Effect of the Annealing Temperature of Lithiophilic Ag–Cu Co-Deposition on the Cycling Performance of Li-Metal Anodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Different CCs
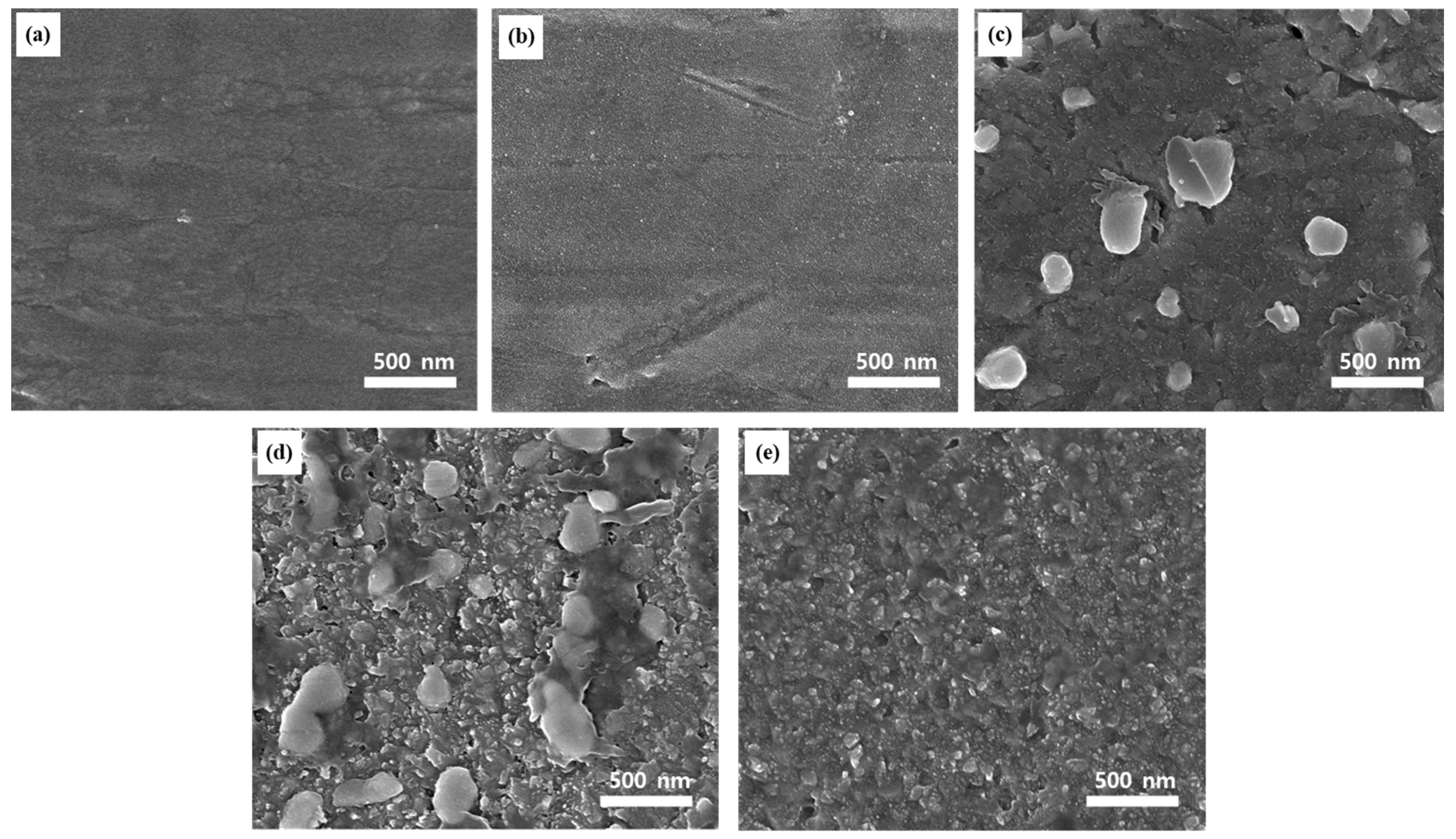
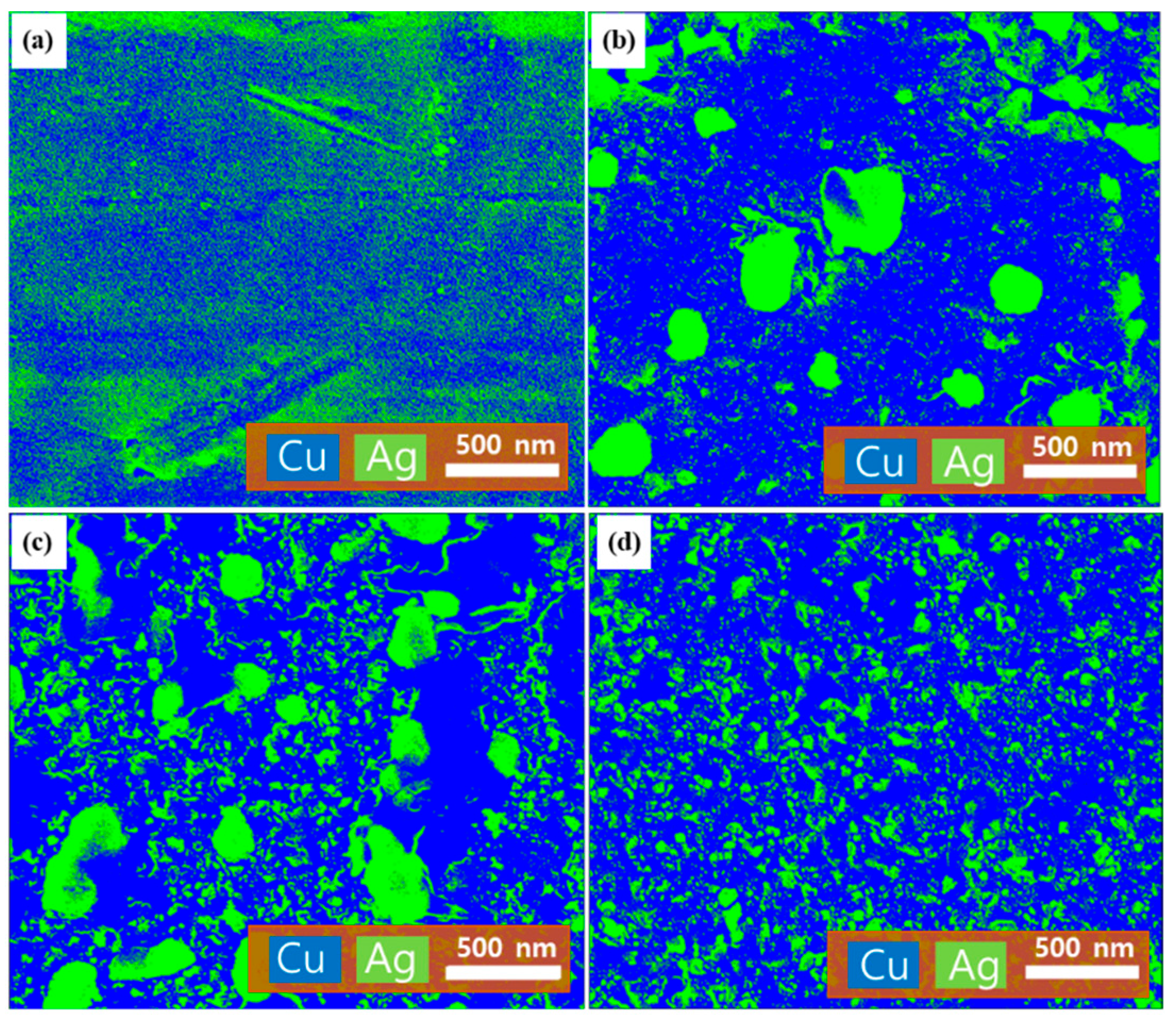
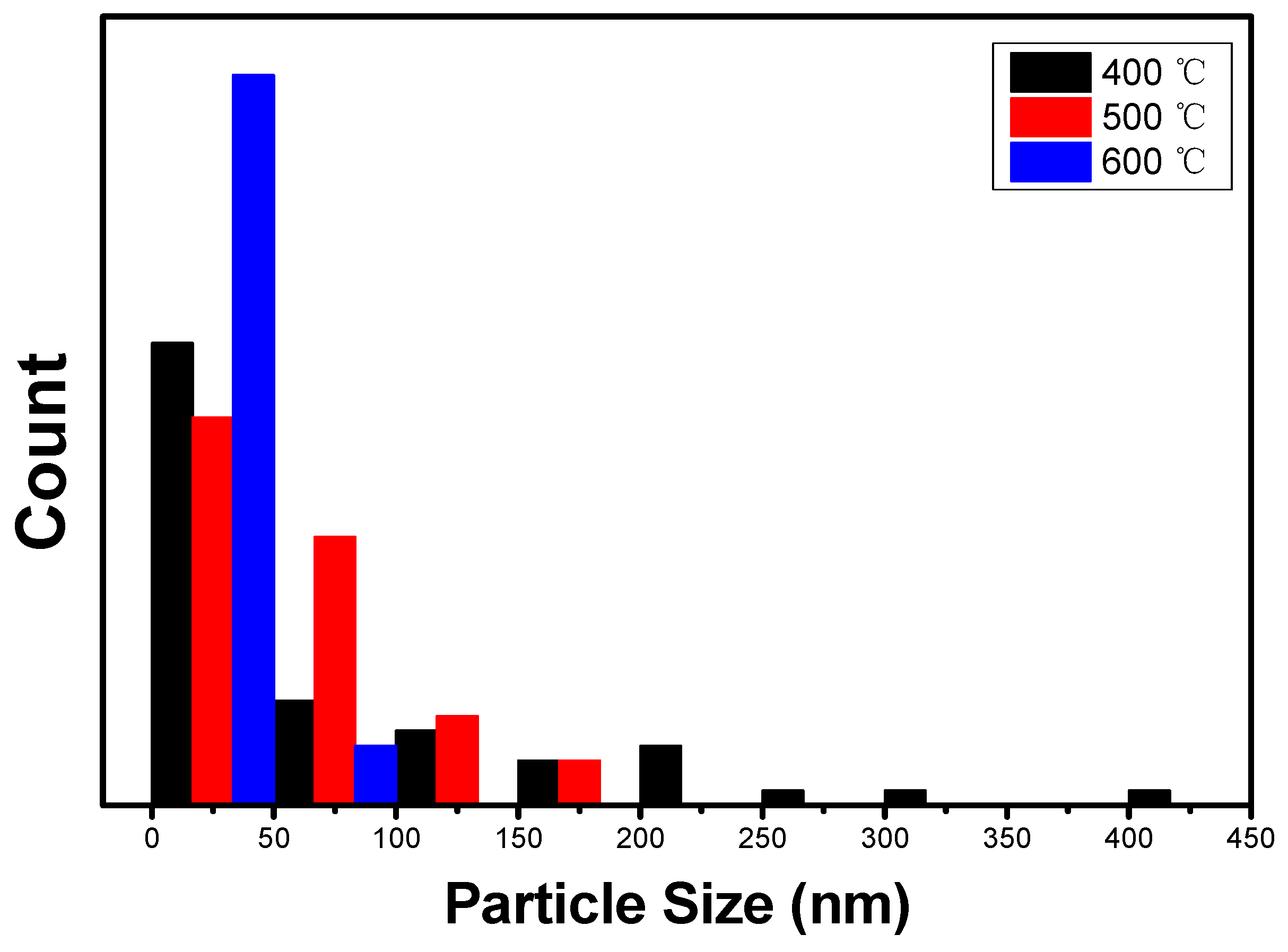
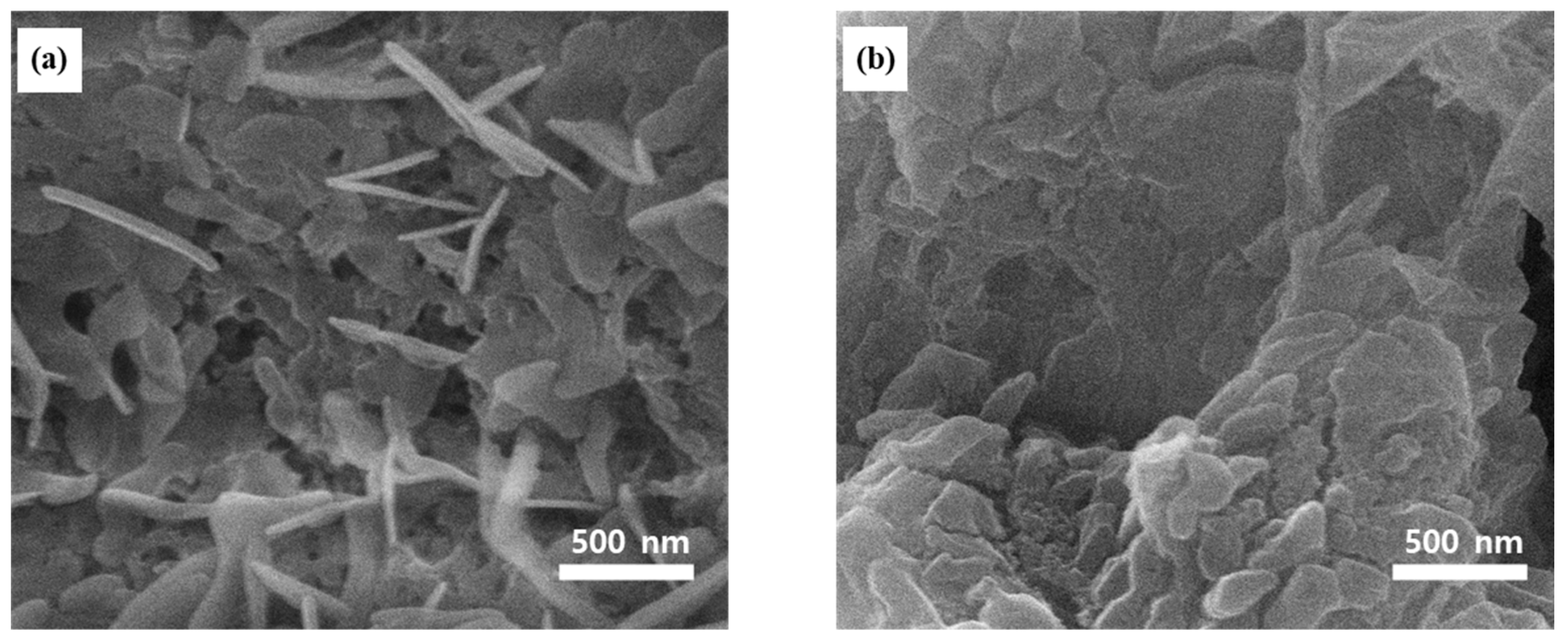
2.2. Surface Morphology and Elemental Composition Analyses of the Ag–Cu CCs under Different Annealing Conditions
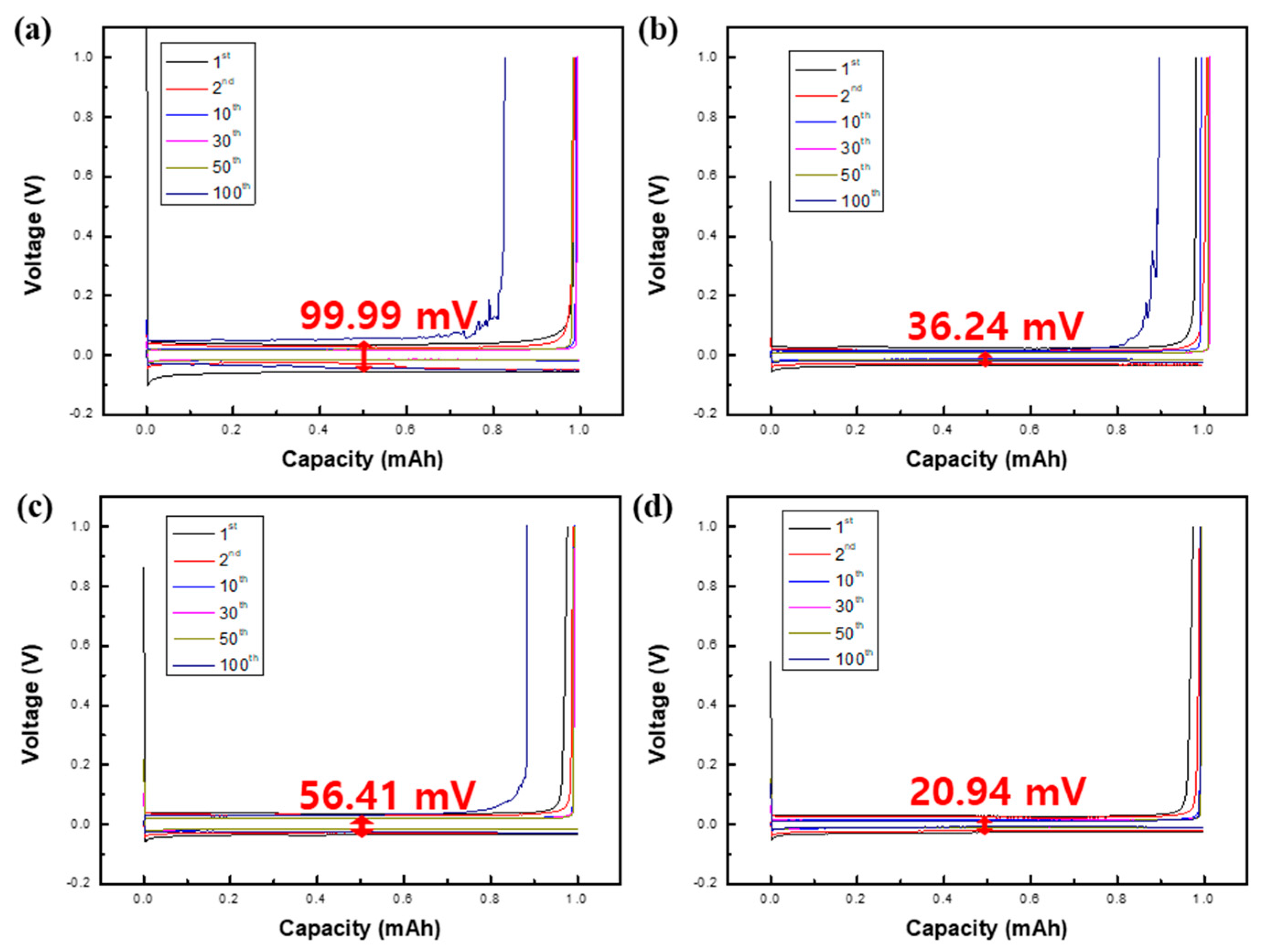
2.3. Evaluation of the Electrochemical Properties of LMAs
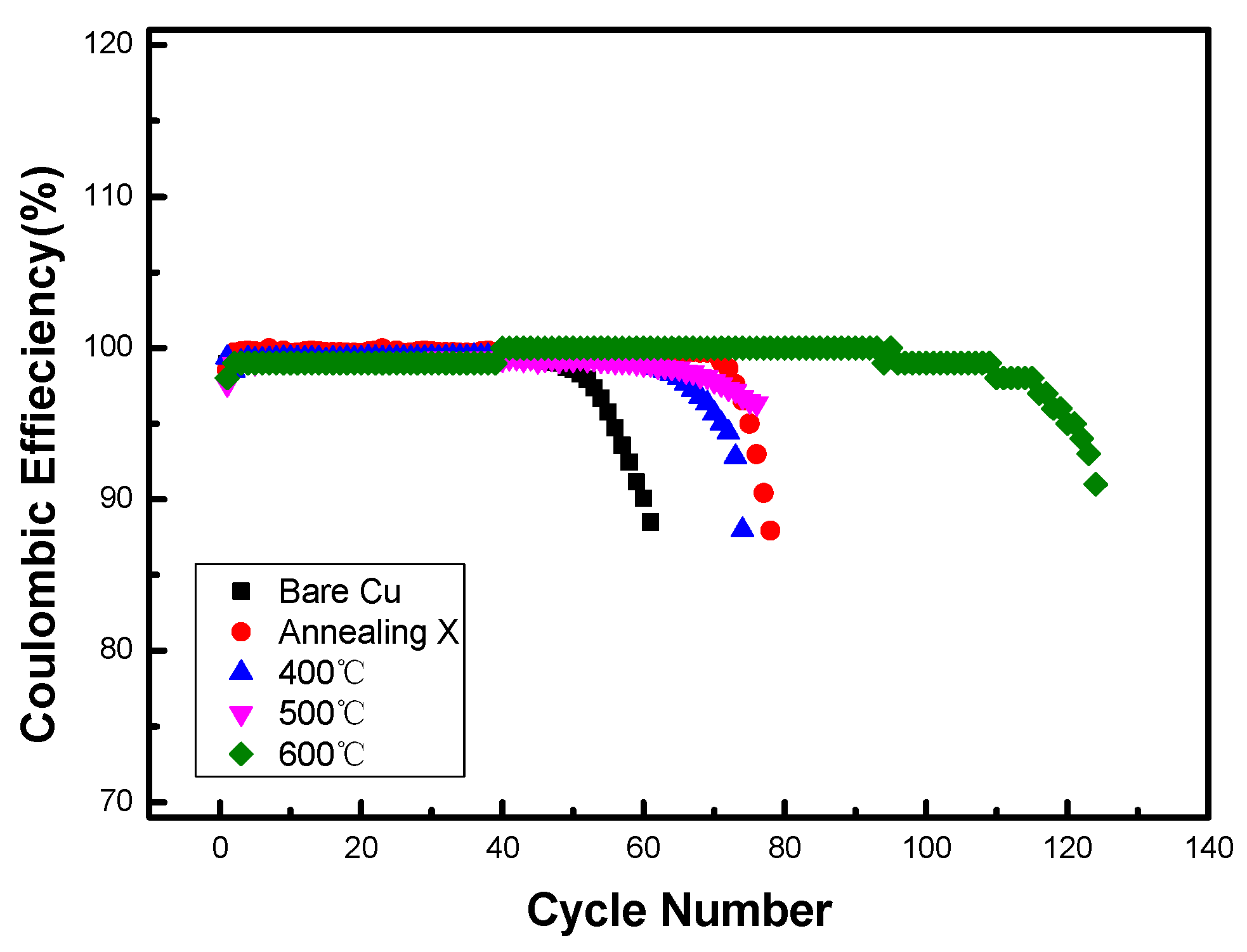
2.4. Evaluation of the Cycling Performance of LMA
3. Materials and Methods
