Photocatalytic Degradation of Emerging Contaminants with N-Doped TiO2 Using Simulated Sunlight in Real Water Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD and UV-VIS Characterization
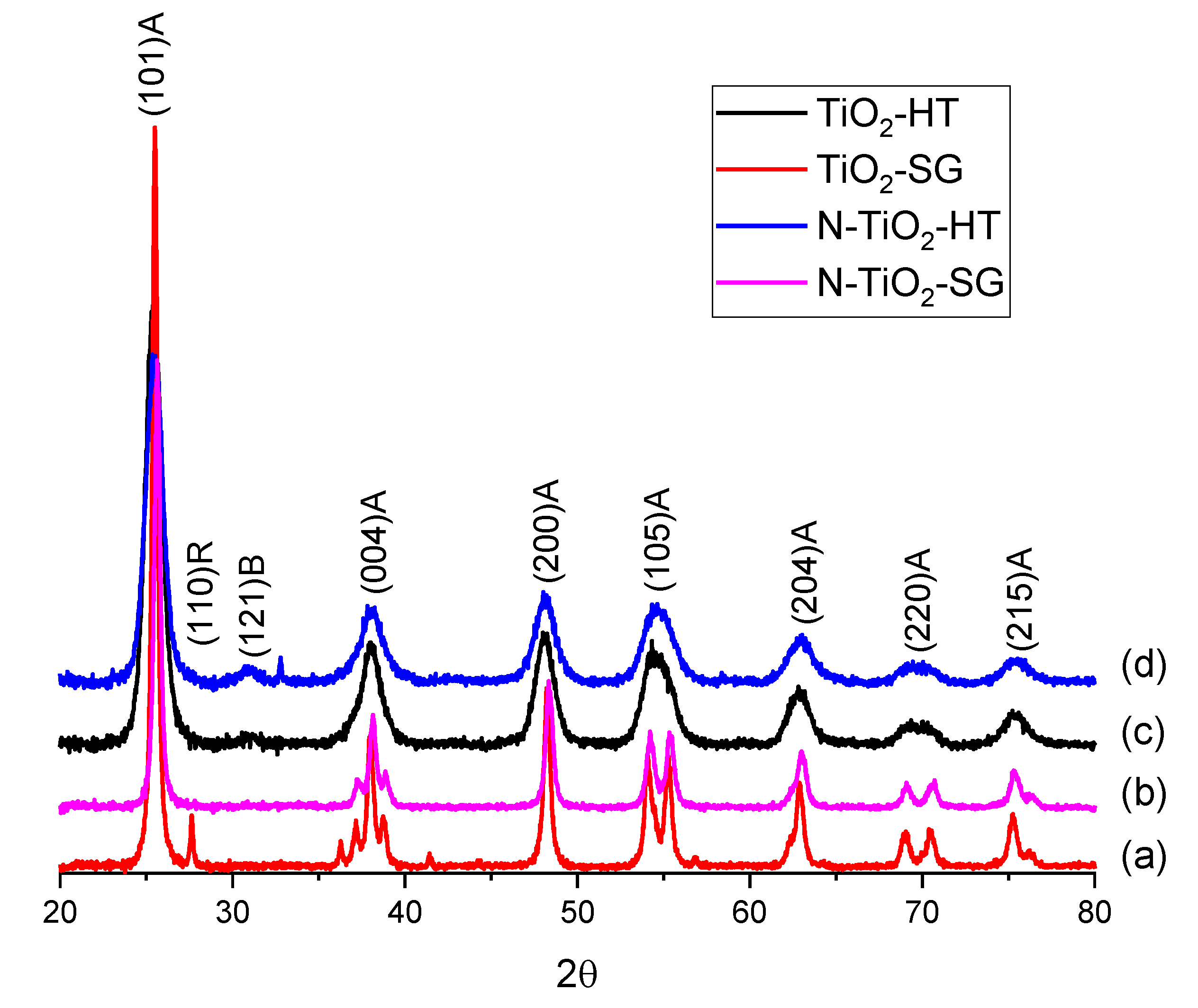
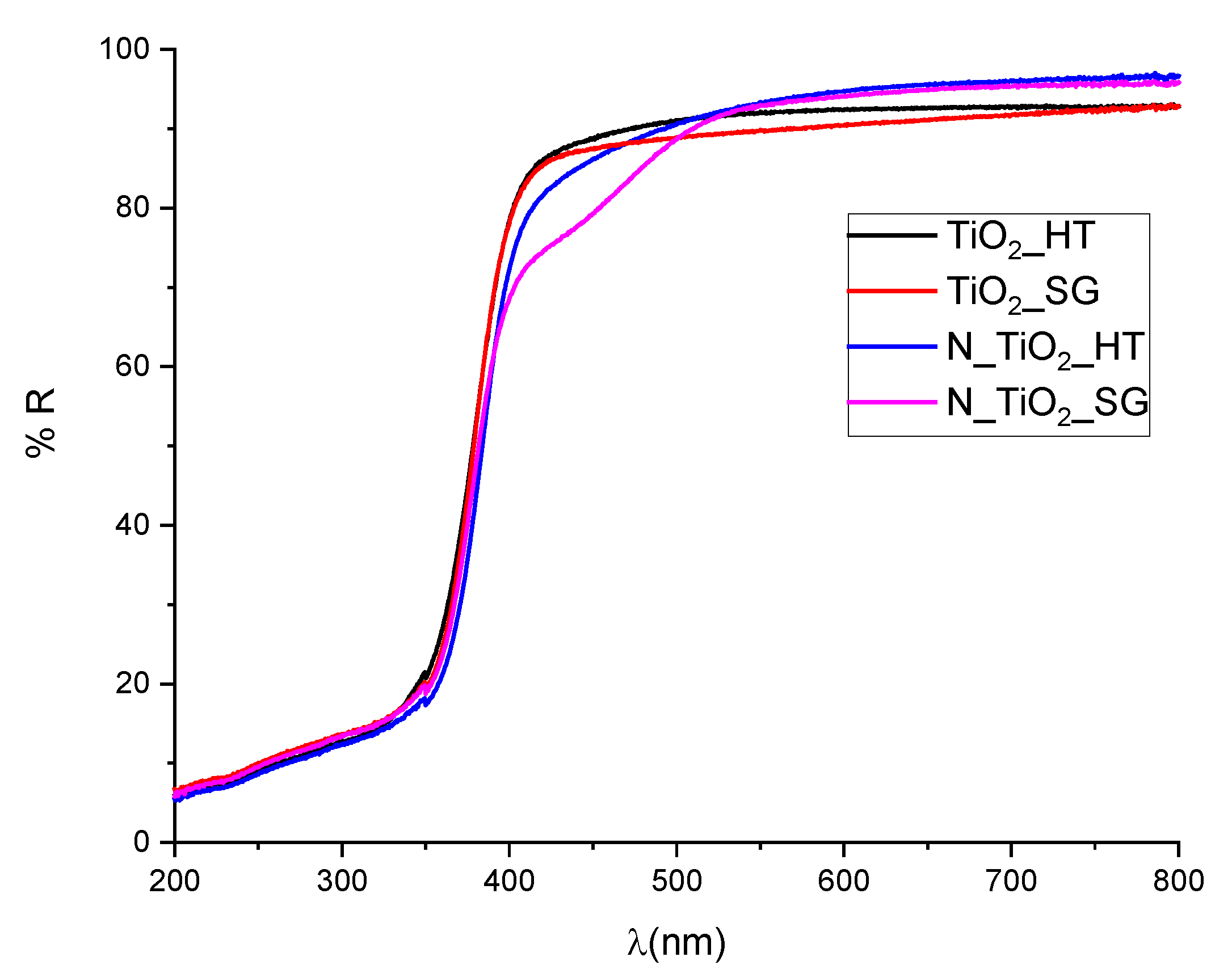
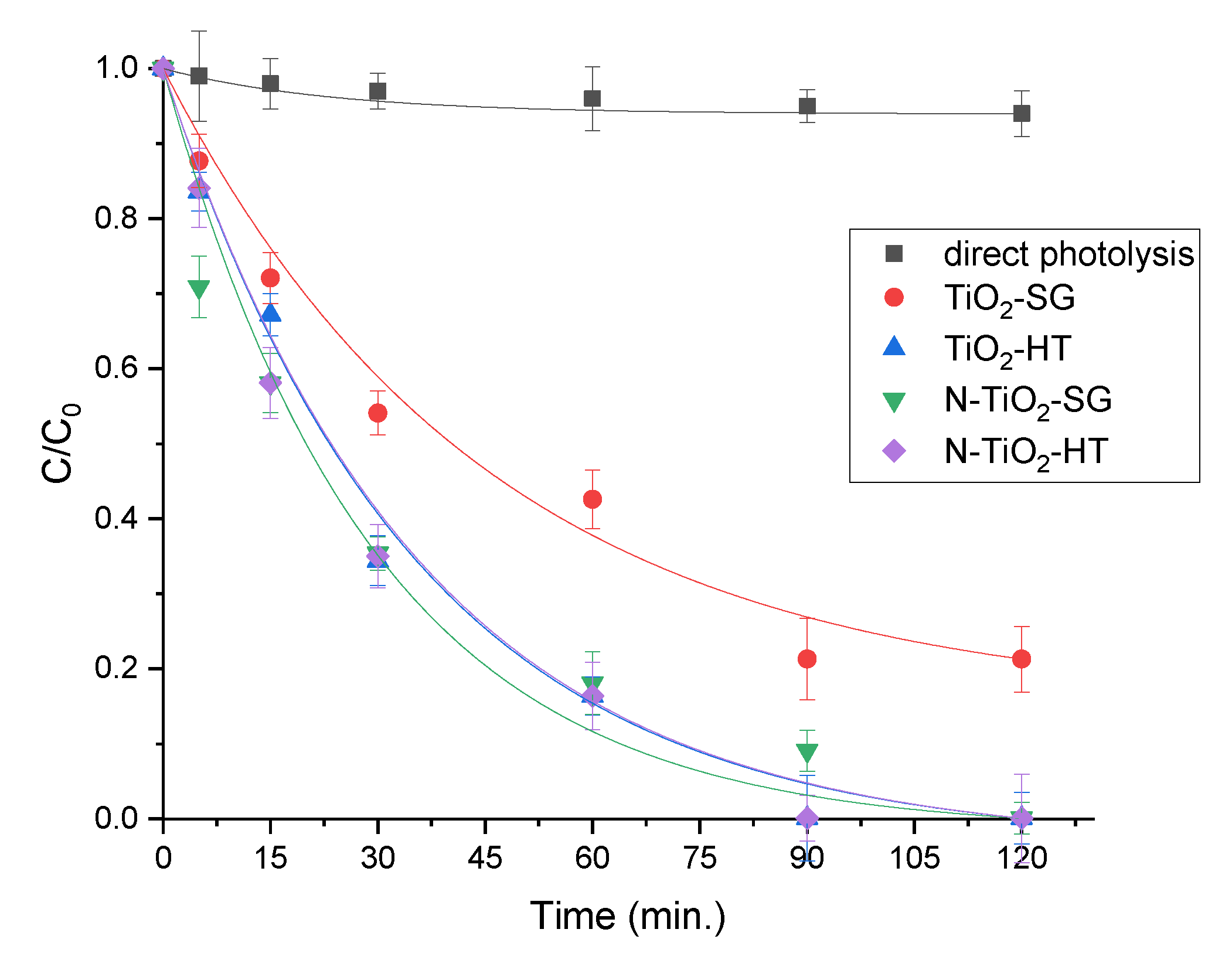
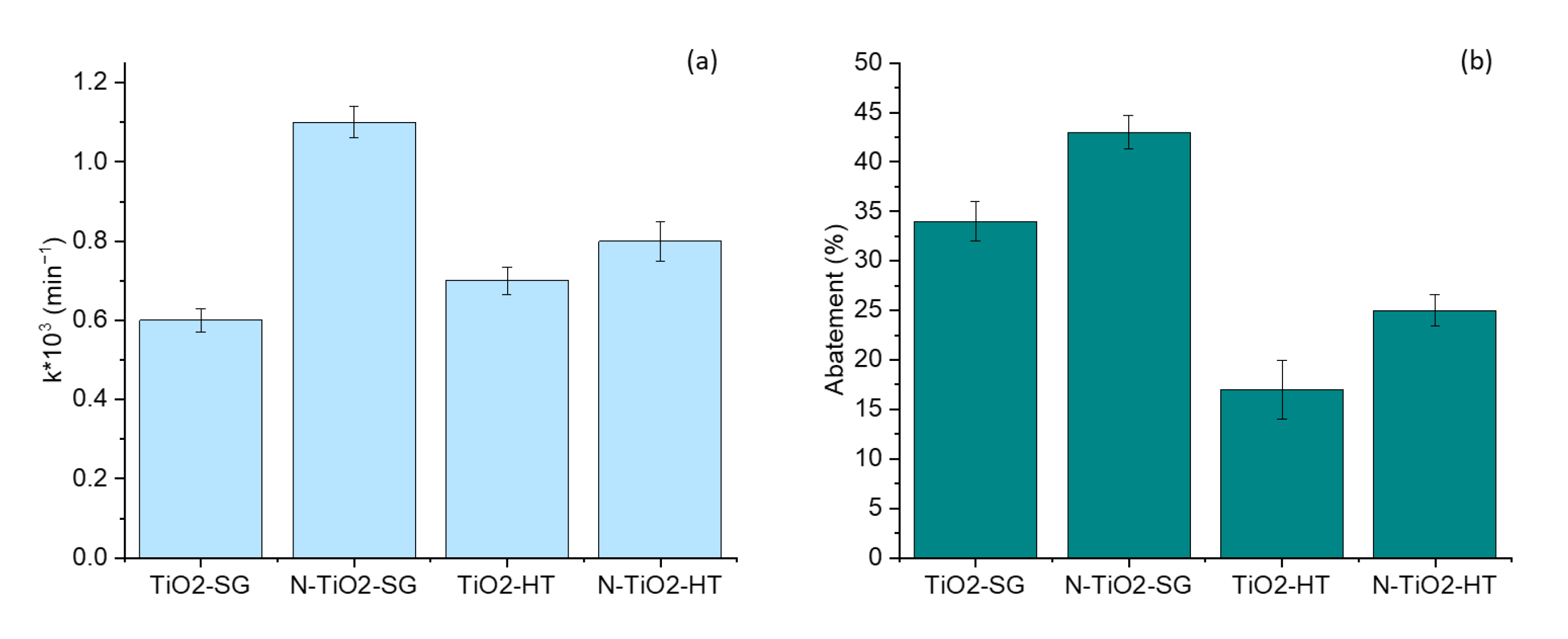
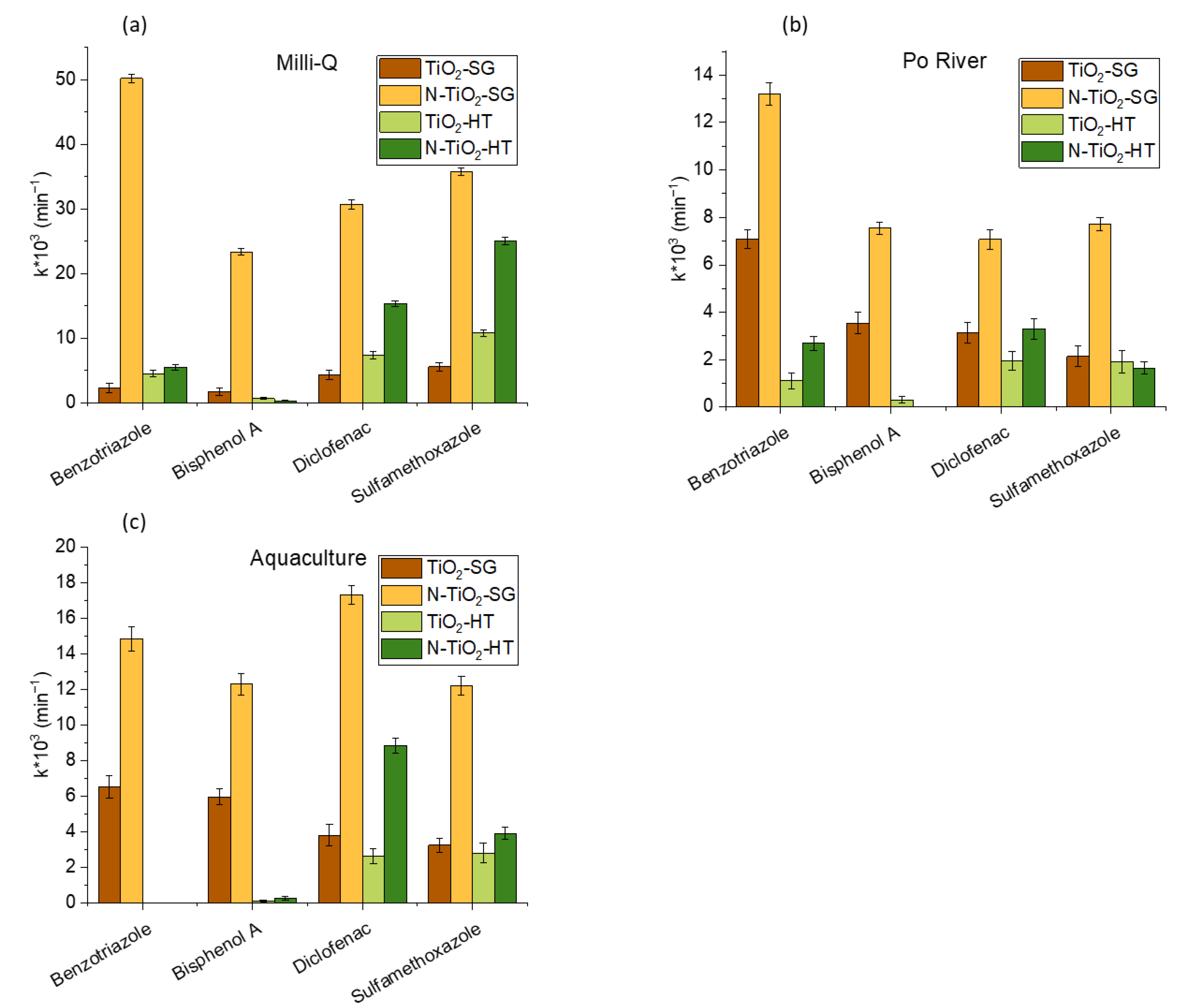
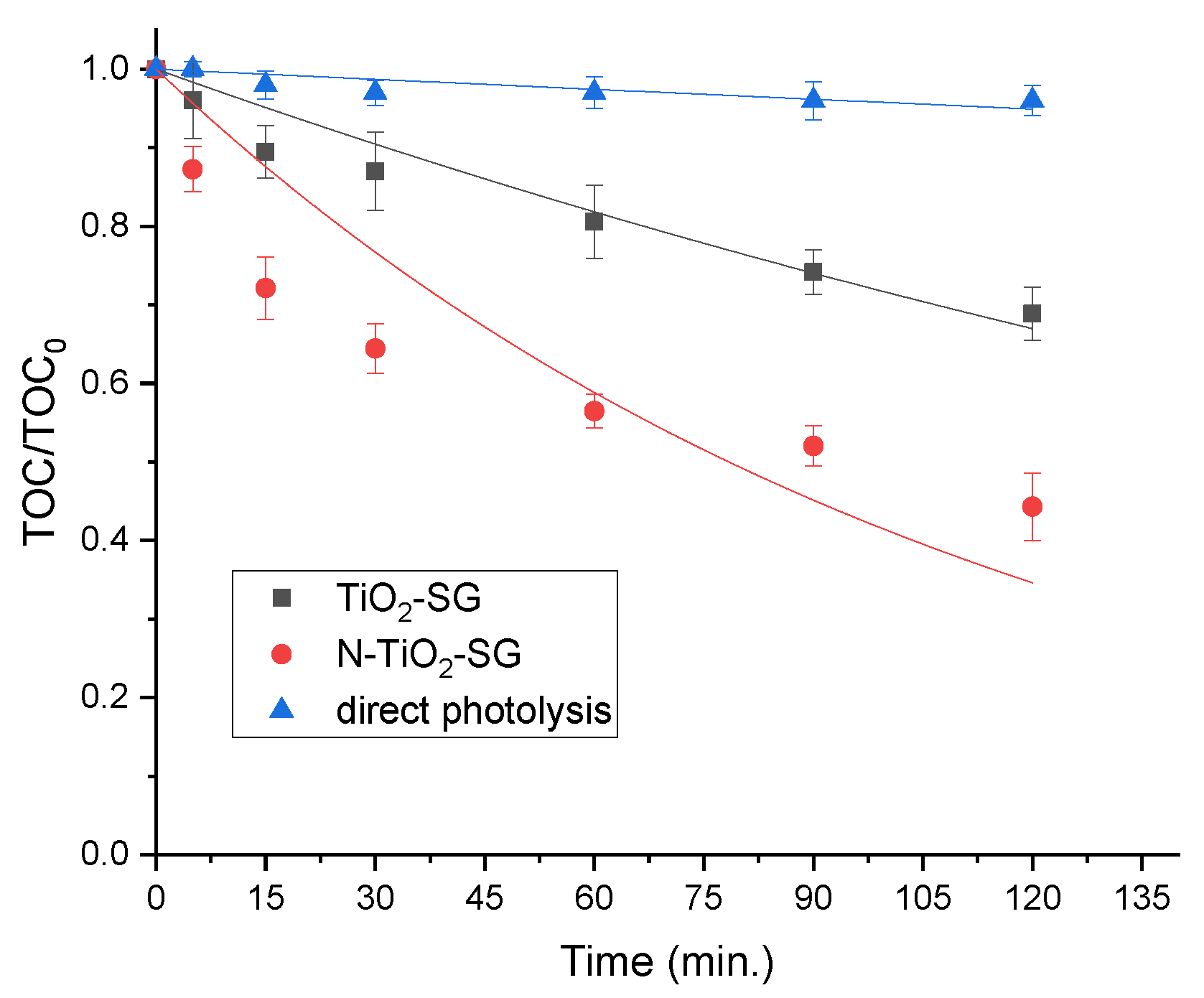
2.2. Photocatalytic Degradation of Organic Pollutants
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of TiO2 Using Sol–Gel
3.1.2. Synthesis of TiO2 Using Hydrothermal Method
3.1.3. Synthesis of N-TiO2 Using Sol–Gel Method
3.1.4. Synthesis of N-TiO2 Using Hydrothermal Method
3.2. Characterization
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gleick, P.H. Global Freshwater Resources: Soft-Path Solutions for the 21st Century. Science 2003, 302, 1524–1528. [Google Scholar] [CrossRef]
- Konar, M.; Evans, T.P.; Levy, M.; Scott, C.A.; Troy, T.J.; Vörösmarty, C.J.; Sivapalan, M. Water resources sustainability in a globalizing world: Who uses the water? Hydrol. Process. 2016, 30, 3330–3336. [Google Scholar] [CrossRef]
