Effects of Boron-Containing Compounds on Liposoluble Hormone Functions
Abstract
1. Introduction
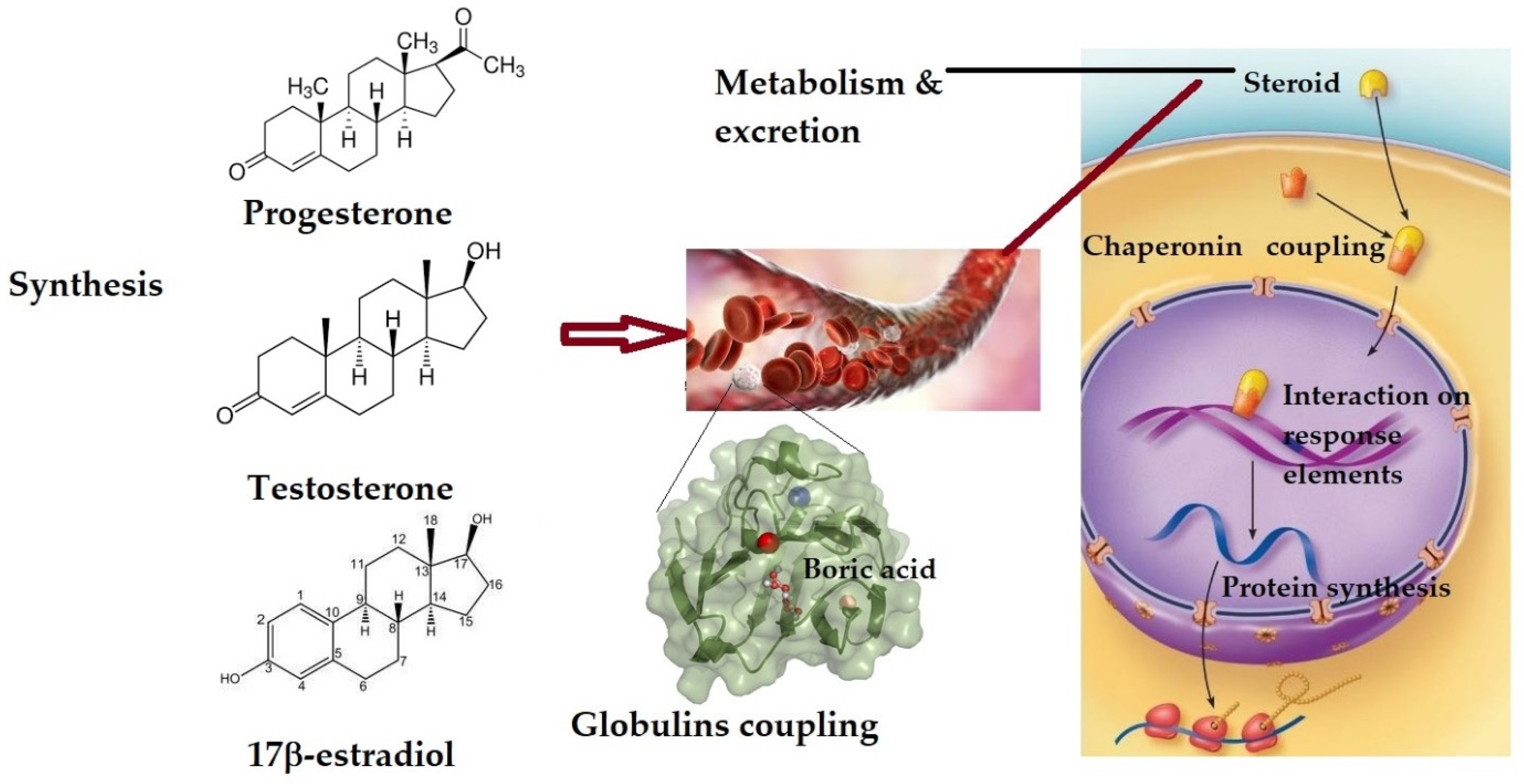
2. The Effect on Sex Hormones and Their Receptors
2.1. Changes in Sex Hormones’ Plasma Level
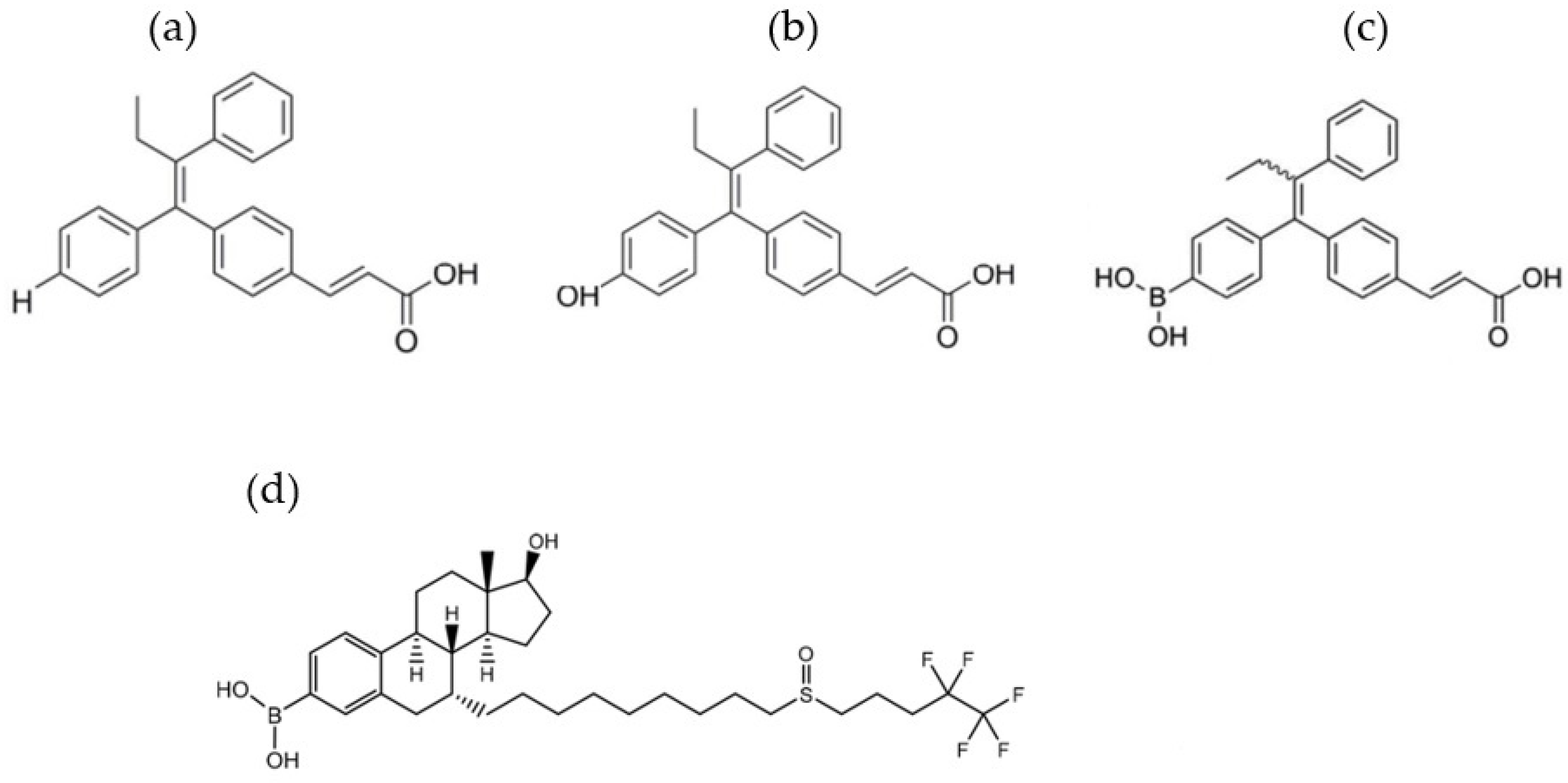
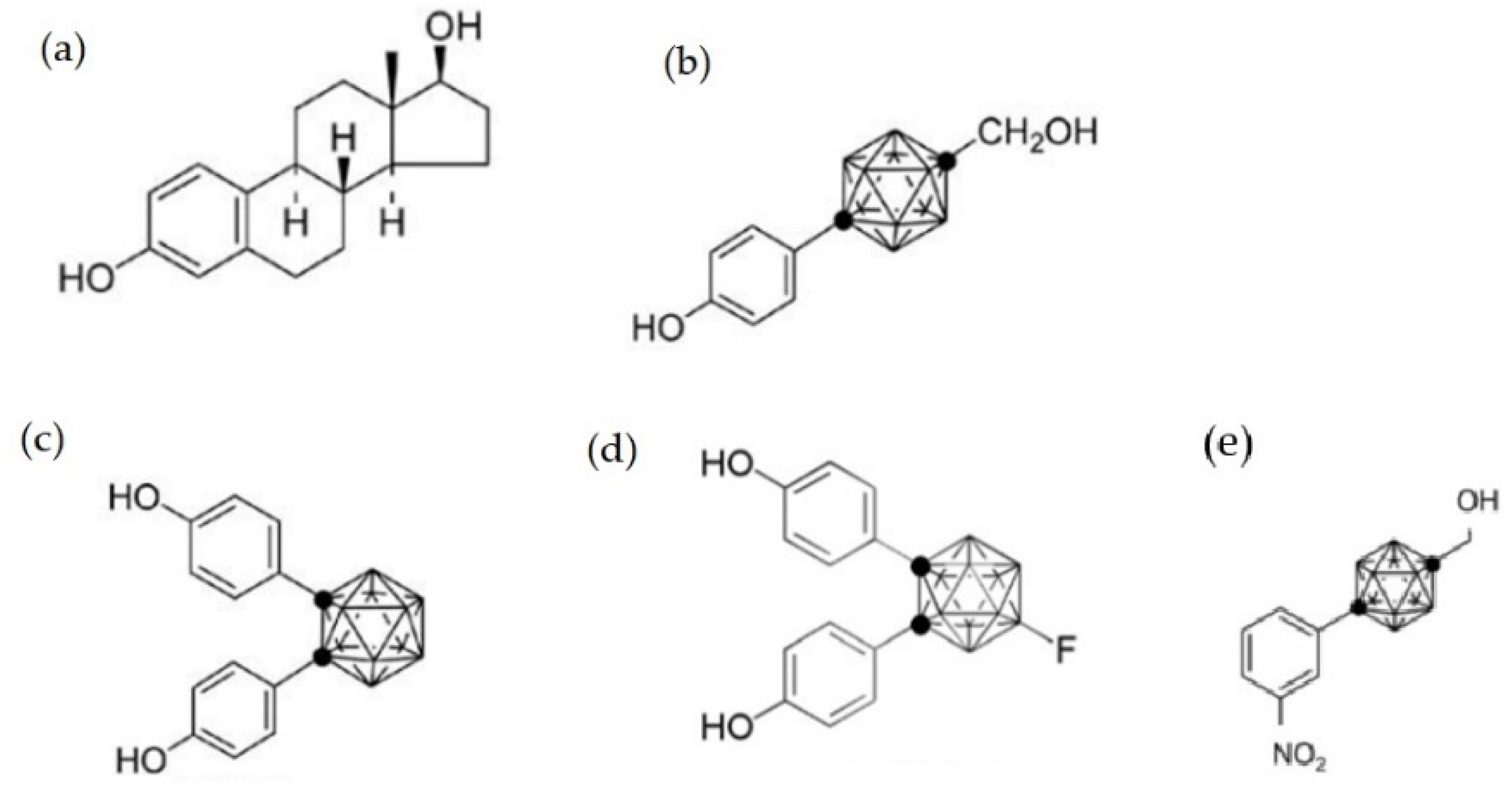
2.2. The Effect on Sex Hormone Receptors
2.3. The Effect on Sex Hormone-Dependent Human Metabolism
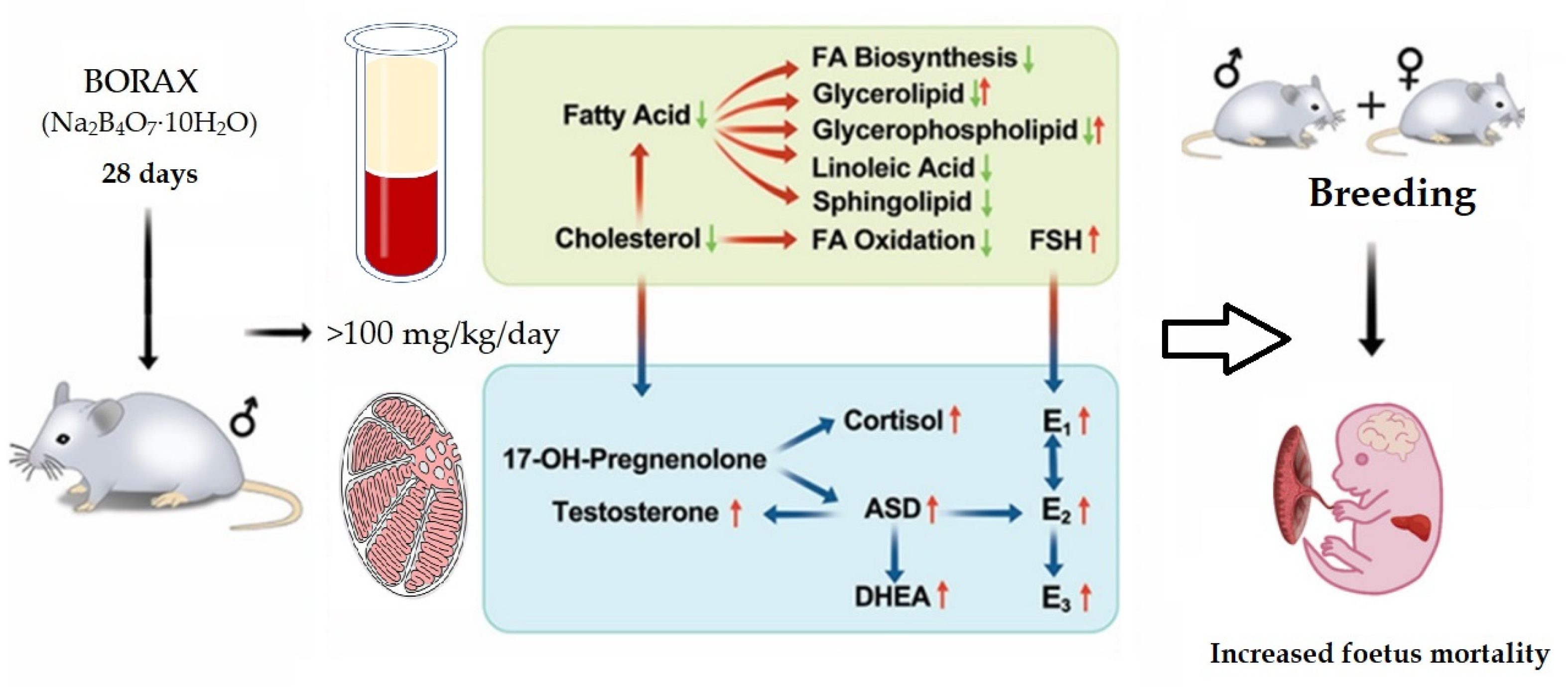
2.4. Regarding Fertility
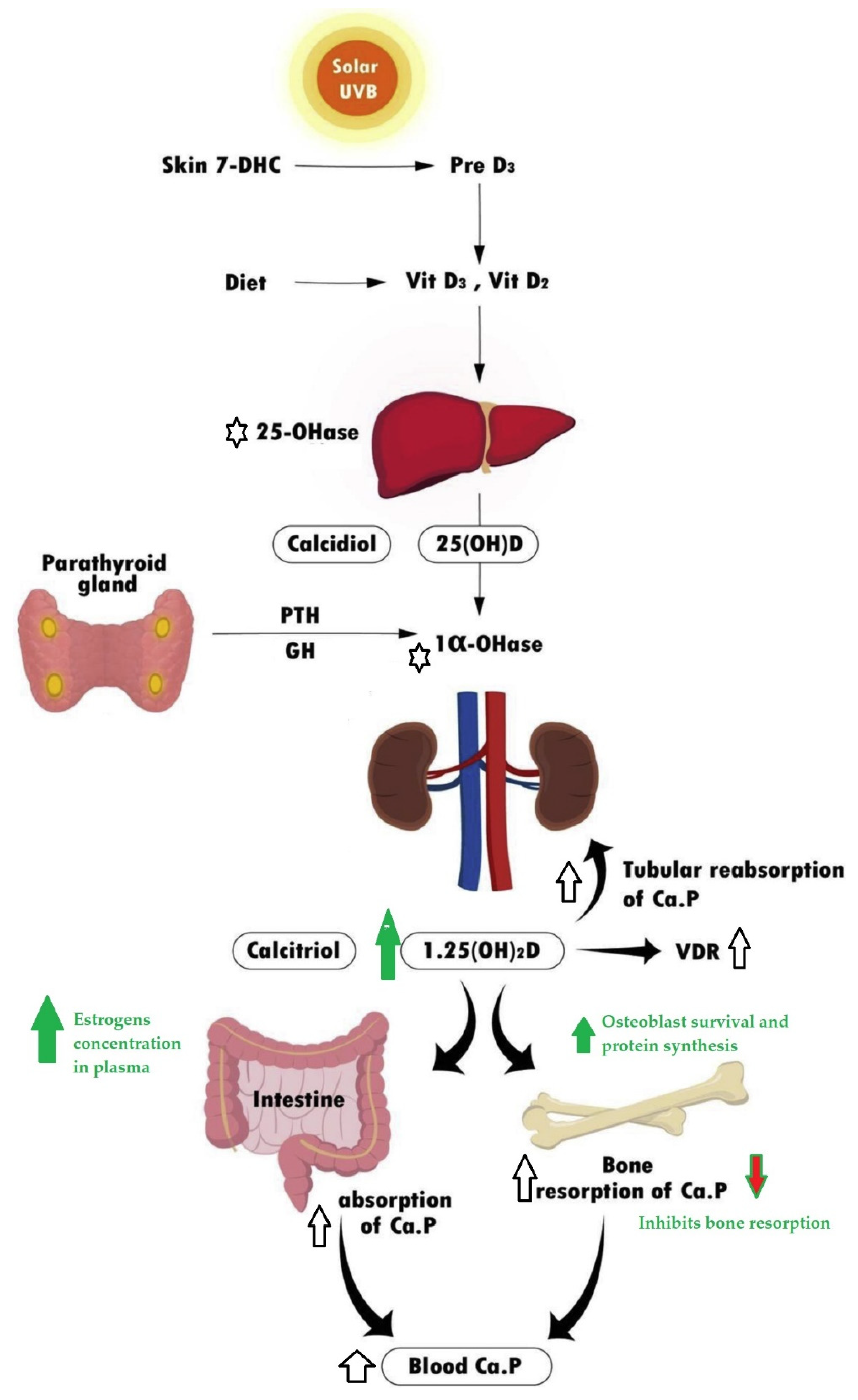
3. The Effect in Vitamin D Plasma Levels and Actions
4. Effects in Thyroid Hormones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Wang, M.; Pallarés, N.; Ferrer, E.; Berrada, H.; Barba, F.J. Sterols and fat-soluble vitamins. In Food Lipids; Academic Press: Cambridge, MA, USA, 2022; pp. 323–348. [Google Scholar]
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. In Proceedings of the Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2019; Volume 24, pp. 170–175. [Google Scholar]
- Rafeeq, H.; Ahmad, S.; Tareen, M.B.K.; Shahzad, K.A.; Bashir, A.; Jabeen, R.; Shehzadi, I. Biochemistry of Fat Soluble Vitamins, Sources, Biochemical Functions and Toxicity. Haya Saudi J. Life Sci. 2020, 5, 188–196. [Google Scholar] [CrossRef]
- Imig, J.D. Eicosanoid blood vessel regulation in physiological and pathological states. Clin. Sci. 2020, 134, 2707–2727. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ursúa, M.A.; Farfán-García, E.D.; Geninatti-Crich, S. Turning Fear of Boron Toxicity into Boron-containing Drug Design. Curr. Med. Chem. 2019, 26, 5005–5018. [Google Scholar] [CrossRef] [PubMed]
- Hosmane, N.S. Boron Science: New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Topnikova, A.P.; Belokoneva, E.L. The structure and classification of complex borates. Russ. Chem. Rev. 2019, 88, 204. [Google Scholar] [CrossRef]
- Muetterties, E. Boron Hydride Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 032314649X. [Google Scholar]
- Hey-Hawkins, E.; Teixidor, C.V. Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Grimes, R.N. Carboranes; Academic Press: Cambridge, MA, USA, 2016; ISBN 0128019050. [Google Scholar]
- Mogoşanu, G.D.; Biţă, A.; Bejenaru, L.E.; Bejenaru, C.; Croitoru, O.; Rău, G.; Rogoveanu, O.-C.; Florescu, D.N.; Neamţu, J.; Scorei, I.D.; et al. Calcium Fructoborate for Bone and Cardiovascular Health. Biol. Trace Elem. Res. 2016, 172, 277–281. [Google Scholar] [CrossRef]
- Gizer, M.; Köse, S.; Karaosmanoglu, B.; Taskiran, E.Z.; Berkkan, A.; Timuçin, M.; Korkusuz, F.; Korkusuz, P. The effect of boron-containing nano-hydroxyapatite on bone cells. Biol. Trace Elem. Res. 2020, 193, 364–376. [Google Scholar] [CrossRef]
- Sizmaz, O.; Koksal, B.; Tekeli, A.; Yildiz, G. Effects of boron supplementation alone or in combination with different vitamin D-3 levels on laying performance, eggshell quality, and mineral content and fatty acid composition of egg yolk in laying hens. J. Anim. Feed Sci. 2021, 30, 288–294. [Google Scholar] [CrossRef]
- Naghii, M.R.; Mofid, M.; Asgari, A.R.; Hedayati, M.; Daneshpour, M.S. Comparative effects of daily and weekly boron supplementation on plasma steroid hormones and proinflammatory cytokines. J. Trace Elem. Med. Biol. 2011, 25, 54–58. [Google Scholar] [CrossRef]
- Ri, C.-C.; Mf, C.-R.; IR, S.; MA, S.-U. Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders. Biol. Trace Elem. Res. 2022, online ahead of print. [Google Scholar] [CrossRef]
