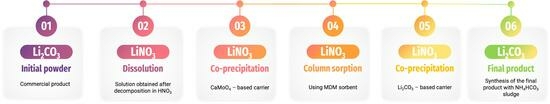
Purification of Lithium Carbonate from Radioactive Contaminants Using a MnO2-Based Inorganic Sorbent
Abstract
:1. Introduction
2. Materials and Methods
3. Lithium Nitrate Purification Via Co-Precipitation
4. Lithium Nitrate Sorption Purification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peiro, L.T.; Méndez, G.V.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Oliviera, G.A.D.; Bustillos, J.O.; Ferreira, J.C.; Bergamaschi, V.S.; Moraes, R.M.D.; Gimenez, M.P.; Miyamoto, F.K.; Seneda, J.A. Applications of lithium in nuclear energy. In Proceedings of the International Nuclear Atlantic Conference—INAC 2017, Belo Horizonte, Brazil, 22–27 October 2017. [Google Scholar]
- Heroy, W.B. Disposal of radioactive waste in salt cavities. In The Disposal of Radioactive Waste on Land; National Research Council (US) Committee on Waste Disposal; National Academies Press: Washington, DC, USA, 1957. [Google Scholar]
- Forsberg, C.W.; Lam, S.; Carpenter, D.M.; Whyte, D.G.; Scarlat, R.; Contesu, C.; Wei, L.; Stempien, J.; Blandford, E. Tritium Control and Capture in Salt-Cooled Fission and Fusion reactors: Status, Challenges, and Path Forward. Nucl. Technol. 2017, 197, 119–139. [Google Scholar] [CrossRef]
- Halfon, S.; Paul, M.; Arenshtam, A.; Berkovits, D.; Bisyakoev, M.; Eliyahu, I.; Feinberg, G.; Hazenshprung, N.; Kijel, D.; Nagler, A.; et al. High-power liquid-lithium target prototype for accelerator-based boron neutron capture therapy. Appl. Radiat. Isot. 2011, 69, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rahaman, S.; Elomaa, V.-V.; Eronen, T.; Hakala, J.; Jokinen, A.; Julin, J.; Kankainen, A.; Saastamoinen, A.; Suhonen, J.; Weber, C.; et al. Q values of the 76Ge and 100Mo double-beta decays. Phys. Lett. B 2008, 662, 111–116. [Google Scholar] [CrossRef]
- Augier, C.; Barabash, A.S.; Bellini, F.; Benato, G.; Beretta, M.; Bergé, L.; Billard, J.; Borovlev, Y.A.; Cardani, L.; Casali, N.; et al. Final results on the 0νββ decay half-life limit of 100Mo from the CUPID-Mo experiment. Eur. Phys. J. C 2022, 82, 1033. [Google Scholar] [CrossRef]
- Kim, B.H.; Ha, D.H.; Jeon, J.A.; Jo, S.H.; Kang, W.G.; Kang, C.S.; Kang, W.G.; Kim, H.S.; Kim, S.C.; Kim, S.G.; et al. Status and Performance of the AMoRE-I Experiment on Neutrinoless Double Beta Decay. J. Low Temp. Phys. 2022, 209, 962–970. [Google Scholar] [CrossRef]
- Yoomin, O. AMoRE. In Proceedings of the NEUTRINO 2022, Virtual, 30 May–4 June 2022. [Google Scholar]
- Alenkov, V.; Aryal, P.; Beyer, J.; Boiko, R.S.; Boonin, K.; Buzanov, O.; Chanthima, N.; Chernyak, M.K.; Chernyak, D.M.; Choi, J.; et al. Technical Design Report for the AMoRE 0νββ Decay Search Experiment. arXiv 2015, arXiv:1512.05957. [Google Scholar]
- Son, J.K.; Choe, J.S.; Gileva, O.; Hahn, I.S.; Kang, W.G.; Kim, D.Y.; Kim, G.W.; Kim, H.J.; Kim, Y.D.; Lee, C.H.; et al. Growth and development of pure Li2MoO4 crystals for rare event experiment at CUP. JINST 2020, 15, C07035. [Google Scholar] [CrossRef]
- Grigorieva, V.; Shlegel, V.; Bekker, T.; Ivannikova, N.; Giuliani, A.; Marcillac, P.D.; Marnieros, S.; Novati, V.; Olivieri, E.; Poda, D.; et al. Li2MoO4 Crystals Grown by Low-Thermal-Gradient Czochralski Technique. J. Mater. Sci. Eng. B 2017, 7, 63–70. [Google Scholar]
- Yeon, H.; Choe, J.S.; Gileva, O.; Hahn, K.I.; Kang, W.G.; Kim, G.W.; Kim, H.J.; Kim, Y.; Kim, Y.; Lee, E.K.; et al. Preparation of low-radioactive high-purity enriched 100MoO3 powder for AMoRE-II experiment. Front. Phys. 2023, 11, 1142136. [Google Scholar] [CrossRef]
- Armengaud, A.; Augier, C.; Barabash, A.S.; Beeman, J.W.; Bekker, T.B.; Bellini, F.; Benoît, A.; Bergé, L.; Bergmann, T.; Billard, J.; et al. Development of 100Mo-containing scintillating bolometers for a high-sensitivity neutrinoless double-beta decay search. Eur. Phys. J. C 2017, 77, 785. [Google Scholar] [CrossRef] [PubMed]
- CUP Center for Underground Physics. Available online: https://centers.ibs.re.kr/html/cup_en/ (accessed on 11 July 2023).
