Abstract
The Schiff base reaction of imidazole-2-carboxaldehydes with the anion of alanine, leucine and phenylalanine in the presence of nickel(II) ion gives the neutral NiL2 complexes. The Schiff base ligand, L, binds through an imidazole nitrogen, NIm, the amino acid nitrogen, NAA, and a carboxylate oxygen, O, atom. The two N2O ligands bind to the nickel(II) in a meridional fashion with the NIm and O of each ligand in trans positions. These ligands can exist as the anticipated aldimine, Im − CH = NAA − CH(R) − CO2−, or the ketimine, Im − CH2NAA = C(R) − CO2−, tautomer. Tautomerization of the initially formed aldimine Schiff base results in movement of the hydrogen atom of the alpha carbon of the amino acid to the aldehyde carbon, CAld, atom of the imidazole carboxaldehyde with resultant relocation of the imine double bond in the reverse direction. Ten structures of the structurally unprecedented ketimine tautomer, prepared from imidazole-2-carboxaldehydes and a pyrazole-3-carboxaldehyde, were presented. The structural data supported the formation of the ketimines in each case, while the aldimine tautomer was observed with imidazole-4-carboxaldehydes. A rationale of this can be explained on the basis of charge distribution in the likely intermediate in the tautomerization.
1. Introduction
Schiff bases are one of the most commonly encountered ligands in synthetic inorganic chemistry due to their ease of synthesis and flexibility in choice of donor atoms. Schiff-base complexes have been used in diverse research areas including spin crossover [1,2,3,4,5], models of enzyme systems [6,7,8] and supramolecular complexes [9,10]. Two types of tautomerism in Schiff bases have been a subject of inquiry: enol imine/keto enamine and aldimine/ketimine. Both types have been observed in imines derived from aromatic aldehydes with ortho hydroxy groups, such as salicylaldehyde.
An enol imine/keto enamine tautomerism involves the transfer of the acidic hydrogen atom of the hydroxyl group to the nitrogen atom of the imine, as shown in Figure 1.
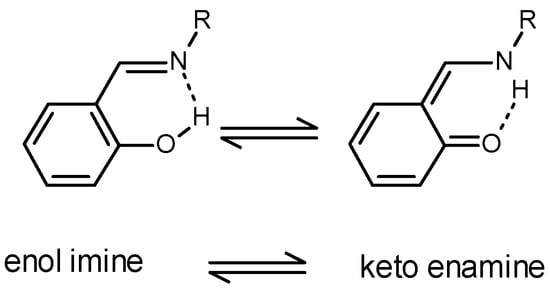
Figure 1.
Enol imine—keto enamine tautomerism in Schiff bases.
Studies of Schiff bases between salicylaldehyde derivatives and 1° amines or amino acids have confirmed the existence of this equilibrium in solution using nmr [11,12,13,14,15], solution IR [16] and UV-vis spectral data [15,16,17,18] and crystal structures of many Schiff base complexes verify the existence of the keto enamine tautomer [11,19]. Recent work details the photo- and thermo-responsive behavior of the two forms for the Schiff base formed between salicylaldehyde derivatives and aniline derivatives and their potential as a molecular switch between the enol imine and the keto enamine forms [11].
The aldimine–ketimine tautomerism has been reported in solution for metal complexes of Schiff bases of o-hydroxy aromatic aldehydes with amino acids, as shown in Figure 2. The aldimine–ketimine tautomers made from pyridoxal and alanine and pyridoxamine and pyruvate, respectively, have been observed by 1H nmr. However, the tautomerization between the aldimine and ketimine isomers was only observed in Zn(II) and Al(III) containing systems [20]. The aldimine-to-ketimine tautomerization involves the transfer of the hydrogen atom from Cα of the amino acid to the aldehyde carbon atom. This transfer is significant, as it involves the loss of the stereogenic center at Cα of the amino acid. On the other hand, the ketimine to aldimine tautomerization creates a stereogenic center at this carbon atom. If this transfer is stereoselective or stereospecific, it provides a mechanism for enantiomeric enrichment. The origins of homochirality of amino acids and other prebiotic molecules is an important topic [21,22,23].
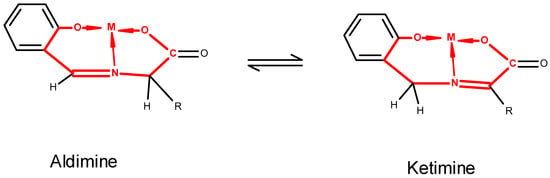
Figure 2.
Example of aldimine–ketimine tautomerism. The complex backbone, defined by OCA-Cca-Cα-NAA-CALD-C1-C2-Ophenoxy-M, is largely planar. The aldimine and ketimine are defined as the condensates of a deprotonated amino acid with an aldehyde or of a methanamine with a 2-oxo carboxylate, respectively.
The ketimine and aldimine complexes of Figure 2 (R = Me and M = Cu) were prepared from the Schiff-base condensates of o-hydroxybenzylamine with sodium pyruvate and salicylaldehyde with alanine, respectively [24]. It was found that in basic ethanol solution, the aldimine complex was more stable than the ketimine complex as solutions of the latter were observed by visible spectroscopy to convert to the aldimine complex [25]. No structural data for the ketimine are available.
Early work reported the metal catalyzed racemization, transamination, and decarboxylation of amino acids by pyridoxal found in pyridoxal-5-phosphate (PLP) and other o-hydroxy-aromatic aldehydes [26,27,28], which led to a flurry of effort centered around the activity of metal Schiff-base complexes to catalyze the reactions of pyridoxal containing enzymes. Currently, it is thought that formation of the ketimine plays a major role in the reactions of PLP and amino acid substrates. [29,30] Consequently, the aldimine–ketimine tautomerism reaction has been extensively studied in solution for coordination complexes containing Schiff bases via UVvis spectroscopy [24,31,32], magnetic measurements [24], and proton nmr [20,25]. More recently, it has been reported that the presence of a metal ion has no effect on the rate of decarboxylation or transamination and that the dependence observed earlier may instead result from an enhancement of Schiff base formation in these systems [33]. Since structural observation of the aldimine–ketimine equilibrium has not been seen in the free Schiff bases of aldehydes, the observation of the aldimine and ketimine forms of metal bound Schiff bases may be the only method for study of this important type of tautomerism.
Although numerous Schiff base complexes have been synthesized for the study of the aldimine/ketimine tautomerism, no structural evidence has been provided for the existence of a ketimine complex in these systems. A search of the Cambridge Structural Database (CSD) for an aldimine structure formed from the Schiff base condensate of any substituted salicylaldehyde or pyridine-4-carboxaldehyde with an α-amino acid, other than glycine, provides 731 and 30 examples, respectively, the majority with metals. In contrast, there are no examples of the ketimine tautomer. An analogous search for a Schiff base derived from any substituted pyridine-2-carboxaldehyde provides 29 examples of the aldimine tautomer and one example of a ketimine as shown in Figure 3. However, this product is not a result of an aldimine/ketimine tautomerization but of an unexpected oxidation of an amine [34].
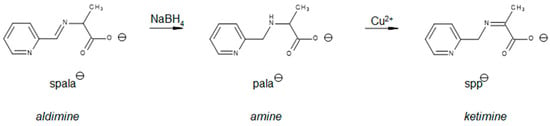
Figure 3.
The Schiff base formed from the condensation of pyridine-2-carboxaldehyde with alanine, spala-, is reduced to the amine pala-. Subsequent reaction with copper(II) salts gives the ketimine, spp-. While spp- is a ketimine, it is not formed from the tautomerization of the aldimine.
To date, the only structural report of a ketimine formed by tautomerization of the aldimine is that of the nickel(II) complex of the Schiff base formed from alanine and N-methylimidazole-2-carboxaldehyde that we reported earlier [35]. In the present work, we report the synthesis and structural characterization of ten new nickel(II) complexes of a tridentate ligand formed by the Schiff base condensation of the anion of alanine(A), leucine(L) or phenylalanine(F) with several imidazole-2-carboxaldehydes and a pyrazole-3-carboxaldehyde. All ten complexes exhibit the ketimine form of the ligand. It is proposed for these reactions that the aldimine complex is formed first and tautomerizes to give the ketimine product. These complexes have been characterized by IR, UVvis, ESI MS (all in Supplemental Information) and single crystal structure determination.
2. Results and Discussion
2.1. Choice of Amino Acids and Imidazole Carboxaldehydes
The structurally reported Schiff base complexes of an imidazole-4-carboxaldehyde with an amino acid give the anticipated aldimine product with copper(II) [36,37], nickel(II), [38], zinc(II) [39], and several lanthanide(III) ions [40,41]. The only example with an imidazole-2-carboxaldehyde gave the unexpected ketimine product [35]. The formation of the ketimine is significant, as the alpha carbon atom of the amino acid is no longer a stereogenic center. The structural determination of ten related Schiff-base complexes of imidazole-2-carboxaldehydes and a pyrazole-3-carboxaldehyde were investigated to determine whether ketimine formation and resultant loss of chirality is a general observation or unique to our previously reported complex. The aldehydes used and labeling scheme employed are shown in Figure 4.
