of selected hydrogen supply chains—Factors determining the overall GHG emissions. Hydrog. Supply Chain 2018, 2018, 81–109. [Google Scholar]
- Crowl, D.A.; Jo, Y.-D. The hazards and risks of hydrogen. J. Loss Prevent. Proc. 2007, 20, 158–164. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, S.; Sun, F. Research Progress in Vehicular High Mass Density Solid Hydrogen Storage Materials. Chin. J. Rare Met. 2022, 46, 796–812. [Google Scholar]
- Cheng, H.-M.; Yang, Q.-H.; Liu, C. Hydrogen storage in carbon nanotubes. Carbon 2001, 39, 1447–1454. [Google Scholar] [CrossRef]
- Zhevago, N.K.; Denisov, E.I.; Glebov, V.I. Experimental investigation of hydrogen storage in capillary arrays. Int. J. Hydrogen Energy 2010, 35, 169–175. [Google Scholar] [CrossRef]
- Jordá-Beneyto, M.; Suárez-García, F.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen storage on chemically activated carbons and carbon nanomaterials at high pressures. Carbon 2007, 45, 293–303. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and Potential as Hydrogen Storage Medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Luo, H.; Barrio, J.; Sunny, N.; Li, A.; Steier, L.; Shah, N.; Stephens, I.E.L.; Titirici, M.-M. Progress and Perspectives in Photo- and Electrochemical-Oxidation of Biomass for Sustainable Chemicals and Hydrogen Production. Adv. Energy Mater. 2021, 11, 2101180. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.-J. Graphitic carbon nitride materials for photocatalytic hydrogen production via water splitting: A short review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef]
- McEvoy, J.P.; Brudvig, G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006, 106, 4455–4483. [Google Scholar] [CrossRef]
- Srinivasan, N.K.; Michael, J.V. The thermal decomposition of water. Int. J. Chem. Kinet. 2006, 38, 211–219. [Google Scholar] [CrossRef]
- Baykara, S. Hydrogen production by direct solar thermal decomposition of water, possibilities for improvement of process efficiency. Int. J. Hydrogen Energy 2004, 29, 1451–1458. [Google Scholar] [CrossRef]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Wang, X.; Bi, S.; Zhang, J.; Tao, H. Towards the Rational Design of Stable Electrocatalysts for Green Hydrogen Production. Catalysts 2022, 12, 204. [Google Scholar] [CrossRef]
- Bokareva, O.S.; Mohle, T.; Neubauer, A.; Bokarev, S.I.; Lochburnner, S.; Kuhn, O. Chemical Tuning and Absorption Properties of Iridium Photosensitizers for Photocatalytic Applications. Inorganics 2017, 5, 23. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Hetterscheid, D.G.; Reek, J.N. Mononuclear water oxidation catalysts. Angew. Chem. Int. Ed. 2012, 51, 9740–9747. [Google Scholar] [CrossRef] [PubMed]
- Sameera, W.M.; McKenzie, C.J.; McGrady, J.E. On the mechanism of water oxidation by a bimetallic manganese catalyst: A density functional study. Dalton Trans. 2011, 40, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, K.; Yao, J.; Shaik, S.; Chen, H. Probing ligand effects on O-O bond formation of Ru-catalyzed water oxidation: A computational survey. Inorg. Chem. 2014, 53, 7130–7136. [Google Scholar] [CrossRef]