- Chaudhry, F.N.; Malik, M. Factors affecting water pollution: A review. J. Ecosyst. Ecography 2017, 7, 225–231. [Google Scholar]
- Kumar, N.M.; Sudha, M.C.; Damodharam, T.; Varjani, S. Chapter 3-Micro-pollutants in surface water: Impacts on the aquatic environment and treatment technologies. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–62. [Google Scholar] [CrossRef]
- Ashraf, M.A. Persistent Organic Pollutants (POPs): A Global Issue, a Global Challenge; Springer: Berlin, Germany, 2017; pp. 4223–4227. [Google Scholar]
- Lofrano, G.; Libralato, G.; Meric, S.; Vaiano, V.; Sacco, O.; Venditto, V.; Guida, M.; Carotenuto, M. 1-Occurrence and potential risks of emerging contaminants in water. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants; Sacco, O., Vaiano, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–25. [Google Scholar]
- Goswami, L.; Kumar, R.V.; Borah, S.N.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: A review. J. Water Process Eng. 2018, 26, 314–328. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Alpana, A.; Shrikant, M.; Pratyush, K.; Abhibnav, B.; Ruchita, T. Review on synthetic study of benzotriazole. GSC Biol. Pharm. Sci. 2020, 11, 215–225. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Microbial degradation of sulfamethoxazole in the environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, W.; Sun, B.; Li, H.; Qiao, P.; Xu, Y.; Wu, J.; Lin, K.; Fu, H. Defects-engineering of magnetic γ-Fe2O3 ultrathin nanosheets/mesoporous black TiO2 hollow sphere heterojunctions for efficient charge separation and the solar-driven photocatalytic mechanism of tetracycline degradation. Appl. Catal. B Environ. 2019, 240, 319–328. [Google Scholar] [CrossRef]
- Rahman, M.U.; Qazi, U.Y.; Hussain, T.; Nadeem, N.; Zahid, M.; Bhatti, H.N.; Shahid, I. Solar driven photocatalytic degradation potential of novel graphitic carbon nitride based nano zero-valent iron doped bismuth ferrite ternary composite. Opt. Mater. 2021, 120, 111408. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, Y.; Chen, T.; Guo, Q.; Wei, W.; Ni, B. Bi2O3@ carbon nanocomposites for solar-driven photocatalytic degradation of chlorophenols. ACS Appl. Nano Mater. 2019, 2, 2308–2316. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen doped titanium dioxide (N-TiO2): Synopsis of synthesis methodologies, doping mechanisms, property evaluation and visible light photocatalytic applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Varma, R.; Yadav, M.; Tiwari, K.; Makani, N.; Gupta, S.; Kothari, D.C.; Miotello, A.; Patel, N. Roles of Vanadium and Nitrogen in Photocatalytic Activity of VN-Codoped TiO2 Photocatalyst. Photochem. Photobiol. 2018, 94, 955–964. [Google Scholar] [CrossRef] [PubMed]
- El Koura, Z.; Cazzanelli, M.; Bazzanella, N.; Patel, N.; Fernandes, R.; Arnaoutakis, G.E.; Gakamsky, A.; Dick, A.; Quaranta, A.; Miotello, A. Synthesis and Characterization of Cu and N Codoped RF-Sputtered TiO2 Films: Photoluminescence Dynamics of Charge Carriers Relevant for Water Splitting. J. Phys. Chem. C 2016, 120, 12042–12050. [Google Scholar] [CrossRef]
- Bazzanella, N.; Bajpai, O.P.; Fendrich, M.; Guella, G.; Miotello, A.; Orlandi, M. Ciprofloxacin degradation with a defective TiO2-x nanomaterial under sunlight. MRS Commun. 2023, 13, 1252–1259. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for Visible Light Responses in Anodic Photocurrents at N-Doped TiO2 Film Electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven N− F− codoped TiO2 photocatalysts. 