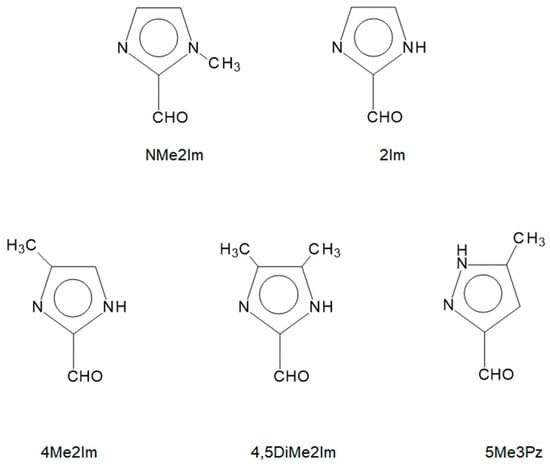
Figure 4.
Line drawings of the imidazole-2-carboxaldehydes and one pyrazole carboxaldehyde used in this study and their abbreviations.
The deprotonated anions of the amino acids used in these complexes are alanine (A), leucine (L) and phenylalanine (F), as these side chains lack other donor atoms which could bind to a metal. The specific Schiff-base condensates of the anions of the amino acids and aldehydes are designated by a code for the anion of the amino acid, A, L or F, followed by the code for the aldehyde. Figure 5 shows three specific ligands employed and the codes by which they are designated. Since the ligands, made from the Schiff base condensation of the amino acid anions, are negatively charged, the divalent nickel complexes, Ni(ligand)2, are neutral.
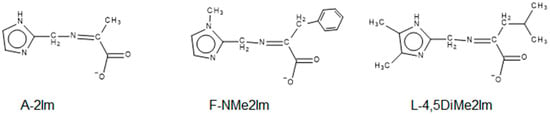
Figure 5.
Line drawings and codes for three of the ligands employed in this study. The first letter stands for the anion of the amino acid, A (alanine), L (leucine) and F (phenylalanine). The subsequent portion is the azole carboxaldehyde used, as pictured in Figure 4.
2.2. Synthesis and Preliminary Characterization of Complexes
The complexes were prepared by reaction of the potassium salt of the amino acid with an imidazole-2-carboxaldehyde or pyrazole-3-carboxaldehyde in refluxing methanol followed by addition of nickel(II) acetate. The reaction mixtures deposited suitable crystals after several days. The use of nickel(II) perchlorate is also effective but may result in co-precipitation of potassium perchlorate. Preliminary characterization of the NiL2 complexes consisted of FTIR, positive ion ESIMS and UVvis spectroscopy. All complexes have a strong imine signal between 1650 and 1610 cm−1. Ni(F-NMe2Im)2 has a split imine which may be due to two crystallographic modifications, as discussed in Section 2.7. All display broad peaks in the 3500–3100 cm−1 region due to the presence of water solvates and N-H stretch for complexes having this feature. In the positive ion ESIMS, the following m/e values, NiL2H+, NiL2Na+, NiL2K+ and Ni2L3+, were observed for all complexes, which affords a rapid method for identifying reaction products. Specific observed values are provided in the Experimental Methods section.
UVvis spectra were recorded for the octahedral nickel(II) amino acid-imidazole complexes reported here and are provided in Supplemental Information. The λMAX values of the spin allowed d to d transitions depend significantly on the donor atoms of the ligands. The crystal field splitting parameter varies with the donor set, N6 > N3O3 > O6, and is not greatly influenced by ligand substituents, the hydrocarbon side chains of the three amino acids used. A strong donor set will shift the λMAX values to shorter wavelengths. The nickel(II) Schiff-base condensate of salicylaldehyde with triethylenetetraamine, Ni(trien)(sal)2 was used as a spectral reference for the present imidazole complexes. Both reference compound and the present complexes use nickel(II), have an N4O2 donor set with two imines and both bind the oxygen atoms cis. A structurally characterized Ni(trien)(sal)2 complex exhibits broad λMAX at 385 and 585 nm [42]. The present complexes are soluble in the reaction mixture but the resultant crystals are only sparingly soluble in neat methanol, which makes obtaining reliable extinction coefficients difficult. The spectra were obtained by adding a few crystals to a cuvette of methanol and then allowing time for dissolution to occur. The visible spectra consisted of two broad peaks with λMAX between 370–380 and 580–595 nm. The former peak was a shoulder on a much larger peak with a max below 300 nm. This larger peak is not a nickel(II) d to d band and is thought to be associated with the two substituted imidazoles, as has been reported for imidazole-2-carboxaldehyde (λMAX at 280 nm, a = 12,000) [43,44]. The observed peaks with λMAX at 370–380 and 580–595 nm are consistent with those of the spectral standard, Ni(trien)(sal)2, at 385 and 585 nm. In solution, the present complexes vary in color from green to a greenish blue. It cannot be stated if the observed color and reported λMAX values are attributed to the ketimine or to an equilibrium mixture of aldimine/ketimine that on standing will produce ketimine crystals.
2.3. Background Information on Structure of Tridentate Schiff Bases of AA
There are 585 structurally characterized metal complexes of tridentate ligands formed from the Schiff-base condensates of an amino acid (other than glycine) with substituted salicylaldehydes in the CSD. All of these are of the aldimine tautomer. These ligands are fairly inflexible due to the rings and double bonds resulting in meridional coordination of the essentially planar ligands with trans O-M-O angles peaking at 156° and some approaching 180°, as shown in Figure 6. There are nine backbone atoms, M-O-CCA–α−NAA−Caldehyde−Csal−Csal−Osal, as shown in Figure 2. The number of structurally characterized Schiff-base complexes of pyridine-4-carboxaldehydes (30), pyridine-2-carboxaldehydes (9) and imidazole-4-carboxaldehydes (24) with an amino acid are less than those with salicylaldehyde and also reveal meridional coordination in octahedral complexes. All of these complexes are aldimine.
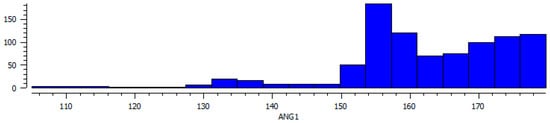
Figure 6.
Histogram illustrating the distribution of the O-M-O angle of the Schiff bases of salicylaldehyde and amino acids (see Figure 2). Note the predominance of cases with a trans angle reflecting meridional coordination.
2.4. Organization of Structural Data
The crystallographic details of the ten Schiff-base complexes reported here are given in Table S1, which is in the Supplemental Information along with ORTEPs of all complexes. Selected structural parameters of nine of these complexes and Ni(A-NMe2Im)2, reported previously, [35] are given in Table 1 in Section 2.5. The structural parameters of the complexes themselves exhibit little variation regardless of the aldehyde or amino acid that is used. These are discussed in Section 2.5. The structures of the ketimine ligands themselves will be discussed in Section 2.6 and bond parameters are provided there as well as in Table 2. This includes the important change in coordination number of Cα with significant changes in both its bond distances and angles. One of the ten new complexes, Ni(L-NMe2Im)2, crystallizes in both monoclinic and triclinic forms. The crystallographic parameters of both are provided in Table S1. The structural parameters of the monoclinic form are provided in Table 1 (complex, Section 2.5) and 3 (ligand, Section 2.6). The structure of the triclinic form is discussed in Section 2.7 and its bond parameters are also given in Table 3. A sketch of the ketimine ligand system and atom labelling employed here is shown in Figure 7. The same labeling scheme is used in the structural data provided in Table 1, Table 2 and Table 3.
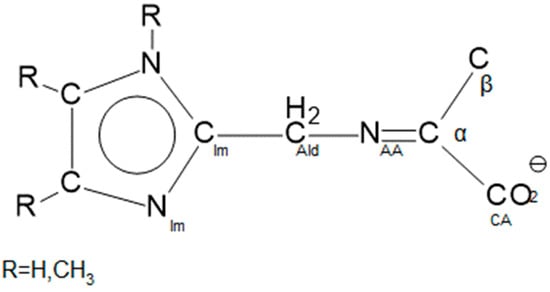
Figure 7.
Line drawing illustrating the seven labelled atoms of the backbone of the ketimine ligand, O-CCA-Cα-NAA-CAld-CIm-NIm. The O, NAA and NIm atoms bind to the nickel(II). Cα, NAA and CAld are labelled as they are to indicate their chemical origin prior to the observed tautomerization.
