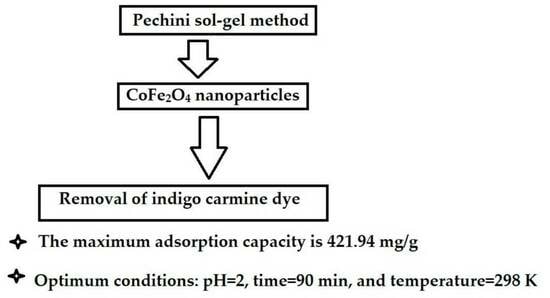
Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of CoFe2O4 Nanoparticles
2.3. Characterization
2.4. Adsorption of Indigo Carmine Dye from Aqueous Solutions
3. Results and Discussion
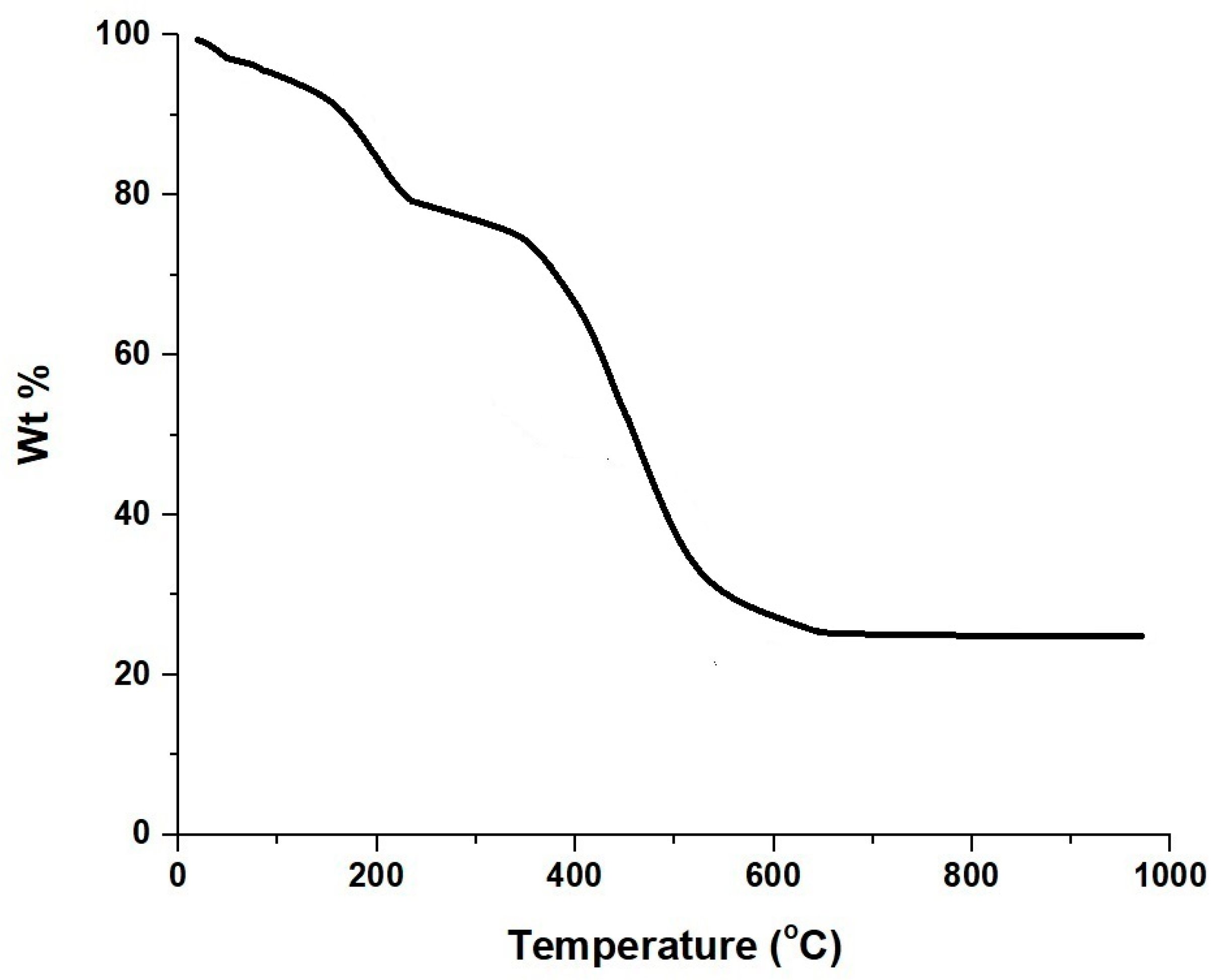
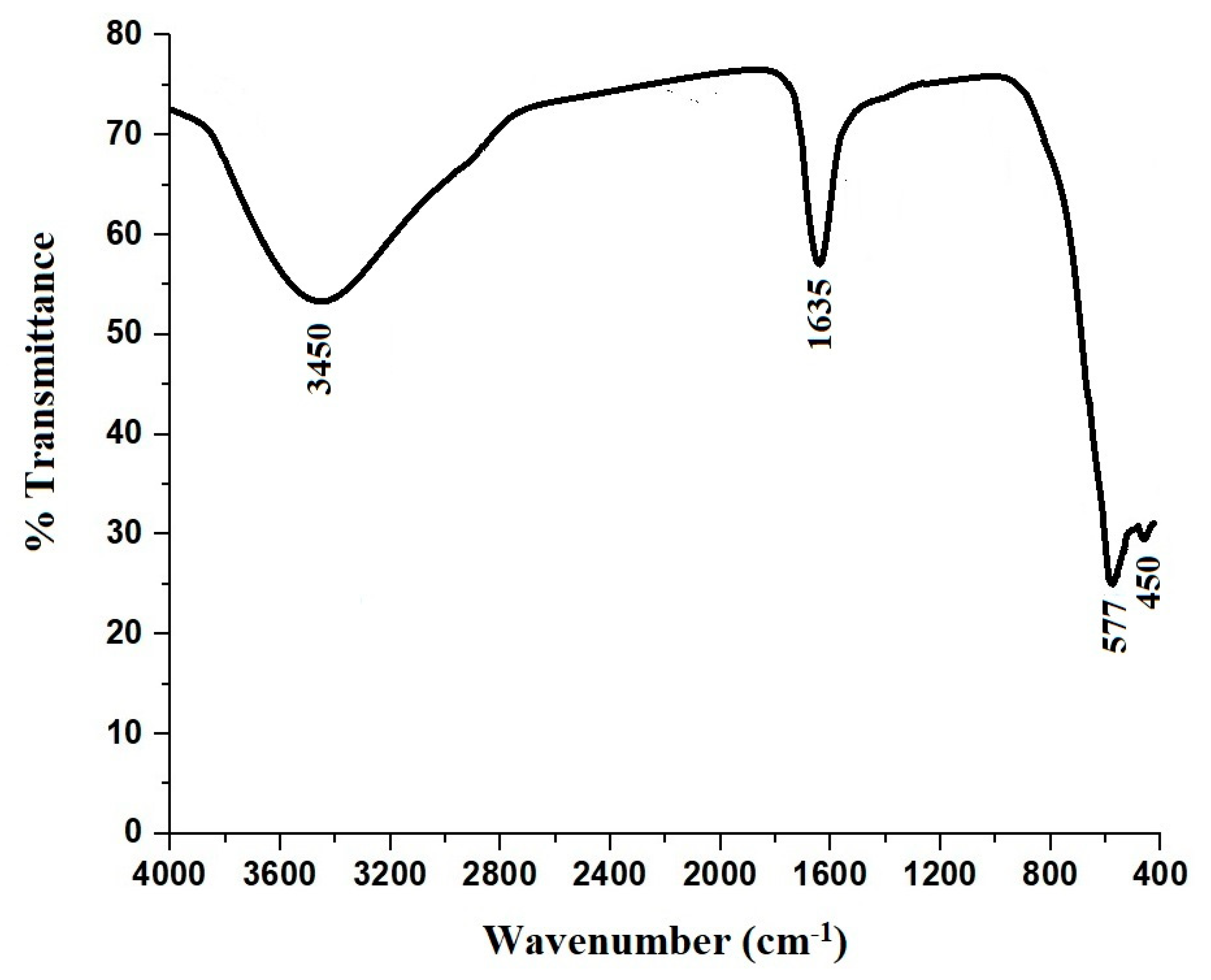
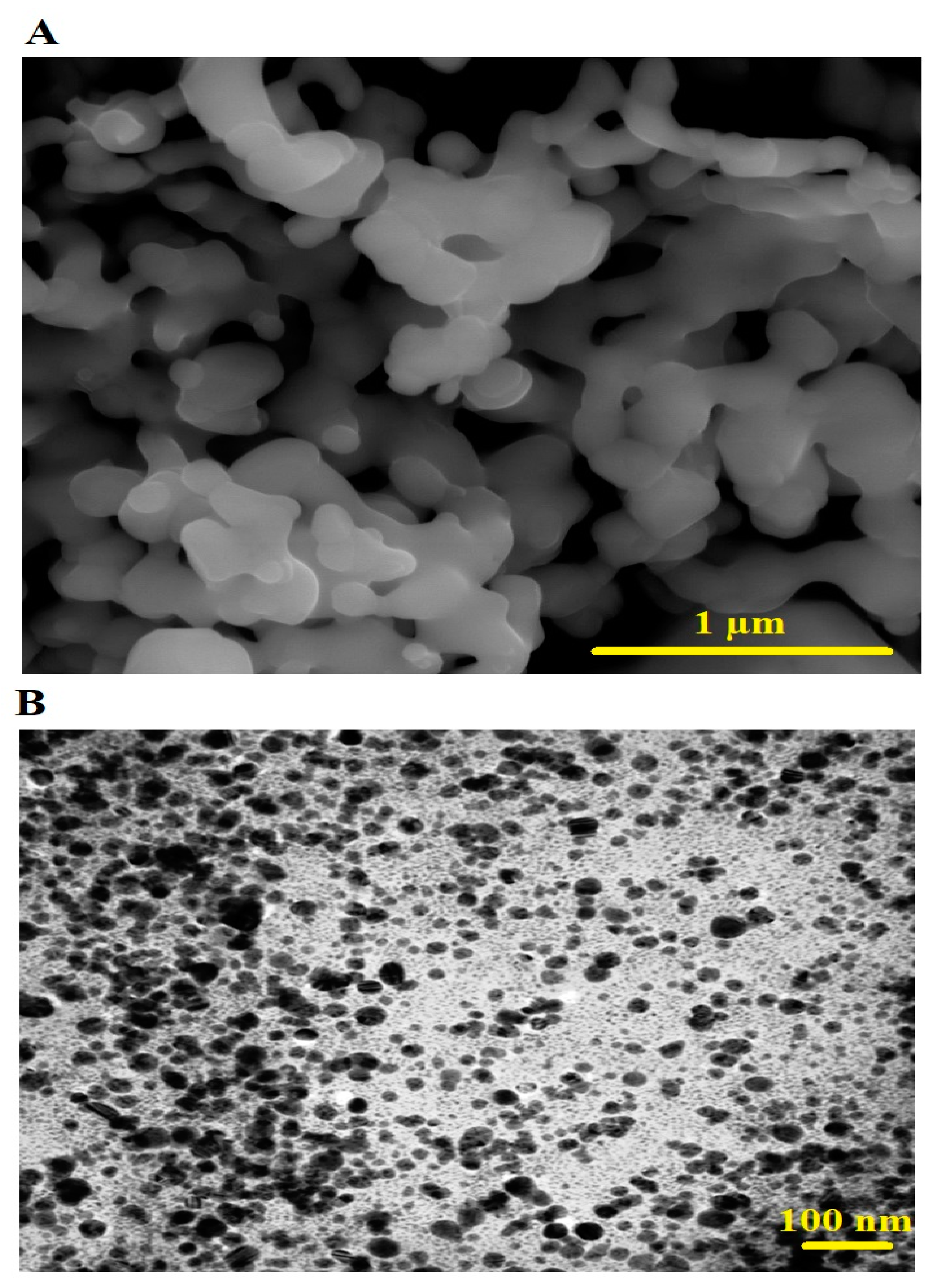
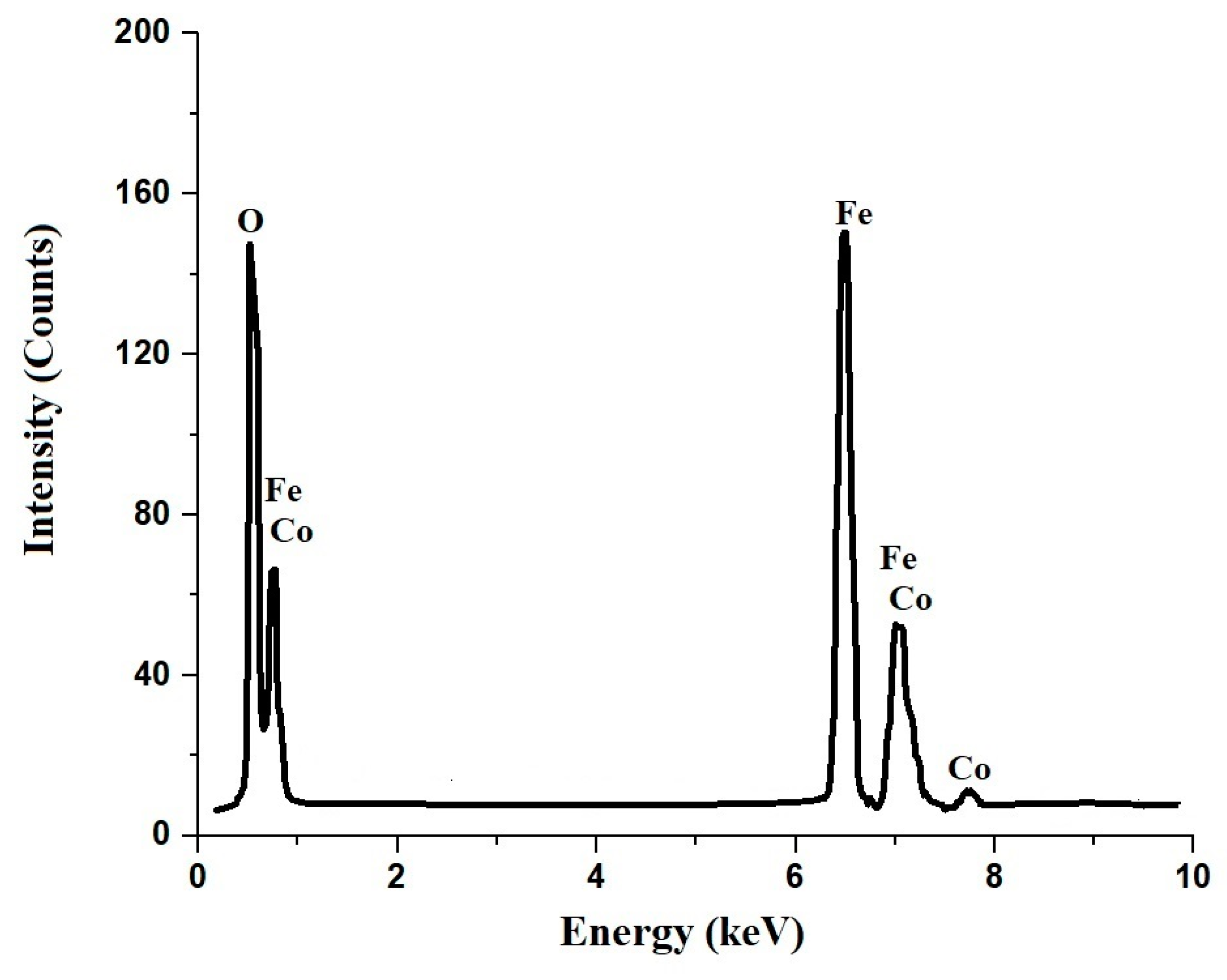
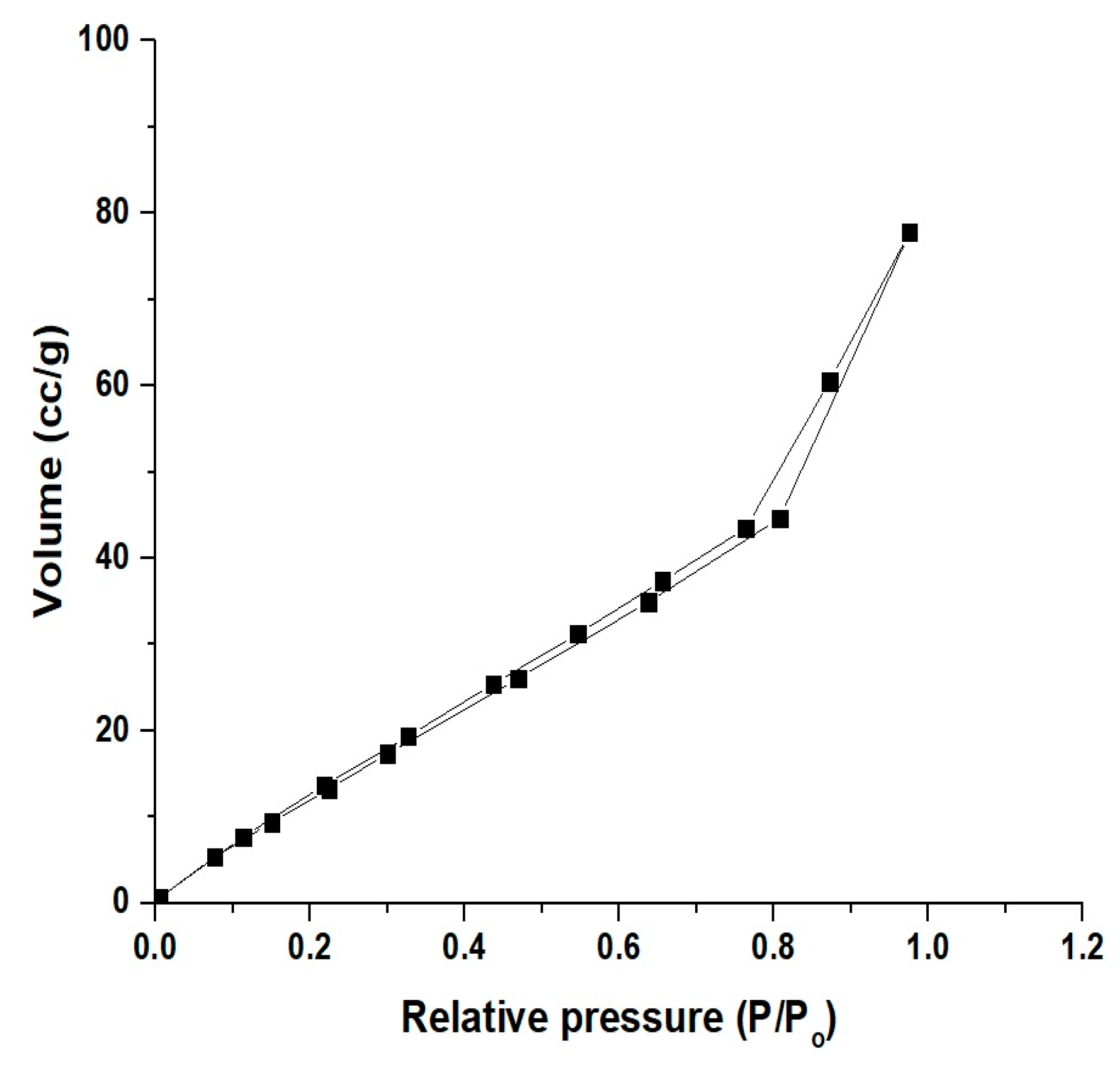
3.1. Synthesis and Characterization of CoFe2O4 Nanoparticles
3.2. Adsorption of Indigo Carmine Dye from Aqueous Solutions
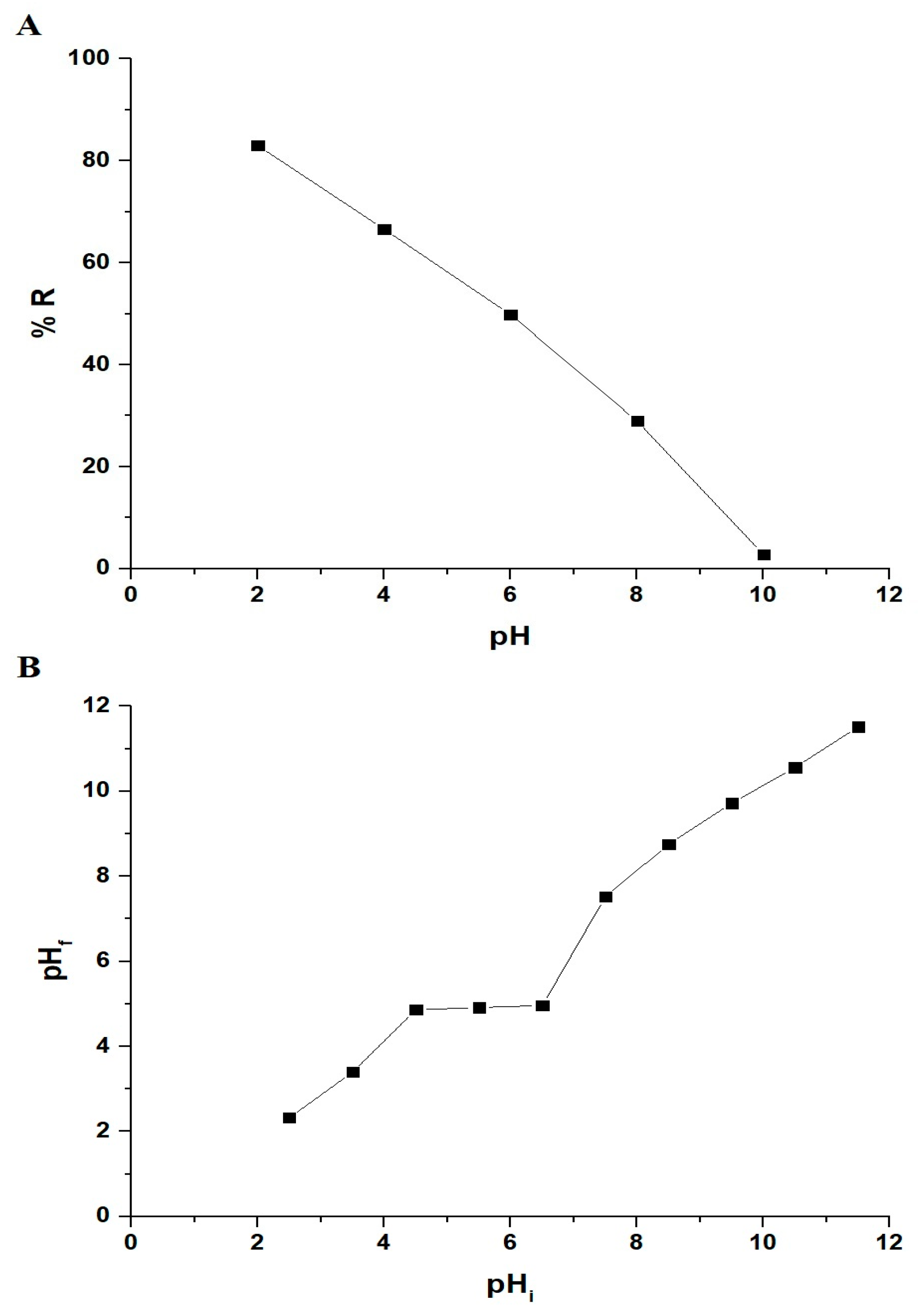
3.2.1. Influence of pH
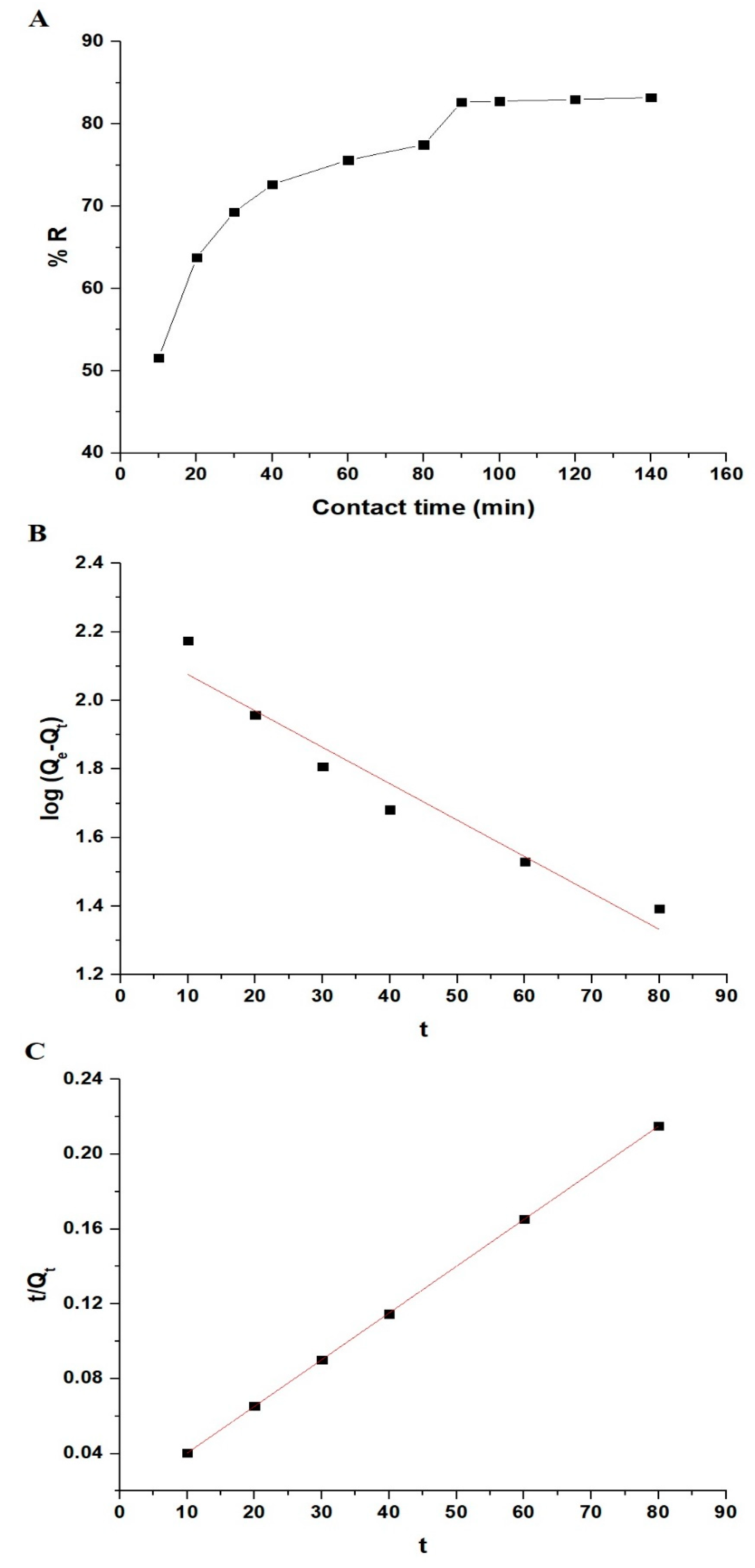
3.2.2. Influence of Adsorption Time and Adsorption Kinetics
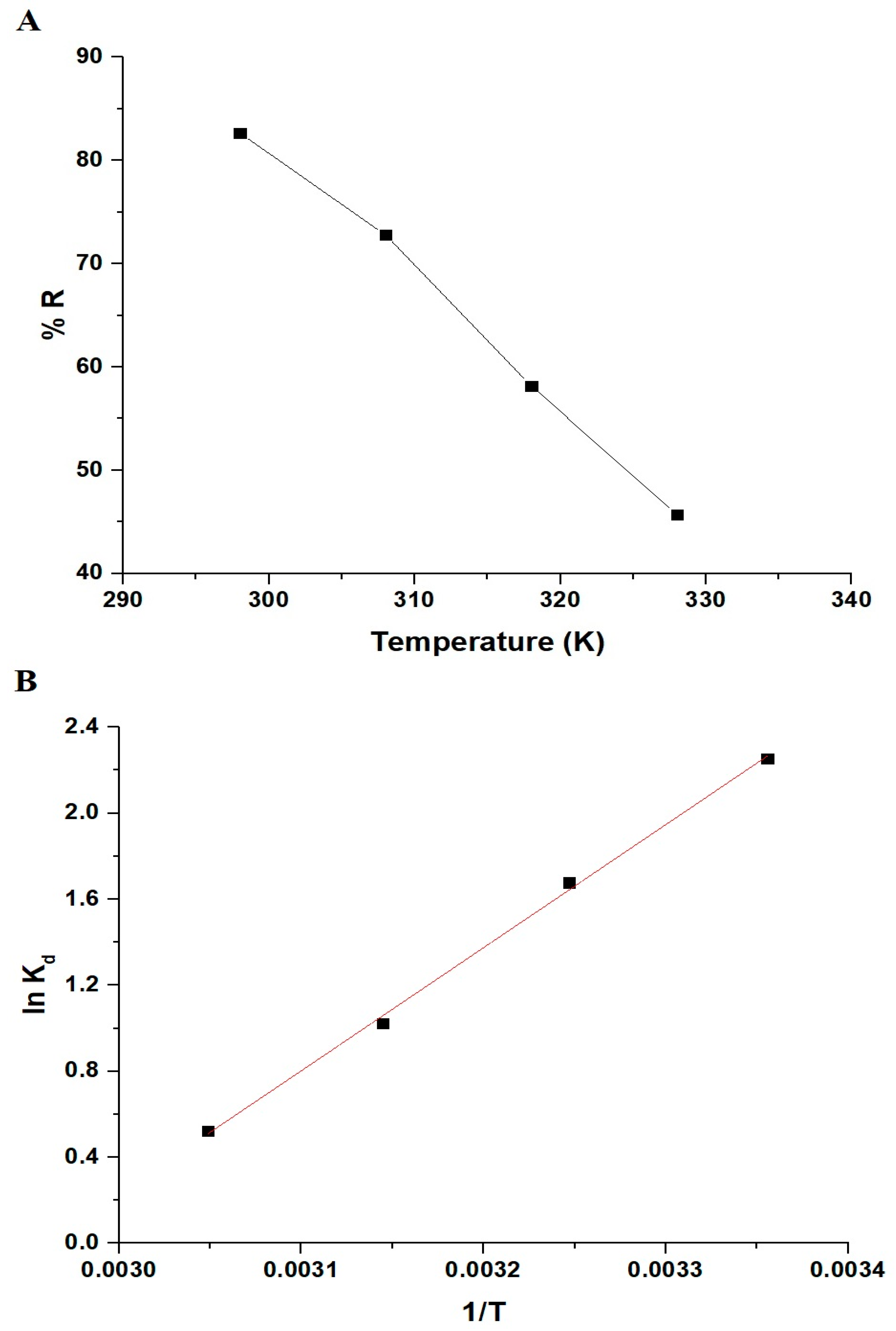
3.2.3. Influence of Solution Temperature and Thermodynamic Parameters
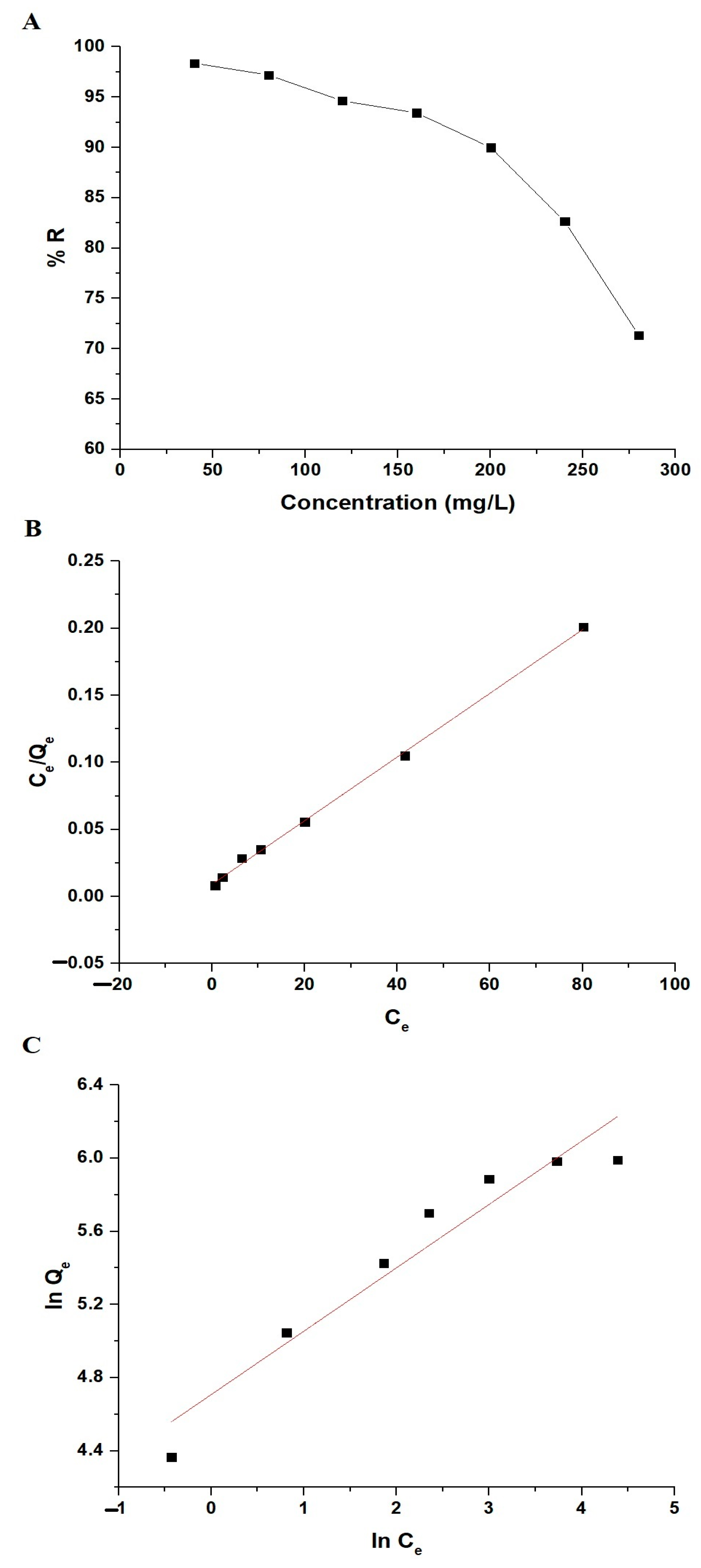
3.2.4. Influence of Concentration and Adsorption Isotherms
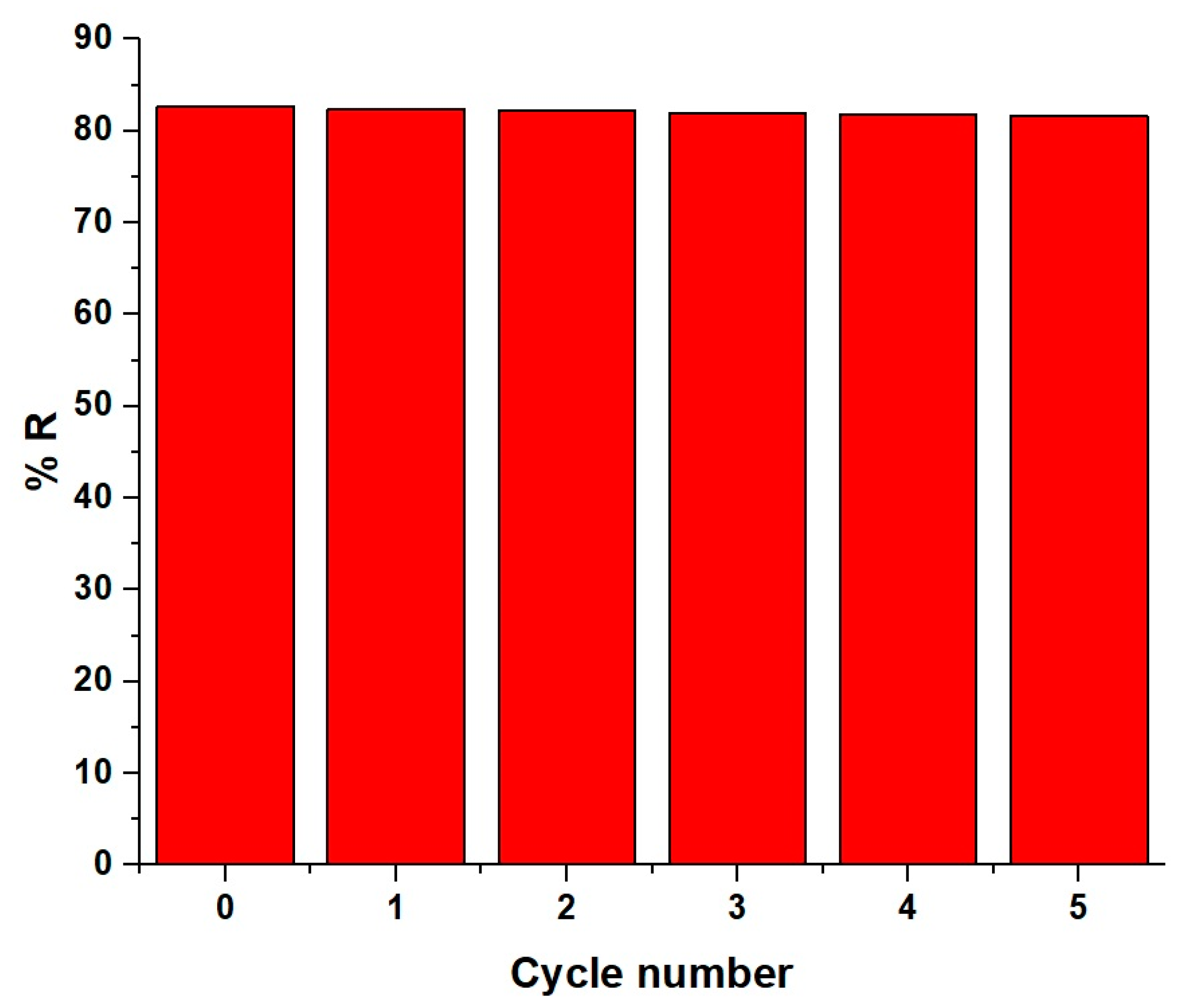
3.2.5. Influence of Regeneration and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Kordy, A.; Elgamouz, A.; Lemdek, E.M.; Tijani, N.; Alharthi, S.S.; Kawde, A.N.; Shehadi, I. Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents. Membranes 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Akrami, M.; Danesh, S.; Eftekhari, M. Comparative Study on the Removal of Cationic Dyes Using Different Graphene Oxide Forms. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1785–1797. [Google Scholar] [CrossRef]
- Ghiasi, E.; Malekzadeh, A. Removal of Various Textile Dyes Using LaMn(Fe)O3 and LaFeMn0.5O3 Nanoperovskites; RSM Optimization, Isotherms and Kinetics Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2789–2804. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Y.; Liu, N.; Wu, Y. Iron Manganese Oxide Modified Multi-Walled Carbon Nanotube as Efficient Adsorbent for Removal of Organic Dyes: Performance, Kinetics and Mechanism Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4027–4042. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Algethami, F.K.; Alsalem, H.S.; Binkadem, M.S. Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media. Inorganics 2023, 11, 339. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Algethami, F.K.; AlSalem, H.S.; Binkadem, M.S.; Khairy, M.; Saad, F.A.; El-Sayyad, G.S.; Alqahtani, Z. Efficient Disposal of Rhodamine 6G and Acid Orange 10 Dyes from Aqueous Media Using ZrO2/CdMn2O4/CdO as Novel and Facilely Synthesized Nanocomposites. Inorganics 2023, 11, 333. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Cheng, W.; Zhang, T. Targeted Reclaiming Cationic Dyes from Dyeing Wastewater with a Dithiocarbamate-Functionalized Material through Selective Adsorption and Efficient Desorption. J. Colloid Interface Sci. 2020, 579, 766–777. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Vigneshwaran, S.; Nikitha, M.; Hassan, C.A.A.; Meenakshi, S. Ce(III) Networked Chitosan/β-Cyclodextrin Beads for the Selective Removal of Toxic Dye Molecules: Adsorption Performance and Mechanism. Carbohydr. Polym. Technol. Appl. 2020, 1, 100018. [Google Scholar] [CrossRef]
- Rafiq, A.; Imran, M.; Aqeel, M.; Naz, M.; Ikram, M.; Ali, S. Study of Transition Metal Ion Doped CdS Nanoparticles for Removal of Dye from Textile Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1915–1923. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Elshahawy, M.F.; Mohammed, R.D.; Mahmoud, G.A. Removal of Astrazon Red Dye from Wastewater Using Eggshell/Graphene Oxide Embed in (Gum Acacia/Acrylamide) Hydrogel Nanocomposites Synthesized by Gamma Irradiation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3617–3637. [Google Scholar] [CrossRef]