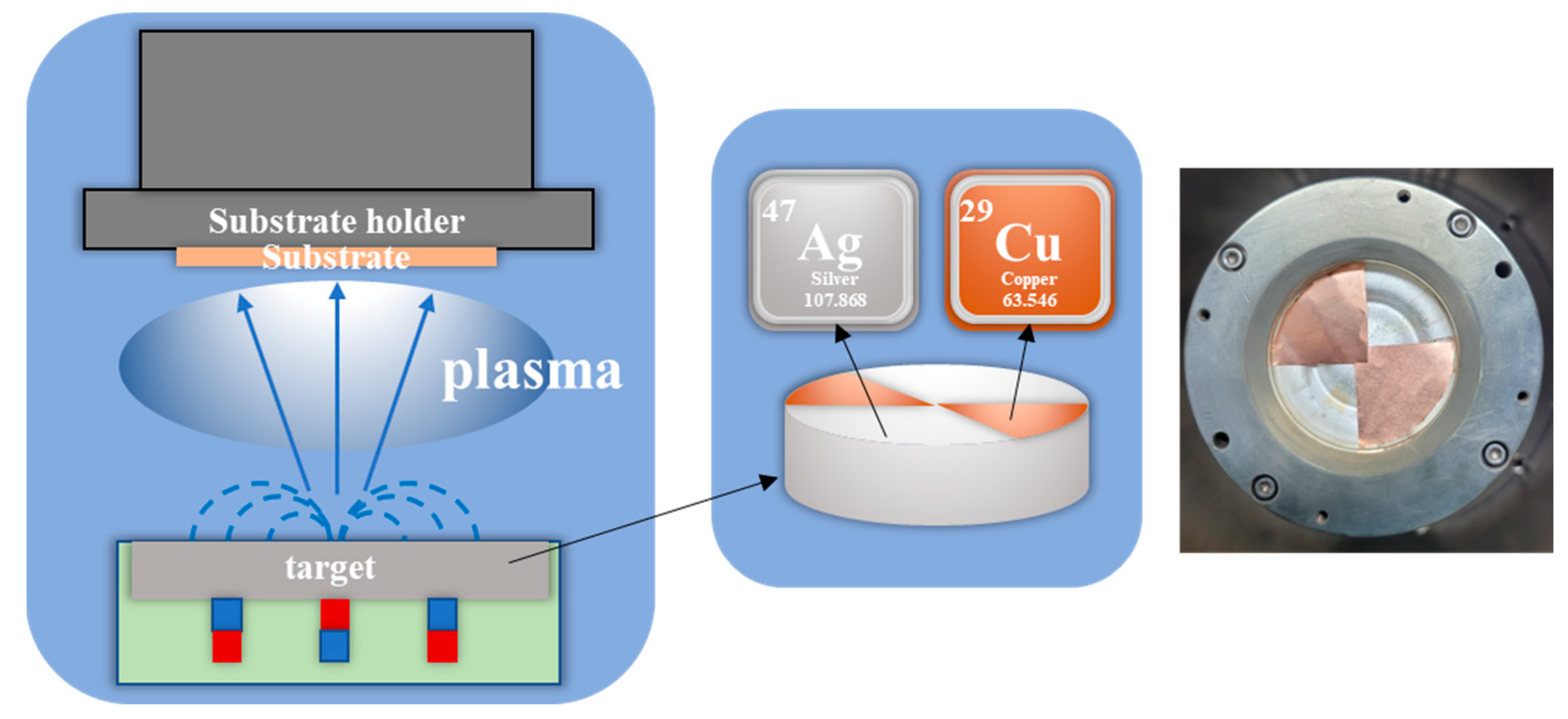
3.1. Fabrication of Ag–Cu CCs
3.2. Characterization of the Ag–Cu CCs
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zubi, G.; Dufo-Lopez, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Jun, M.; Bingbing, C.; Longlong, W.; Guanglei, C. Progress and prospect on effects of potassium substitution failure mechanisms of solid-state lithium batteries. J. Power Source 2018, 392, 94–115. [Google Scholar]
- Yang-kook, S. Promising all-solid-state batteries for future electric vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar]
- Liu, B.; Zhang, J.G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angew. Chem. 2017, 129, 7872–7876. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Fang, C.; Wang, X.; Meng, Y.S. Key issues hindering a practical lithium-metal anode. Trends Chem. 2019, 1, 152–158. [Google Scholar] [CrossRef]
- Li, X.; Yang, G.; Zhang, S.; Wang, Z.; Chen, L. Improved lithium deposition on silver plated carbon fiber paper. Nano Energy 2019, 66, 104144. [Google Scholar] [CrossRef]
- Li, H. Practical evaluation of Li-ion batteries. Joule 2019, 3, 911–914. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, modeling, detection, and prevention of the internal short circuit in lithium-ion batteries: Recent advances and perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.G. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Hagos, T.T.; Huang, C.J.; Teshager, M.A.; Cheng, J.H.; Su, W.N.; Hwang, B.J. Nucleation and growth mechanism of lithium metal electroplating. J. Am. Chem. Soc. 2019, 141, 18612–18623. [Google Scholar] [CrossRef]
- Wang, S.H.; Yue, J.; Dong, W.; Zuo, T.T.; Li, J.Y.; Liu, X.; Zhang, X.D.; Liu, L.; Shi, J.-L.; Yin, Y.-X.; et al. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 2019, 10, 4930. [Google Scholar] [CrossRef]
- Yan, K.; Lee, H.W.; Gao, T.; Zheng, G.; Yao, H.; Wang, H.; Lu, Z.; Zhou, Y.; Liang, Z.; Liu, Z.; et al. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 2014, 14, 6016–6022. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Z.; Lee, H.W.; Xiong, F.; Hsu, P.C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Cho, K.Y.; Hong, S.H.; Kwon, J.; Song, H.; Kim, S.; Jo, S.; Eom, K. Effects of a nanometrically formed lithiophilic silver@copper CC on the electrochemical nucleation and growth behaviors of lithium metal anodes. Appl. Surf. Sci. 2021, 554, 149578. [Google Scholar] [CrossRef]
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Suryanarayana, C. Phase formation under non-equilibrium processing conditions: Rapid solidification processing and mechanical alloying. J. Mater. Sci. 2018, 53, 13364–13379. [Google Scholar] [CrossRef]
- Ohring, M. Materials Science of Thin Films: Deposition and Structure; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- McNaught, A.D. Compendium of Chemical Terminology; Blackwell Science: Oxford, UK, 1997; Volume 1669. [Google Scholar]



| Annealing Temperature | Sheet Resistance (ohm/sq) |
|---|---|
| w/o annealing | 55.51 |
| 25.24 | |
| X | |
| X |
| Annealing Temperature | Nucleation Overpotential (mV) | Plateau Overpotential (mV) |
|---|---|---|
| Bare Cu | 37.31 mV | 14.98 mV |
| Annealing X | 0.62 mV | 13.60 mV |
| 12.70 mV | 9.78 mV | |
| 18.04 mV | 8.41 mV | |
| 15.60 mV | 9.78 mV |
| Annealing Temperature | Nucleation Overpotential (mV) | Plateau Overpotential (mV) |
|---|---|---|
| Bare Cu | 36.84 mV | 65.59 mV |
