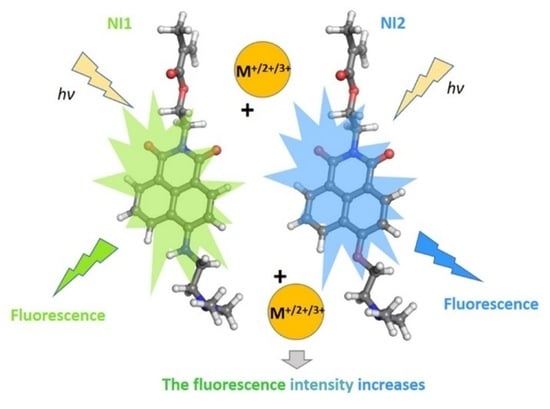
Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations
Abstract
1. Introduction
2. Results
2.1. Synthesis of Compounds NI1 and NI2
2.2. Photophysical Characterization in Different Organic Solvents
2.3. Computational Studies
3. Materials and Methods
3.1. Synthesis of 1,8-Naphthalimides
3.2. Synthesis of 2-(6-(2-(N,N-Dimethylaminoethylamino)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate (NI1)
3.3. Synthesis of 2-(6-(2-(N,N-Dimethylaminoethoxy)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate (NI2)
3.4. Synthesis of 2-(6-bromo)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate
3.5. Analysis
3.6. DFT Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Finney, N.S. Small-Molecule Fluorescent Probes and Their Design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Wang, X.-L.; Tusong; Xu, L.-H. Studies on the Synthesis and Spectral Properties of Novel 4-Benzofuranyl-1,8-Naphthalimide Derivatives. Dye. Pigment. 2005, 67, 27–33. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, M.; Shi, G.; Wang, L.; Yin, H.; Mei, C. A Novel Fluorescent Molecule Based on 1,8-Naphthalimide: Synthesis, Spectral Properties, and Application in Cell Imaging. Res. Chem. Intermed. 2010, 36, 1021–1026. [Google Scholar] [CrossRef]
- Poteau, X.; Brown, A.I.; Brown, R.G.; Holmes, C.; Matthew, D. Fluorescence Switching in 4-Amino-1,8-Naphthalimides: “On–off–on” Operation Controlled by Solvent and Cations. Dye. Pigment. 2000, 47, 91–105. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Qi, J.; Liu, X.-Y.; He, H.-R.; Chen, J.-T.; Yang, G.-M. 4-Amino-1,8-Naphthalimide-Based Fluorescent Sensor with High Selectivity and Sensitivity for Zn2+ Imaging in Living Cells. Inorg. Chem. Commun. 2014, 43, 173–178. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New Fluorescent Chemosensors for Metal Ions in Solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Kelly, L.A.; Roll, M.; Joseph, J.; Seenisamy, J.; Barrett, J.; Kauser, K.; Warner, K.S. Solvent-Dependent Photophysics and Reactivity of Monomeric and Dimeric 4-Amino-1,8-Naphthalimides. J. Phys. Chem. A 2021, 125, 2294–2307. [Google Scholar] [CrossRef]
- Grabtchev, I.; Konstantinov, T.; Guittonneau, S.; Meallier, P. Photochemistry of Some 1,8-Naphthalic Anhydride Derivatives. Dye. Pigment. 1997, 35, 361–366. [Google Scholar] [CrossRef]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-Naphthalimide Derivatives as Dyes for Textile and Polymeric Materials: A Review. Fibers Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. Chemical Modification of Cotton Fabric with 1,8-Naphthalimide for Use as Heterogeneous Sensor and Antibacterial Textile. J. Photochem. Photobiol. A Chem. 2019, 382, 111924. [Google Scholar] [CrossRef]
- Staneva, D.; Angelova, S.; Grabchev, I. Spectral Characteristics and Sensor Ability of a New 1,8-Naphthalimide and Its Copolymer with Styrene. Sensors 2020, 20, 3501. [Google Scholar] [CrossRef]
- Jawale Patil, P.D.; Ingle, R.D.; Wagalgave, S.M.; Bhosale, R.S.; Bhosale, S.V.; Pawar, R.P.; Bhosale, S.V. A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection. Chemosensors 2019, 7, 38. [Google Scholar] [CrossRef]