- Fathi, E.; Derakhshanfard, F.; Gharbani, P.; Ghazi Tabatabaei, Z. Facile Synthesis of MgO/C3N4 Nanocomposite for Removal of Reactive Orange 16 Under Visible Light. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2234–2240. [Google Scholar] [CrossRef]
- Noreen, S.; Khalid, U.; Ibrahim, S.M.; Javed, T.; Ghani, A.; Naz, S.; Iqbal, M. ZnO, MgO and FeO Adsorption Efficiencies for Direct Sky Blue Dye: Equilibrium, Kinetics and Thermodynamics Studies. J. Mater. Res. Technol. 2020, 9, 5881–5893. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.; Rosy; Sharma, Y.C. A Review on Sustainable Mesoporous Activated Carbon as Adsorbent for Efficient Removal of Hazardous Dyes from Industrial Wastewater. J. Water Process Eng. 2023, 54, 104054. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating Polymer Network Hydrogels for Removal of Synthetic Dyes: A Comprehensive Review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef]
- Singh, B.; Singh, P.; Siddiqui, S.; Singh, D.; Gupta, M. Wastewater Treatment Using Fe-Doped Perovskite Manganites by Photocatalytic Degradation of Methyl Orange, Crystal Violet and Indigo Carmine Dyes in Tungsten Bulb/Sunlight. J. Rare Earths 2023, 41, 1311–1322. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; El-Shall, M.S.; Awad, F.S. Facile Synthesis of Amorphous Zirconium Phosphate Graphitic Carbon Nitride Composite and Its High Performance for Photocatalytic Degradation of Indigo Carmine Dye in Water. J. Mater. Res. Technol. 2022, 20, 1456–1469. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Khan, M.A.J.R.; Al-Mamun, M.R.; Islam, S.Z.; Khaleque, M.A.; Hossain, M.I.; Khan, M.Z.H.; Islam, M.S.; Marwani, H.M.; et al. Toxic Dye Removal, Remediation, and Mechanism with Doped SnO2-Based Nanocomposite Photocatalysts: A Critical Review. J. Water Process Eng. 2023, 54, 104069. [Google Scholar] [CrossRef]
