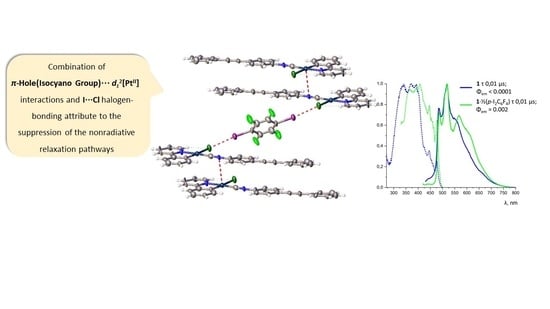
Solid State Phosphorescence Enhancement of PtII-Based Emitters via Combination of π-Hole(Isocyano Group)⋅⋅⋅ dz2[PtII] and I···Cl Halogen-Bonding Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Instrumentation, and Methods
2.2. X-Ray Diffraction Study
2.3. Computational Details
3. Results
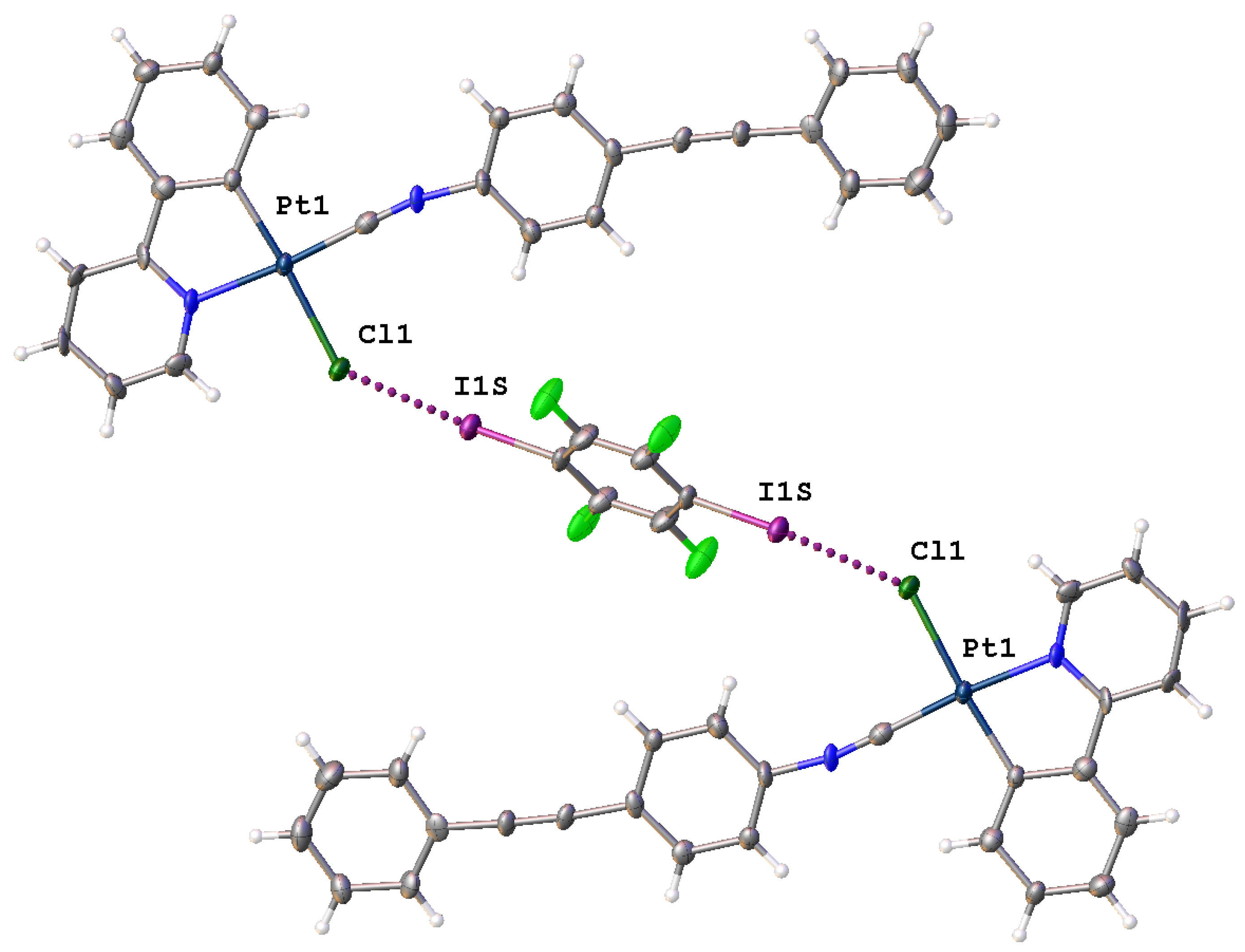
3.1. General Description of the X-Ray Structures
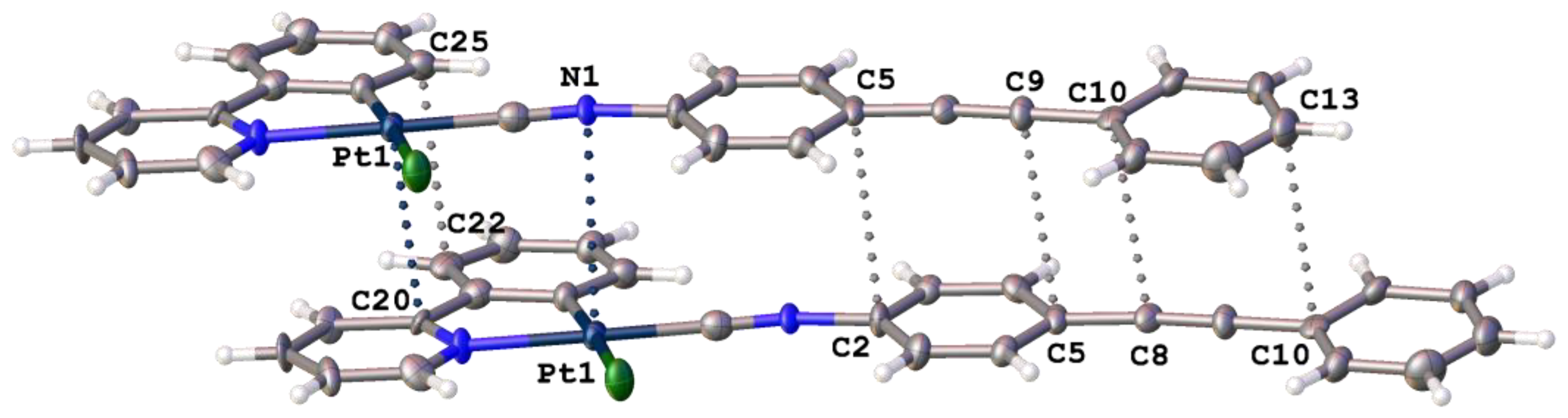
| Contact | Distance, Å | Angle, ° | Ra/b/c |
|---|---|---|---|
| I···Cl | 3.204(3) | ∠(C–I···Cl) 174.6(4) | 0.86/0.82/0.83 |
| NCN⋯Pt | 3.536(10) | ∠(C–N···Pt) 86.7(4)° | 1.08/0.97/0.90 |
| C25···C22 | 3.438(19) | ∠(C21–C22···C25) 87.7(9)° | 1.01/1.01/0.97 |
| Pt1···C20 | 3.612(13) | ∠(C21–C20···Pt) 86.7(4)° | 1.06/0.96/0.89 |
| C5···C2 | 3.462(19) | ∠(N1–C2···C5) 83.7(7)° | 1.02/1.02/0.98 |
| C9···C5 | 3.504 (19) | ∠(C8–C9···C5) 96.7(9)° | 1.03/1.03/0.99 |
| C10···C8 | 3.487(19) | ∠(C9–C8···C10) 96.9(9)° | 1.03/1.03/0.99 |
| C13···C10 | 3.520(2) | ∠(C9–C10···C13) 86.4(8)° | 1.04/1.04/0.99 |
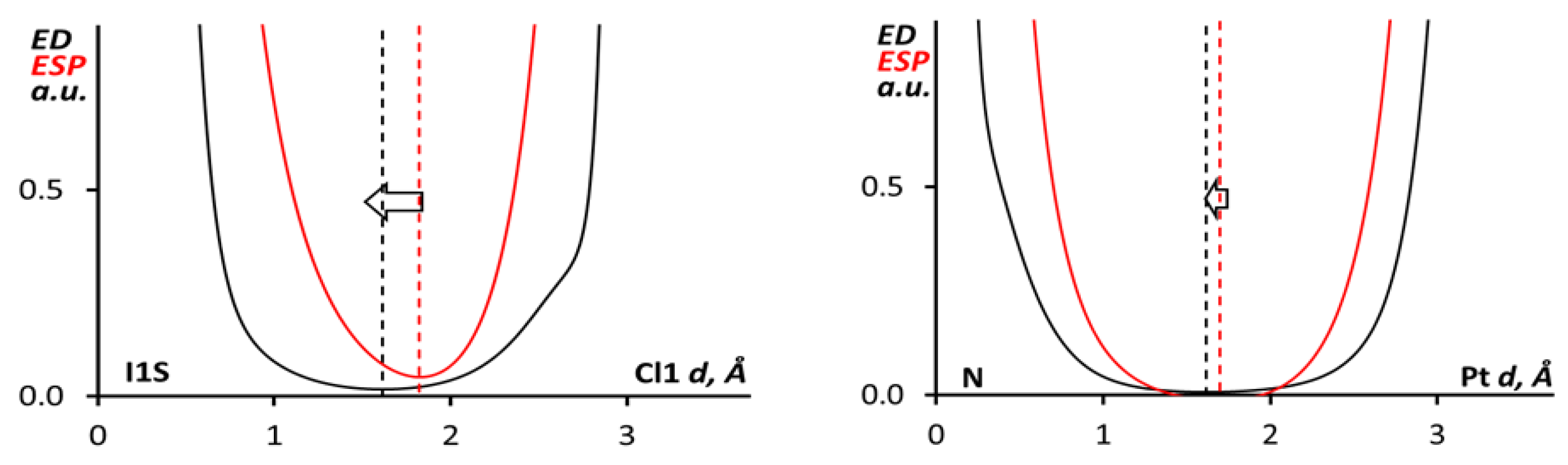
3.2. Theoretical Study

| Supramolecular Associate | Contact | Sign(λ2)ρ(r) | ∇2ρ(r) | G(r) | V(r) | H(r) | WBI | Einta/b/c/d | ΔE, kcal/mol |
|---|---|---|---|---|---|---|---|---|---|
| A | I1S···Cl1 | −0.017 | 0.0481 | 0.011 | −0.010 | 0.001 | 0.07 | 4.3/4.6/3.1/3.0 | −8.0 |
| I1S···H7 | −0.005 | 0.0123 | 0.002 | −0.002 | 0.001 | 0.002 | −/−/0.6/0.7 | ||
| B | Pt1···N1 | −0.007 | 0.0229 | 0.005 | −0.004 | 0.001 | 0.002 | − | −23.5 |
| C25···C22 | −0.005 | 0.0144 | 0.003 | −0.002 | 0.001 | 0.002 | − | ||
| Pt1···C20 | −0.007 | 0.0193 | 0.004 | −0.003 | 0.001 | 0.002 | − | ||
| C5···C2 | −0.004 | 0.0149 | 0.003 | −0.002 | 0.001 | 0.001 | − | ||
| C9···C5 | −0.004 | 0.0144 | 0.003 | −0.002 | 0.001 | 0.001 | − | ||
| C10···C8 | −0.004 | 0.0146 | 0.003 | −0.002 | 0.001 | 0.001 | − | ||
| C13···C10 | −0.004 | 0.0137 | 0.003 | −0.002 | 0.001 | 0.001 | − |

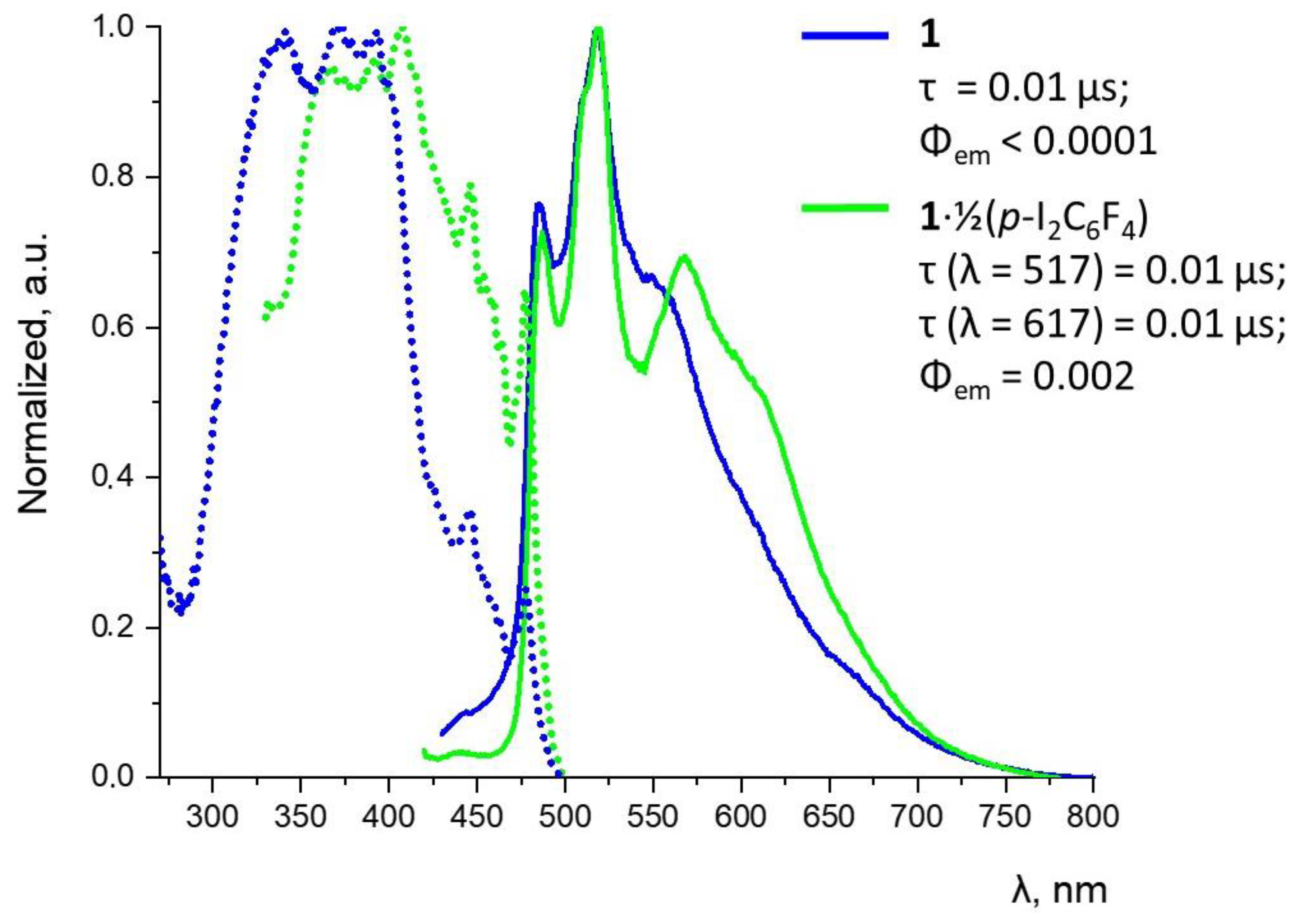