2.5. Structure of the Ni(ketimine)2 Complexes, Table 1
The ten NiL2 ketimine complexes exhibit Ni-NIm (or Ni-NPz), Ni-NAA and Ni-O bond distances that vary between 2.0 and 2.1 Å and are all unremarkable. The three trans angles, unique NAA-Ni-NAA′ and two NAA-Ni-O, NAA′-Ni-O′ angles show some distortion from 180°. The NAA-Ni-NAA angles exhibit the least distortion at ~176° due to the fact that NAA and NAA′ are not on the same ligand and, therefore, are less constrained. The meridional coordination of two tridentate ligands requires that the terminal donor atoms of each ligand, NIm (or NPz in one case) and O, be trans to one another. The observed NIm-Ni-O bond angles exhibit more distortion, ~157°, due to the fact that the positions of NIm and O are constrained as they are on the same ligand. This trans angle of ~157° in these complexes is smaller than the O-M-O trans angle of the salicylaldehyde complexes pictured in Figure 6, with none approaching 180°. The extra carbon atom in the backbone of the salicylaldehyde complexes allows greater flexibility with examples of the trans angle approaching 180°. None of the present complexes has a NIm-Ni-O angle approaching 180°. There are two five membered rings, Ni-O-CCA-Cα-NAA and Ni-NIm-CIm-CAld-NAA that define the eight backbone atoms of one ketimine ligand. The reduction in the number of backbone atoms from nine to eight in the sal-AA vs. the Im-AA (or Pz-AA in one case) complexes and the presence of two five membered rings rather than a six and a five membered ring explains the greater distortion of the NIm-Ni-O trans angle observed in the present AA-Im series.

Table 1.
Selected structural data for the Ni(ketimine)2 complexes. Bond distances in Å and angles in °. Data for Ni(A-NMe2Im)2 taken from reference [35].
Table 1.
Selected structural data for the Ni(ketimine)2 complexes. Bond distances in Å and angles in °. Data for Ni(A-NMe2Im)2 taken from reference [35].
| Compound | Distance (Å) | Value 1 | Value 2 | Angle (°) | Value 1 | Value 2 |
|---|---|---|---|---|---|---|
| Ni-Nim | 2.0782(9) | 2.0870(9) | NAA-Ni-NAA | 177.68(3) | ||
| Ni(A-NMe2Im)2 | Ni-NAA | 2.0259(9) | 2.0212(9) | NIm-Ni-O | 157.05(3) | 157.19(3) |
| Ni-O | 2.0901(8) | 2.1175(8) | ||||
| Ni-Nim | 2.0768(15) | 2.0531(14) | NAA-Ni-NAA | 179.34(6) | ||
| Ni(L-NMe2Im)2 | Ni-NAA | 2.0335(13) | 2.0388(13) | NIm-Ni-O | 157.59(5) | 157.56(5) |
| monoclinic | Ni-O | 2.0977(12) | 2.0838(12) | |||
| Ni-Nim | 2.07(2) | 2.09(2) | NAA-Ni-NAA | 176.3(7) | ||
| Ni(F-NMe2Im)2 | Ni-NAA | 2.085(18) | 1.957(15) | NIm-Ni-O | 155.5(7) | 157.2(7) |
| Ni-O | 2.029(17) | 2.065(18) | ||||
| Ni-Nim | 2.065(2) | 2.069(2) | NAA-Ni-NAA | 177.12(10) | ||
| Ni(A-2Im)2 | Ni-NAA | 2.025(2) | 2.031(2) | NIm-Ni-O | 157.70(8) | 156.57(7) |
| Ni-O | 2.100(2) | 2.1146(19) | ||||
| Ni-Nim | 2.0968(19) | 2.0592(19) | NAA-Ni-NAA | 170.24(8) | ||
| Ni(L-2Im)2 | Ni-NAA | 2.0347(19) | 2.0258(19) | NIm-Ni-O | 157.60(7) | 158.13(7) |
| Ni-O | 2.1011(16) | 2.1153(16) | ||||
| Ni-Nim | 2.093(4) | 2.072(4) | NAA-Ni-NAA | 174.44(16) | ||
| Ni(F-2Im)2 | Ni-NAA | 2.028(4) | 2.028(4) | NIm-Ni-O | 157.05(15) | 158.38(18) |
| Ni-O | 2.107(3) | 2.073(4) | ||||
| Ni-Nim | 2.094(4) | 2.099(5) | NAA-Ni-NAA | 174.8(2) | ||
| Ni(L-4Me2Im)2 | Ni-NAA | 2.043(5) | 2.091(6) | NIm-Ni-O | 157.12(18) | 156.74(19) |
| Ni-O | 2.094(4) | 2.099(5) | ||||
| Ni-Nim | 2.077(13) | 2.085(14) | NAA-Ni-NAA | 178.43(5) | ||
| Ni(A-4,5DiMe2Im)2 | Ni-NAA | 2.0210(13) | 2.0248(13) | NIm-Ni-O | 157.63(5) | 156.75(5) |
| Ni-O | 2.1301(12) | 2.1193(12) | ||||
| Ni-Nim | 2.0947(19) | 2.0822(19) | NAA-Ni-NAA | 174.80(8) | ||
| Ni(L-4,5DiMe2Im)2 | Ni-NAA | 2.043(2) | 2.0251(18) | NIm-Ni-O | 154.92(2) | 156.54(8) |
| Ni-O | 2.097(9) | 2.1207(16) | ||||
| Ni-Nim | 2.082(2) | 2.060(2) | NAA-Ni-NAA | 176.41(8) | ||
| Ni(L-5Me3Pz)2 | Ni-NAA | 2.029(2) | 2.028(2) | NIm-Ni-O | 156.58(9) | 156.53(8) |
| Ni-O | 2.076(2) | 2.1000(18) | ||||
All ten complexes exhibit nearly perpendicular coordination of the two meridionally bound ligands. Eight backbone atoms trace the bonding on one ligand to the nickel(II). These are the Ni-O-CCA-Cα-NAA-Caldehyde-CIm-NIm atoms. These two groupings of eight atoms, one for each ligand, are planar and perpendicular to one another. This is illustrated for Ni(F-NMe2Im)2 in Figure 8. The two azole planes are bent ~4° out of their respective backbone planes.
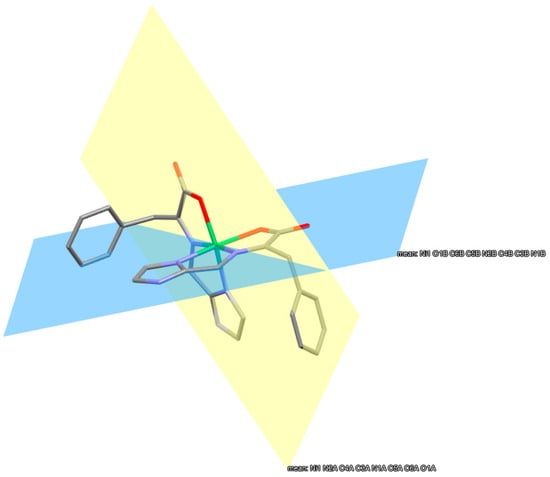
Figure 8.
Structure of Ni(F-NMe2Im)2. Hydrogen atoms have been omitted for clarity. The yellow and blue planes are at 86.20° to one another and are the best planes of the two groupings of eight backbone atoms of each F-2Im ligand. The out-of-plane displacement in Å of each backbone atom is provided as pairs of numbers, as there are two ligands. Ni (0.054, 0.101), O (0.013, 0.005), CCA (0.002, 0.074) Cα (0.004, 0.027), NAA (0.035, 0.029), CAld (0.041, 0.076), CIm (0.005, 0.044), NIm (0.037, 0.061).
Each of the Ni(ketimine)2 complexes except those involving NMe2Im has a hydrogen bonding donor, NIm-H (or NPz-H), and a hydrogen bonding acceptor, a carboxylate oxygen atom, on each ligand. All of the complexes crystallize as water solvates except for Ni(L-2Im)2, which is anhydrous. This complex exhibits an interesting supramolecular feature as it can only hydrogen bond to its neighboring molecules. The core of this is two identical hydrogen bonds that link two adjacent complexes, a hydrogen bound dimer. These two hydrogen bonds are (1) a NIm-H on the first ligand of the first complex to a nickel bound carboxylate oxygen atom on the first ligand of the second complex and (2) a nickel bound carboxylate atom of the second ligand on the first complex to an NIm–H on the second ligand of the second complex. Two identical NIm-H…ONi bound bonds link two adjacent molecules. The planes of the imidazole ring of the first ligand of the first complex and the imidazole ring of the second ligand of the second complex are parallel, with an interplanar angle of 0.00°. The two imidazole rings that are in parallel planes are not superimposed. Thus, simple π to π stacking of the imidazole rings is not responsible for this arrangement but π to π interactions of the two delocalized planar ligands may contribute to this. The remaining four hydrogen bonding sites of the parent hydrogen bound dimer extend the supramolecular array, as shown in Figure 9. The carboxylate oxygen atoms that are not bound to the nickel atom are not involved in the hydrogen bonding.
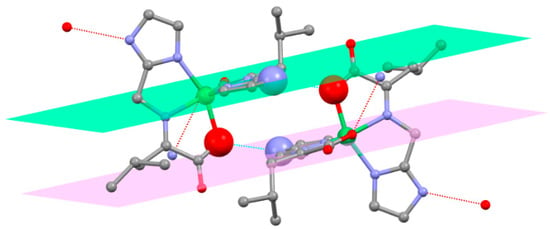
Figure 9.