- Liu, T.; Li, G.; Shen, N.; Wang, L.; Timmer, B.J.J.; Kravchenko, A.; Zhou, S.; Gao, Y.; Yang, Y.; Yang, H.; et al. Promoting Proton Transfer and Stabilizing Intermediates in Catalytic Water Oxidation via Hydrophobic Outer Sphere Interactions. Chem. Eur. J. 2022, 28, e202104562. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Eggleston, D.S.; Murphy, W.R.; Geselowitz, D.A.; Gersten, S.W.; Hodgson, D.J.; Meyer, T.J. Structure and redox properties of the water-oxidation catalyst [(bpy)2(OH2)RuORu(OH2)(bpy)2]4+. J. Am. Chem. Soc. 1985, 107, 3855–3864. [Google Scholar] [CrossRef]
- Gersten, S.W.; Samuels, G.J.; Meyer, T.J. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 1982, 104, 4029–4030. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Ru-bda: Unique Molecular Water-Oxidation Catalysts with Distortion Induced Open Site and Negatively Charged Ligands. J. Am. Chem. Soc. 2019, 141, 5565–5580. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Duan, L.; Xu, Y.; Privalov, T.; Sun, L. Structural modifications of mononuclear ruthenium complexes: A combined experimental and theoretical study on the kinetics of ruthenium-catalyzed water oxidation. Angew. Chem. Int. Ed. 2011, 50, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Rahimi, F.; Aro, E.M.; Lee, C.H.; Allakhverdiev, S.I. Nano-sized manganese oxides as biomimetic catalysts for water oxidation in artificial photosynthesis: A review. J. R. Soc. Interface 2012, 9, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Kaneko, M. Molecular catalysts for water oxidation. Chem. Rev. 2001, 101, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Grotjahn, D.B.; Brown, D.B.; Martin, J.K.; Marelius, D.C.; Abadjian, M.C.; Tran, H.N.; Kalyuzhny, G.; Vecchio, K.S.; Specht, Z.G.; Cortes-Llamas, S.A.; et al. Evolution of iridium-based molecular catalysts during water oxidation with ceric ammonium nitrate. J. Am. Chem. Soc. 2011, 133, 19024–19027. [Google Scholar] [CrossRef]
- Zong, R.; Thummel, R.P. 2,9-Di-(2′-pyridyl)-1,10-phenanthroline: A tetradentate ligand for Ru(II). J. Am. Chem. Soc. 2004, 126, 10800–10801. [Google Scholar] [CrossRef]
- Sens, C.; Romero, I.; Rodriguez, M.; Llobet, A.; Parella, T.; Benet-Buchholz, J. A new Ru complex capable of catalytically oxidizing water to molecular dioxygen. J. Am. Chem. Soc. 2004, 126, 7798–7799. [Google Scholar] [CrossRef]
- Zong, R.; Thummel, R.P. A new family of Ru complexes for water oxidation. J. Am. Chem. Soc. 2005, 127, 12802–12803. [Google Scholar] [CrossRef]
- Xu, Y.; Akermark, T.; Gyollai, V.; Zou, D.; Eriksson, L.; Duan, L.; Zhang, R.; Akermark, B.; Sun, L. A new dinuclear ruthenium complex as an efficient water oxidation catalyst. Inorg. Chem. 2009, 48, 2717–2719. [Google Scholar] [CrossRef]
- Duan, L.; Fischer, A.; Xu, Y.; Sun, L. Isolated seven-coordinate Ru(IV) dimer complex with [HOHOH]- bridging ligand as an intermediate for catalytic water oxidation. J. Am. Chem. Soc. 2009, 131, 10397–10399. [Google Scholar] [CrossRef]
- Nyhlen, J.; Duan, L.; Akermark, B.; Sun, L.; Privalov, T. Evolution of O2 in a seven-coordinate Ru(IV) dimer complex with a [HOHOH]− bridge: A computational study. Angew. Chem. Int. Ed. 2010, 49, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Matheu, R.; Ertem, M.Z.; Gimbert-Suriñach, C.; Sala, X.; Llobet, A. Seven Coordinated Molecular Ruthenium-Water Oxidation Catalysts: A Coordination Chemistry Journey. Chem. Rev. 2019, 119, 3453–3471. [Google Scholar] [CrossRef] [PubMed]
- Luque-Urrutia, J.A.; Gimferrer, M.; Casals-Cruañas, È.; Poater, A. In silico switch from second- to first-row transition metals in olefin metathesis: From Ru to Fe and from Rh to Co. Catalysts 2017, 7, 389. [Google Scholar] [CrossRef]
