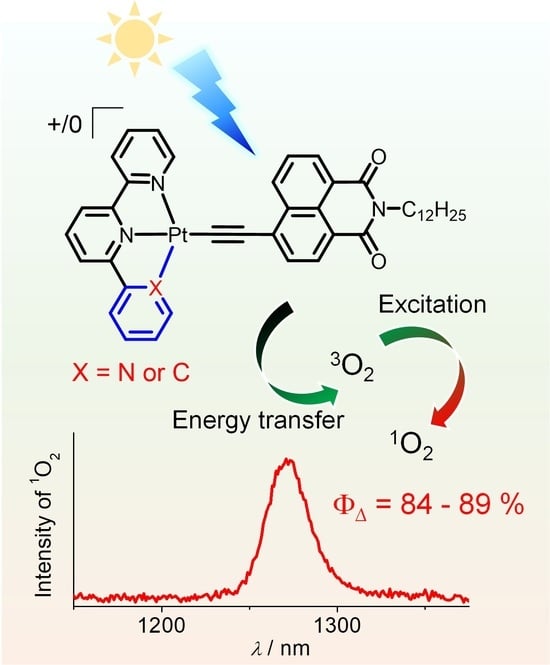
Naphthalimide-Modified Tridentate Platinum(II) Complexes: Synthesis, Characterization, and Application in Singlet Oxygen Generation
Abstract
:1. Introduction
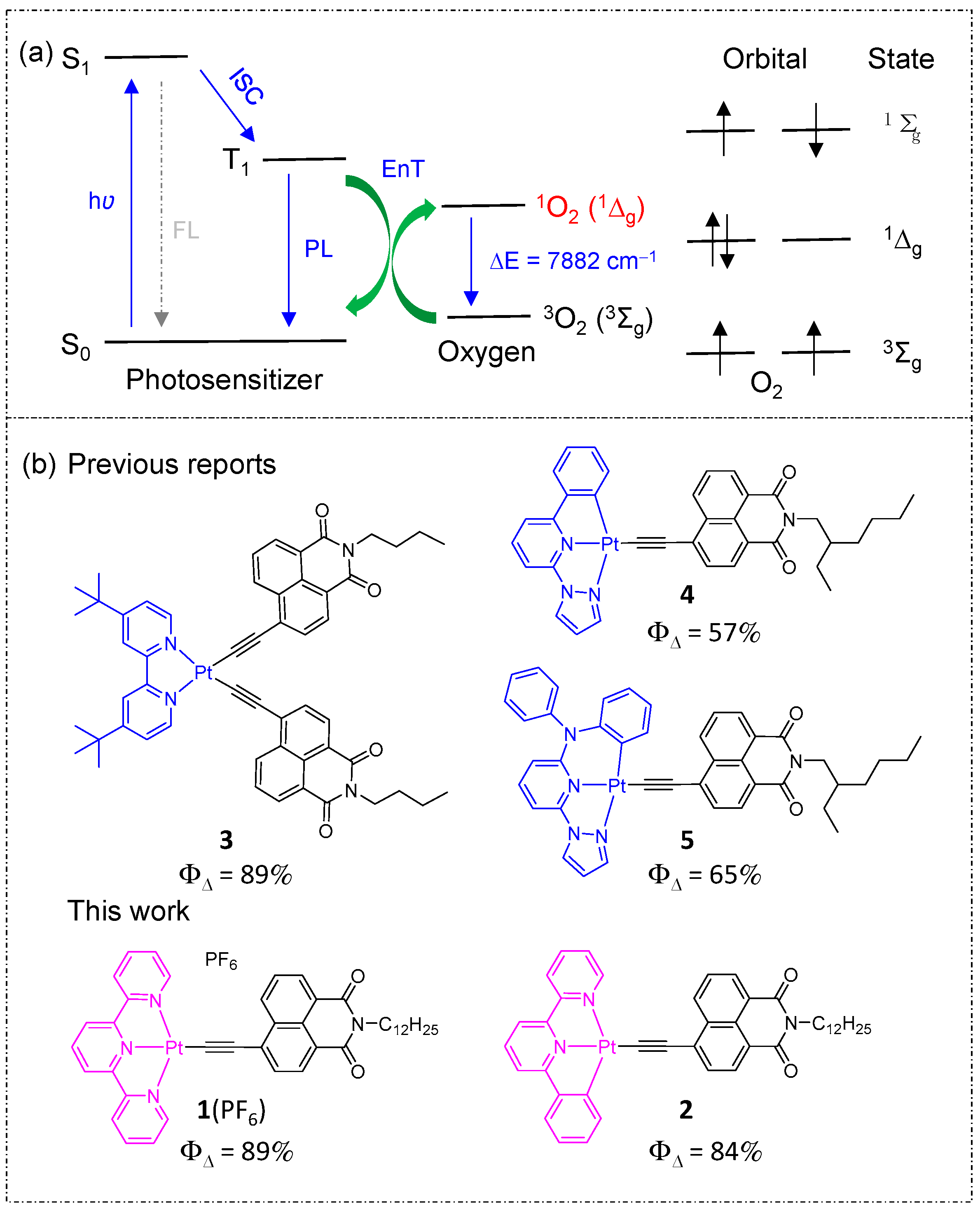
2. Results and Discussion
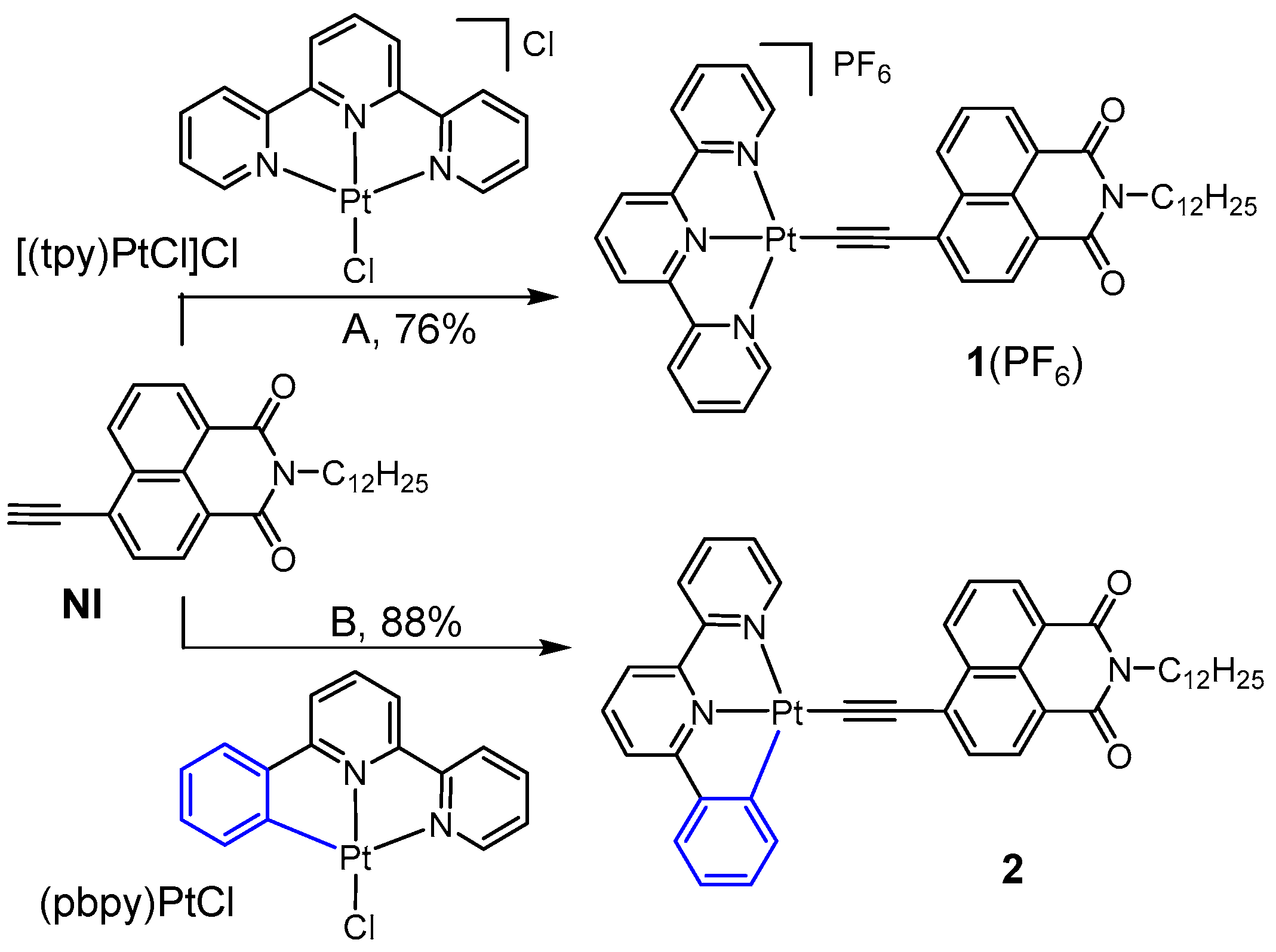
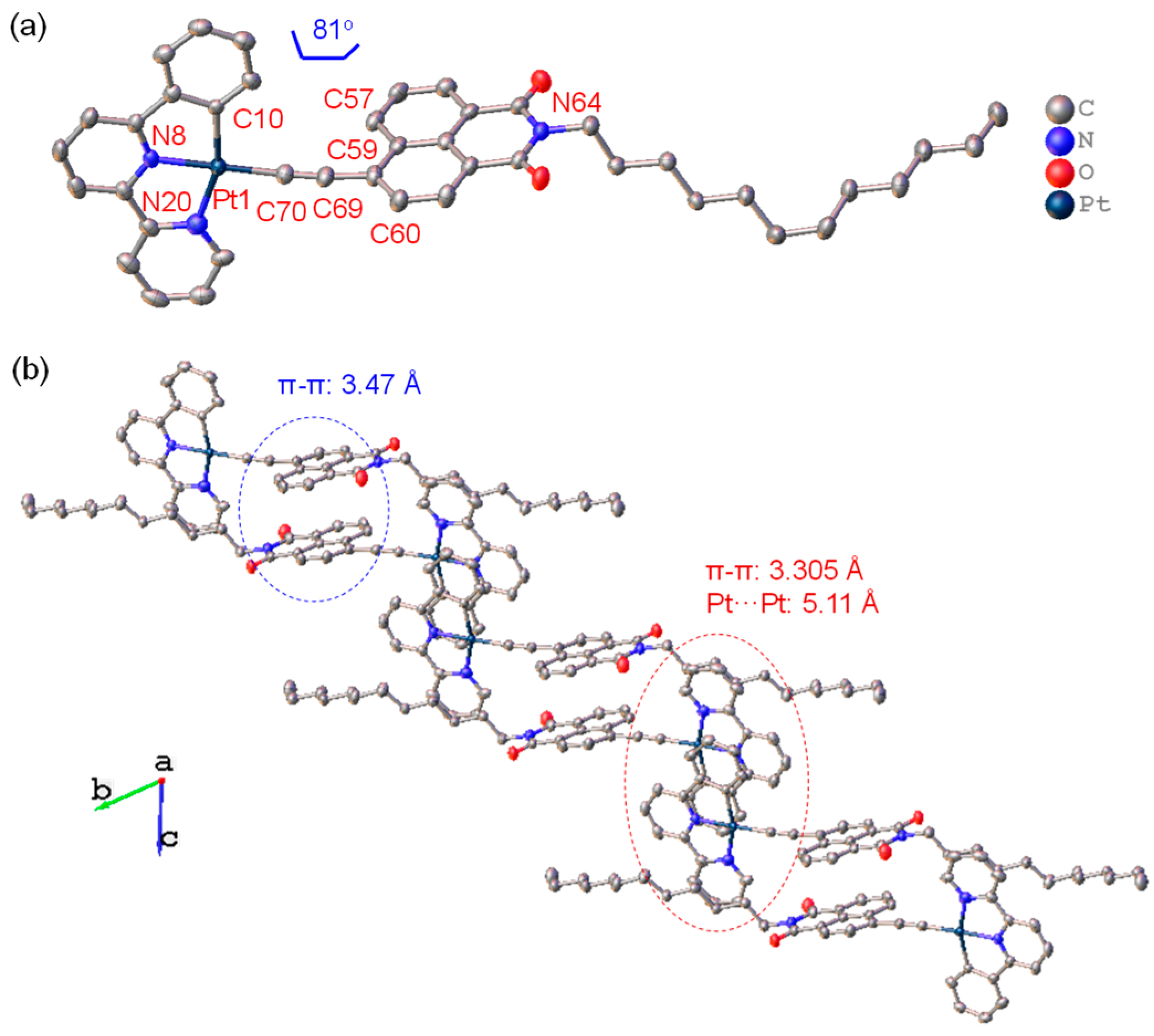
2.1. Synthesis and X-ray Single-Crystal Diffraction Analysis
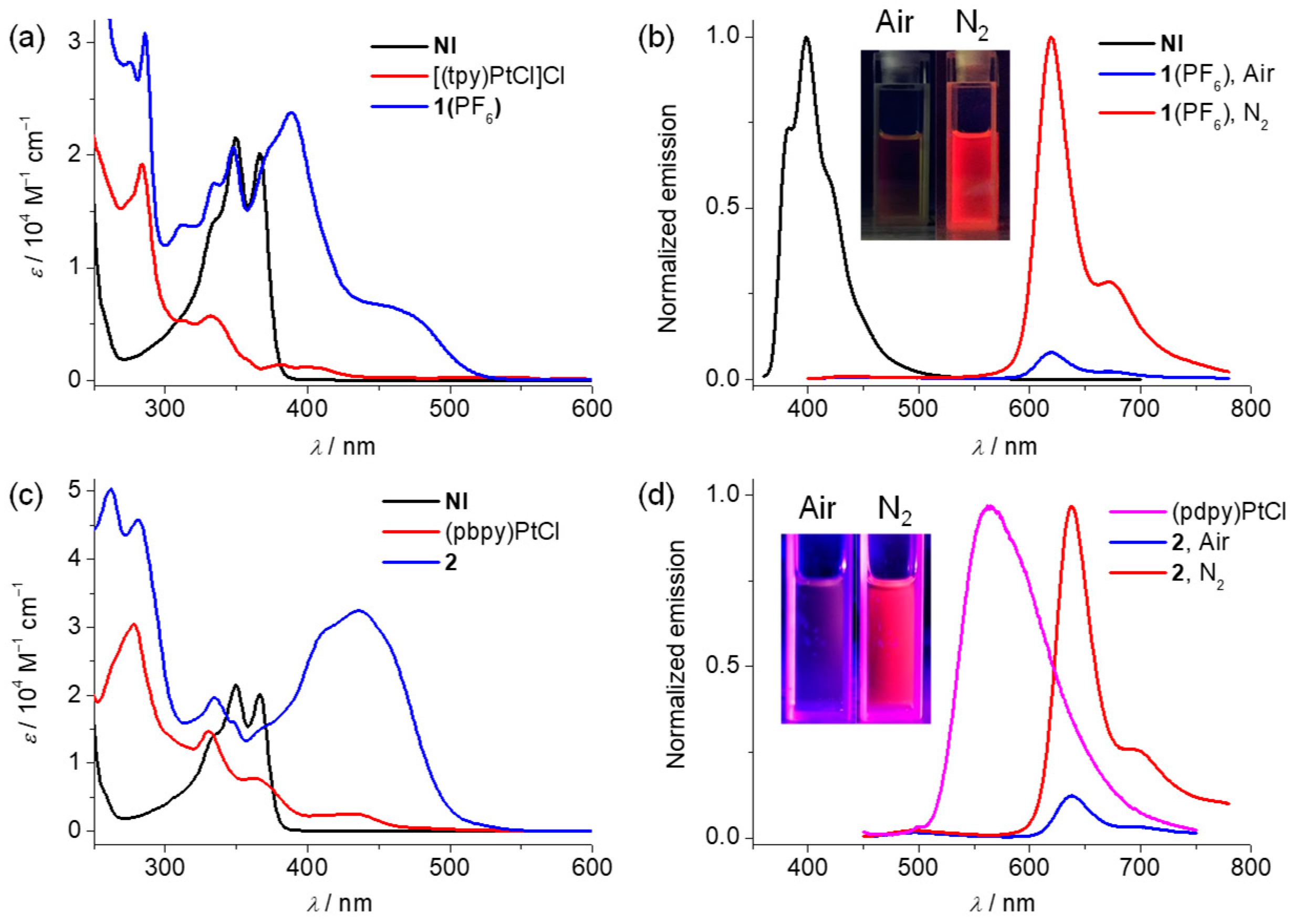
2.2. Steady-State Absorption and Emission Spectroscopies
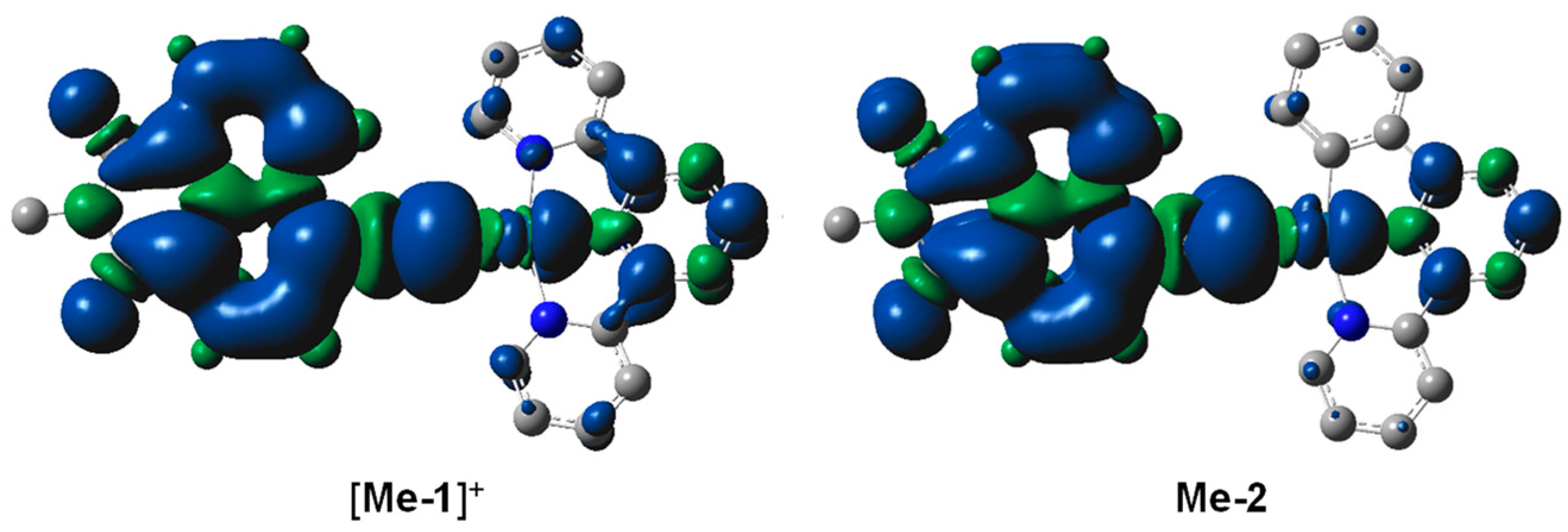
2.3. Theoretical Calculations
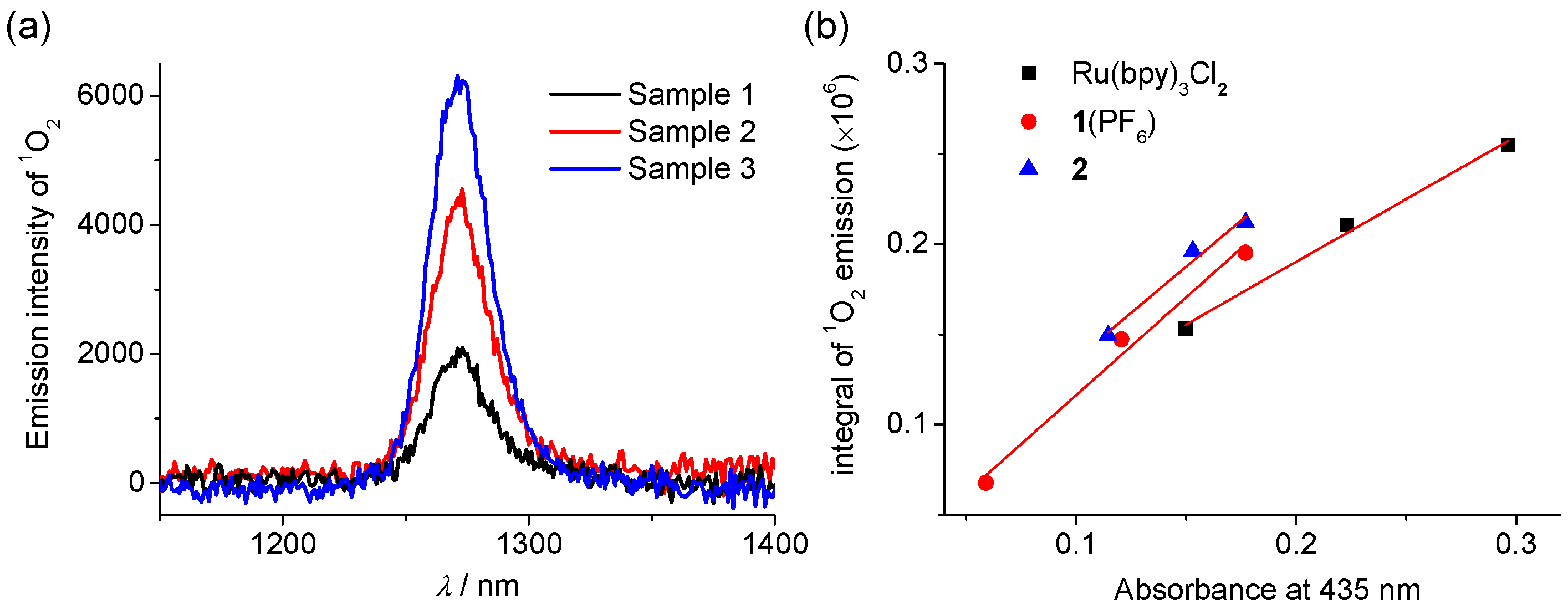
2.4. Singlet Oxygen Generation
3. Experimental Section
3.1. General Information for Synthesis and Characterization
3.2. Information on Other Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Ashen-Garry, D.; Selke, M. Singlet Oxygen Generation by Cyclometalated Complexes and Applications. Photochem. Photobiol. 2014, 90, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, M.; Xu, Y.; Wang, Q.; Liu, J.; Zhou, X.; Ji, H.; Tang, Q.; Gu, X.; Liu, S.; et al. Dual-Phosphorescent Iridium(III) Complexes Extending Oxygen Sensing from Hypoxia to Hyperoxia. Coord. Chem. Rev. 2023, 478, 214979–214998. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially Resolved Cellular Responses to Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Qu, J.; Lin, J.; Huang, P.; Xia, X.-H. Inorganic Nanomaterials with Intrinsic Singlet Oxygen Generation for Photodynamic Therapy. Adv. Sci. 2021, 8, 2102587. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, N.; Zhao, D.; Ma, Y. New cyclometalated transition-metal based photosensitizers for singlet oxygen generation and photodynamic therapy. Sci. China Chem. 2016, 59, 40–52. [Google Scholar] [CrossRef]