| Annealing X | 18.81 mV | 41.58 mV |
| 20.49 mV | 41.89 mV | |
| 19.88 mV | 39.90 mV | |
| 19.84 mV | 32.41 mV |
| Resistance [Ω cm2] | Rs | RSEI | Rct |
|---|---|---|---|
| 500 °C | 9.1 | 67.2 | 210.4 |
| 600 °C | 7.9 | 38.8 | 55.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Kang, S.G.; Kim, B.J.; Lee, H.; Kim, J.; Yoon, C.-B. Effect of the Annealing Temperature of Lithiophilic Ag–Cu Co-Deposition on the Cycling Performance of Li-Metal Anodes. Inorganics 2023, 11, 440. https://doi.org/10.3390/inorganics11110440
Kim DH, Kang SG, Kim BJ, Lee H, Kim J, Yoon C-B. Effect of the Annealing Temperature of Lithiophilic Ag–Cu Co-Deposition on the Cycling Performance of Li-Metal Anodes. Inorganics. 2023; 11(11):440. https://doi.org/10.3390/inorganics11110440
Chicago/Turabian StyleKim, Dae Hyun, Seul Gi Kang, Bo Jung Kim, Heegyoun Lee, Jinmo Kim, and Chang-Bun Yoon. 2023. "Effect of the Annealing Temperature of Lithiophilic Ag–Cu Co-Deposition on the Cycling Performance of Li-Metal Anodes" Inorganics 11, no. 11: 440. https://doi.org/10.3390/inorganics11110440
APA StyleKim, D. H., Kang, S. G., Kim, B. J., Lee, H., Kim, J., & Yoon, C.-B. (2023). Effect of the Annealing Temperature of Lithiophilic Ag–Cu Co-Deposition on the Cycling Performance of Li-Metal Anodes. Inorganics, 11(11), 440. https://doi.org/10.3390/inorganics11110440