- Kan, F.; Kucukkurt, I. Investigation of the effect of boron on thyroid functions and biochemical parameters in hypothyroid induced-rats. J. Biochem. Mol. Toxicol. 2022, 36, e23186. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, Z.; Duan, L.; Deng, F.; Chen, Y.; Quan, S.; Liu, X.; Cha, Y.; Gong, Y.; Wang, C. Reproductive toxicity and metabolic perturbations in male rats exposed to boron. Sci. Total Environ. 2021, 785, 147370. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing boring about boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- Ghanizadeh, G.; Babaei, M.; Naghii, M.R.; Mofid, M.; Torkaman, G.; Hedayati, M. The effect of supplementation of calcium, vitamin D, boron, and increased fluoride intake on bone mechanical properties and metabolic hormones in rat. Toxicol. Ind. Health 2014, 30, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Samman, S.; Naghii, M.R.; Lyons Wall, P.M.; Verus, A.P. The nutritional and metabolic effects of Boron in humans and animals. Biol. Trace Elem. Res. 1998, 66, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Naghii, M.R.; Samman, S. The effect of boron supplementation on the distribution of boron in selected tissues and on testosterone synthesis in rats. J. Nutr. Biochem. 1996, 7, 507–512. [Google Scholar] [CrossRef]
- Naghii, M.R.; Samman, S. The effect of boron supplementation on its urinary excretion and selected cardiovascular risk factors in healthy male subjects. Biol. Trace Elem. Res. 1997, 56, 273–286. [Google Scholar] [CrossRef]
- Lee, I.P.; Sherins, R.J.; Dixon, R.L. Evidence for induction of germinal aplasia in male rats by environmental exposure to boron. Toxicol. Appl. Pharmacol. 1978, 45, 577–590. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Gallagher, S.K.; Johnson, L.K.; Nielsen, E.J. Boron enhaces and mimics some effects of estrogen therapy in psotmenopausal women. J. Trace Elem. Exp. Med. 1992, 5, 237–246. [Google Scholar]
- Bello, M.; Guadarrama-García, C.; Velasco-Silveyra, L.M.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Several effects of boron are induced by uncoupling steroid hormones from their transporters in blood. Med. Hypotheses 2018, 118, 78–83. [Google Scholar] [CrossRef]
- Avvakumov, G.V.; Grishkovskaya, I.; Muller, Y.A.; Hammond, G.L. Resolution of the human sex hormone-binding globulin dimer interface and evidence for two steroidbinding sites per homodimer. J. Biol. Chem. 2001, 276, 34453–34457. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef] [PubMed]
- Breuner, C.W.; Orchinik, M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002, 175, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Artem, C.; Zheng, S.; Magid, F.; Hammond, G.L. Successful in silico discovery of novelnonsteroidal ligands for human sex hormone binding globulin. J. Med. Chem. 2005, 48, 3203–3213. [Google Scholar]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef]
- Gardill, B.R.; Vogl, M.R.; Lin, H.Y.; Hammond, G.L.; Muller, Y.A. Corticosteroid-binding globulin: Structure-function implications from species differences. PLoS ONE 2012, 7, e52759. [Google Scholar] [CrossRef]
- Esther, M.Y.; Subramaniyan, V.; Kumar, A.P.; Subramanian, M.; Palani, M. Molecular docking, ADMET analysis and dynamics approach to potent natural inhibitors against sex hormone binding globulin in male infertility. Pharmacogn. J. 2017, 9, s35–s43. [Google Scholar] [CrossRef]
- Başaran, N.; Duydu, Y.; Bolt, H.M. Reproductive toxicity in boron exposed workers in Bandirma, Turkey. J. Trace Elem. Med. Biol. 2012, 26, 165–167. [Google Scholar] [CrossRef]
- Green, N.R.; Ferrando, A.A. Plasma boron and the effects of boron supplementation in males. Environ. Health Perspect. 1994, 102, 73–77. [Google Scholar]