Hydrogen bonding arrangement in Ni(L-2Im)2. The hydrogen atoms are omitted for clarity. The hydrogen bonding partners, (1) the nitrogen atom of the first ligand of the first complex and the oxygen atom of the first ligand of the second complex and (2) the oxygen atom of the second ligand of the first complex and the nitrogen atom of the second ligand of the second complex, are exaggerated in size. The two NIm-H…..ONi bound distances are 2.723(3)Å. The planes of the imidazole rings of the first ligand of the first complex (green) and the second ligand of the second complex (violet) are parallel. The four remaining hydrogen bonds (dashed lines) are identical with a NIm-H…..ONi bound distance of 2.804(3)Å.
2.6. Structure of the Ketimine Ligand, Table 2
Structural changes on the ligand itself are extremely significant, as this results in the loss of a stereogenic center, Cα. The tautomerization results in movement of the hydrogen atom on the Cα to CAld and the movement of the double bond in the opposite direction from between Cald and NAA to between NAA and Cα. The structure of Ni(A-2Im)2 is shown in Figure 10 and is illustrative of all complexes. The bond distances and angles on the important Cα atom are provided for all ten complexes in Table 2. The only reasonable explanation of this is loss of the hydrogen atom and formation of a double bond to NAA. This is supported by the short NAA-Cα (~1.28 Å) and long CAld-NAA (~1.47 Å) bond distances. The bond angles around Cα all now average ~120°, consistent with the change in hybridization from sp3 to sp2. The opposite changes to CAld consistent with a change from sp2 to sp3 hybridization are also observed and discussed in Section 2.7. Despite the loss of chirality at Cα, the nickel complexes are still chiral, Δ or Λ, due to the biscoordination of the two tridentate ligands. None of the complexes crystallized in a Sohncke space group, and so all complexes are racemic at the level of the crystal.
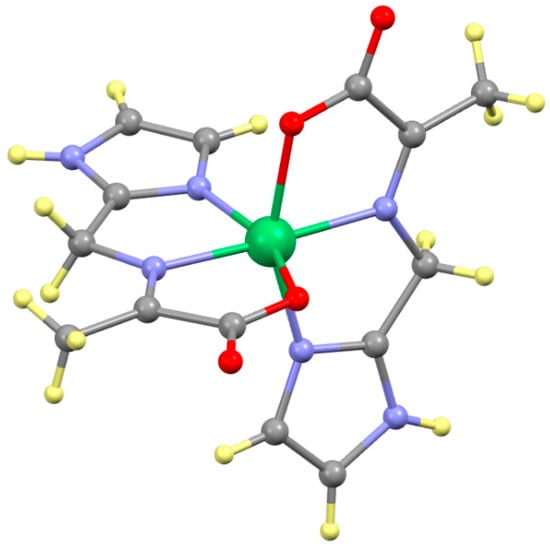
Figure 10.
Structure of Ni(A-2Im)2. The hydrogen atoms are shown in pale yellow to be visible. Note the lack of a hydrogen atom on Cα, now with a CN = 3, which results in loss of a stereogenic center and the new position of the hydrogen atom on CAld, now with a CN = 4.

Table 2.
Selected structural data of the ketimine ligand for the Ni(ketimine)2 complexes Bond distances in Å and angles in °. Data for Ni(A-NMe2Im)2 taken from reference [35].
Table 2.
Selected structural data of the ketimine ligand for the Ni(ketimine)2 complexes Bond distances in Å and angles in °. Data for Ni(A-NMe2Im)2 taken from reference [35].
| Compound | Distance (Å) | Value 1 | Value 2 | Angle (°) | Value 1 | Value 2 |
|---|---|---|---|---|---|---|
| Cα-NAA | 1.2805(13) | 1.2796(14) | NAA-Cα-Cβ | 127.16(10) | 126.27(9) | |
| Ni(A-NMe2Im)2 | Cα-Cβ | 1.4857(15) | 1.4824(15) | NAA-Cα-CCA | 113.00(9) | 112.86(9) |
| Cα-CCA | 1.5357(14) | 1.5390(14) | Cβ -Cα-CCA | 119.84(9) | 120.86(9) | |
| Cα-NAA | 1.281(2) | 1.280(8) | NAA-Cα-Cβ | 126.61(15) | 126.7(10) | |
| Ni(L-NMe2Im)2 | Cα-Cβ | 1.494(2) | 1.511(7) | NAA-Cα-CCA | 112.91(14) | 112.4(5) |
| monoclinic | Cα-CCA | 1.546(2) | 1.549(3) | Cβ -Cα-CCA | 120.48(14) | 119.9(11) |
| Cα-NAA | 1.255(16) | 1.259(16) | NAA-Cα-Cβ | 127.1(18) | 124.6(17) | |
| Ni(F-NMe2Im)2 | Cα-Cβ | 1.50(3) | 1.54(3) | NAA-Cα-CCA | 113.0(15) | 113.8(14) |
| Cα-CCA | 1.537(19) | 1.535(19) | Cβ -Cα-CCA | 119.8(17) | 121.4(16) | |
| Cα-NAA | 1.269(4) | 1.283(4) | NAA-Cα-Cβ | 126.9(3) | 126.4(2) | |
| Ni(A-2Im)2 | Cα-Cβ | 1.486(4) | 1.476(4) | NAA-Cα-CCA | 113.0(2) | 113.2(2) |
| Cα-CCA | 1.534(5) | 1.541(3) | Cβ -Cα-CCA | 120.0(3) | 120.5(2) | |
| Cα-NAA | 1.272(3) | 1.278(3) | NAA-Cα-Cβ | 126.9(2) | 125.7(4) | |
| Ni(L-2Im)2 | Cα-Cβ | 1.490(3) | 1.485(9) | NAA-Cα-CCA | 114.5(2) | 113.8(2) |
| Cα-CCA | 1.546(3) | 1.540(3) | Cβ -Cα-CCA | 118.6(2) | 120.4(4) | |
| Cα-NAA | 1.304(7) | 1.285(8) | NAA-Cα-Cβ | 121.0(10) | 129.7(10) | |
| Ni(F-2Im)2 | Cα-Cβ | 1.43(4) | 1.48(2) | NAA-Cα-CCA | 112.4(5) | 113.4(5) |
| Cα-CCA | 1.537(7) | 1.50(2) | Cβ -Cα-CCA | 125.7(9) | 114.9(9) | |
| Cα-NAA | 1.251(8) | 1.267(8) | NAA-Cα-Cβ | 126.1(10) | 125.9(1) | |
| Ni(L-4Me2Im)2 | Cα-Cβ | 1.530(10) | 1.48(2) | NAA-Cα-CCA | 113.9(6) | 112.5(6) |
| Cα-CCA | 1.537(9) | 1.551(9) | Cβ -Cα-CCA | 119.5(10) | 121.6(12) | |
| Cα-NAA | 1.277(2) | 1.272(2) | NAA-Cα-Cβ | 125.76(15) | 126.35(16) | |
| Ni(A-4,5DiMe2Im)2 | Cα-Cβ | 1.492(2) | 1.492(2) | NAA-Cα-CCA | 113.35(14) | 113.99(14) |
| Cα-CCA | 1.535(2) | 1.535(2) | Cβ -Cα-CCA | 120.88(14) | 119.95(15) | |
| Cα-NAA | 1.176(9) | 1.294(3) | NAA-Cα-Cβ | 128.6(7) | 125.1(2) | |
| Ni(L-4,5DiMe2Im)2 | Cα-Cβ | 1.520(9) | 1.487(4) | NAA-Cα-CCA | 112.4(6) | 112.6(2) |
| Cα-CCA | 1.542(8) | 1.534(3) | Cβ -Cα-CCA | 118.8(7) | 122.3(2) | |
| Cα-NAA | 1.261(4) | 1.279(3) | NAA-Cα-Cβ | 126.0(3) | 127.0(2) | |
| Ni(L-5Me3Pz)2 | Cα-Cβ | 1.505(4) | 1.494(4) | NAA-Cα-CCA | 113.9(3) | 112.9(2) |
| Cα-CCA | 1.534(4) | 1.530(4) | Cβ -Cα-CCA | 120.1(3) | 120.1(2) |
2.7. Structure of Triclinic Ni(L-NMe2Im)2, Table 3
The reaction of nickel(II) with L-NMe2Im gives two crystalline products: monoclinic and triclinic. The crystallographic parameters of both are given in Table S1. The monoclinic compound has both ligands in the ketimine form with structural parameters quite similar to the nine other ketimine products given in Table 1 and Table 2. The triclinic product is quite distinct. It is best described as Ni(L)(L′). The asymmetric unit gives a unique position for all sixteen non-hydrogen atoms of one ligand that exhibits bond parameters of the ketimine tautomer. The second ligand is disordered, giving two different sets of sixteen non-hydrogen atoms in the asymmetric units. This is distinguished from two sets of coordinates for only the side chain atoms of the amino acid, which reflects two different conformations. This disorder extends to the entire second ligand, indicating something more significant. The dominant set (0.691) is the ketimine tautomer. Its structural parameters are nearly identical to those of the monoclinic form of Ni(L-NMe2Im)2 as described in Table 1 and Table 2 and will not be discussed further. The minority (0.309) tautomer is, surprisingly, the aldimine. The structure of the Ni(ketimine)(aldimine) complex of Ni(L-NMe2Im)2 is shown in Figure 11 and selected structural parmeters are given in Table 3.