2. Optical characterization, photocatalysis, and potential application to air purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Lin, Z.; Orlov, A.; Lambert, R.M.; Payne, M.C. New Insights into the Origin of Visible Light Photocatalytic Activity of Nitrogen-Doped and Oxygen-Deficient Anatase TiO2. J. Phys. Chem. B 2005, 109, 20948–20952. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Morikawa, T.; Ohwaki, T.; Taga, Y. Band-gap narrowing of TiO2 films induced by N-doping. Phys. B Condens. Matter 2006, 376–377, 823–826. [Google Scholar] [CrossRef]
- Emeline, A.; Kuzmin, G.; Serpone, N. Wavelength-dependent photostimulated adsorption of molecular O2 and H2 on second generation titania photocatalysts: The case of the visible-light-active N-doped TiO2 system. Chem. Phys. Lett. 2008, 454, 279–283. [Google Scholar] [CrossRef]
- Emeline, A.V.; Sheremetyeva, N.V.; Khomchenko, N.V.; Ryabchuk, V.K.; Serpone, N. Photoinduced Formation of Defects and Nitrogen Stabilization of Color Centers in N-Doped Titanium Dioxide. J. Phys. Chem. C 2007, 111, 11456–11462. [Google Scholar] [CrossRef]
- Ohsawa, T.; Lyubinetsky, I.; Du, Y.; Henderson, M.A.; Shutthanandan, V.; Chambers, S.A. Crystallographic dependence of visible-light photoactivity in epitaxial TiO2−x Nx anatase and rutile. Phys. Rev. 2009, 79, 085401. [Google Scholar] [CrossRef]