- Leifke, E.; Gorenoi, V.; Wichers, C.; Von Zur Mühlen, A.; Von Büren, E.; Brabant, G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: Cross-sectional data from a healthy male cohort. Clin. Endocrinol. (Oxf.) 2000, 53, 689–695. [Google Scholar] [CrossRef]
- Hunt, C.D. Dietary Boron: An Overview of the Evidence for Its Role in Immune Function. J. Trace Elem. Exp. Med. 2003, 16, 291–306. [Google Scholar] [CrossRef]
- Beattie, J.H.; Peace, H.S. The influence of a low-boron diet and boron supplementation on bone, major mineral and sex steroid metabolism in postmenopausal women. Br. J. Nutr. 1993, 69, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Boyacioglu, O.; Orenay-Boyacioglu, S.; Yildirim, H.; Korkmaz, M. Boron intake, osteocalcin polymorphism and serum level in postmenopausal osteoporosis. J. Trace Elem. Med. Biol. 2018, 48, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Orenay-Boyacioglu, S.; Korkmaz, M.; Kahraman, E.; Yildirim, H.; Bora, S.; Ataman, O.Y. Biological effects of tolerable level chronic boron intake on transcription factors. J. Trace Elem. Med. Biol. 2017, 39, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.A.; Flowers, W.L.; Spears, J.W.; Nielsent, F.H. Long-term effects of boron supplementation on reproductive characteristics and bone mechanical properties in gilts. J. Anim. Sci. 2002, 80, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.A.; Spears, J.W. Effect of boron supplementation of pig diets on the production of tumor necrosis factor- α and interferon- γ 1, 2. Am. Soc. Anim. Sci. 2003, 81, 2552–2561. [Google Scholar] [CrossRef]
- Hunt, C.D.; Herbel, J.L.; Nielsen, F.H. Metabolic responses of postmenopausal women to supplemental dietary boron and aluminum during usual and low magnesium intake: Boron, calcium, and magnesium absorption and retention and blood mineral concentrations. Am. J. Clin. Nutr. 1997, 65, 803–813. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, S.; Akerstrom, V.L.; Yuan, C.; Ma, Y.; Zhong, Q.; Zhang, C.; Zhang, Q.; Guo, S.; Ma, P. Fulvestrant-3 boronic acid (ZB716): An orally bioavailable selective estrogen receptor downregulator (SERD). J. Med. Chem. 2016, 59, 8134–8140. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, S.; Guo, S.; Zhang, C.; Zhong, Q.; Zhang, Q.; Ma, P.; Skripnikova, E.V.; Bratton, M.R.; Wiese, T.E. Rational design of a boron-modified triphenylethylene (GLL398) as an oral selective estrogen receptor downregulator. ACS Med. Chem. Lett. 2017, 8, 102–106. [Google Scholar] [CrossRef]
- Raghava, N.; Das, B.C.; Ray, S.K. Neuroprotective effects of estrogen in CNS injuries: Insights from animal models. Neurosci. Neuroeconomics 2017, 6, 15–29. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically Active Compounds Based on Membranotropic Cage Carriers–Derivatives of Adamantane and Polyhedral Boron Clusters. Russ. J. Inorg. Chem. 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Das, B.C.; Trujillo-Ferrara, J.G. Boron-containing compounds: Chemico-biological properties and expanding medicinal potential in prevention, diagnosis and therapy. Expert Opin. Ther. Pat. 2014, 24, 485–500. [Google Scholar] [CrossRef]
- Thirumamagal, B.T.S.; Zhao, X.B.; Bandyopadhyaya, A.K.; Narayanasamy, S.; Johnsamuel, J.; Tiwari, R.; Golightly, D.W.; Patel, V.; Jehning, B.T.; Backer, M.V. Receptor-targeted liposomal delivery of boron-containing cholesterol mimics for boron neutron capture therapy (BNCT). Bioconjug. Chem. 2006, 17, 1141–1150. [Google Scholar] [CrossRef]
- Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. [Google Scholar] [CrossRef]