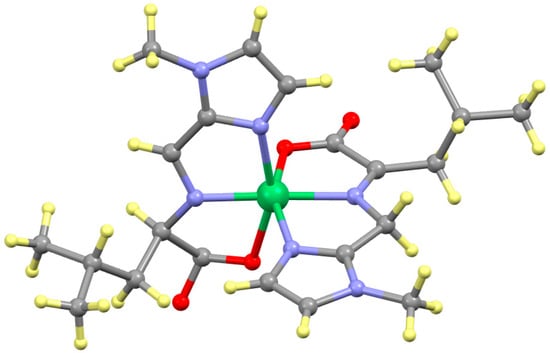
Figure 11.
Structure of triclinic Ni(L-NMe2Im)2. The ligand on the left is the aldimine. Note the presence of a hydrogen atom on Cα and only one hydrogen atom on CAld. The ligand on the right is the ketamine form of that shown on the left. Note the absence of a hydrogen atom on Cα and two hydrogen atoms on CAld.
The three non-hydrogen atoms that best differentiate the two forms of the second ligand are CAld, NAA and Cα, as movement of the hydrogen atom between Cald and Cα alters the coordination number and, hence, bond angles of these two atoms significantly. The coordination number of NAA is three in either tautomer. All of the bond distances and angles of these three critical atoms for the Ni(ketimine)(aldimine) complex of Ni(L-NMe2Im)2 are given in Table 3. In the simplest approach, this can be regarded simply as a difference in the position of NAA. In the aldimine form, it slides closer to CAld and away from Cα, while in the ketimine form, the movement is in the opposite direction. In either case, the sum of the NAA-Cα and NAA-CAld distances is only slightly different, 2.71 for aldimine and 2.745 Å for the ketimine. However, this neglects the movement of the hydrogen atom and resultant changes in the coordination number of CAld and Cα. The positions of the other thirteen non-hydrogen atoms of the second ligand are different from one another but none involve a change in coordination number and will not be discussed further. This is the only complex presented that retains a stereogenic center at Cα as one ligand is in the aldimine tautomer. The stereochemical assignment of the two stereogenic atoms, Cα and Ni, is CRNiɅ for the structure shown in Figure 11. However, as this complex crystallized in a non-Sohncke space group, the sample consists of equal amounts of both enantiomers: CRNiɅ and CSNiΔ. It is not possible to state whether or not the observed pairing is a general correlation for Ni(aldimine)(ketimine) complexes, as, to date, this is the only complex of this type with two stereogenic centers [45].
The presence of a complex that contains both the aldimine and ketimine form of the same ligand suggests that there is only a small difference in the energies of these tautomers, at least in the triclinic form of Ni(L-NMe2Im)2. However, it should be pointed out that this product crystallized in both a monoclinic and triclinic form. In the monoclinic form, both ligands are ketimine and in the triclinic form, one ligand is ketimine, while the second ligand is disordered between the two forms with the majority form being ketimine. The energies of the aldimine and ketimine tautomers must be very close in the minority portion of the triclinic form of Ni(L-NMe2Im)2, as both are observed. However, even for this complex, the ketimine form is the sole or dominant form observed in both the monoclinic and triclinic forms. It cannot be stated that the other complexes lack a similar polymorph, but only that none were observed.

Table 3.
Selected structural data for the aldimine and ketimine ligands of the triclinic form of. Ni(L-NMe2Im)2. Bond distances are in Å and bond angles are in °. xxxxxx means no value is possible.
Table 3.
Selected structural data for the aldimine and ketimine ligands of the triclinic form of. Ni(L-NMe2Im)2. Bond distances are in Å and bond angles are in °. xxxxxx means no value is possible.
| Distance | Aldimine | Ketimine | Angle | Aldimine | Ketimine |
|---|---|---|---|---|---|
| CAld-CIm | 1.478(19) | 1.491(7) | CIm CAld-NAA | 114.6(12) | 104.3(4) |
| CAld-NAA | 1.34(2) | 1.465(8) | CIm CAld-H | 122.2 | 110.9 |
| CAld-H | 0.9500 | 0.9900 | CIm CAld-H′ | xxxxxx | 110.9 |
| CAld-H | xxxxxx | 0.9900 | NAA-CAld-H | 122.2 | 110.9 |
| NAA-CAld-H′ | xxxxxx | 110.9 | |||
| H-CAld-H′ | xxxxxx | 108.9 | |||
| NAA-CAld | 1.34(2) | 1.465(8) | Ni-NAA-CAld | 116.7(10) | 116.1(3) |
| NAA-Cα | 1.375(13) | 1.280(5) | Ni-NAA-Cα | 123.6(9) | 115.8(3) |
| NAA-Ni | 1.921(11) | 2.077(4) | Cα-NAA-CAld | 114.1(11) | 127.1(3) |
| Cα-NAA | 1.375(13) | 1.280(5) | NAA-Cα-Cβ | 118.8(6) | 121.5(3) |
| Cα-Cβ | 1.4905(13) | 1.4905(13) | NAA-Cα-CCA | 104.3(7) | 114.4(2) |
| Cα-CCA | 1.690(13) | 1.5341(13) | NAA-Cα-H | 108.7 | xxxxxxx |
| Cα-H | 1.000 | xxxxxx | Cβ-Cα-CCA | 106.2(5) | 122.8(3) |
| Cβ-Cα-H | 108.7 | xxxxxx | |||
| CCA-Cα-H | 108.7 | xxxxxx |
2.8. Isomeric Justification of Outcome of Aldimine to Ketimine Tautomerism
The reaction of 5-methyl-4-imidazole carboxaldehyde with an amino acid results in isolation of the aldimine complex with copper(II) [20], nickel(II) [21], zinc(II) [22] and La(III) [23]. The present complexes show that the ketimine is formed under the same reaction conditions when nickel(II) and an imidazole-2-carboxaldeyde is used. The extension of conjugation does not explain the isomeric dependence on outcome, as, in either case, the newly formed C = NAA is conjugated. If aldimine, the CAld = NAA bond is conjugated with the imidazole ring. If ketimine, the NAA = Cα bond is conjugated with the carboxylate group.
However, stabilization of an intermediate does show an isomeric difference. The racemization of amino acids can be achieved through deprotonation of Cα and delocalization of the negative charge through a ketimine structure. A possible mechanism for the aldimine to ketimine tautomerization reaction involves deprotonation of Cα followed by reprotonation at a different site. Three resonance structures of the deprotonated ligand of an amino acid Schiff-base condensate of an imidazole-2-carboxaldehyde are shown in Figure 12. Resonance structure III places a negative charge on the electronegative nitrogen atom of the imidazole ring, which should stabilize the deprotonated species. The subsequent reprotonation at CAld gives the observed ketimine product. Charge stabilization/delocalization is also facilitated if a metal ion is in the preformed binding pocket. It is not known if ketimine formation in these examples requires a metal ion or not, as all reactions were carried out with nickel(II).
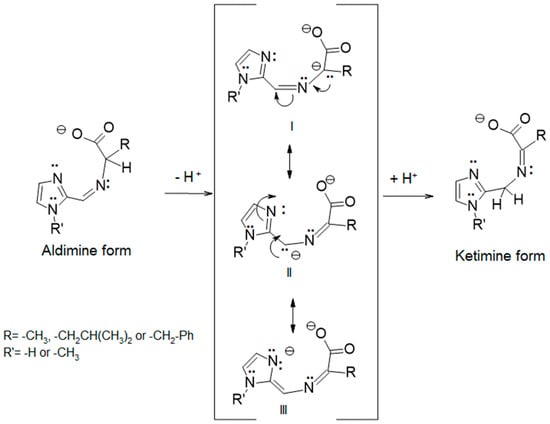
Figure 12.
Deprotonation of Cα of the aldimine tautomer of the Schiff base formed from an imidazole-2-carboxaldehyde with an amino acid results in an intermediate which has several resonance forms. Resonance form III may stabilize the intermediate, as it places a negative charge on the electronegative nitrogen atom. Reprotonation at CAld results in formation of the observed ketimine. The presence of a metal ion in the binding pocket may also facilitate tautomerization.
The formation of the resonance stabilized carbanion shown in Figure 12 requires a base to deprotonate Cα of the aldimine tautomer. In our system, there are two obvious bases present for abstraction of the proton–acetate ion from nickel (II) acetate and the imidazole ring from imidazole-2-carboxaldehyde. It has been demonstrated in solid phase peptide synthesis that the imidazole moiety is basic enough to deprotonate Cα of an amino acid, especially of histidine, which is being incorporated into a peptide [46,47].
Similar resonance structures can be shown for the Schiff base of an imidazole-4-carboxaldehyde with an amino acid. Those for I′ and II′ of an imidazole-4-carboxaldehyde are identical to those of I and II for an imidazole-2-carboxaldehyde in terms of location of the negative charge. However, resonance structure III′, as shown in Figure 13, has a negative charge on a carbon atom of the imidazole ring as compared to the electronegative nitrogen atom of III.