- Codolà, Z.; Gamba, I.; Acuna-Pares, F.; Casadevall, C.; Clemancey, M.; Latour, J.M.; Luis, J.M.; Lloret-Fillol, J.; Costas, M. Design of Iron Coordination Complexes as Highly Active Homogenous Water Oxidation Catalysts by Deuteration of Oxidation-Sensitive Sites. J. Am. Chem. Soc. 2019, 141, 323–333. [Google Scholar] [CrossRef]
- Gimbert-Suriñach, C.; Moonshiram, D.; Francàs, L.; Planas, N.; Bernales, V.; Bozoglian, F.; Guda, A.; Mognon, L.; López, I.; Hoque, M.A.; et al. Structural and Spectroscopic Characterization of Reaction Intermediates Involved in a Dinuclear Co-Hbpp Water Oxidation Catalyst. J. Am. Chem. Soc. 2016, 138, 15291–15294. [Google Scholar] [CrossRef]
- Poater, A. Environmental friendly Fe substitutive of Ru in water oxidation catalysis. Catal. Commun. 2014, 44, 2–5. [Google Scholar] [CrossRef]
- Gil-Sepulcre, M.; Garrido-Barros, P.; Oldengott, J.; Funes-Ardoiz, I.; Bofill, R.; Sala, X.; Benet-Buchholz, J.; Llobet, A. Consecutive Ligand-Based Electron Transfer in New Molecular Copper-Based Water Oxidation Catalysts. Angew. Chem. Int. Ed. 2021, 60, 18639–18644. [Google Scholar] [CrossRef]
- Ezhov, R.; Karbakhsh Ravari, A.; Page, A.; Pushkar, Y. Water Oxidation Catalyst cis-[Ru(bpy)(5,5′-dcbpy)(H2O)2]2+ and Its Stabilization in Metal–Organic Framework. ACS Catal. 2020, 10, 5299–5308. [Google Scholar] [CrossRef]
- De Palo, A.; La Ganga, G.; Nastasi, F.; Guelfi, M.; Bortoluzzi, M.; Pampaloni, G.; Puntoriero, F.; Campagna, S.; Marchetti, F. Ru(ii) water oxidation catalysts with 2,3-bis(2-pyridyl)pyrazine and tris(pyrazolyl)methane ligands: Assembly of photo-active and catalytically active subunits in a dinuclear structure. Dalton Trans. 2020, 49, 3341–3352. [Google Scholar] [CrossRef]
- Masllorens, E.; Rodriguez, M.; Romero, I.; Roglans, A.; Parella, T.; Benet-Buchholz, J.; Poyatos, M.; Llobet, A. Can the disproportion of oxidation state III be favored in RuII−OH2/RuIV=O systems? J. Am. Chem. Soc. 2006, 128, 5306–5307. [Google Scholar] [CrossRef] [PubMed]
- Vereshchuk, N.; Matheu, R.; Benet-Buchholz, J.; Pipelier, M.; Lebreton, J.; Dubreuil, D.; Tessier, A.; Gimbert-Surinach, C.; Ertem, M.Z.; Llobet, A. Second Coordination Sphere Effects in an Evolved Ru Complex Based on Highly Adaptable Ligand Results in Rapid Water Oxidation Catalysis. J. Am. Chem. Soc. 2020, 142, 5068–5077. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.W.; Xie, Y.; Szalda, D.J.; Concepcion, J.J. Lability and Basicity of Bipyridine-Carboxylate-Phosphonate Ligand Accelerate Single-Site Water Oxidation by Ruthenium-Based Molecular Catalysts. J. Am. Chem. Soc. 2017, 139, 15347–15355. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shaffer, D.W.; Lewandowska-Andralojc, A.; Szalda, D.J.; Concepcion, J.J. Water Oxidation by Ruthenium Complexes Incorporating Multifunctional Bipyridyl Diphosphonate Ligands. Angew. Chem. Int. Ed. 2016, 55, 8067–8071. [Google Scholar] [CrossRef]
- Kamdar, J.M.; Marelius, D.C.; Moore, C.E.; Rheingold, A.L.; Smith, D.K.; Grotjahn, D.B. Ruthenium Complexes of 2,2′-Bipyridine-6,6′-diphosphonate Ligands for Water Oxidation. ChemCatChem 2016, 8, 3045–3049. [Google Scholar] [CrossRef]
- Matheu, R.; Ertem, M.Z.; Benet-Buchholz, J.; Coronado, E.; Batista, V.S.; Sala, X.; Llobet, A. Intramolecular Proton Transfer Boosts Water Oxidation Catalyzed by a Ru Complex. J. Am. Chem. Soc. 2015, 137, 10786–10795. [Google Scholar] [CrossRef]