- Sedlak, D.; Wilson, T.A.; Tjarks, W.; Radomska, H.S.; Wang, H.; Kolla, J.N.; Lesnikowski, Z.J.; Spicakova, A.; Ali, T.; Ishita, K. Structure–Activity Relationship of para-Carborane Selective Estrogen Receptor β Agonists. J. Med. Chem. 2021, 64, 9330–9353. [Google Scholar] [CrossRef]
- Machuki, J.O.; Zhang, H.Y.; Harding, S.E.; Sun, H. Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol. 2018, 222, e12978. [Google Scholar] [CrossRef]
- Ohta, K.; Ogawa, T.; Oda, A.; Kaise, A.; Endo, Y. Design and synthesis of carborane-containing estrogen receptor-beta (ERβ)-selective ligands. Bioorganic Med. Chem. Lett. 2015, 25, 4174–4178. [Google Scholar] [CrossRef]
- Endo, Y.; Iijima, T.; Yamakoshi, Y.; Fukasawa, H.; Miyaura, C.; Inada, M.; Kubo, A.; Itai, A. Potent estrogen agonists based on carborane as a hydrophobic skeletal structure: A new medicinal application of boron clusters. Chem. Biol. 2001, 8, 341–355. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Hunt, C.D.; Mullen, L.M.; Hunt, J.R. Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women 1. FASEB J. 1987, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Inada, M.; Matsumoto, C.; Takita, M.; Ogawa, T.; Endo, Y.; Miyaura, C. A novel carborane analog, BE360, with a carbon-containing polyhedral boron-cluster is a new selective estrogen receptor modulator for bone. Biochem. Biophys. Res. Commun. 2009, 380, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Ogawa, T.; Kaise, A.; Endo, Y. Enhanced estrogen receptor beta (ERβ) selectivity of fluorinated carborane-containing ER modulators. Bioorg. Med. Chem. Lett. 2013, 23, 6555–6558. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Ogawa, T.; Endo, Y. Estrogenic activity of B-fluorinated o-carborane-1,2-bisphenol synthesized via SNAr reaction. Bioorg. Med. Chem. Lett. 2012, 22, 4728–4730. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gustafsson, J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.-A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336. [Google Scholar] [CrossRef]
- Murphy, N.; McCarthy, E.; Dwyer, R.; Farràs, P. Boron clusters as breast cancer therapeutics. J. Inorg. Biochem. 2021, 218, 111412. [Google Scholar] [CrossRef]
- Watanabe, K.; Hirata, M.; Tominari, T.; Matsumoto, C.; Endo, Y.; Murphy, G.; Nagase, H.; Inada, M.; Miyaura, C. BA321, a novel carborane analog that binds to androgen and estrogen receptors, acts as a new selective androgen receptor modulator of bone in male mice. Biochem. Biophys. Res. Commun. 2016, 478, 279–285. [Google Scholar] [CrossRef]
- Romero-Aguilar, K.S.; Arciniega-Martínez, I.M.; Farfán-García, E.D.; Campos-Rodríguez, R.; Reséndiz-Albor, A.A.; Soriano-Ursúa, M.A. Effects of boron-containing compounds on immune responses: Review and patenting trends. Expert Opin. Ther. Pat. 2019, 29, 339–351. [Google Scholar] [CrossRef]
- Jin, E.; Pei, Y.; Liu, T.; Ren, M.; Hu, Q.; Gu, Y.; Li, S. Effects of boron on the proliferation, apoptosis and immune function of splenic lymphocytes through ERα and ERβ. Food Agric. Immunol. 2019, 30, 743–761. [Google Scholar] [CrossRef]
- Mori, S.; Tsuemoto, N.; Kasagawa, T.; Nakano, E.; Fujii, S.; Kagechika, H. Development of Boron-Cluster-Based Progesterone Receptor Antagonists Bearing a Pentafluorosulfanyl (SF5) Group. Chem. Pharm. Bull. 2019, 67, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Takagaki, R.; Fujii, S.; Urushibara, K.; Tanatani, A.; Kagechika, H. Novel Non-steroidal Progesterone Receptor Ligands Based on m-Carborane Containing a Secondary Alcohol: Effect of Chirality on Ligand Activity. Chem. Pharm. Bull. 2017, 65, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Nakano, E.; Yanagida, N.; Mori, S.; Masuno, H.; Kagechika, H. Development of p-carborane-based nonsteroidal progesterone receptor antagonists. Bioorg. Med. Chem. 2014, 22, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Zargham, E.O.; Mason, C.A.; Lee, M.W., Jr. The use of carboranes in cancer drug development. Int. J. Cancer Clin. Res 2019, 6, 110–113. [Google Scholar]