- Novosibirsk Rare Metal Plant. Available online: http://cesium.ru (accessed on 11 July 2023).
- Gileva, O.; Aryal, P.; Karki, S.; Kim, H.J.; Kim, Y.; Milyutin, V.; Park, H.K.; Shin, K.A. Investigation of the molybdenum oxide purification for the AMoRE experiment. J. Radioanal. Nucl. Chem. 2017, 314, 1695–1700. [Google Scholar] [CrossRef]
- Nekrasova, N.A.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A. Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants. Inorganics 2023, 11, 126. [Google Scholar] [CrossRef]
- Seastar Chemicals. Available online: https://www.seastarchemicals.com/our-development/specialized-analysis/ (accessed on 12 July 2023).
- Lee, M.H. Radioassay and Purification for Experiments at Y2L and Yemilab in Korea. J. Phys. Conf. Ser. 2020, 1468, 012249. [Google Scholar] [CrossRef]
- Ryabtsev, A.D.; Kotsupalo, N.P.; Kurakov, A.A.; Menzheres, L.T.; Titarenko, V.I. Theoretical Foundations of Technology for the Production of Lithium Carbonate by the Ammonia Method. Theor. Found. Chem. Eng. 2018, 53, 815–820. [Google Scholar] [CrossRef]
- Mühr-Ebert, E.L.; Wagner, F.; Walther, C. Speciation of uranium: Compilation of a thermodynamic database and its experimental evaluation using different analytical techniques. Appl. Geochem. 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Koyanaka, H.; Matsubaya, O.; Koyanaka, Y.; Hatta, N. Quantitative correlation between Li absorption and H content in manganese oxide spinel λ-MnO2. J. Electroanal. Chem. 2003, 559, 77–81. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ concentration aqueous solutions. Front. Chem. Eng. 2002, 4, 1008680. [Google Scholar] [CrossRef]
- Kozlovskaia, O.N.; Shibetskaia, I.G.; Bezhin, N.A.; Tananaev, I.G. Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea. Materials 2023, 16, 1935. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Prozorovich, V.G.; Kouznetsova, T.F.; Radkevich, A.V.; Krivoshapkin, P.V.; Krivoshapkina, E.F.; Sillanpää, M. Sorption behavior of 85Sr onto manganese oxides with tunnel structure. J. Radioanal. Nucl. Chem. 2018, 316, 673–683. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Kelly, J.C.; Wang, M.; Dai, Q.; Winjobi, Q. Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium-ion battery cathodes and lithium-ion batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Andrade, M.A.; Oliveira, G.C.; Cotrim, M.E.B.; Seneda, J.A.; Bustillos, O.V. Use of the Ion Exchange Technique for Purifiation of Lithium Carbonate for Nuclear Industry. In Proceedings of the 2021 International Nuclear Atlantic Conference—INAC 2021, Rio de Janeiro, Brazil, 29 November–2 December 2021. [Google Scholar]
- Peng, C.; Liu, F.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium(Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]

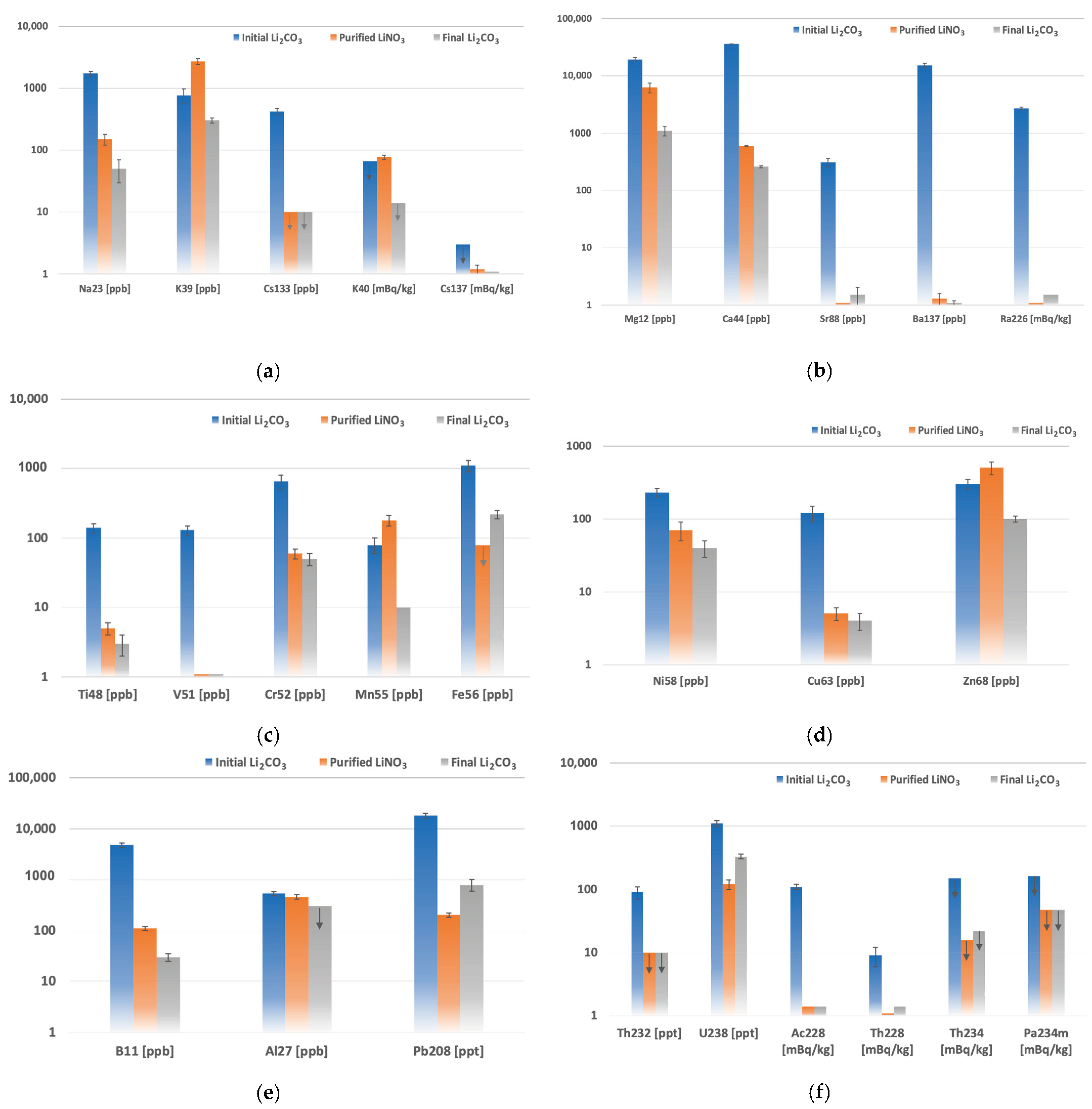
| Element | Concentration [ppb] | Element | Concentration [ppb] | Radionuclides | Activity [mBq/kg] |
|---|---|---|---|---|---|
| Al | 530 ± 50 | Mn | 80 ± 20 | 40K | ≤66 |
| B | 4800 ± 500 | Na | 1720 ± 150 | 137Cs | ≤3 |
| Ba | 15,200 ± 1500 | Ni | 230 ± 30 | 234Th | ≤151 |
| Ca | 36,000 ± 5000 | Pb | 18 ± 2 | 234mPa | ≤162 |
| Cr | 650 ± 150 | Sr | 310 ± 50 | 226Ra | 2730 ± 140 |
| Cs | 420 ± 50 | 232Th | 0.09 ± 0.02 | 228Ac | 110 ± 10 |
| Cu | 120 ± 30 | Ti | 140 ± 20 | 228Th | 9 ± 3 |
| Fe | 1100 ± 200 | 238U | 1.1 ± 0.1 | ||
| K | 770 ± 70 | V | 130 ± 20 | ||
| Mg | 19,500 ± 1500 | Zn | 300 ± 50 |
| K [ppb] | Sr [ppb] | Ba [ppb] | Ca [ppm] | Fe [ppb] | Pb [ppb] | Th [ppt] | U [ppt] | |
|---|---|---|---|---|---|---|---|---|
| Initial material | 770 ± 70 | 310 ± 50 | 15,200 ± 1500 | 36 ± 5 | 1100 ± 200 | 18 ± 2 | 90 ± 20 | 1100 ± 100 |
| Co-prec. with Li2CO3 | 430 ± 50 | 160 ± 30 | 1200 ± 150 | 30 ± 5 | 200 ± 50 | 10 ± 1 | ≤6 | 200 ± 30 |
| Co-prec. with CaCO3 | 270 ± 30 | 9350 ± 500 | 1400 ± 150 | 50 ± 5 | 200 ± 50 | 0.7 ± 0.2 | ≤6 | 150 ± 20 |
| Co-prec. with CaMoO4 | 300 ± 30 | 1100 ± 100 | 900 ± 100 | 45 ± 5 | <100 | <0.1 | ≤6 | ≤6 |
| Separation Factor | K | Sr | Ca | Ba | Pb | Th | U |
|---|---|---|---|---|---|---|---|
| 1 mol L−1 LiNO3 | 0.1 | 210 | 60 | 2100 | >50 | ≥1 | ≥1 |
| 4 mol L−1 LiNO3 | 0.1 | 200 | 70 | 1900 | >50 | ≥1 | ≥1 |
| 7 mol L−1 LiNO3 | 0.3 | 4 | 10 | 60 | >40 | ≥1 | ≥1 |