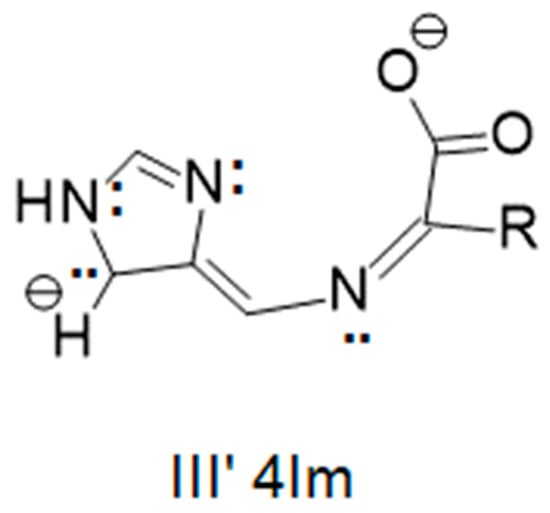
Figure 13.
Resonance structure III′ for the Schiff base of an imidazole-4-carboxaldehyde with an amino acid. In this case, the negative charge is on a carbon atom of the imidazole ring rather than the more electronegative nitrogen atom as shown in Figure 12. Even if a metal ion were in the binding pocket, it would not greatly stabilize the negative charge on the carbon atom of the imidazole ring. Tautomerization is not observed in this system.
The long-known ability of pyridoxal to racemize amino acids has been attributed to the ability of the nitrogen atom of the pyridine ring to stabilize charge based on its ring position. The argument here is similar, favorable charge stabilization in an imidazole-2-carboxaldehyde intermediate over that of an analogous imidazole-4-carboxaldehyde intermediate may explain the reactivity difference. Resonance structures III and III′ provide a logical explanation, but not proof, for the isomeric dependency that is experimentally observed: imidazole-4-carboxaldehydes give aldimines when reacted with an amino acid but imidazole-2-carboxaldehydes give ketimines under the same conditions.
2.9. Structure of Ni(L-5Me3Pz)2
The isomeric pyrazole ring provides another opportunity to examine the outcome of the aldimine/ketimine tautomerism. Will the product of a Schiff-base condensation with a pyrazole-3-carboxaldehyde and an amino acid be aldimine or ketimine? The reaction of 5-methylpyrazole-3-carboxaldehyde with amino acids was investigated to determine if its nickel(II) Schiff-base complex would crystallize in the aldimine (analogous to an imidazole-4-carboxaldehyde) or the ketimine (analogous to an imidazole-2-carboxaldehyde) form of the ligand. A resonance structure similar to III for this aldehyde, Figure 14, shows that it has a similar charge stabilization suggesting that ketimine should be formed, as experimentally observed and shown in Figure 15.
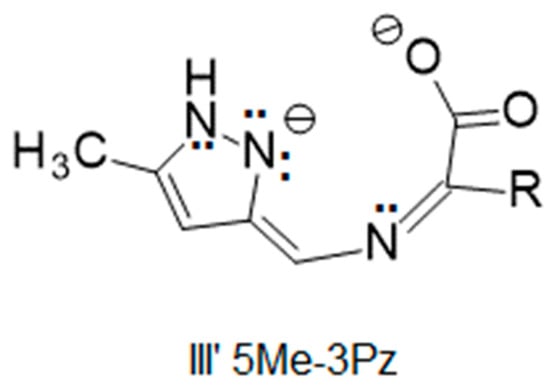
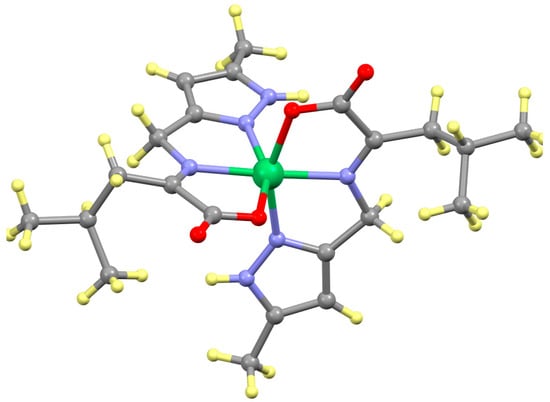
Figure 15.
Structure of Ni(L-5Me3Pz)2. Ligands are in the ketimine tautomer as observed for the 2Im complexes not the aldimine form as observed for the Schiff bases of 4Im.
2.10. Reactivity of Valine and Isoleucine
All of the reactions described here for A, L and F were also conducted for valine(V). In every case, the reaction resulted in the formation of a large amount of a green non-crystalline product within 12–24 h. The product was insoluble in methanol, other common solvents and DMF and DMSO, which may suggest a higher order aggregate, as has been observed in copper(II) complexes of an imidazole-4-carboxaldehyde condensate with phenylalanine. [48] Variations of the reaction were carried out that included altering the source of nickel(II), exclusion of water and exclusion of base to deprotonate the V zwitterion. None resulted in any improvement. In one reaction mixture, a few crystals were found in the bulk precipitate. These were analyzed and found to be the aldimine Schiff base. A repeat of this reaction did not result in observation of crystals. This structure is not reported here, as, to date, we have failed to come up with reproducible synthetic conditions to obtain the product even in low yield. V does form well behaved crystalline Schiff-base condensates (aldimine) with substituted salicylaldehydes, [49] pyridine-4-carboxaldehydes, [50] a pyrazole carboxaldehyde [51] and 5-methyl-4-imidazole carboxaldehyde. [52] A few reactions of isoleucine(I) with imidazole-2-carboxaldehydes were also investigated as both V and I are branched at Cβ and perhaps this feature alters reactivity [53] in an unanticipated manner. The results were the same as with V, non-crystalline insoluble product. Clearly, the reactivity of V and I differs greatly from that of A, L and F and requires more work.
3. Experimental Methods
3.1. General
DL alanine, leucine. phenylalanine, imidazole-2-carboxaldehyde, 4-methylimidazole-2-carboxaldehyde, 4,5-dimethylimidazole-2-carboxaldehyde, nickel(II) acetate tetrahydrate, methanol and 0.10 M potassium hydroxide in methanol were obtained from Aldrich. 5-methyl-1H-pyrazole-3-carboxaldehyde was obtained from ChemBridge. All solvents were of reagent grade and used without further purification. IR and UVvis spectra were obtained on a Perkin Elmer Spectrum Two FT IR and Lambda 365 spectrometer, respectively.
3.2. ESI-MS Were Obtained by Axis Pharm Laboratory, San Diego, CA
The positive ion mode ESI MS of all samples were recorded and the results are in the Supplemental Information.
3.3. X-ray Crystallography
Crystal data for all complexes were collected on a Bruker Smart Apex II 1000 CCD or on an Oxford Gemini diffractometer. All structures were solved using the direct methods program SHELXS-97. [54] All nonsolvent heavy atoms were located using subsequent difference Fourier syntheses. The structures were refined against F2 with the program SHELXL, [55,56] in which all data collected were used including negative intensities. All nonsolvent heavy atoms were refined anisotropically. All hydrogen atoms were located by Fourier difference. Complete crystallographic details are given in Table S1 in the Supplemental Information.
3.4. Synthesis
A standard procedure was used for the synthesis of all complexes illustrated below for Ni(A-2Im)2.
A solution of potassium hydroxide in methanol (10.0 mL, 0.100 M, 0.001 mol) was added to a suspension of DL alanine (0.089 g, 0.001 mol) and water (5.0 mL) in a 100 mL round bottom flask. Methanol (15.0 mL) and imidazole-2-carboxaldehyde (0.096 g, 0.001 mol) were added and the mixture was refluxed for 30 min. The resulting clear to pale yellow solution was poured into a 50 mL beaker and nickel(II)acetate (in methanol) was added (10.0 mL, 0.0500 M nickel(II) acetate tetrahydrate, 0.000500 mol). The solution turned green and deposited Ni(A-2Im)2 in 3–4 days. The sample was gravity filtered and left to air dry (0.118 g, 60% yield).
3.5. Available Data
The following is a list of nickel complexes that provides their CCDC deposition numbers (all structural information is available free of charge from the Cambridge Structural Database) and observed m/e values in the positive ion mode ESIMS to nearest mass. Observed m/e values in the molecular ion region correspond to [NiL2H+], [NiL2Na+], [NiL2K+] and [Ni2L3+]. The IR, UVvis and positive ion ESI MS of each complex is included in the Supplemental Information.