- Ishii, K. Functional singlet oxygen generators based on phthalocyanines. Coord. Chem. Rev. 2012, 256, 1556–1568. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The triplet excited state of Bodipy: Formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.; Mosinger, J.; Wagnerová, D.M. Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules; models for photodynamic therapy. Coord. Chem. Rev. 2004, 248, 321–350. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Mahammed, A.; Gross, Z. Corroles as triplet photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. [Google Scholar] [CrossRef]
- Kar, B.; Das, U.; Roy, N.; Paira, P. Recent advances on organelle specific Ru(II)/Ir(III)/Re(I) based complexes for photodynamic therapy. Coord. Chem. Rev. 2023, 474, 214860–214936. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; An, J.; Ma, X.; Yang, J.; Luo, L.; Deng, Y.; Kim, J.S.; Sun, Y. Construction of a 980 nm laser-activated Pt(II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization. Sci. China Chem. 2023, 66, 155–163. [Google Scholar] [CrossRef]
- Chen, H.; Wen, K.; Lu, Y.; Zhang, X.; Shi, Y.; Shi, Q.; Ma, H.; Peng, Q.; Huang, H. White-light-driven fluorescence switch for super-resolution imaging guided photodynamic and photoacid therapy. Sci. China Chem. 2022, 65, 2528–2537. [Google Scholar] [CrossRef]
- Tian, S.; Lu, Y.; He, Z.; Yue, Q.; Zhuang, Z.; Wang, Y.; Meng, F.; Luo, L. Polydiacetylene-based poly-ion complex enabling aggregation-induced emission and photodynamic therapy dual turn-on for on-demand pathogenic bacteria elimination. Sci. China Chem. 2022, 65, 1782–1790. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, R.; Zhang, C.; Huang, J.; Ji, L.; Chao, H. Two-photon luminescent metal complexes for bioimaging and cancer phototherapy. Coord. Chem. Rev. 2016, 310, 16–40. [Google Scholar] [CrossRef]
- Stacey, O.J.; Pope, S.J.A. New avenues in the design and potential application of metal complexes for photodynamic therapy. RSC Adv. 2013, 3, 25550–25564. [Google Scholar] [CrossRef]
- Ruggi, A.; van Leeuwen, F.W.B.; Velders, A.H. Interaction of dioxygen with the electronic excited state of Ir(III) and Ru(II) complexes: Principles and biomedical applications. Coord. Chem. Rev. 2011, 255, 2542–2554. [Google Scholar] [CrossRef]