3.3. Photophysical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, M.C.; Chan, A.K.W.; Chan, M.Y.; Yam, V.W.W. Platinum and Gold Complexes for OLEDs. Top. Curr. Chem. 2016, 374, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.L.; Guo, S.; Deng, Y.J.; Liu, S.J.; Zhao, Q. Electroluminochromic Materials and Devices Based on Metal Complexes. Chem. Asian J. 2019, 14, 3791–3802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-C.; Xiao, H.; Zhang, X.; Xu, L.-J.; Chen, Z.-N. Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Chem. Soc. Rev. 2019, 378, 121–133. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent Advances on Metal-Based Near-Infrared and Infrared Emitting OLEDs. Molecules 2019, 24, 1412. [Google Scholar] [CrossRef] [PubMed]
- Mahoro, G.U.; Fernandez-Cestau, J.; Renaud, J.-L.; Coto, P.B.; Costa, R.D.; Gaillard, S. Recent Advances in Solid-State Lighting Devices Using Transition Metal Complexes Exhibiting Thermally Activated Delayed Fluorescent Emission Mechanism. Adv. Opt. Mater. 2020, 8, 2000260. [Google Scholar] [CrossRef]
- Lee, S.; Han, W.-S. Cyclometalated Ir(III) complexes towards blue-emissive dopant for organic light-emitting diodes: Fundamentals of photophysics and designing strategies. Inorg. Chem. Front. 2020, 7, 2396. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. Diversity of Luminescent Metal Complexes in OLEDs: Beyond Traditional Precious Metals. Chem. Asian J. 2021, 16, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Abd Elhameid, M.K.; Ryad, N.; My, A.-S.; Mohammed, M.R.; Ismail, M.M.; El Meligie, S. Design, Synthesis and Screening of 4,6-Diaryl Pyridine and Pyrimidine Derivatives as Potential Cytotoxic Molecules. Chem. Pharm. Bull. 2018, 66, 939–952. [Google Scholar] [CrossRef]
- Ma, D.-L.; Wong, S.-Y.; Kang, T.-S.; Ng, H.-P.; Han, Q.-B.; Leung, C.-H. Iridium(III)-based chemosensors for the detection of metal ions. Methods 2019, 168, 3–17. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Arias-Rotondo, D.M.; McCusker, J.K. The photophysics of photoredox catalysis: A roadmap for catalyst design. Chem. Soc. Rev. 2016, 45, 5803–5820. [Google Scholar] [CrossRef] [PubMed]
- Glaser, F.; Wenger, O.S. Recent progress in the development of transition-metal based photoredox catalysts. Coord. Chem. Rev. 2020, 405, 213129. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2021, 122, 1485–1542. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Chou, P.T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(ii) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef] [PubMed]