Ni(A-NMe2Im)2 [19]: PADKIR, [NiL2H+] = 419, [NiL2Na+] = 441, [NiL2K+] = 457, [Ni2L3+] = 656
Ni(L-NMe2Im)2: Monoclinic(967170) and Triclinic(967169), [NiL2H+] = 503, [NiL2Na+] = 525. [NiL2K]+ = 541, [Ni2L3+] = 782
Ni(F-NMe2Im)2: 2192114. [NiL2H+] = 571, [NiL2Na+] = 593. [NiL2K+] = 609, [Ni2L3+] = 884
Ni(A-2Im)2: 970635. [NiL2H+] = 391, [NiL2Na+] = 413. [NiL2K+] = 429, [Ni2L3+] = 614
Ni(L-2Im)2: 970637, [NiL2H+] = 475, [NiL2Na+] = 497, [NiL2K+] = 513, [Ni2L3+] = 740
Ni(F-2Im)2: 970636, [NiL2H+] = 543, [NiL2Na+] = 565, [NiL2K+] = not obs, [Ni2L3+] = 842
Ni(L-4Me2Im)2: 2015581, [NiL2H+] = not obs., [NiL2Na+] = 525. [NiL2K+] = 541, [Ni2L3+] = 782
Ni(A-4,5DiMe2Im)2: 2015577, [NiL2H+] = not obs., [NiL2Na+] = 469. [NiL2K+] = 485, [Ni2L3+]= 698
Ni(L-4,5DiMe2Im)2: 2015578. [NiL2H+] = 531, [NiL2Na+]=553. [NiL2K+] = 569, [Ni2L3+] = 824
Ni(L-5Me3Pz)2: 2015579, [NiL2H+] = not obs., [NiL2Na+] = 525. [NiL2K+] = 541, [Ni2L3+] = 782
4. Conclusions
The reaction of several imidazole-2-carboxaldehydes and a pyrazole-3-carboxaldehyde with the anions of A, L and F amino acids results in the ketimine Schiff-base tautomer isolated as its nickel(II) complex. Since the reactions initially produce the aldimine Schiff base (reaction of an AA anion with an aldehyde), the aldimine–ketimine equilibria clearly favors ketimine in these cases, as that is the isolated product. This family of amino acid complexes contains the only known structurally characterized ketimines formed as a result of aldimine to ketimine tautomerization. This Schiff base condensation shows significant isomer selectivity for product as the analogous reactions with an imidazole-4-carboxaldehyde result in formation of the aldimine tautomer. The reaction outcome dependence on the isomer of imidazole carboxaldehyde used, aldimines from imidazole-4-carboxaldehyde and ketimines from imidazole-2-carboxaldehydes, may be attributed to the ability of the latter to better stabilize the likely intermediate, the deprotonated aldimine complex. The reversal of the aldimine to ketimine conversion would regenerate the aldimine. If the required proton transfer occurred in a stereospecific or selective process, it provides a pathway to produce homochiral material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics11100381/s1, Table S1: Crystallographic Data for new Nickel Ketimine Complexes.
Author Contributions
Conceptualization, G.B.; methodology, G.B.; formal analysis, R.J.B. and P.Z.; investigation, G.B. and C.B.; resources, G.B.; writing—original draft preparation, G.B. and C.B.; writing—review and editing, G.B. and C.B.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NASA, NNX15AM13A.
Data Availability Statement
The Supplemental information contains IR, UVvis and ESI MS data for all complexes. Structural data is available free of charge from the CCDC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ohta, H.; Kojima, M.; Iijima, S.; Hayami, S.; Maeda, Y.; Kaizaki, S.; Dahan, F.; et al. An Unprecedented Homochiral Mixed-Valence Spin-Crossover Compound. Angew. Chem. Int. Ed. 2003, 42, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A New Family of Spin Crossover Complexes with a Tripod Ligand Containing Three Imidazoles: Synthesis, Characterization, and Magnetic Properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = Tris [2-(((2-methylimidazol-4-yll)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar] [PubMed]
- Yamada, M.; Ooidemizu, M.; Ikuta, Y.; Osa, S.; Matsumoto, N.; Iijima, S.; Kojima, M.; Dahan, F.; Tuchagues, J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X− = ClO4−, BF4−, PF6−, AsF6−, and SbF6−; H3LMe = Tris [2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 8406–8416. [Google Scholar] [CrossRef] [PubMed]
- Sunatsuki, Y.; Ohta, H.; Kojima, M.; Ikuta, Y.; Goto, Y.; Matsumoto, N.; Iijima, S.; Akashi, H.; Kaizaki, S.; Dahan, F.; et al. Supramolecular Spin-Crossover Iron Complexes Based on Imidazole−Imidazolate Hydrogen Bond. Inorg. Chem. 2004, 43, 4154–4171. [Google Scholar] [CrossRef]
- Brewer, G. Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amones. Magnetochemistry 2020, 6, 28. [Google Scholar] [CrossRef]
- Shahraki, S. Schiff base compounds as artificial metalloenzymes. Colloids Surf. B Biointerfaces 2022, 218, 112727–112743. [Google Scholar] [CrossRef]
- Bos, J.; Roelfes, G. Artificial metalloenzymes for enantioselective catalysis. Curr. Opin. Chem. Biol. 2014, 19, 135–143. [Google Scholar] [CrossRef]
- Erxleben, A. Transition metal salen complexes in bioinorganic and medicinal chemistry. Inorg. Chim. Acta 2018, 472, 40–57. [Google Scholar] [CrossRef]
- Yoshida, N.; Oshio, H.; Ito, T. Supramolecular motifs in metal complexes of Schiff bases. Part 6. Topology of two types of self-assembly of bis-N,O-bidentate Schiff baseligands by copper(II) ions. J. Chem. Soc. Perkin Trans. 2 2001, 1674–1678. [Google Scholar] [CrossRef]
- Alcazar, J.J.; Geue, N.; Valladares, V.; Canete, A.; Perez, E.G.; Garcia-Rio, L.; Santos, J.G.; Aliaga, M.E. Supramolecular Control of Reactivity toward Hydrolysis of 7-Diethylaminocoumarin Schiff Bases by Cucurbit [7]uril Encapsulation. ACS Omega 2021, 6, 10333–10342. [Google Scholar] [CrossRef]
- Hulushe, S.T.; Malan, F.P.; Hosten, E.C.; Lobb, K.A.; Khanye, S.D.; Watkins, G.M. Photo-and thermoresponsive N-salicylideneaniline derivatives: Solid-state studies and structural aspect. New J. Chem. 2022, 46, 20940–20950. [Google Scholar] [CrossRef]
- Martinez, R.F.; Avalos, M.; Babiano, R.; Cintas, P.; Jimenez, J.L.; Light, M.E.; Palacios, J.C. Tautomerism in Schiff bases. The cases of 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-naphthaldehyde investigated in solution and the solid state. J. Org. Biomol. Chem. 2011, 9, 8268–8275. [Google Scholar] [CrossRef] [PubMed]
- Gallant, A.J.; Yun, M.; Sauer, M.; Yeung, C.S.; MacLachlan, M.J. Tautomerization in naphthalenediimines: A keto-enamine Schiff base macrocycle. Org. Lett. 2005, 7, 4827–4830. [Google Scholar] [CrossRef] [PubMed]
- Perona, A.; Sanz, D.; Claramunt, R.M.; Elguero, J. NMR studies of novel Schiff bases derived from L-α-amino methyl esters and 3-hydroxypyridin-4-carboxaldehyde. J. Magn. Reson. Chem. 2008, 46, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Dudek, G.O.; Dudek, E.P. Spectroscopic Studies of Keto Enol Equilibria. IX. N15 Substituded Anilides. J. Am. Chem. Soc. 1966, 88, 2407. [Google Scholar] [CrossRef]
- Herscovitch, R.; Charette, J.J.; De Hoffmann, E. Physicochemical properties of Schiff Bases. II. Tautomeric equilibrium of substituted N-Salicylidene-2-aminopropanes and p-hydroxy-N-benzylidene-2-aminopropane. J. Am. Chem. Soc. 1973, 95, 5135–5140. [Google Scholar] [CrossRef]
- Metzler, C.M.; Cahill, A.; Metzler, D.E. Equilibriums and absorption spectra of Schiff bases. J. Am. Chem. Soc. 1980, 102, 6075–6082. [Google Scholar] [CrossRef]
- Heinert, D.D.; Martell, A.E. Pyridoxine and Pyridoxal Analogs. VI. Electronic Absorption Spectra of Schiff Bases. J. Am. Chem. Soc. 1963, 85, 183–188. [Google Scholar] [CrossRef]
- Blagus, A.; Cincic, D.; Friscic, T.; Kaitner, B.; Stilinovic, V. Schiff bases derived from hydroxyaryl aldehydes: Molecular and crystal structure, tautomerism, quinoid effect, coordination compounds. Maced. J. Chem. Chem. Eng. 2010, 29, 117–138. [Google Scholar] [CrossRef]
- Gasnow, O.A.; Holm, R.H. A proton resonance investigation of equilibria, solute structures, and transamination in the aqueous systems pyridoxamine-pyruvate-zinc(II) and –aluminum(III). J. Am. Chem. Soc. 1969, 91, 5984–5993. [Google Scholar]
- Viedma, C.; Ortiz, J.E.; de Torres, T.; Izumi, T.; Blackmond, D.G. Evolution of solid phase homochirality for a proteinogenic amino acid. J. Am. Chem. Soc. 2008, 130, 15274–15275. [Google Scholar] [CrossRef]
- Klussman, M.; Iwamura, H.; Mathew, S.P.; Wells, D.H., Jr.; Pandya, U.; Armstrong, A.; Blackmond, D.G. Thermodynamic control, of asymmetric amplification in amino acid catalysis. Nature 2006, 441, 621–623. [Google Scholar] [CrossRef]