- Zhong, Y.-W. Photofunction-Directed Coordination Molecular Engineering. Chin. J. Chem. 2021, 39, 543–549. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-L.; Tang, K.; Zhong, Y.-W. A Carbazole-Bridged Biscyclometalated Diplatinum Complex: Synthesis, Characterization, and Dual-Mode Aggregation-Enhanced Phosphorescence. Inorg. Chem. 2021, 60, 6607–6615. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhong, Y.-W.; Yao, J. Regulation of intra- and intermolecular Pt–Pt and π–π interactions of a U-shaped diplatinum complex to achieve pseudo-polymorphic emissions in solution and crystalline states. J. Mater. Chem. C 2017, 5, 7222–7229. [Google Scholar] [CrossRef]
- Djurovich, P.I.; Murphy, D.; Thompson, M.E.; Hernandez, B.; Gao, R.; Hunt, P.L.; Selke, M. Cyclometalated iridium and platinum complexes as singlet oxygen photosensitizers: Quantum yields, quenching rates and correlation with electronic structures. Dalton Trans. 2007, 3763–3770. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Adams, H.; Best, J.; Edge, R.; Navaratnam, S.; Weinstein, J.A. Deep-Red Luminescence and Efficient Singlet Oxygen Generation by Cyclometalated Platinum(II) Complexes with 8-Hydroxyquinolines and Quinoline-8-thiol. Inorg. Chem. 2006, 45, 9410–9415. [Google Scholar] [CrossRef]
- Wu, W.; Yang, P.; Ma, L.; Lalevée, J.; Zhao, J. Visible-Light Harvesting PtII Complexes as Singlet Oxygen Photosensitizers for Photooxidation of 1,5-Dihydroxynaphthalene. Eur. J. Inorg. Chem. 2013, 2013, 228–231. [Google Scholar] [CrossRef]
- Potocny, A.M.; Pistner, A.J.; Yap, G.P.A.; Rosenthal, J. Electrochemical, Spectroscopic, and 1O2 Sensitization Characteristics of Synthetically Accessible Linear Tetrapyrrole Complexes of Palladium and Platinum. Inorg. Chem. 2017, 56, 12703–12711. [Google Scholar] [CrossRef] [PubMed]