- Venkatesh, D.; Pavalamalar, S.; Anbalagan, K. Selective Photodegradation on Dual Dye System by Recoverable Nano SnO2 Photocatalyst. J. Inorg. Organomet. Polym. Mater. 2019, 29, 939–953. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Cheng, Z.; Liu, S.; Ren, Y.; Chen, X.; Fan, M.; Shen, Z. Quantitative Structure-Activity Relationship (QSAR) Guides the Development of Dye Removal by Coagulation. J. Hazard. Mater. 2022, 438, 129448. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Jin, K.; Fan, Z.; Du, P.; Ji, Y.; Wang, J.; Zhao, Y.; Yao, C.; Cai, Z. Facile Fabrication of Fabric-Based Membrane for Adjustable Oil-in-Water Emulsion Separation, Suspension Filtration and Dye Removal. Sep. Purif. Technol. 2023, 323, 124467. [Google Scholar] [CrossRef]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-Based Hollow Nanofiber Membranes with Polyvinylpyrrolidone and Polyvinyl Alcohol for Efficient Removal and Filtration of Organic Dyes and Heavy Metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Choi, Y.K.; Kim, H.J.; Song, H.S.; Lee, S.M.; Lee Park, S.; Lee, H.S.; Koh, J.; et al. Application of Macroalgal Biomass Derived Biochar and Bioelectrochemical System with Shewanella for the Adsorptive Removal and Biodegradation of Toxic Azo Dye. Chemosphere 2021, 264, 128539. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Khairy, M.; Abdulkhair, B.Y.; Abdelrahman, E.A. Efficient Disposal of Basic Fuchsin Dye from Aqueous Media Using ZrO2/MgMn2O4/Mg(Mg0.333Mn1.333)O4 as a Novel and Facilely Synthesized Nanocomposite. Inorganics 2023, 11, 363. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent Advances in Al(Iii)/In(Iii)-Based MOFs for the Detection of Pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of Hazardous Dyes on Functionalized Multiwalled Carbon Nanotubes in Single and Binary Systems: Experimental Study and Physicochemical Interpretation of the Adsorption Mechanism. Chem. Eng. J. 2020, 389, 124467. [Google Scholar] [CrossRef]