- Goto, T.; Ohta, K.; Fujii, S.; Ohta, S.; Endo, Y. Design and synthesis of androgen receptor full antagonists bearing ap-carborane cage: Promising ligands for anti-androgen withdrawal syndrome. J. Med. Chem. 2010, 53, 4917–4926. [Google Scholar] [CrossRef]
- Azad, N.; Sakla, N.; Bahn, G. The effect of testosterone replacement therapy on glycemic control in hypogonadal men with type 2 diabetes mellitus. J. Clin. Diabetes 2018, 1, 1–5. [Google Scholar]
- Gambineri, A.; Pelusi, C. Sex hormones, obesity and type 2 diabetes: Is there a link? Endocr. Connect. 2019, 8, R1–R9. [Google Scholar] [CrossRef]
- Schiffer, L.; Kempegowda, P.; Arlt, W.; O’Reilly, M.W. Mechanisms in endocrinology: The sexually dimorphic role of androgens in human metabolic disease. Eur. J. Endocrinol. 2017, 177, R125–R143. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Morgentaler, A. Testosterone for Life: Recharge Your Vitality, Sex Drive, Muscle Mass, and Overall Health; McGraw-Hill: New York, NY, USA, 2009; ISBN 9780071642514. [Google Scholar]
- Nielsen, F.H. Is boron nutritionally relevant? Nutr. Rev. 2008, 66, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Farfán-García, E.D.; Castillo-Mendieta, N.T.; Ciprés-Flores, F.J.; Padilla-Martínez, I.I.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Current data regarding the structure-toxicity relationship of boron-containing compounds. Toxicol. Lett. 2016, 258, 115–125. [Google Scholar] [CrossRef]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin kills mycobacterial persisters without detectable resistance. Front Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef]
- Rogoveanu, O.C.; Mogoşanu, G.D.; Bejenaru, C.; Bejenaru, L.E.; Croitoru, O.; Neamţu, J.; Pietrzkowski, Z.; Reyes-Izquierdo, T.; Biţă, A.; Scorei, I.D.; et al. Effects of calcium fructoborate on levels of C-reactive protein, total cholesterol, low-density lipoprotein, triglycerides, IL-1β, IL-6, and MCP-1: A double-blind, placebo-controlled clinical study. Biol. Trace Elem. Res. 2015, 163, 124–131. [Google Scholar] [CrossRef]
- Bita, A.; Mogosanu, G.D.; Bejenaru, L.E.; Oancea, C.N.; Bejenaru, C.; Croitoru, O.; Rau, G.; Neamtu, J.; Scorei, I.D.; Scorei, I.R.; et al. Simultaneous quantitation of boric acid and calcium fructoborate in dietary supplements by HPTLC–densitometry. Anal. Chem. Res. 2017, 33, 743–746. [Google Scholar]
- Naghii, M.R.; Mofid, M. Elevation of biosynthesis of endogenous 17-B oestradiol by boron supplementation: One possible role of dietary boron consumption in humans. J. Nutr. Environ. Med. 2008, 17, 127–135. [Google Scholar] [CrossRef]
- Naghii, M.R.; Samman, S. Role of boron in nutrition and metabolism. Prog. Fd. Nutr. Sci. 1993, 17, 331–349. [Google Scholar]
- Brewster, J.H.; Negishi, E. Brown: Passes through the mountains. Science 1980, 207, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Granner, D.K. Hormones of the Gonads, 21st ed.; Murray, R.K., Granner, D.K., Mayes, P.A., Rodwell, V.W., Eds.; Appleton & Lange: New York, NY, USA, 1988. [Google Scholar]