- To, W.P.; Wan, Q.Y.; Tong, G.S.M.; Che, C.M. Recent Advances in Metal Triplet Emitters with d(6), d(8), and d(10) Electronic Configurations. Trends Chem. 2020, 2, 796–812. [Google Scholar] [CrossRef]
- Yam, V.W.W.; Law, A.S.Y. Luminescent d(8) metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Wang, J.; Yang, C. Diverse emission properties of transition metal complexes beyond exclusive single phosphorescence and their wide applications. Coord. Chem. Rev. 2021, 433, 213755. [Google Scholar] [CrossRef]
- Sutton, G.D.; Olumba, M.E.; Nguyen, Y.H.; Teets, T.S. The diverse functions of isocyanides in phosphorescent metal complexes. Dalton Trans. 2021, 50, 17851. [Google Scholar] [CrossRef]
- Jain, V.K. Cyclometalated group-16 compounds of palladium and platinum: Challenges and opportunities. Coord. Chem. Rev. 2021, 427, 213546. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Lu, B.; Liu, S.; Yan, D. Recent advances in photofunctional polymorphs of molecular materials. Chin. Chem. Lett. 2019, 30, 1908–1922. [Google Scholar] [CrossRef]
- Ohno, K.; Hasebe, M.; Nagasawa, A.; Fujihara, T. Change in Luminescence Induced by Solution-Mediated Phase-Transition of Cyclometalated Platinum(II) Complex with Isoquinoline Carboxylate. Inorg. Chem. 2017, 56, 12158–12168. [Google Scholar] [CrossRef]
- Norton, A.E.; Abdolmaleki, M.K.; Liang, J.M.; Sharma, M.; Golsby, R.; Zoller, A.; Krause, J.A.; Connick, W.B.; Chatterjee, S. Phase transformation induced mechanochromism in a platinum salt: A tale of two polymorphs. Chem. Comm. 2020, 56, 10175–10178. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, Z.; Wang, C.; Zhong, R.; Wang, F. Recent progress on supramolecular assembly of organoplatinum(II) complexes into long-range ordered nanostructures. Coord. Chem. Rev. 2020, 414, 213300. [Google Scholar] [CrossRef]
- Kimura, M.; Yoshida, M.; Fujii, S.; Miura, A.; Ueno, K.; Shigeta, Y.; Kobayashi, A.; Kato, M. Liquid–liquid interface-promoted formation of a porous molecular crystal based on a luminescent platinum(ii) complex. Chem. Commun. 2020, 56, 12989–12992. [Google Scholar] [CrossRef]
- Katkova, S.A.; Mikherdov, A.S.; Sokolova, E.V.; Novikov, A.S.; Starova, G.L.; Kinzhalov, M.A. Intermolecular (Isocyano group)···PtII interactions involving coordinated isocyanides in cyclometalated PtII complexes. J. Mol. Struct. 2022, 1253, 132230. [Google Scholar] [CrossRef]