- Barolo, G.; Livraghi, S.; Chiesa, M.; Paganini, M.C.; Giamello, E. Mechanism of the Photoactivity under Visible Light of N-Doped Titanium Dioxide. Charge Carriers Migration in Irradiated N-TiO2 Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2012, 116, 20887–20894. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Yamanaka, K.-I.; Morikawa, T. Charge Separation and Trapping in N-Doped TiO2 Photocatalysts: A Time-Resolved Microwave Conductivity Study. J. Phys. Chem. Lett. 2010, 1, 3261–3265. [Google Scholar] [CrossRef]
- Yamanaka, K.-i.; Morikawa, T. Charge-carrier dynamics in nitrogen-doped TiO2 powder studied by femtosecond time-resolved diffuse reflectance spectroscopy. J. Phys. Chem. C 2012, 116, 1286–1292. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Y.; Yang, L.; Wen, Y.; Xiong, Z.; Lei, L.; Wang, L.; Zeng, Q. Branched core-shell a-TiO2@ N-TiO2 nanospheres with gradient-doped N for highly efficient photocatalytic applications. Chin. Chem. Lett. 2023, 34, 107628. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, S.; Xu, H.; Ma, C.; Sun, J.; Li, H.; Pei, H. Application of N-TiO2 for visible-light photocatalytic degradation of Cylindrospermopsis raciborskii—More difficult than that for photodegradation of Microcystis aeruginosa? Environ. Pollut. 