- Shafikov, M.Z.; Suleymanova, A.F.; Kutta, R.J.; Gorski, A.; Kowalczyk, A.; Gapin’ska, M.; Kowalski, K.; Czerwieniec, R. Ligand design and nuclearity variation towards dual emissive Pt(II) complexes for singlet oxygen generation, dual channel bioimaging, and theranostics. J. Mater. Chem. C 2022, 10, 5636–5647. [Google Scholar] [CrossRef]
- Garai, A.; Villa, M.; Marchini, M.; Patra, S.K.; Pain, T.; Mondal, S.; Ceroni, P.; Kar, S. Synthesis, Structure, Photophysics, and Singlet Oxygen Sensitization by a Platinum(II) Complex of Meso-Tetra-Acenaphthyl Porphyrin. Eur. J. Inorg. Chem. 2021, 2021, 4089–4095. [Google Scholar] [CrossRef]
- Subedi, D.R.; Reid, R.; D’Souza, P.F.; Nesterov, V.N.; D’Souza, F. Singlet Oxygen Generation in Peripherally Modified Platinum and Palladium Porphyrins: Effect of Triplet Excited State Lifetimes and meso-Substituents on 1O2 Quantum Yields. ChemPlusChem 2022, 87, e202200010. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Zhao, J.; Hayvali, M.; Elmali, A.; Karatay, A. Effect of Molecule Conformation Restriction on the Photophysical Properties of N^N Platinum(II) Bis(ethynylnaphthalimide) Complexes Showing Close-Lying 3MLCT and 3LE Excited States. Inorg. Chem. 2019, 58, 1850–1861. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.; Zhao, J.; Wu, M. Synergetic effect of C*N^N/C^N^N coordination and the arylacetylide ligands on the photophysical properties of cyclometalated platinum complexes. J. Mater. Chem. C 2015, 3, 2291–2301. [Google Scholar] [CrossRef]
- Lu, W.; Mi, B.-X.; Chan, M.C.W.; Hui, Z.; Che, C.-M.; Zhu, N.; Lee, S.-T. Light-Emitting Tridentate Cyclometalated Platinum(II) Complexes Containing σ-Alkynyl Auxiliaries: Tuning of Photo- and Electrophosphorescence. J. Am. Chem. Soc. 2004, 126, 4958–4971. [Google Scholar] [CrossRef]
- Schneider, J.; Du, P.; Jarosz, P.; Lazarides, T.; Wang, X.; Brennessel, W.W.; Eisenberg, R. Cyclometalated 6-Phenyl-2,2′-bipyridyl (CNN) Platinum(II) Acetylide Complexes: Structure, Electrochemistry, Photophysics, and Oxidativeand Reductive-Quenching Studies. Inorg. Chem. 2009, 48, 4306–4316. [Google Scholar] [CrossRef]