- Feng, C.; Ren, P.; Huo, M.; Dai, Z.; Liang, D.; Jin, Y.; Ren, F. Facile Synthesis of Trimethylammonium Grafted Cellulose Foams with High Capacity for Selective Adsorption of Anionic Dyes from Water. Carbohydr. Polym. 2020, 241, 116369. [Google Scholar] [CrossRef]
- Tambat, S.N.; Ahirrao, D.J.; Pandit, A.B.; Jha, N.; Sontakke, S.M. Hydrothermally Synthesized N2-UiO-66 for Enhanced and Selective Adsorption of Cationic Dyes. Environ. Technol. Innov. 2020, 19, 101021. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Vigneshwaran, S.; Meenakshi, S. Synthesis and Characterization of La(III) Supported Carboxymethylcellulose-Clay Composite for Toxic Dyes Removal: Evaluation of Adsorption Kinetics, Isotherms and Thermodynamics. Int. J. Biol. Macromol. 2020, 161, 1117–1126. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Chen, Y.; Cai, L.; Wang, Y.; Song, W. An Abundant Porous Biochar Material Derived from Wakame (Undaria pinnatifida) with High Adsorption Performance for Three Organic Dyes. Bioresour. Technol. 2020, 318, 124082. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Ou, L. Removal of Indigo Carmine from Aqueous Solution by Microwave-Treated Activated Carbon from Peanut Shell. Desalin. Water Treat. 2016, 57, 718–727. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawasaki, N.; Tanada, S.; Tamura, T.; Shimizu, Y. Indigo Carmine Removal by Charcoal from Rice Bran as an Agricultural By-Product. Toxicol. Environ. Chem. 2005, 87, 321–327. [Google Scholar] [CrossRef]
- Kesraoui, A.; Selmi, T.; Seffen, M.; Brouers, F. Influence of Alternating Current on the Adsorption of Indigo Carmine. Environ. Sci. Pollut. Res. 2017, 24, 9940–9950. [Google Scholar] [CrossRef] [PubMed]