- Mohr, G.J. Synthesis of Naphthalimide-Based Indicator Dyes with a 2-Hydroxyethylsulfonyl Function for Covalent Immobilisation to Cellulose. Sensors Actuators B Chem. 2018, 275, 439–445. [Google Scholar] [CrossRef]
- Callan, J.F.; de Silva, A.P.; Magri, D.C. Luminescent Sensors and Switches in the Early 21st Century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Grabchev, I.; Qian, X.; Xiao, Y.; Zhang, R. Novel Heterogeneous PET Fluorescent Sensors Selective for Transition Metal Ions or Protons: Polymers Regularly Labelled with Naphthalimide. New J. Chem. 2002, 26, 920–925. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and Colorimetric Chemosensors for Cations Based on 1,8-Naphthalimide Derivatives: Design Principles and Optical Signalling Mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Jain, N.; Kaur, N. A Comprehensive Compendium of Literature of 1,8-Naphthalimide Based Chemosensors from 2017 to 2021. Coord. Chem. Rev. 2022, 459, 214454. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Betcheva, R. Fluorescent Dendrimers as Sensors for Biologically Important Metal Cations. Curr. Med. Chem. 2012, 19, 4976–4983. [Google Scholar] [CrossRef]
- Temiz, H.T.; Boyaci, I.H.; Grabchev, I.; Tamer, U. Surface Enhanced Raman Spectroscopy as a New Spectral Technique for Quantitative Detection of Metal Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 339–347. [Google Scholar] [CrossRef]
- Oshchepkov, A.S.; Oshchepkov, M.S.; Oshchepkova, M.V.; Al-Hamry, A.; Kanoun, O.; Kataev, E.A. Naphthalimide-Based Fluorescent Polymers for Molecular Detection. Adv. Opt. Mater. 2021, 9, 2001913. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent Hydrogel–Textile Composite Material Synthesized by Photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef]
- Sali, S.; Guittonneau, S.; Grabchev, I. A Novel Blue Fluorescent Chemosensor for Metal Cations and Protons, Based on 1,8-Naphthalimide and Its Copolymer with Styrene. Polym. Adv. Technol. 2006, 17, 180–185. [Google Scholar] [CrossRef]
- Grabchev, I.; Dumas, S.; Chovelon, J.-M. Studying the Photophysical Properties of a Polymerizable 1,8-Naphthalimide Dye and Its Copolymer with Styrene as Potential Fluorescent Sensors for Metal Cations. Polym. Adv. Technol. 2008, 19, 316–321. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Grabchev, I.; Meallier, P.; Konstantinova, T.; Popova, M. Synthesis of Some Unsaturated 1,8-Naphthalimide Dyes. Dye. Pigment. 1995, 28, 41–46. [Google Scholar] [CrossRef]
- Grabchev, I.; Petkov, C.; Bojinov, V. 1,8-Naphthalimides as Blue Emitting Fluorophores for Polymer Materials. Macromol. Mater. Eng. 2002, 287, 904–908. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hehre, W.J.; Lathan, W.A. Self-Consistent Molecular Orbital Methods. XIV. An Extended Gaussian-Type Basis for Molecular Orbital Studies of Organic Molecules. Inclusion of Second Row Elements. J. Chem. Phys. 1972, 56, 5255–5257. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 5 October 2022).