2019, 245, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bao, W.; Li, L.; Cheng, H.; Huang, W.; Kong, H.; Li, Y. The synergistic effect of nitrogen-doped titanium dioxide/mercaptobenzoic acid/silver nanocomplexes for surface-enhanced Raman scattering. J. Nanopart. Res. 2018, 20, 82. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H.E. Standard X-ray Diffraction Powder Patterns; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1971; Volume 25. [Google Scholar]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Fernandes, E.; Martins, R.C.; Gomes, J. Photocatalytic ozonation of parabens mixture using 10% N-TiO2 and the effect of water matrix. Sci. Total. Environ. 2020, 718, 137321. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.-K.; Hu, L.; Zheng, J.; Prabhakaran, D.; Wu, S.; Puchtler, T.J.; Li, M.; Wong, K.-Y.; Taylor, R.A.; et al. Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Tauc, J.; Scott, T.A. The optical properties of solids. Phys. Today 1967, 20, 105–107. [Google Scholar] [CrossRef]

| Sample | Band Gap (eV) |
|---|---|
| TiO2-SG | 3.38 |
| N-TiO2-SG | 3.37 |
| TiO2-HT | 3.37 |
| N-TiO2-HT | 3.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggero, E.; Giovagnoni, A.; Zollo, A.; Calza, P.; Paganini, M.C. Photocatalytic Degradation of Emerging Contaminants with N-Doped TiO2 Using Simulated Sunlight in Real Water Matrices. Inorganics 2023, 11, 439. https://doi.org/10.3390/inorganics11110439
Gaggero E, Giovagnoni A, Zollo A, Calza P, Paganini MC. Photocatalytic Degradation of Emerging Contaminants with N-Doped TiO2 Using Simulated Sunlight in Real Water Matrices. Inorganics. 2023; 11(11):439. https://doi.org/10.3390/inorganics11110439
Chicago/Turabian StyleGaggero, Elisa, Arianna Giovagnoni, Alessia Zollo, Paola Calza, and Maria Cristina Paganini. 2023. "Photocatalytic Degradation of Emerging Contaminants with N-Doped TiO2 Using Simulated Sunlight in Real Water Matrices" Inorganics 11, no. 11: 439. https://doi.org/10.3390/inorganics11110439
APA StyleGaggero, E., Giovagnoni, A., Zollo, A., Calza, P., & Paganini, M. C. (2023). Photocatalytic Degradation of Emerging Contaminants with N-Doped TiO2 Using Simulated Sunlight in Real Water Matrices. Inorganics, 11(11), 439. https://doi.org/10.3390/inorganics11110439