- Wang, X.; Goeb, S.; Ji, Z.; Castellano, F.N. Excited State Absorption Properties of Pt(II) Terpyridyl Complexes Bearing π-Conjugated Arylacetylides. J. Phys. Chem. B 2010, 114, 14440–14449. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, R.; Shi, H.; Shu, M.; Hu, J.; Li, H.; Zhu, H. Synthesis, tunable photophysics and nonlinear absorption of terpyridyl Pt(II) complexes bearing different acetylide ligands. Dyes Pigm. 2016, 126, 165–172. [Google Scholar] [CrossRef]
- Lai, S.-W.; Chan, M.C.-W.; Cheung, T.-C.; Peng, S.-M.; Che, C.-M. Probing d8-d8 Interactions in Luminescent Mono- and Binuclear Cyclometalated Platinum(II) Complexes of 6-Phenyl-2,2′-bipyridines. Inorg. Chem. 1999, 38, 4046–4055. [Google Scholar] [CrossRef]
- Shi, H.; Clarkson, G.J.; Sadler, P.J. Dual action photosensitive platinum(II) anticancer prodrugs with photoreleasable azide ligands. Inorg. Chim. Acta 2019, 489, 230–235. [Google Scholar] [CrossRef]
- Hobert, S.E.; Carney, J.T.; Cummings, S.D. Synthesis and luminescence properties of platinum(II) complexes of 4′-chloro-2,2′:6′,2′′-terpyridine and 4,4′,4′′-trichloro-2,2′:6′,2′′-terpyridine. Inorg. Chim. Acta 2001, 318, 89–96. [Google Scholar] [CrossRef]
- Martínez-Martínez, V.; Llano, R.S.; Furukawa, S.; Takashima, Y.; Arbeloa, I.L.; Kitagawa, S. Enhanced Phosphorescence Emission by Incorporating Aromatic Halides into an Entangled Coordination Framework Based on Naphthalenediimide. ChemPhysChem 2014, 15, 2517–2521. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Li, Y.; Zhu, H.; Sun, W. Photophysics and Nonlinear Absorption of Cyclometalated 4,6-Diphenyl-2,2′-bipyridyl Platinum(II) Complexes with Different Acetylide Ligands. J. Phys. Chem. A 2010, 114, 12639–12645. [Google Scholar] [CrossRef]