- Beattie, J.H.; Weersink, E. Borate and molybdate inhibitory of catechol estrogen and pyrocatechol methylation by catechol-o-methyltransferase. J. Inorg. Biochem. 1992, 46, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Naghii, M.R.; Samman, S. The effect of boron on plasma testosterone and plasma lipids in rats. Nutr. Res. 1997, 17, 523–532. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Meacham, S.L. Growing Evidence for Human Health Benefits of Boron. J. Evid. Based. Complementary Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- Kilic, A.; Savci, A.; Alan, Y.; Beyazsakal, L. The synthesis of novel boronate esters and N-Heterocyclic carbene (NHC)-stabilized boronate esters: Spectroscopy, antimicrobial and antioxidant studies. J. Organomet. Chem. 2020, 917, 121268. [Google Scholar] [CrossRef]
- Barrón-González, M.; Montes-Aparicio, A.V.; Cuevas-Galindo, M.E.; Orozco-Suárez, S.; Barrientos, R.; Alatorre, A.; Querejeta, E.; Trujillo-Ferrara, J.G.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Boron-containing compounds on neurons: Actions and potential applications for treating neurodegenerative diseases. J. Inorg. Biochem. 2022, 238, 112027. [Google Scholar] [CrossRef]
- Kilic, A.; Söylemez, R.; Okumuş, V. Design, spectroscopic properties and effects of novel catechol spiroborates derived from Schiff bases in the antioxidant, antibacterial and DNA binding activity. J. Organomet. Chem. 2022, 960, 122228. [Google Scholar] [CrossRef]
- Kilic, A.; Savci, A.; Alan, Y.; Birsen, H. Synthesis and spectroscopic properties of 4, 4′-bipyridine linker bioactive macrocycle boronate esters: Photophysical properties and antimicrobial with antioxidant studies. J. Organomet. Chem. 2021, 941, 121807. [Google Scholar] [CrossRef]
- Korkmaz, M. Boron. In Boron and Human Health; Korkmaz, M., Ed.; Nobel Akademik Yayıncılık: Çankaya, Türkiye, 2020; pp. 47–66. ISBN 978-625-402-341-5. [Google Scholar]
- Sayli, B.S.; Tüccar, E.; Elhan, A.H. An assessment of fertility in boron-exposed Turkish subpopulations. Reprod. Toxicol. 1998, 12, 297–304. [Google Scholar] [CrossRef]
- Bolt, H.M.; Başaran, N.; Duydu, Y. Effects of boron compounds on human reproduction. Arch. Toxicol. 2020, 94, 717–724. [Google Scholar] [CrossRef]
- Smallwood, C. International Program in Chemical Safety. In Boron; World Health Organization: Geneva, Switzerland, 1998; pp. 192–201. [Google Scholar]
- Robbins, W.; Xun, L.; Jia, J.; Kennedy, N.; Elashoff, D.; Ping, L. Chronic boron exposure and human semen parameters. Reprod. Toxicol. 2010, 29, 184–190. [Google Scholar] [CrossRef]
- Weir, R.J.; Fisher, R.S. Toxicologic studies on borax and boric acid. Toxicol. Appl. Pharmacol. 1972, 23, 351–364. [Google Scholar] [CrossRef]
- Scialli, A.R.; Bonde, J.P.; Brüske-Hohlfeld, I.; Culver, B.D.; Li, Y.; Sullivan, F.M. An overview of male reproductive studies of boron with an emphasis on studies of highly exposed Chinese workers. Reprod. Toxicol. 2010, 29, 10–24. [Google Scholar] [CrossRef]
- European Commission. OPINION ON Boron Compounds; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Whorton, M.D.; Haas, J.L.; Trent, L.; Wong, O. Reproductive effects of sodium borates on male employees: Birth rate assessment. Occup. Environ. Med. 1994, 51, 761–767. [Google Scholar] [CrossRef]
- Marat, I.; Arstan, M.; Galymzhan, Y.; Timur, J.; Yerbolat, I.; Almasbek, Y. Impact of chromium and boron compounds on the reproductive function in rats. Toxicol. Ind. Health 2018, 34, 365–374. [Google Scholar] [CrossRef]
- Medicines Adverse Reactions Comitte. Boron-Containing Excipients and Fertility Concerns; Medsafe Pharmacovigilance Team: Wellington, New Zealand, 2021. Available online: https://www.medsafe.govt.nz/committees/MARC/reports/186-3.2.1-Boron.pdf (accessed on 14 February 2023).