- Aliprandi, A.; Genovese, D.; Mauro, M.; Cola, L.D. Recent Advances in Phosphorescent Pt(II) Complexes Featuring Metallophilic Interactions: Properties and Applications. Chem. Lett. 2015, 44, 1152–1169. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef]
- Gray, H.B.; Záliš, S.; Vlček, A. Electronic structures and photophysics of d8-d8 complexes. Coord. Chem. Rev. 2017, 345, 297–317. [Google Scholar] [CrossRef]
- Ravotto, L.; Ceroni, P. Aggregation induced phosphorescence of metal complexes: From principles to applications. Coord. Chem. Rev. 2017, 346, 62–76. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, M. Regulation of metal–metal interactions and chromic phenomena of multi-decker platinum complexes having π-systems. Coord. Chem. Rev. 2018, 355, 101–115. [Google Scholar] [CrossRef]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.-T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.-T.; Koshevoy, I.O. Halogen Bonding to Amplify Luminescence: A Case Study Using a Platinum Cyclometalated Complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Sivchik, V.; Sarker, R.K.; Liu, Z.Y.; Chung, K.Y.; Grachova, E.V.; Karttunen, A.J.; Chou, P.T.; Koshevoy, I.O. Improvement of the Photophysical Performance of Platinum-Cyclometalated Complexes in Halogen-Bonded Adducts. Chem. Eur. J. 2018, 24, 11475–11484. [Google Scholar] [CrossRef] [PubMed]
- Katkova, S.A.; Luzyanin, K.V.; Novikov, A.S.; Kinzhalov, M.A. Modulation of luminescence properties for [cyclometalated]-PtII(isocyanide) complexes upon co-crystallisation with halosubstituted perfluorinated arenes. New J. Chem. 2021, 45, 2948–2952. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-covalent intramolecular interactions through ligand-design promoting efficient photoluminescence from transition metal complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.J. Halogen bonding in room-temperature phosphorescent materials. Coord. Chem. Rev. 2020, 404, 213107. [Google Scholar] [CrossRef]