- Schaffner-Hamann, C.; von Zelewsky, A.; Barbieri, A.; Barigelletti, F.; Muller, G.; Riehl, J.P.; Neels, A. Diastereoselective Formation of Chiral Tris-Cyclometalated Iridium (III) Complexes: Characterization and Photophysical Properties. J. Am. Chem. Soc. 2004, 126, 9339–9348. [Google Scholar] [CrossRef]
- Murov, S.L.; Carmichael, I.; Hug, G.L. Handbook of Photochemistry, 3rd ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 424–545. [Google Scholar]
- Shafikov, M.Z.; Suleymanova, A.F.; Kutta, R.J.; Brandl, F.; Gorski, A.; Czerwieniec, R. Dual emissive dinuclear Pt(II) complexes and application to singlet oxygen generation. J. Mater. Chem. C 2021, 9, 5808–5818. [Google Scholar] [CrossRef]
- Abdel-Shafi, A.A.; Beerb, P.D.; Mortimera, R.J.; Wilkinson, F. Photosensitized Generation of Singlet Oxygen from (Substituted Bipyridine)ruthenium(II) Complexes. Helv. Chim. Acta 2001, 84, 2784–2795. [Google Scholar] [CrossRef]
- Abdel-Shafi, A.A.; Bourdelandeb, J.L.; Ali, S.S. Photosensitized generation of singlet oxygen from rhenium(I) and iridium(III) complexes. Dalton Trans. 2007, 2510–2516. [Google Scholar] [CrossRef]
- Kearns, D.R. Physical and chemical properties of singlet molecular oxygen. Chem. Rev. 1971, 71, 395–427. [Google Scholar] [CrossRef]
- Abdel-Shafi, A.A.; Beer, P.D.; Mortimera, R.J.; Wilkinson, F. Photosensitized generation of singlet oxygen from ruthenium(II)-substituted benzoaza-crown-bipyridine complexes. Phys. Chem. Chem. Phys. 2000, 2, 3137–3144. [Google Scholar] [CrossRef]
- Ji, Z.; Li, Y.; Sun, W. 4′-(5′′-R-Pyrimidyl)-2,2′:6′,2′′-terpyridyl (R = H, OEt, Ph, Cl, CN) Platinum(II) Phenylacetylide Complexes: Synthesis and Photophysics. Inorg. Chem. 2008, 47, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, A.; Cunha, C.; Bosque, R.; Pina, J.; Ward, J.S.; Truong, K.-N.; Rissanen, K.; Lima, J.C.; Crespo, M.; de Melo, J.S.S.; et al. Room-Temperature Phosphorescence and Efficient Singlet Oxygen Production by Cyclometalated Pt(II) Complexes with Aromatic Alkynyl Ligands. Inorg. Chem. 2020, 59, 8220–8230. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, J.E.; Chakraborty, A.; Myahkostupov, M.; Wright, K.M.; Castellano, F.N. Long-lived triplet excited state in a platinum(II) perylene monoimide complex. Dalton Trans. 2018, 47, 15071–15081. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Zhao, J.; Zhuang, Y.; Liu, C.; Zhu, Y.; Jia, H.; Wu, K.; Shen, J.; Fu, X.; et al. Molecular Dipole-Induced Photoredox Catalysis for Hydrogen Evolution over Self-Assembled Naphthalimide Nanoribbons. Angew. Chem. Int. Ed. 2022, 61, e2021176. [Google Scholar]
- Hofmann, A.; Dahlenburg, L.; van Eldik, R. Cyclometalated Analogues of Platinum Terpyridine Complexes: Kinetic Study of the Strong σ-Donor Cis and Trans Effects of Carbon in the Presence of a π-Acceptor Ligand Backbone. Inorg. Chem. 2003, 42, 6528–6538. [Google Scholar] [CrossRef]
- Laurila, E.; Oresmaa, L.; Niskanen, M.; Hirva, P.; Haukka, M. Metal-Metal Interactions in Stacked Mononuclear and Dinuclear Rhodium 2,20-Biimidazole Carbonyl Complexes. Cryst. Growth Des. 2010, 10, 3775–3786. [Google Scholar] [CrossRef]
- Mirzaei, S.; Ivanov, M.V.; Timerghazin, Q.K. Improving Performance of the SMD Solvation Model: Bondi Radii Improve Predicted Aqueous Solvation Free Energies of Ions and pKa Values of Thiols. J. Phys. Chem. A 2019, 123, 9498–9504. [Google Scholar] [CrossRef]

| Compound | 1(PF6) | 2 | NI | |||
|---|---|---|---|---|---|---|
| Parameter | DCE | CH3CN | DCE | CH3CN | DCE | |
| λabs (nm)/ε(104 M−1 cm−1) | 465/0.6 389/2.4 348/2.1 286/3.1 | 435/1.3 385/2.6 343/2.1 282/3.4 | 437/3.2 412/2.9 335/2.0 282/4.6 | 440/1.7 405/1.3 330/0.9 279/2.1 | 366/2.0 350/2.2 | |
| λem (nm) | 620 | 618 | 640 | 636 | 400 | |
| τ (μs) air/N2 | 0.6/4.8 | 0.27/3.2 | 0.72/7.0 | 0.24/1.65 | 3 × 10−4/-- | |
| Φ 2 (%) air/N2 | 0.3/1.9 | --/0.4 | 0.3/1.9 | 0.1/0.9 | 8.1/-- | |
| kr 3 (103 s−1) air/N2 | 5.0/4.0 | --/1.2 | 4.2/2.7 | 4.2/5.4 | 2.7 × 105/-- | |
| knr 4 (105 s−1) air/N2 | 16.6/2.0 | --/3.1 | 13.8/1.4 | 41.6/6.0 | 30.6 × 103/-- | |
| kq 5 (108 M−1 s−1) | 9.1 | 18 | 7.8 | 19 | -- | |