- Hein, J.E.; Blackmond, D.G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.N.; Holm, R.H. Synthesis of Tautomeric Schiff Base Complexes and Ketimine Aldimine Conversion Rates of Copper(II) Complexes. Inorg. Chem. 1972, 11, 2553–2556. [Google Scholar] [CrossRef]
- Weinstein, G.N.; O’Connor, M.J.; Holm, R.H. Preparation, Properties, and Racemization Kinetics of Copper(II)-Schiff Base-Amino Acid Complexes Related to Vitamin B6 Catalysis. Inorg. Chem. 1970, 9, 2104–2112. [Google Scholar] [CrossRef]
- Yamada, S.; Hongo, C.; Yoshioka, R.; Chibata, I. Method for the Racemization of Optically Active Amino Acids. J. Org. Chem. 1983, 48, 843–846. [Google Scholar] [CrossRef]
- Metzler, D.; Snell, E. Some Transamination Reactions Involving Vitamin B6. J. Am. Chem. Soc. 1952, 74, 979–983. [Google Scholar] [CrossRef]
- Longenecker, J.; Snell, E. The Comparative Activities of Metal Ions in Promoting Pyridoxal-catalyzed Reactions of Amino Acids1. J. Am. Chem. Soc. 1957, 79, 142–145. [Google Scholar] [CrossRef]
- Jung, M.J. Enzyme Inhibition by Amino Acids and their Derivatives. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Chapman and Hall Ltd.: London, UK, 1985; pp. 228–234. [Google Scholar]
- Voet, D.; Voet, J.G.; Pratt, C.W. Amino Acid Metabolism. In Fundamentals of Biochemistry; John Wiley and Sons: New York, NY, USA, 2002; pp. 616–619. [Google Scholar]
- Eichhorn, G.; Dawes, J. The Metal Complexes of Vitamin B6 and Schiff’s Base Derivatives. J. Am. Chem. Soc. 1954, 76, 5663–5667. [Google Scholar] [CrossRef]
- Matsushima, Y.; Martell, A. Pyridoxal Analogs. X. Zinc(II)-Chelate Catalysis of Transamination in Methanol Solution. J. Am. Chem. Soc. 1967, 89, 1331–1335. [Google Scholar] [CrossRef]
- Zabinski, R.F.; Toney, M.D. Metal Ion Inhibition of Nonenzymatic Pyridoxal Phosphate Catalyzed Decarboxylation and Transamination. J. Am. Chem. Soc. 2001, 123, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, T.; Hu, B.; Li, G.; Zhang, H.; Cao, R. Homochiral Supramolecular Compounds Constructed from Amino Acid Derivatives: Syntheses, Structures, Chiroptical, and Photoluminescence Properties. Cryst. Growth Des. 2010, 10, 3051–3059. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Zemba, M. Structural evidence of a ketimine as the product of an amino acid with an aldehyde. An intermediate in the racemization and transamination of amino acids. Inorg. Chem. Commun. 2016, 64, 35–38. [Google Scholar] [CrossRef]
- Iihoshi, T.; Sato, T.; Towatari, M.; Matsumoto, N.; Kojima, M. Enantioselective Assembly Structures of Copper(II) and Zinc(II) Complexes with the 1:1 Condensation Products of Imidazole-4-carbaldehyde Derivatives and DL-Phenylalanine. Bull. Chem. Soc. Jpn. 2009, 82, 458–466. [Google Scholar] [CrossRef]
- Cruz, C.; Gonzalez, C.; Rubio, F.; Erices, J.; Wrighton-Araneda, K.; Cortés-Arriagada, D.; Venegas-Yazigi, D.; Audebrand, N.; Paredes-García, V. Chiral 1D Metal–Organic Materials Based on Cu(II) and Amino Acid Schiff Bases. Cryst. Growth Des. 2022, 22, 237–250. [Google Scholar] [CrossRef]
- Shintoya, S.; Yamauchi, S.; Oishi, T.; Matsumoto, N.; Fujiami, T. Assembly structures of 1:2 nickel(II) complex of 5-methylimidazol-4-yl-methylidene-l-phenylalanine and 1:2 nickel(II) complex of its racemic ligand. Inorg. Chim. Acta 2013, 406, 59–64. [Google Scholar] [CrossRef]
- Brewer, G.; Butcher, R.J.; Zemba, M. Experimental Crystal Structure Determination CCDC 961032, HOVRAM01; CSD Communication: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Yamouchi, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatski, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; Re, N. Synthesis, Structure, Luminescence, and Magnetic Properties of a Single-Ion Magnet“mer”-[Tris(N-[(imidazol-4-yl)-methylidene]-DL-phenylalaninato)terbium(III) and Related“fac”-DL-Alaninato Derivative. Inorg. Chem. 2014, 53, 5961–5971. [Google Scholar] [CrossRef]
- Hamada, D.; Fujinami, T.; Yamauchi, S.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatski, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; et al. Luminescent DyIII single ion magnets with same N6O3 donor atoms but different donor atom arrangements, ‘fac’-[DyIII(HLDL-ala)3]·8H2O and ‘mer’-[DyIII(HLDL-phe)3]·7H2O. Polyhedron 2016, 109, 120–128. [Google Scholar] [CrossRef]
- Sakar, S.; Biswas, S.; Liao, M.S.; Kar, T.; Aydogu, Y.; Dagdelen, F.; Mostafa, G.; Chattopadhyay, A.P.; Yap, G.P.A.; Xie, R.H.; et al. An attempt towards coordination supramolecularity from Mn(II), Ni(II) and Cd(II) with a new hexadentate [N4O2] symmetrical Schiff base ligand: Syntheses, crystal structures, electrical conductivity and optical properties. Polyhedron 2008, 27, 3359–3370. [Google Scholar] [CrossRef]
- Felber, T.; Schafer, T.; Herrmann, H. Five-Membered Heterocycles as Potential Photosensitizers in the Tropospheric Aqueous Phase: Photophysical Properties of Imidazole-2-carboxaldehyde, 2-Furaldehyde, and 2-acetylfuran. J. Phys. Chem. A 2020, 124, 10029–10039. [Google Scholar] [CrossRef]
- Lin, X.; Huang, M.; Lu, T.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Characterization of Imidazole Compounds in Aqueous Secondary Organic Aerosol Generated from Evaporation of Droplets Containing Pyruvaldehyde and Inorganic Ammonium. Atmosphere 2022, 12, 970. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Robichaux, G.T.; Viragh, C. Correlation of nitrogen chirality, R or S, with metal chelate chirality, Δ or Λ, in a series of reduced tripodal Schiff base complexes. A route to total spontaneous resolution. Inorg. Chim. Acta 2014, 410, 171–177. [Google Scholar] [CrossRef]
- Yang, Y.; Hansen, L.; Baldi, A. Suppression of simultaneous Fmoc-His-(Trt)-OH racemization and Nα-DIC endcapping in solid phase peptide synthesis through design of experiments and its implication for an amino acid activation strategy in peptide synthesis. Org. Process Res. Dev. 2022, 26, 2464–2474. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Huang, Y.; Li, J.; Deng, G.; Chen, G.; Xi, Z.; Zhou, C. Suppression of alpha-carbon racemization in peptide synthesis based on a thiol-labile amino protecting group. Nat. Commun. 2023, 14, 5324–5334. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Hashibe, T.; Takahashi, S.; Hagiwara, H.; Matsumoto, N.; Sunatsuki, Y. Enantioselective assembling into tetra- and octanuclear structures by deprotonation of copper(II) complexes of N-[(5-methylimidazol-4-yl)methylidene]-dl-phenylalanine and its l-form ligand. Polyhedron 2012, 33, 209–217. [Google Scholar] [CrossRef]
- Schwarzer, S.; Bohme, U.; Fels, S.; Gunther, B.; Brendler, E. (S)-N-[(2-hydroxynaphthalen-1-yl)methylidene]valine—A valuable ligand for the preparation of chiral complexes. Inorg. Chim. Acta 2018, 483, 136–147. [Google Scholar] [CrossRef]
- Capasso, S.; Giordano, F.; Mattia, C.; Mazzarella, L.; Ripamonti, A. Stereochemistry of model compounds for pyridoxal-catalysed reactions. Crystal structures of the hydrated complexes bis(pyridoxylidene-DL-valinato)nickel(II) and bis(pyridoxylidene-L-valinato)zinc(II). J. Chem. Soc. Dalton Trans. 1974, 2228–2233. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.R.; Sun, C.; Huang, M.; Zhang, H. Synthesis, crystal structures, antibacterial activities, and fluorescence properties of Schiff base ligand and Its nickel(II) complex. Russ. J. Coord. Chem. 2014, 40, 432–438. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J. Catholic University of America: Washington, DC, USA, 2023; manuscript in preparation.
- Wanders, R.J.A.; Duran, M.; Loupatty, F.J. Enzymology of the branched-chain amino acid oxidation disorders: The valine pathway. J. Inherit. Metab. Dis. 2012, 35, 5–12. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELXL—90: Direct methods for larger molecules. Acta Crystallogr. 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M.; Sheldrick, G.M.; Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Refinement; ScienceOpen, Inc.: Burlington, MA, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Analysis; Göttingen University, Instit. Fr Anorganisheche Chemie der Universitat, Tammanstrasse 4, D-3400: Göttingen, Germany, 1998. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).