- Jiwalak, N.; Rattanaphani, S.; Bremner, J.B.; Rattanaphani, V. Equilibrium and Kinetic Modeling of the Adsorption of Indigo Carmine onto Silk. Fibers Polym. 2010, 11, 572–579. [Google Scholar] [CrossRef]
- Yazdi, M.G.; Ivanic, M.; Mohamed, A.; Uheida, A. Surface Modified Composite Nanofibers for the Removal of Indigo Carmine Dye from Polluted Water. RSC Adv. 2018, 8, 24588–24598. [Google Scholar] [CrossRef]
- Geyikçi, F. Factorial Design Analysis for Adsorption of Indigo Carmine onto Montmorillonite-Evaluation of the Kinetics and Equilibrium Data. Prog. Org. Coat. 2016, 98, 28–34. [Google Scholar] [CrossRef]
- De Luna, M.S.; Ascione, C.; Santillo, C.; Verdolotti, L.; Lavorgna, M.; Buonocore, G.G.; Castaldo, R.; Filippone, G.; Xia, H.; Ambrosio, L. Optimization of Dye Adsorption Capacity and Mechanical Strength of Chitosan Aerogels through Crosslinking Strategy and Graphene Oxide Addition. Carbohydr. Polym. 2019, 211, 195–203. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Removal of Ciprofloxacin and Indigo Carmine from Water by Carbon Nanotubes Fabricated from a Low-Cost Precursor: Solution Parameters and Recyclability. Ain Shams Eng. J. 2022, 14, 101844. [Google Scholar] [CrossRef]
- Kanwal, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Basic Dye Adsorption onto Clay/MnFe2O4 Composite: A Mechanistic Study. Water Environ. Res. 2017, 89, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.I. Removal of Methylene Blue Dye from Aqueous Solution Using Activated Charcoal Modified Manganese Ferrite (AC-MnFe2O4): Kinetics, Isotherms, and Thermodynamics Studies. Part. Sci. Technol. 2020, 38, 756–767. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Algethami, F.K.; Saad, F.A.; Abdelrahman, E.A. Remarkable High Adsorption of Methylene Blue Dye from Aqueous Solutions Using Facilely Synthesized MgFe2O4 Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2035–2045. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Synthesis and Characterization of Magnetically Separable and Recyclable Crumbled MgFe2O4/Reduced Graphene Oxide Nanoparticles for Removal of Methylene Blue Dye from Aqueous Solutions. J. Phys. Chem. Solids 2021, 149, 109760. [Google Scholar] [CrossRef]