- Duydu, Y.; Başaran, N.; Yalçın, C.Ö.; Üstündağ, A.; Aydın, S.; Anlar, H.G.; Bacanlı, M.; Aydos, K.; Atabekoğlu, C.S.; Golka, K. Boron-exposed male workers in Turkey: No change in sperm Y: X chromosome ratio and in offspring’s sex ratio. Arch. Toxicol. 2019, 93, 743–751. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef]
- El-Dakdoky, M.H.; Abd El-Wahab, H.M.F. Impact of boric acid exposure at different concentrations on testicular DNA and male rats fertility. Toxicol. Mech. Methods 2013, 23, 360–367. [Google Scholar] [CrossRef]
- Hunt, C.D.; Nielsen, F.H. Interaction between boron and cholecalciferol in the chick. In Proceedings of the Trace Element Metabolism in Man and Animals, Canberra, Australia, 11–15 May 1981; pp. 597–600. [Google Scholar]
- Hunt, C.D. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Env. Health Perspect. 1994, 102, 35–43. [Google Scholar]
- Rosen, V.; Wozney, J.M. Bone morphogenetic proteins. In Principles of Bone Biology; Academic Press: Cambridge, MA, USA, 2002; Volume 2, pp. 919–928. [Google Scholar]
- Naghii, M.R.; Torkaman, G.; Mofid, M. Effects of boron and calcium supplementation on mechanical properties of bone in rats. BioFactors 2006, 28, 195–201. [Google Scholar] [CrossRef]
- Hunter, J.M.; Nemzer, B.V.; Rangavajla, N.; Biţă, A.; Rogoveanu, O.C.; Neamţu, J.; Scorei, I.R.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; et al. The fructoborates: Part of a family of naturally occurring sugar–borate complexes—Biochemistry, physiology, and impact on human health: A review. Biol. Trace Elem. Res. 2019, 188, 11–25. [Google Scholar] [CrossRef]
- Sheng, M.H.; Taper, L.J.; Veit, H.; Qian, H.; Ritchey, S.J.; Lau, K.H. Dietary boron supplementation enhanced the action of estrogen, but not that of parathyroid hormone, to improve trabecular bone quality in ovariectomized rats. Biol. Trace Elem. Res. 2001, 82, 109–123. [Google Scholar] [CrossRef]
- Dupre, J.N.; Keenan, M.J.; Hegsted, M.; Brudevold, A.M. Effects of dietary boron in rats fed a vitamin D-deficient diet. Environ. Health Perspect. 1994, 102, 55–58. [Google Scholar]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef]
- Cauley, J.A.; Robbins, J.; Chen, Z. Effect of boron supplementation of pig diets on the production of tumor necrosis factor-alpha and interferon-gamma. Jama 2003, 290, 1729–1738. [Google Scholar] [CrossRef]
- Horowitz, M.C. Cytokines and estrogen in bone: Anti-osteoporotic effects. Science 1993, 260, 626–628. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Peng, X.; Lingxia, Z.; Schrauzer, G.N.; Xiong, G. Selenium, boron, and germanium deficiency in the etiology of Keshan-Beck disease. Biol. Trace Elem. Res. 2000, 77, 193–197. [Google Scholar] [CrossRef]
- Fang, W.; Wu, P.; Hu, R.; Huang, Z. Environmental Se-Mo-B deficiency and its possible effects on crops and Keshan-Beck disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ. Geochem. Health 2003, 25, 267–280. [Google Scholar] [CrossRef]
- Hunt, C.D.; Idso, J.P. Dietary boron as a physiological regulator of the normal inflammatory response: A review and current research progress. J. Trace Elem. Exp. Med. 1999, 12, 221–233. [Google Scholar] [CrossRef]
- Volpe, S.L.; Taper, L.J.; Meacham, S. The relationship between boron and magnesium status and bone mineral density in the human: A review. Magnes Res. 1993, 6, 291–296. [Google Scholar]
- Nielsen, F.H.; Mullen, L.M.; Gallagher, S.K. Effect of boron depletion and repletion on blood indicators of calcium status in humans fed a magnesium-low diet. J. Trace Elem. Exp. Med. 1990, 3, 45–54. [Google Scholar]
- Miljkovic, D.; Scorei, R.I.; Cimpoiaşu, V.M.; Scorei, I.D. Calcium fructoborate: Plant-based dietary boron for human nutrition. J. Diet Suppl. 2009, 6, 211–226. [Google Scholar] [CrossRef]
- Miljkovic, D.; Miljkovic, N.; McCarty, M.F. Up-regulatory impact of boron on vitamin D function–Does it reflect inhibition of 24-hydroxylase? Med. Hypotheses 2004, 63, 1054–1056. [Google Scholar] [CrossRef]
- Hegsted, M.; Keenan, M.J.; Siver, F.; Wozniak, P. Effect of boron on vitamin D deficient rats. Biol. Trace Elem. Res. 1991, 28, 243–255. [Google Scholar] [CrossRef]
- Hunt, C.D.; Herbel, J.L.; Idso, J.P. Dietary boron modifies the effects of vitamin D3 nutriture on indices of energy substrate utilization and mineral metabolism in the chick. J. Bone Min. Res. 1994, 9, 171–181. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Ge, C.; Xiao, G.; Roca, H.; Jiang, D. Transcriptional regulation of osteoblasts. Cells Tissues Organs. 2009, 189, 144–152. [Google Scholar] [CrossRef]
- Korolev, I.N.; Panova, L.N.; Bobkova, A.S.; Korovkina, E.G. Morphofunctional characteristics of the thyroid and a change in the level of thyroid hormones in the blood from the internal use of boron-containing waters. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 1989, 3, 28–31. [Google Scholar]
- Popova, E.V.; Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Skalny, A.V. Boron–A potential goiterogen? Med. Hypotheses 2017, 104, 63–67. [Google Scholar] [CrossRef]
- Kucukkurt, I.; Akbel, E.; Karabag, F.; Ince, S. The effects of dietary boron compounds in supplemented diet on hormonal activity and some biochemical parameters in rats. Toxicol. Ind. Health 2015, 31, 255–260. [Google Scholar] [CrossRef]