| Element | Concentration [ppb] | Element | Concentration [ppb] | ||
|---|---|---|---|---|---|
| Purified LiNO3 | Final Li2CO3 | Purified LiNO3 | Final Li2CO3 | ||
| Al | 460 ± 50 | ≤300 | Mn | 180 ± 30 | ≤10 |
| B | 110 ± 10 | 30 ± 5 | Na | 150 ± 30 | 50 ± 20 |
| Ba | 1.3 ± 0.3 | 1.0 ± 0.1 | Ni | 70 ± 20 | 40 ± 10 |
| Ca | 600 ± 120 | 260 ± 30 | Pb | 0.20 ± 0.02 | 0.8 ± 0.2 |
| Cr | 60 ± 10 | 50 ± 10 | Sr | ≤0.2 | 1.5 ± 0.5 |
| Cs | ≤10 | ≤10 | 232Th | ≤0.01 | ≤0.01 |
| Cu | 5 ± 1 | 4 ± 1 | Ti | 5 ± 1 | 3 ± 1 |
| Fe | ≤80 | 220 ± 30 | 238U | 0.12 ± 0.02 | 0.33 ± 0.03 |
| K | 2700 ± 300 | 300 ± 30 | V | ≤1 | ≤1 |
| Mg | 6.3 ± 1.2 | 1.0 ± 0.2 | Zn | 500 ± 100 | 100 ± 10 |
| Activity [mBq/kg] | ||
|---|---|---|
| Purified LiNO3 | Final Li2CO3 | |
| 40K | 77 ± 6 | ≤14 |
| 137Cs | 1.2 ± 0.2 | ≤1.0 |
| 234Th | ≤16 | ≤22 |
| 234mPa | ≤47 | ≤47 |
| 226Ra | ≤1.0 | ≤1.5 |
| 228Ac | ≤1.4 | ≤1.4 |
| 228Th | ≤1.0 | ≤1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gileva, O.; Aryal, P.; Choe, J.; Kim, Y.; Kim, Y.; Lee, E.; Lee, M.H.; Milyutin, V.; Shin, K.; Yeon, H. Purification of Lithium Carbonate from Radioactive Contaminants Using a MnO2-Based Inorganic Sorbent. Inorganics 2023, 11, 410. https://doi.org/10.3390/inorganics11100410
Gileva O, Aryal P, Choe J, Kim Y, Kim Y, Lee E, Lee MH, Milyutin V, Shin K, Yeon H. Purification of Lithium Carbonate from Radioactive Contaminants Using a MnO2-Based Inorganic Sorbent. Inorganics. 2023; 11(10):410. https://doi.org/10.3390/inorganics11100410
Chicago/Turabian StyleGileva, Olga, Pabitra Aryal, JunSeok Choe, Yena Kim, Yeongduk Kim, Eunkyung Lee, Moo Hyun Lee, Vitaly Milyutin, KeonAh Shin, and Hyojin Yeon. 2023. "Purification of Lithium Carbonate from Radioactive Contaminants Using a MnO2-Based Inorganic Sorbent" Inorganics 11, no. 10: 410. https://doi.org/10.3390/inorganics11100410
APA StyleGileva, O., Aryal, P., Choe, J., Kim, Y., Kim, Y., Lee, E., Lee, M. H., Milyutin, V., Shin, K., & Yeon, H. (2023). Purification of Lithium Carbonate from Radioactive Contaminants Using a MnO2-Based Inorganic Sorbent. Inorganics, 11(10), 410. https://doi.org/10.3390/inorganics11100410