- Vergis, B.R.; Kottam, N.; Hari Krishna, R.; Nagabhushana, B.M. Removal of Evans Blue Dye from Aqueous Solution Using Magnetic Spinel ZnFe2O4 Nanomaterial: Adsorption Isotherms and Kinetics. Nano-Struct. Nano-Objects 2019, 18, 100290. [Google Scholar] [CrossRef]
- Algarni, T.S.; Al-Mohaimeed, A.M.; Al-Odayni, A.B.; Abduh, N.A.Y. Activated Carbon/ZnFe2O4 Nanocomposite Adsorbent for Efficient Removal of Crystal Violet Cationic Dye from Aqueous Solutions. Nanomaterials 2022, 12, 3224. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of Congo Red and Methylene Blue Dyes on an Ashitaba Waste and a Walnut Shell-Based Activated Carbon from Aqueous Solutions: Experiments, Characterization and Physical Interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Khalil, M.M.H.; Algethami, F.K.; Khairy, M.; Abou El-Reash, Y.G.; Saad, F.A.; Shah, R.K.; Ammar, A.M. Facile Synthesis of MgO/CuO and MgO/Cu3MgO4 Binary Nanocomposites as Promising Adsorbents for the Disposal of Zn(II) Ions. J. Inorg. Organomet. Polym. Mater. 2023, in press. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Li, F.; Khaimanov, S.; Tsidaeva, N.; Lahoubi, M. PEG-Assisted Hydrothermal Synthesis of CoFe2O4 Nanoparticles with Enhanced Selective Adsorption Properties for Different Dyes. Appl. Surf. Sci. 2016, 389, 1003–1011. [Google Scholar] [CrossRef]



| Effect | Concentration of Dye (mg/L) | Volume of Dye (mL) | Amount of Adsorbent (g) | pH | Time (min) | Temperature (K) |
|---|---|---|---|---|---|---|
| pH | 240 | 120 | 0.06 | 2–10 | 240 | 298 |
| Time | 240 | 120 | 0.06 | 2 | 10–140 | 298 |