- Hoque, M.A.; Gil-Sepulcre, M.; de Aguirre, A.; Elemans, J.; Moonshiram, D.; Matheu, R.; Shi, Y.; Benet-Buchholz, J.; Sala, X.; Malfois, M.; et al. Water oxidation electrocatalysis using ruthenium coordination oligomers adsorbed on multiwalled carbon nanotubes. Nat. Chem. 2020, 12, 1060–1066. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Solà, M.; Poater, A. WOC Mechanism by a Ru(bda) catalyst switches with pH. Catal. Today 2020, 358, 278–283. [Google Scholar] [CrossRef]
- Richmond, C.J.; Escayola, S.; Poater, A. Axial Ligand effects of Ru-BDA Complexes in the O-O Bond Formation via the I2M Bimolecular Mechanism in Water Oxidation Catalysis. Eur. J. Inorg. Chem. 2019, 2019, 2101–2108. [Google Scholar] [CrossRef]
- Richmond, C.J.; Matheu, R.; Poater, A.; Falivene, L.; Benet-Buchholz, J.; Sala, X.; Cavallo, L.; Llobet, A. Supramolecular water oxidation with Ru–bda-based catalysts. Chem. Eur. J. 2014, 20, 17282–17286. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Kamdar, J.M.; Grotjahn, D.B.; Solà, M.; Poater, A. Understanding the Performance of a Bisphosphonate Ru Water Oxidation Catalyst. Dalton Trans. 2020, 49, 14052–14060. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.J.; Zhong, D.K.; Szalda, D.J.; Muckerman, J.T.; Fujita, E. Mechanism of water oxidation by [Ru(bda)(L)2]: The return of the “blue dimer”. Chem. Commun. 2015, 51, 4105–4108. [Google Scholar] [CrossRef]
- Huynh, M.H.; Meyer, T.J. Proton-coupled electron transfer. Chem. Rev. 2007, 107, 5004–5064. [Google Scholar] [PubMed]
- Bucci, A.; Menendez Rodriguez, G.; Bellachioma, G.; Zuccaccia, C.; Poater, A.; Cavallo, L.; Macchioni, A. An Alternative Reaction Pathway for Iridium-Catalyzed Water Oxidation Driven by Cerium Ammonium Nitrate (CAN). ACS Catal. 2016, 6, 4559–4563. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Gimbert-Suriñach, C.; Matheu, R.; Sala, X.; Llobet, A. How to make an efficient and robust molecular catalyst for water oxidation. Chem. Soc. Rev. 2017, 46, 6088–6098. [Google Scholar] [CrossRef] [PubMed]
- Matheu, R.; Garrido-Barros, P.; Gil-Sepulcre, M.; Ertem, M.Z.; Sala, X.; Gimbert-Suriñach, C.; Llobet, A. The development of molecular water oxidation catalysts. Nat. Rev. Chem. 2019, 3, 331–341. [Google Scholar] [CrossRef]
- Creus, J.; De Tovar, J.; Romero, N.; García-Antón, J.; Bofill, R.; Sala, X. Ruthenium Nanoparticles for Catalytic Water Splitting. ChemSusChem 2019, 12, 2493–2514. [Google Scholar] [PubMed]
- Li, C.-J.; Li, Z. Green chemistry: The development of cross-dehydrogenative coupling (CDC) for chemical synthesis. Pure Appl. Chem. 2006, 78, 935–945. [Google Scholar] [CrossRef]
- Fuse, H.; Mitsunuma, H.; Kanai, M. Catalytic Acceptorless Dehydrogenation of Aliphatic Alcohols. J. Am. Chem. Soc. 2020, 142, 4493–4499. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Fu, H.; Zheng, X.; Chen, H.; Li, R.; Yu, X. Synthesis of Unsymmetrical N-Heterocyclic Carbene–Nitrogen–Phosphine Chelated Ruthenium(II) Complexes and Their Reactivity in Acceptorless Dehydrogenative Coupling of Alcohols to Esters. Organometallics 2019, 38, 1750–1760. [Google Scholar] [CrossRef]