- Armstrong, T.A.; Spears, J.W.; Lloyd, K.E. Inflammatory response, growth, and thyroid hormone concentrations are affected by long-term boron supplementation in gilts. J. Anim. Sci. 2001, 79, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.; Ibrahim, S.S.; El-Anwar, A.H.; Mabrook, E.A.; Ibrahim, T.B.; Abdel-Razik, A.-R.H. Effects of dietary boron supplementation on the testicular function and thyroid activity in male goats: Involvement of CYP17A1 gene. Reprod. Domest. Anim. 2022, 57, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.B.; Abdel-Wahab, A.; Aziz, R.L.A.; El-Anwar, A.H.; Ibrahim, S.S. Dietary boron supplementation and its impact on testicular function, thyroid activity and serum calcium in rams. Small Rumin. Res. 2019, 174, 156–162. [Google Scholar] [CrossRef]
- Mirzaei, H.R.; Sahebkar, A.; Salehi, R.; Nahand, J.S.; Karimi, E.; Jaafari, M.R.; Mirzaei, H. Boron neutron capture therapy: Moving toward targeted cancer therapy. J. Cancer Res. Ther. 2016, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Pisarev, M.A.; Dagrosa, M.A.; Thomasz, L.; Juvenal, G. Boron neutron capture therapy applied to undifferentiated thyroid carcinoma. Medicina (B. Aires) 2006, 66, 569–573. [Google Scholar] [PubMed]
- Luca, E.; Fici, L.; Ronchi, A.; Marandino, F.; Rossi, E.D.; Caristo, M.E.; Malandrino, P.; Russo, M.; Pontecorvi, A.; Vigneri, R. Intake of Boron, Cadmium, and Molybdenum enhances rat thyroid cell transformation. J. Exp. Clin. Cancer Res. 2017, 36, 73. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carpano, M.; Curotto, P.; Thorp, S.; Casal, M.; Juvenal, G.; Pisarev, M.; Dagrosa, M.A. In vitro studies of DNA damage and repair mechanisms induced by BNCT in a poorly differentiated thyroid carcinoma cell line. Radiat. Environ. Biophys. 2018, 57, 143–152. [Google Scholar] [CrossRef]
- Pan, Y.-Y.; Yao, S.-F.; Lin, K.-H.; Chou, F.-I.; Lee, J.-C.; Tai, S.-K.; Huang, W.-S.; Lan, K.-L.; Chao, Y.; Chen, Y.-W. Boron neutron capture therapy as salvage treatment for recurrent papillary thyroid carcinoma—A case report. Ther. Radiol. Oncol. 2020, 4, 21. [Google Scholar] [CrossRef]
- Perona, M.; Majdalani, M.E.; Rodríguez, C.; Nievas, S.; Carpano, M.; Rossini, A.; Longhino, J.M.; Cabrini, R.; Pisarev, M.A.; Juvenal, G.J.; et al. Experimental studies of boron neutron capture therapy (BNCT) using histone deacetylase inhibitor (HDACI) sodium butyrate, as a complementary drug for the treatment of poorly differentiated thyroid cancer (PDTC). Appl. Radiat. Isot. 2020, 164, 109297. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossío, U.; Llop, J. Gold nanoparticles as boron carriers for boron neutron capture therapy: Synthesis, radiolabelling and in vivo evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Salimi Beni, A.; Eskandari, R.; Karami, M.; Khorram, M. Interaction of propylthiouracil, an anti-thyroid drug with boron nitride nanotube: A DFT study. Adsorption 2020, 26, 1385–1396. [Google Scholar] [CrossRef]
- Ou, M.; Wang, X.; Yu, L.; Liu, C.; Tao, W.; Ji, X.; Mei, L. The emergence and evolution of borophene. Adv. Sci. 2021, 8, 2001801. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sadique, M.A.; Kaushik, A.; Ranjan, P.; Khan, R.; Srivastava, A.K. Borophene as an emerging 2D flatland for biomedical applications: Current challenges and future prospects. J. Mater. Chem. B 2022, 10, 1146–1175. [Google Scholar] [CrossRef] [PubMed]

| Compound | IC50 (μM) 1 |
|---|---|
| GW5638 | 0.39 |
| GW7604 | 0.0017 |
| GLL398 | 1.14 |
| ZB716 | 4.1 |
| Compound | t ½ (h) | Cmax (μg/mL) | AUC (μg·h/mL) |
|---|---|---|---|
| GLL398 | 3.9 | 3.51 | 36.9 |
| ZB716 | 23.5 | 0.16 | 2.5 |
| Compound | RBA | Selectivity ERβ/ERα | IC50 (μM) | EC50 (μM) | |
|---|---|---|---|---|---|
| ERα | ERβ | ||||
| 17β-E2 | 100 | 100 | 1.0 | 1.0 | 1.0 |
| BE360 | 68 | 46 | 0.68 | 0.58 | 0.024 |
| BE310 | 15 | 16 | 1.1 | 0.63 | 0.85 |
| Control | B-125 | B-250 | B-500 | |
|---|---|---|---|---|
| Final body weight (g) | 299.6 ± 5.36 | 300.5 ± 7.86 | 281.3 ± 8.90 | 267.7 ± 3.88 a,b |
| Body weight gain (g) | 120.5 ± 3.63 | 123.0 ± 6.56 | 102.3 ± 7.43 a,b | 96.3 ± 5.48 a,b |
| R. testis weight (g) | 1.57 ± 0.03 | 1.49 ± 0.04 | 1.38 ± 0.10 a | 0.46 ± 0.02 a,b,c |
| R. epididymis weight (g) | 0.58 ± 0.02 | 0.57 ± 0.02 | 0.52 ± 0.04 | 0.35 ± 0.02 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez-Fregoso, E.; Kilic, A.; Rodríguez-Vera, D.; Nicanor-Juárez, L.E.; Romero-Rizo, C.E.M.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Effects of Boron-Containing Compounds on Liposoluble Hormone Functions. Inorganics 2023, 11, 84. https://doi.org/10.3390/inorganics11020084
Estevez-Fregoso E, Kilic A, Rodríguez-Vera D, Nicanor-Juárez LE, Romero-Rizo CEM, Farfán-García ED, Soriano-Ursúa MA. Effects of Boron-Containing Compounds on Liposoluble Hormone Functions. Inorganics. 2023; 11(2):84. https://doi.org/10.3390/inorganics11020084
Chicago/Turabian StyleEstevez-Fregoso, Elizabeth, Ahmet Kilic, Diana Rodríguez-Vera, Luis E. Nicanor-Juárez, C. Elena M. Romero-Rizo, Eunice D. Farfán-García, and Marvin A. Soriano-Ursúa. 2023. "Effects of Boron-Containing Compounds on Liposoluble Hormone Functions" Inorganics 11, no. 2: 84. https://doi.org/10.3390/inorganics11020084
APA StyleEstevez-Fregoso, E., Kilic, A., Rodríguez-Vera, D., Nicanor-Juárez, L. E., Romero-Rizo, C. E. M., Farfán-García, E. D., & Soriano-Ursúa, M. A. (2023). Effects of Boron-Containing Compounds on Liposoluble Hormone Functions. Inorganics, 11(2), 84. https://doi.org/10.3390/inorganics11020084