- Yan, D.; Evans, D.G. Molecular crystalline materials with tunable luminescent properties: From polymorphs to multi-component solids. Mater. Horiz. 2014, 1, 46–57. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Ananyev, I.V.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. π-Hole·dz2[PtII] Interactions with Electron-Deficient Arenes Enhance the Phosphorescence of PtII-Based Luminophores. Inorg. Chem. 2020, 59, 9308–9314. [Google Scholar] [CrossRef]
- Samandar Sangari, M.; Golbon Haghighi, M.; Nabavizadeh, S.M.; Pfitzner, A.; Rashidi, M. Influence of ancillary ligands on the photophysical properties of cyclometalated organoplatinum(ii) complexes. New J. Chem. 2018, 42, 8661–8671. [Google Scholar] [CrossRef]
- Martínez-Junquera, M.; Lara, R.; Lalinde, E.; Moreno, M.T. Isomerism, aggregation-induced emission and mechanochromism of isocyanide cycloplatinated(ii) complexes. J. Mater. Chem. C 2020, 8, 7221–7233. [Google Scholar] [CrossRef]
- Martinez-Junquera, M.; Lalinde, E.; Moreno, M.T.; Alfaro-Arnedo, E.; Lopez, I.P.; Larrayoz, I.M.; Pichel, J.G. Luminescent cyclometalated platinum(ii) complexes with acyclic diaminocarbene ligands: Structural, photophysical and biological properties. Dalton Trans. 2021, 50, 4539–4554. [Google Scholar] [CrossRef]
- Horiuchi, S.; Umakoshi, K. Recent advances in pyrazolato-bridged homo- and heterometallic polynuclear platinum and palladium complexes. Coord. Chem. Rev. 2023, 476, 214924. [Google Scholar] [CrossRef]
- Amouri, H. Luminescent Complexes of Platinum, Iridium, and Coinage Metals Containing N-Heterocyclic Carbene Ligands: Design, Structural Diversity, and Photophysical Properties. Chem. Rev. 2023, 123, 230–270. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Grachova, E.V.; Luzyanin, K.V. Tuning the Luminescence of Transition Metal Complexes with Acyclic Diaminocarbene Ligands. Inorg. Chem. Front. 2022, 9, 417. [Google Scholar] [CrossRef]
- Paziresh, S.; Babadi Aghakhanpour, R.; Fuertes, S.; Sicilia, V.; Niroomand Hosseini, F.; Nabavizadeh, S.M. A double rollover cycloplatinated(II) skeleton: A versatile platform for tuning emission by chelating and non-chelating ancillary ligand systems. Dalton Trans. 2019, 48, 5713. [Google Scholar] [CrossRef]
- Fornies, J.; Sicilia, V.; Borja, P.; Casas, J.M.; Diez, A.; Lalinde, E.; Larraz, C.; Martin, A.; Moreno, M.T. Luminescent Benzoquinolate-Isocyanide Platinum(II) Complexes: Effect of Pt···Pt and π···π Interactions on their Photophysical Properties. Chem. Asian J. 2012, 7, 2813–2823. [Google Scholar] [CrossRef]