- Azofra, L.M.; Poater, A. Diastereoselective diazenyl formation: The key for manganese-catalysed alcohol conversion into (E)-alkenes. Dalton Trans. 2019, 48, 14122–14127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhu, M.; Wang, C.; Li, J.; Jiang, X.; Wei, Y.; Tang, W.; Xue, D.; Xiao, J. Chemoselective dehydrogenative esterification of aldehydes and alcohols with a dimeric rhodium(II) catalyst. Chem. Sci. 2016, 7, 4428–4434. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.; Ackermann, L.; Gelman, D. Dehydrogenative Cross-Coupling of Primary and Secondary Alcohols. Adv. Synth. Catal. 2013, 355, 3077–3080. [Google Scholar]
- Taniguchi, K.; Jin, X.; Yamaguchi, K.; Nozaki, K.; Mizuno, N. Versatile routes for synthesis of diarylamines through acceptorless dehydrogenative aromatization catalysis over supported gold-palladium bimetallic nanoparticles. Chem. Sci. 2017, 8, 2131–2142. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gellrich, U.; Diskin-Posner, Y.; Leitus, G.; Avram, L.; Milstein, D. Manganese-Catalyzed N-Formylation of Amines by Methanol Liberating H2: A Catalytic and Mechanistic Study. Angew. Chem. Int. Ed. 2017, 56, 4229–4233. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, U.K.; Ben-David, Y.; Milstein, D. Manganese Catalyzed α-Olefination of Nitriles by Primary Alcohols. J. Am. Chem. Soc. 2017, 139, 11710–11713. [Google Scholar]
- Guchhait, S.K.; Sisodiya, S.; Saini, M.; Shah, Y.V.; Kumar, G.; Daniel, D.P.; Hura, N.; Chaudhary, V. Synthesis of Polyfunctionalized Pyrroles via a Tandem Reaction of Michael Addition and Intramolecular Cyanide-Mediated Nitrile-to-Nitrile Condensation. J. Org. Chem. 2018, 83, 5807–5815. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Wei, Y.; Shi, M. Base-Promoted Tandem Cyclization for the Synthesis of Benzonitriles by C−C Bond Construction. Adv. Synth. Catal. 2018, 360, 808–813. [Google Scholar] [CrossRef]
- Sharma, K.; Shrivastava, A.; Mehra, R.N.; Deora, G.S.; Alam, M.M.; Zaman, M.S.; Akhter, M. Synthesis of novel benzimidazole acrylonitriles for inhibition of Plasmodium falciparum growth by dual target inhibition. Arch. Pharm. Res. 2018, 351, 1700251. [Google Scholar] [CrossRef]
- Garbe, M.; Junge, K.; Walker, S.; Wei, Z.; Jiao, H.; Spannenberg, A.; Bachmann, S.; Scalone, M.; Beller, M. Manganese(I)-Catalyzed Enantioselective Hydrogenation of Ketones Using a Defined Chiral PNP Pincer Ligand. Angew. Chem. Int. Ed. 2017, 56, 11237–11241. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Tanaka, H.; Kamaru, N.; Yoshizawa, K.; Nishibayashi, Y. Catalytic formation of ammonia from molecular dinitrogen by use of dinitrogen-bridged dimolybdenum-dinitrogen complexes bearing PNP-pincer ligands: Remarkable effect of substituent at PNP-pincer ligand. J. Am. Chem. Soc. 2014, 136, 9719–9731. [Google Scholar] [CrossRef]
- Weng, W.; Guo, C.; Celenligil-Cetin, R.; Foxman, B.M.; Ozerov, O.V. Skeletal change in the PNP pincer ligand leads to a highly regioselective alkyne dimerization catalyst. Chem. Commun. 2006, 2, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Foxman, B.M.; Ozerov, O.V. N−H Cleavage as a Route to Palladium Complexes of a New PNP Pincer Ligand. Organometallics 2004, 23, 326–328. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Solà, M.; Milstein, D.; Poater, A. Mechanism of the Manganese-Pincer Catalyzed Acceptorless Dehydrogenative Coupling of Nitriles and Alcohols. J. Am. Chem. Soc. 2019, 141, 2398–2403. [Google Scholar] [CrossRef]