| Temperature (298–328 K) | 240 | 120 | 0.06 | 2 | 90 | 298–328 |
| Concentration (40–280 mg/L) | 40–280 | 120 | 0.06 | 2 | 90 | 298 |
| Experimental | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| Qexp (mg/g) | Qe (mg/g) | kFirst (1/min) | R2 | Qe (mg/g) | kSecond (g/mg·min) | R2 |
| 396.74 | 152.06 | 0.0245 | 0.9300 | 400.00 | 0.00041 | 0.9999 |
| ΔH° (KJ/mol) | ΔS° (KJ/mol K) | ΔG° (KJ/mol) | |||
|---|---|---|---|---|---|
| −47.82 | 0.1415 | 298 | 308 | 318 | 328 |
| −89.99 | −91.41 | −92.83 | −94.24 | ||
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Qmax (mg/g) | kL (L/mg) | R2 | Qmax (mg/g) | kF (mg/g)(L/mg)1/n | R2 |
| 421.94 | 0.2596 | 0.9985 | 740.01 | 110.85 | 0.9148 |
| Adsorbent | Maximum Adsorption Capacity (mg/g) | pH | Time | Ref. |
|---|---|---|---|---|
| Polyacrylonitrile/Fe3O4/3-mercaptopropionic acid composite | 154.50 | 5 | 25 | [37] |
| Activated carbon | 87.80 | 2 | 80 | [35] |
| Montmorillonite | 40.00 | 2 | 20 | [38] |
| Chitosan aerogels | 168.60 | 2 | 100 | [39] |
| Carbon nanotubes | 93.00 | 2 | 50 | [40] |
| CoFe2O4 nanoparticles | 421.94 | 2 | 90 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wasidi, A.S.; Abdelrahman, E.A. Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media. Inorganics 2023, 11, 453. https://doi.org/10.3390/inorganics11120453
Al-Wasidi AS, Abdelrahman EA. Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media. Inorganics. 2023; 11(12):453. https://doi.org/10.3390/inorganics11120453
Chicago/Turabian StyleAl-Wasidi, Asma S., and Ehab A. Abdelrahman. 2023. "Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media" Inorganics 11, no. 12: 453. https://doi.org/10.3390/inorganics11120453
APA StyleAl-Wasidi, A. S., & Abdelrahman, E. A. (2023). Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media. Inorganics, 11(12), 453. https://doi.org/10.3390/inorganics11120453