- Sicilia, V.; Arnal, L.; Escudero, D.; Fuertes, S.; Martin, A. Chameleonic Photo- and Mechanoluminescence in Pyrazolate-Bridged NHC Cyclometalated Platinum Complexes. Inorg. Chem. 2021, 60, 12274–12284. [Google Scholar] [CrossRef]
- Dobrynin, M.V.; Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Starova, G.L.; Kukushkin, V.Y.; Islamova, R.M. Cyclometalated Platinum(II) Complexes Simultaneously Catalyze the Cross-Linking of Polysiloxanes and Function as Luminophores. ACS Appl. Polym. Mater. 2021, 3, 857. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Cheranyova, A.M.; Ivanov, D.M.; Kukushkin, V.Y.; Bokach, N.A. Polymorph-Dependent Phosphorescence of Cyclometalated Platinum(II) Complexes and Its Relation to Non-covalent Interactions. ACS Omega 2022, 7, 34454–34462. [Google Scholar] [CrossRef] [PubMed]
- Katkova, S.A.; Kozina, D.O.; Kisel, K.S.; Sandzhieva, M.A.; Tarvanen, D.A.; Makarov, S.V.; Porsev, V.V.; Tunik, S.P.; Kinzhalov, M.A. Cyclometalated platinum(II) complexes with acyclic diaminocarbene ligands for OLED application. Dalton Trans. 2023, 52, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Eremina, A.A.; Kinzhalov, M.A.; Katlenok, E.A.; Smirnov, A.S.; Andrusenko, E.V.; Pidko, E.A.; Suslonov, V.V.; Luzyanin, K.V. Phosphorescent Iridium(III) Complexes with Acyclic Diaminocarbene Ligands as Chemosensors for Mercury. Inorg. Chem. 2020, 59, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Avdontceva, M.S.; Kukushkin, V.Y. Ligation-Enhanced π-Hole·π Interactions Involving Isocyanides: Effect of π-Hole·π Noncovalent Bonding on Conformational Stabilization of Acyclic Diaminocarbene Ligands. Inorg. Chem. 2018, 57, 6722–6733. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef] [PubMed]
- Katkova, S.A.; Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Zolotarev, A.A.; Boyarskiy, V.P.; Kukushkin, V.Y. (Isocyano Group π-Hole) dz2-M-II Interactions of (Isocyanide) M-II Complexes, in which Positively Charged Metal Centers (d8-M=Pt, Pd) Act as Nucleophiles. Chem. Eur. J. 2019, 25, 8590–8598. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Ivanov, D.M.; Melekhova, A.A.; Bokach, N.A.