- Masdemont, J.; Luque-Urrutia, J.A.; Gimferrer, M.; Milstein, D.; Poater, A. Mechanism of Coupling of Alcohols and Amines To Generate Aldimines and H2 by a Pincer Manganese Catalyst. ACS Catal. 2019, 9, 1662–1669. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Pèlachs, T.; Solà, M.; Poater, A. Double-Carrousel Mechanism for Mn-catalyzed Dehydrogenative Amide Synthesis from Alcohols and Amines. ACS Catal. 2021, 11, 6155–6161. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef]
- Leigh, G.J. Haber-Bosch and Other Industrial Processes; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
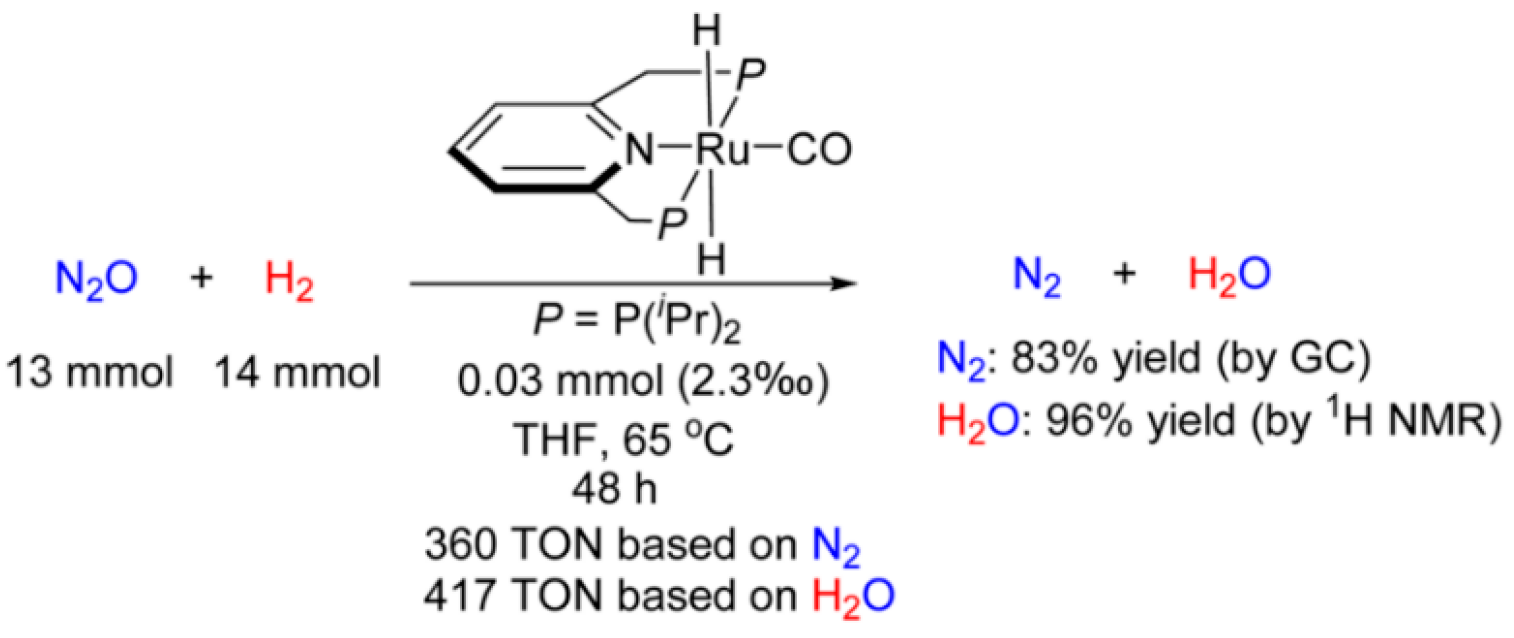
- Luque-Urrutia, J.A.; Poater, A. The Fundamental non Innocent Role of Water for the Hydrogenation of Nitrous Oxide by PNP pincer Ru-based catalysts. Inorg. Chem. 2017, 56, 14383–14387. [Google Scholar] [CrossRef]
- Zeng, R.; Feller, M.; Ben-David, Y.; Milstein, D. Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex. J. Am. Chem. Soc. 2017, 139, 5720–5723. [Google Scholar] [CrossRef]
- Len, T.; Luque, R. Addressing the CO2 challenge through thermocatalytic hydrogenation to carbon monoxide, methanol and methane. Green Chem. 2023, 25, 490–521. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Solà, M.; Poater, A. Carbon Dioxide Conversion on Supported Metal Nanoparticles: A Brief Review. Catalysts 2023, 13, 305. [Google Scholar] [CrossRef]
- Gaona, M.A.; de la Cruz-Martinez, F.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Alonso-Moreno, C.; Rodriguez, A.M.; Rodriguez-Dieguez, A.; Castro-Osma, J.A.; Otero, A.; Lara-Sanchez, A. Synthesis of helical aluminium catalysts for cyclic carbonate formation. Dalton Trans. 2019, 48, 4218–4227. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, J.; Spannenberg, A.; Werner, T. An in situ formed Ca2+–crown ether complex and its use in CO2-fixation reactions with terminal and internal epoxides. Green Chem. 2017, 19, 3769–3779. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2017, 8, 419–450. [Google Scholar] [CrossRef]
- Monassier, A.; D’Elia, V.; Cokoja, M.; Dong, H.; Pelletier, J.D.A.; Basset, J.-M.; Kühn, F.E. Synthesis of Cyclic Carbonates from Epoxides and CO2 under Mild Conditions Using a Simple, Highly Efficient Niobium-Based Catalyst. ChemCatChem 2013, 5, 1321–1324. [Google Scholar]
- Vummaleti, S.V.C.; Nolan, S.P.; Cavallo, L.; Talarico, G.; Poater, A. How easy is CO2 fixation by M–C bond containing complexes (M = Cu, Ni, Co, Rh, Ir)? Org. Chem. Front. 2016, 3, 19–23. [Google Scholar]
- Aomchad, V.; Del Globo, S.; Poater, A.; D’Elia, V. Exploring the potential of Group III salen complexes for the conversion of CO2 under ambient conditions. Catal. Today 2021, 375, 324–334. [Google Scholar]
- Sodpiban, O.; Del Gobbo, S.; Barman, S.; Aomchad, V.; Kidkhunthod, P.; Ould-Chikh, S.; Poater, A.; D’Elia, V.; Basset, J.-M. Synthesis of Well-defined Yttrium-based Lewis Acids by Capture of a Reaction Intermediate and Catalytic Application for cycloaddition of CO2 to Epoxides Under Atmospheric Pressure. Catal. Sci. Technol. 2019, 9, 6152–6165. [Google Scholar] [CrossRef]
- Natongchai, W.; Luque-Urrutia, J.A.; Phungpanya, C.; Solà, M.; D’Elia, V.; Poater, A.; Zipse, H. Cycloaddition of CO2 to epoxides by highly nucleophilic 4-aminopyridines: Establishing a relationship between carbon basicity and catalytic performance by experimental and DFT investigations. Org. Chem. Front. 2021, 8, 613–627. [Google Scholar] [CrossRef]