| ΦΔ 6 (%) | -- | 89 | -- | 84 | -- | |
| PT,O2 7 (%) | -- | 92 | -- | 85 | -- | |
| fT,Δ 8 (%) | -- | 97 | -- | 99 | -- | |
| Complexes | Electronic Transitions | E (eV) | λ (nm) | f | Major Transitions (Percentage) | Characters |
|---|---|---|---|---|---|---|
| [Me-1]+ | S0→S1 | 2.64 | 470 | 0.2487 | HOMO→LUMO (97%) | LNILtpyCT/MLtpyCT |
| S0→S2 | 3.17 | 390 | 0.0114 | HOMO→LUMO+1 (97%) | LNILtpyCT/MLtpyCT | |
| S0→S3 | 3.20 | 388 | 0.4426 | HOMO→LUMO+2 (57%) HOMO−1→LUMO (40%) | LCNI MLtpyCT/LNILtpyCT | |
| S0→S4 | 3.28 | 378 | 0.1013 | HOMO−1→LUMO (55%) HOMO→LUMO+2 (41%) | MLtpyCT/LNILtpyCT LCNI | |
| Me-2 | S0→S1 | 2.81 | 440 | 0.3582 | HOMO→LUMO (93%) | LCNI/LNILbpyCT/MLbpyCT |
| S0→S2 | 2.92 | 424 | 0.0019 | HOMO−1→LUMO (71%) HOMO−1->LUMO+1 (24%) | ILpbpyCT/LbpyLNICT ILpbpyCT/LbpyLNICT | |
| S0→S3 | 3.03 | 408 | 0.4450 | HOMO→LUMO+1 (84%) | LCNI/LNILbpyCT/MLbpyCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.-L.; Pan, Q.-J.; Ma, D.-X.; Zhong, Y.-W. Naphthalimide-Modified Tridentate Platinum(II) Complexes: Synthesis, Characterization, and Application in Singlet Oxygen Generation. Inorganics 2023, 11, 438. https://doi.org/10.3390/inorganics11110438
Gong Z-L, Pan Q-J, Ma D-X, Zhong Y-W. Naphthalimide-Modified Tridentate Platinum(II) Complexes: Synthesis, Characterization, and Application in Singlet Oxygen Generation. Inorganics. 2023; 11(11):438. https://doi.org/10.3390/inorganics11110438
Chicago/Turabian StyleGong, Zhong-Liang, Qing-Jun Pan, Dian-Xue Ma, and Yu-Wu Zhong. 2023. "Naphthalimide-Modified Tridentate Platinum(II) Complexes: Synthesis, Characterization, and Application in Singlet Oxygen Generation" Inorganics 11, no. 11: 438. https://doi.org/10.3390/inorganics11110438
APA StyleGong, Z.-L., Pan, Q.-J., Ma, D.-X., & Zhong, Y.-W. (2023). Naphthalimide-Modified Tridentate Platinum(II) Complexes: Synthesis, Characterization, and Application in Singlet Oxygen Generation. Inorganics, 11(11), 438. https://doi.org/10.3390/inorganics11110438