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. Chameleonic metal-bound isocyanides: A π-donating CuI-center imparts nucleophilicity to the isocyanide carbon toward halogen bonding. Inorg. Chem. Front. 2022, 9, 1655–1665. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Katkova, S.A.; Doronina, E.P.; Novikov, A.S.; Eliseev, I.I.; Ilichev, V.A.; Kukinov, A.A.; Starova, G.L.; Bokach, N.A. Red photo- and electroluminescent half-lantern cyclometalated dinuclear platinum(II) complex. Z. Kristallogr. Cryst. Mater. 2018, 233, 795–802. [Google Scholar] [CrossRef]
- Katkova, S.A.; Leshchev, A.A.; Mikherdov, A.S.; Kinzhalov, M.A. Synthesis of Cyclometalated Platinum(II) Complex with an Alkynyl-Substituted Isocyanide Ligand, Its Structure and Photophysical Properties. Russ. J. Gen. Chem. 2020, 90, 648–654. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SADABS-2008/1-Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Parr, R.G.; Weitao, Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1995; p. 343. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kashina, M.V.; Ivanov, D.M.; Kinzhalov, M.A. The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals 2021, 11, 799. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Nguyen-Dang, T.T. Quantum Theory of Atoms in Molecules–Dalton Revisited. In Advances in Quantum Chemistry; Löwdin, P.-O., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 14, pp. 63–124. [Google Scholar]
- Bader, R.F.W.; Bader, R.F. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Matveychuk, Y.V.; Troitskaya, E.A.; Tsirelson, V.G. Characterizing the multiple non-covalent interactions in N, S-heterocycles–diiodine complexes with focus on halogen bonding. Comput. Theor. Chem. 2014, 1037, 53–62. [Google Scholar] [CrossRef]
- Lamberts, K.; Handels, P.; Englert, U.; Aubert, E.; Espinosa, E. Stabilization of polyiodide chains via anion⋯anion interactions: Experiment and theory. CrystEngComm 2016, 18, 3832–3841. [Google Scholar] [CrossRef]
- Zhurko, G.A. Chemcraft 1.8—Graphical Software for Visualization of Quantum Chemistry Computations; Ivanovo, Russia. 2005. Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2004&author=G.+A.+Zhurko&author=D.+A.+Zhurko&title=Chemcraft+-+Graphical+Program+for+Visualization+of+Quantum+Chemistry+Computations (accessed on 29 August 2023).