- Dabral, S.; Schaub, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’Elia, V.; Kuhn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalton Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Liyanage, W.; Masud, J.; Kapila, S.; Nath, M. Selective electroreduction of CO2 to carbon-rich products with a simple binary copper selenide electrocatalyst. J. Mater. Chem. A 2021, 9, 7150–7161. [Google Scholar] [CrossRef]
- Saxena, A.; Liyanage, W.; Kapila, S.; Nath, M. Nickel selenide as an efficient electrocatalyst for selective reduction of carbon dioxide to carbon-rich products. Catal. Sci. Technol. 2022, 12, 4727–4739. [Google Scholar] [CrossRef]
- Artz, J.; Muller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective, and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Kleij, A.W. Advancing Halide-Free Catalytic Synthesis of CO2 based Heterocycles. Curr. Opin. Green Sustain. Chem. 2020, 24, 72–81. [Google Scholar] [CrossRef]
- Zou, B.; Hu, C. Halogen-free processes for organic carbonate synthesis from CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 11–16. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Alshahrie, A.; Alghamdi, A.A.; Hasan, P.M.Z.; Ahmed, F.; Albalawi, H.M.E.; Umar, A.; Alsulami, A. Enhancement in the Performance of Dye Sensitized Solar Cells (DSSCs) by Incorporation of Reduced Graphene Oxide (RGO) and Carbon Nanotubes (CNTs) in ZnO Nanostructures. Inorganics 2022, 10, 204. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Yi, X.; Zhao, X.J.; Xiang, W.-C.; Abdelmotallieb, M. Electrodeposited PPy@TiO2 and PEDOT@TiO2 Counter Electrodes for [Co(bpy)3]2+/3+ Redox Mediator-Based Dye-Sensitized Solar Cells. Inorganics 2022, 10, 213. [Google Scholar] [CrossRef]
- Gonzalez, T.; Diaz-Herrera, J.; Tucker, A. Computing Handbook: Computer Science and Software Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Stasyuk, A.J.; Stasyuk, O.A.; Solà, M.; Voityuk, A.A. Photoinduced Charge Shift in Li+-Doped Giant Nested Fullerenes. J. Phys. Chem. C 2019, 123, 16525–16532. [Google Scholar] [CrossRef]
- Yarmolenko, O.V.; Baymuratova, G.R.; Jgatmullina, K.G.; Tulibayeva, G.Z.; Yudina, A.V.; Savinykh, T.A.; Yakushchenko, I.K.; Troshin, P.A.; Shestakov, A.F. Influence of the Lithium Cation Desolvation Process at the Electrolyte/Electrode Interface on the Performance of Lithium Batteries. Inorganics 2022, 10, 176. [Google Scholar] [CrossRef]
- Birrozzi, A.; Mullaliu, A.; Eisenmann, T.; Asenbauer, J.; Diemant, T.; Geiger, D.; Kaiser, I.; de Souza, D.O.; Ashton, T.E.; Groves, A.R.; et al. Synergistic Effect of Co and Mn Co-Doping on SnO2 Lithium-Ion Anodes. Inorganics 2022, 10, 46. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar Cells: In Research and Applications—A Review. Mat. Sci. Appl. 2015, 06, 1145–1155. [Google Scholar] [CrossRef]
- Kibria, M.T.; Ahammed, A.; Sony, S.M.; Hossain, F. Shams-Ul-Islam A Review: Comparative Studies on Different Generation Solar Cells Technology. In Proceedings of the 5th International Conference on Environmental Aspects of Bangladesh, Dhaka, Bangladesh, 23–24 May 2014; pp. 51–53. [Google Scholar]
- Vigil-Galán, O.; Courel, M.; Andrade-Arvizu, J.A.; Sánchez, Y.; Espíndola-Rodríguez, M.; Saucedo, E.; Seuret-Jiménez, D.; Titsworth, M. Route towards low cost-high efficiency second generation solar cells: Current status and perspectives. J. Mat. Sci. Mater. Electron. 2014, 26, 5562–5573. [Google Scholar] [CrossRef]