| Solvent | λA nm | ε L mol−1 cm−1 | λF nm | νA–νF cm−1 | ΦF |
|---|---|---|---|---|---|
| Acetonitrile | 436 | 12,400 | 517 | 3593 | 0.022 |
| N,N-dimethylformamide | 429 | 12,900 | 525 | 4262 | 0.010 |
| n-Butanol | 434 | 12,600 | 525 | 3994 | 0.018 |
| Ethanol | 438 | 12,700 | 536 | 4174 | 0.011 |
| Methanol | 439 | 12,400 | 538 | 4192 | 0.010 |
| Chloroform | 423 | 14,300 | 502 | 3720 | 0.716 |
| Dichloromethane | 424 | 14,600 | 504 | 3753 | 0.462 |
| Tetrahydrofuran | 420 | 14,400 | 500 | 3809 | 0.894 |
| λA nm | ε L mol−1 cm−1 | λF nm | νA–νF cm−1 | ΦF | |
|---|---|---|---|---|---|
| Acetonitrile | 366 | 12,300 | 422 | 3625 | 0.021 |
| N,N-dimethylformamide | 367 | 11,900 | 426 | 3773 | 0.015 |
| n-Butanol | 365 | 12,000 | 425 | 3867 | 0.019 |
| Ethanol | 368 | 12,200 | 438 | 4342 | 0.008 |
| Methanol | 369 | 12,400 | 439 | 4321 | 0.007 |
| Chloroform | 360 | 13,900 | 414 | 3623 | 0.624 |
| Dichloromethane | 360 | 13,600 | 413 | 3564 | 0.762 |
| Tetrahydrofuran | 358 | 13,400 | 410 | 3542 | 0.777 |
| Metal Ions | λA/nm | λF/nm | FE | Stokes Shift/cm−1 | ΦF |
|---|---|---|---|---|---|
| Ag+ | 428 | 515 | 8.90 | 3947 | 0.103 |
| Cu2+ | 429 | 516 | 14.29 | 3930 | 0.169 |
| Zn2+ | 429 | 520 | 4.20 | 4079 | 0.048 |
| Fe3+ | 428 | 510 | 34.25 | 3756 | 0.384 |
| Ni2+ | 429 | 521 | 8.40 | 4116 | 0.101 |
| Ca2+ | 428 | 524 | 1.5 | 4280 | 0.016 |
| Mg2+ | 428 | 524 | 1.9 | 4280 | 0.017 |
| Metal Ions | λA/nm | λF/nm | FE | Stokes Shift/cm−1 | ΦF |
|---|---|---|---|---|---|
| Ag+ | 365 | 416 | 9.40 | 3358 | 0.151 |
| Cu2+ | 365 | 415 | 16.23 | 3301 | 0.249 |
| Zn2+ | 367 | 422 | 6.32 | 3551 | 0.101 |
| Fe3+ | 365 | 412 | 38.05 | 3125 | 0.571 |
| Ni2+ | 366 | 415 | 9.9 | 3226 | 0.147 |
| Ca2+ | 365 | 425 | 1.8 | 3867 | 0.027 |
| Mg2+ | 365 | 426 | 1.9 | 3933 | 0.028 |
| Solvent | NI1 | NI2 | ||||
|---|---|---|---|---|---|---|
| calc. | exp. | μ | calc. | exp. | μ | |
| Toluene | 411 (0.34) | - | 9.16 | 364 (0.35) | - | 7.28 |
| Chloroform | 415 (0.34) | 423 | 9.99 | 366 (0.35) | 360 | 7.71 |
| Methanol | 419 (0.33) | 439 | 10.87 | 368 (0.34) | 369 | 8.18 |
| DMF | 420 (0.34) | 429 | 10.89 | 369 (0.35) | 367 | 8.19 |
| Water | 419 (0.33) | - | 10.97 | 368 (0.34) | - | 8.23 |
| Protonation Site | NI1 | NI2 | ||
|---|---|---|---|---|
| ΔG1 | ΔG78 | ΔG1 | ΔG78 | |
| N1 | 18.7 | 42.5 | 22.1 | 42.9 |
| N2/O | 10.9 | 20.9 | 34.8 | 43.9 |
| N3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Position | Mg2+ | Fe3+ |
|---|---|---|
| NI1 (N substituent) | −3.5 | −107.7 |
| NI1 (C-4 substituent) | −7.6 | −98.4 |
| NI2 (N substituent) | 7.0 | −102.7 |
| NI2 (C-4 substituent) | −8.7 | −93.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabchev, I.; Angelova, S.; Staneva, D. Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics 2023, 11, 47. https://doi.org/10.3390/inorganics11020047
Grabchev I, Angelova S, Staneva D. Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics. 2023; 11(2):47. https://doi.org/10.3390/inorganics11020047
Chicago/Turabian StyleGrabchev, Ivo, Silvia Angelova, and Desislava Staneva. 2023. "Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations" Inorganics 11, no. 2: 47. https://doi.org/10.3390/inorganics11020047
APA StyleGrabchev, I., Angelova, S., & Staneva, D. (2023). Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics, 11(2), 47. https://doi.org/10.3390/inorganics11020047