- Katkova, S.A.; Eliseev, I.I.; Mikherdov, A.S.; Sokolova, E.V.; Starova, G.L.; Kinzhalov, M.A. Cyclometalated Platinum(II) Complexes with Nitrile and Isocyanide Ligands: Synthesis, Structure, and Photophysical Properties. Russ. J. Gen. Chem. 2021, 91, 393. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 7, 3814–3816. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Williams, D.E. Representation of the molecular electrostatic potential by a net atomic charge model. J. Comput. Chem. 1981, 2, 304–323. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Phys. Chem. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular hydrogen bond energies in crystals evaluated using electron density properties: DFT computations with periodic boundary conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Tsirelson, V.G. Interplay between non-covalent interactions in complexes and crystals with halogen bonds. Russ. Chem. Rev. 2014, 83, 1181–1203. [Google Scholar] [CrossRef]
- Malenov, D.P.; Janjić, G.V.; Medaković, V.B.; Hall, M.B.; Zarić, S.D. Noncovalent bonding: Stacking interactions of chelate rings of transition metal complexes. Coord. Chem. Rev. 2017, 345, 318–341. [Google Scholar] [CrossRef]
- Ninković, D.B.; Blagojević Filipović, J.P.; Hall, M.B.; Brothers, E.N.; Zarić, S.D. What Is Special about Aromatic–Aromatic Interactions? Significant Attraction at Large Horizontal Displacement. ACS Cent. Sci. 2020, 6, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V. Testing the tools for revealing and characterizing the iodine-iodine halogen bond in crystals. Acta Crystallogr. B 2017, 73, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Masaryk, L.; Moncol, J.; Herchel, R.; Nemec, I. Halogen Bonding in New Dichloride-Cobalt(II) Complex with Iodo Substituted Chalcone Ligands. Crystals 2020, 10, 354. [Google Scholar] [CrossRef]
- Bulatova, M.; Ivanov, D.M.; Haukka, M. Classics Meet Classics: Theoretical and Experimental Studies of Halogen Bonding in Adducts of Platinum(II) 1,5-Cyclooctadiene Halide Complexes with Diiodine, Iodoform, and 1,4-Diiodotetrafluorobenzene. Cryst. Growth Des. 2021, 21, 974–987. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Mata, I.; Molins, E.; Alkorta, I.; Espinosa, E. Topological Properties of the Electrostatic Potential in Weak and Moderate N···H Hydrogen Bonds. J. Phys. Chem. A 2007, 111, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wu, Y.-H.; Luo, J.; Shi, L.-X.; Chen, Z.-N. Aggregation-induced phosphorescence of iridium(iii) complexes with 2,2′-bipyridine-acylhydrazone and their highly selective recognition to Cu2+. Analyst 2013, 138, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, J.; Cocchi, M.; Murphy, L.; Williams, J.A.G.; Fattori, V. Bi-molecular emissive excited states in platinum (II) complexes for high-performance organic light-emitting diodes. Chem. Phys. 2010, 378, 47–57. [Google Scholar] [CrossRef]
- Diez, A.; Forniés, J.; Larraz, C.; Lalinde, E.; López, J.A.; Martín, A.; Moreno, M.T.; Sicilia, V. Structural and luminescence studies on pi...pi and Pt...Pt interactions in mixed chloro-isocyanide cyclometalated platinum(II) complexes. Inorg Chem. 2010, 49, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Díez, Á.; Forniés, J.; Fuertes, S.; Lalinde, E.; Larraz, C.; López, J.A.; Martín, A.; Moreno, M.T.; Sicilia, V. Synthesis and Luminescence of Cyclometalated Compounds with Nitrile and Isocyanide Ligands. Organometallics 2009, 28, 1705–1718. [Google Scholar] [CrossRef]
- Kui, S.C.; Hung, F.-F.; Lai, S.-L.; Yuen, M.-Y.; Kwok, C.-C.; Low, K.-H.; Chui, S.S.-Y.; Che, C.-M. Luminescent organoplatinum(II) complexes with functionalized cyclometalated C^N^C ligands: Structures, photophysical properties, and material applications. Chemistry 2012, 18, 96–109. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katkova, S.A.; Antonova, E.V.; Cheranyova, A.M.; Ivanov, D.M.; Kinzhalov, M.A. Solid State Phosphorescence Enhancement of PtII-Based Emitters via Combination of π-Hole(Isocyano Group)⋅⋅⋅ dz2[PtII] and I···Cl Halogen-Bonding Interactions. Inorganics 2023, 11, 403. https://doi.org/10.3390/inorganics11100403
Katkova SA, Antonova EV, Cheranyova AM, Ivanov DM, Kinzhalov MA. Solid State Phosphorescence Enhancement of PtII-Based Emitters via Combination of π-Hole(Isocyano Group)⋅⋅⋅ dz2[PtII] and I···Cl Halogen-Bonding Interactions. Inorganics. 2023; 11(10):403. https://doi.org/10.3390/inorganics11100403
Chicago/Turabian StyleKatkova, Svetlana A., Elina V. Antonova, Anna M. Cheranyova, Daniil M. Ivanov, and Mikhail A. Kinzhalov. 2023. "Solid State Phosphorescence Enhancement of PtII-Based Emitters via Combination of π-Hole(Isocyano Group)⋅⋅⋅ dz2[PtII] and I···Cl Halogen-Bonding Interactions" Inorganics 11, no. 10: 403. https://doi.org/10.3390/inorganics11100403
APA StyleKatkova, S. A., Antonova, E. V., Cheranyova, A. M., Ivanov, D. M., & Kinzhalov, M. A. (2023). Solid State Phosphorescence Enhancement of PtII-Based Emitters via Combination of π-Hole(Isocyano Group)⋅⋅⋅ dz2[PtII] and I···Cl Halogen-Bonding Interactions. Inorganics, 11(10), 403. https://doi.org/10.3390/inorganics11100403