- Yan, J.; Saunders, B.R. Third-generation solar cells: A review and comparison of polymer:fullerene, hybrid polymer and perovskite solar cells. RSC Adv. 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- Gómez-Ortíz, N.M.; Vázquez-Maldonado, I.A.; Pérez-Espadas, A.R.; Mena-Rejón, G.J.; Azamar-Barrios, J.A.; Oskam, G. Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol. Energy Mater. Sol. Cells 2010, 94, 40–44. [Google Scholar] [CrossRef]
- Syed, T.H.; Wei, W. Technoeconomic Analysis of Dye Sensitized Solar Cells (DSSCs) with WS2/Carbon Composite as Counter Electrode Material. Inorganics 2022, 10, 191. [Google Scholar] [CrossRef]
- Beiley, Z.M.; Christoforo, M.G.; Gratia, P.; Bowring, A.R.; Eberspacher, P.; Margulis, G.Y.; Cabanetos, C.; Beaujuge, P.M.; Salleo, A.; McGehee, M.D. Semi-transparent polymer solar cells with excellent sub-bandgap transmission for third generation photovoltaics. Adv. Mater. 2013, 25, 7020–7026. [Google Scholar] [CrossRef]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef] [PubMed]
- Geisz, J.F.; France, R.M.; Schulte, K.L.; Steiner, M.A.; Norman, A.G.; Guthrey, H.L.; Young, M.R.; Song, T.; Moriarty, T. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 2020, 5, 326–335. [Google Scholar] [CrossRef]
- Arjunan, T.V.; Senthil, T.S. Review: Dye sensitised solar cells. Mat. Tech. 2013, 28, 9–14. [Google Scholar] [CrossRef]
- Bomben, P.G.; Robson, K.C.D.; Koivisto, B.D.; Berlinguette, C.P. Cyclometalated ruthenium chromophores for the dye-sensitized solar cell. Coord. Chem. Rev. 2012, 256, 1438–1450. [Google Scholar] [CrossRef]
- Kroto, H.W.; Allaf, A.W.; Balm, S.P. C60: Buckminsterfullerene. Chem. Rev. 1991, 91, 1213–1235. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, Q.; Shao, Y.; Deng, Y.; Wang, Q.; Shen, L.; Wang, D.; Wei, W.; Huang, J. Enhancing stability and efficiency of perovskite solar cells with crosslinkable silane-functionalized and doped fullerene. Nat. Commun. 2016, 7, 12806. [Google Scholar] [CrossRef]
- Erten-Ela, S.; Chen, H.; Kratzer, A.; Hirsch, A.; Brabec, C.J. Perovskite solar cells fabricated using dicarboxylic fullerene derivatives. New J. Chem. 2016, 40, 2829–2834. [Google Scholar] [CrossRef]
- Masud, J.; Liyanage, W.P.R.; Cao, X.; Saxena, A.; Nath, M. Copper Selenides as High-Efficiency Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 4075–4083. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Q.; Wu, Y.; Lan, Y.-Q.; Chen, B. Metal–Organic Frameworks for Photo/Electrocatalysis. Adv. Energy Sustain. Res. 2021, 2, 2100033. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.; Zhang, Q. Current Progress in 2D Metal–Organic Frameworks for Electrocatalysis. Small Struct. 2023. Online Version of Record. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Urrutia, J.A.; Ortiz-García, T.; Solà, M.; Poater, A. Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics 2023, 11, 88. https://doi.org/10.3390/inorganics11020088
Luque-Urrutia JA, Ortiz-García T, Solà M, Poater A. Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics. 2023; 11(2):88. https://doi.org/10.3390/inorganics11020088
Chicago/Turabian StyleLuque-Urrutia, Jesús Antonio, Thalía Ortiz-García, Miquel Solà, and Albert Poater. 2023. "Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling" Inorganics 11, no. 2: 88. https://doi.org/10.3390/inorganics11020088
APA StyleLuque-Urrutia, J. A., Ortiz-García, T., Solà, M., & Poater, A. (2023). Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics, 11(2), 88. https://doi.org/10.3390/inorganics11020088










